Abstract
Optogenetic dimerizers are modular domains that can be utilized in a variety of versatile ways to modulate cellular biochemistry. Due to their modularity, many applications using these tools can be easily transferred to new targets without extensive engineering. While a number of photodimerizer systems are currently available, the field remains nascent, with new optimizations for existing systems and new approaches to regulating biological function continuing to be introduced at a steady pace.
Introduction
A fast-evolving area in protein engineering involves the development of systems that use photosensory proteins to control biochemical processes with light. Such efforts have taken two main directions. First, a photosensory domain or protein may be tethered or inserted into a target protein domain, resulting in light-mediated changes in the photosensory domain inducing allosteric or steric changes in target protein activity or interactions. While this approach has been highly effective and can provide tight regulation in a single molecule, often significant protein engineering and optimization is required for each target to achieve robust light control of protein function. A second, more modular approach to regulating protein activity with light uses natural or artificially engineered protein pairs that undergo alterations in binding affinity with light. These paired molecules, termed photodimerizers or light-regulated dimerizer systems, can be affixed to target proteins or domains in diverse ways leading to changes in target protein activity.
Types of Photodimerizer Systems
Photodimerization systems developed to date are all derived from natural photoreceptor domains originating from plants, animals, algae, and fungi, although some have additional engineered components. Such systems consist of two proteins that undergo changes in binding affinity upon light illumination. At least one of the partner pairs is comprised of a light-sensitive photoreceptor domain that undergoes a conformational change with light illumination. The conformational change can lead to altered binding affinity to a partner protein, or altered homomeric interactions [1,2]. Central to the light-induced conformational change is the initial absorption of a photon by the photoreceptor. Each photoreceptor is conjugated to a light-responsive chemical, a chromophore that undergoes a local conformation or oxidation state change upon photon absorption, leading to a structural change within the protein [3,4]. Table 1 describes the different photodimerizer systems that have been developed to date for use in optogenetic regulation, highlighting the chromophore used for each system. In some photoreceptors, including cryptochromes, phytochromes, LOV domains, and BLUF domains, the chromophore consists of a small molecule cofactor conjugated to the protein [3]. In other cases, as with UVR8 or Dronpa, the chromophore is intrinsically contained within the protein amino acid sequence [5–7].
Table 1.
Optogenetic modules for controling protein-protein interactions
| System | Photoreceptor | Co- factor |
Typical/peak
excitation wavelength |
Size (amino acids) |
Approx. lifetime (half-life) |
References | |
|---|---|---|---|---|---|---|---|
| Association | Dissociation | ||||||
| phyB/PIF3 | Arabidopsis thaliana phytochrome B | PΦB, PCB | Pr: 660 nm | Pfr: 740 nm | 621/524 | light inducible | [24] |
| phyB/PIF6 | Arabidopsis thaliana phytochrome B | PΦB, PCB | Pr: 660 nm | Pfr: 740 nm | 908/100 | light inducible | [9] |
| BphP1/PpsR2 | Rhodopseudomonas palustris bacteriophytochrome | Bilive rdin | Pfr: 740 nm | Pr: 650 nm | 732/465 | light inducible, dark reversion 15min | [35] |
| CRY2/CIB1 | Arabidopsis thaliana cryptochrome 2 | FAD | 450 nm | dark | 612/335 498/170 (PHR/CIBN) | 5 min | [25] |
| CRY2PHR/ CRY2PHR (clustering) | Arabidopsis thaliana cryptochrome 2 | FAD | 450 nm | dark | 498 | 5 min | [49,50,59] |
| CRY2olig, CRY2clust | Arabidopsis thaliana cryptochrome 2 | FAD | 450nm | dark | 498 | 5 min | [52,54] |
| TULIPs | Avena sativa Phot1 LOV2 domain | FMN | 450 nm | dark | 153/194 | tunable | [10] |
| iLID/SspB | Avena sativa Phot1 LOV2 domain | FMN | 450 nm | dark | 144/110 | tunable | [63,64] |
| FKF1/GI | Arabidopsis thaliana FKF1 | FMN | 450 nm | dark | 619/1173(wt) 178/1173(N-LOV) | 62.5 h | [27] |
| VVD/VVD | Neurospora crassa Vivid (VVD) | FAD | 450 nm | dark | 150 | tunable | [29] |
| nMag/pMag (Magnets) | Neurospora crassa Vivid (VVD) | FAD | 450nm | dark | 150/150 | tunable | [65] |
| LOVTRAP | Avena sativa Phot1 LOV2 domain | FMN | dark | 450 nm | 143/59 | tunable | [58] |
| EL222 | Erythrobacter litoralis HTCC2594 EL222 | FMN | 450 nm | dark | 208 | < 50 s | [28] |
| Aureochrome | Vaucheria frigida Aureochrome 1 LOV | FMN | 450 nm | dark | 136 | 7 min (tunable) | [47] |
| PixD/PixE | Synechocystis sp. PCC6803 PixD, PixE | FAD | dark | 450 nm | 150/380 | s-min | [66,67] |
| Dronpa | Dronpa | None | 405 nm | 488 nm | 257 | light inducible | [60] |
| UVR8/COP1 | Arabidopsis thaliana UVR8 | None | 280-315nm | N/A | 440/340 | [68] | |
| UVR8/UVR8 | Arabidopsis thaliana UVR8 | None | dark | 280-315nm | 440 | >8 hrs | [55] |
In addition to cofactor differences, the photodimerizer systems vary with respect to size, spectral sensitivity, and reversibility kinetics. The rate of dissociation of protein interactions can range from seconds to days, depending on the system. While all photodimerizer systems described in Table 1 revert back to their dark state spontaneously, some such as phytochromes and Dronpa are optically reversible with light illumination, allowing user-defined control of reversibility. Fast reversal kinetics are particularly important in achieving local, spatially-resolved subcellular control of processes: fast turnoff allows maintenance of a gradient of activated protein that counteracts cellular diffusion. Conversely, systems with slow reversal kinetics are useful for other applications that seek to induce more permanent phenotypes. As in vivo use of these tools can be quite varied, the availability of a variety of photodimerizer systems with different parameters and properties allows tunability and optimization for a specific application.
While all photodimerizer systems result in changes in binding protein interactions upon light absorption, light can alter affinity of interactions in different ways (Figure 1), resulting in increased affinity of partners (light-induced dimerization), decreased affinity of partners (light-induced dissociation), increased or decreased affinity depending on the light wavelength (eg., phytochrome), or in some cases, increased or decreased self-association (light-modulated oligomerization). Each of these approaches has been utilized in different ways for downstream applications, and in some cases have been used in conjunction for complex regulation of biological processes.
Figure 1. Controlling protein interaction with light.
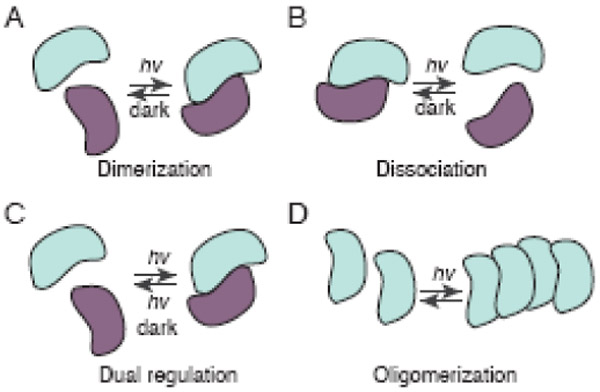
A. Light induced dimerization. Light illumination promotes interaction between two proteins. B. Light induced dissociation. Two proteins interact in dark but are dissociated upon light illumination. C. Light induced dimerization and dissociation. Different wavelengths of light illumination promote either association or dissociation. The ground state is adopted in dark. D. Oligomerization. Light illumination triggers self-association.
Applications of photodimerizer systems
Basic approaches for regulation of protein activity using photodimerizer systems include manipulation of protein localization, reconstitution of split proteins, activation through induced dimerization or oligomerization, and sequestration/release (Fig. 2). Many of these approaches are directly derived from methods originally implemented with chemical dimerization systems (in which protein domains are induced to dimerize by addition of a small molecule that promotes association) [8], but provide advantages of reversibility and spatial control. Below are described some of the central strategies using protein dimerizers for protein photocontrol, highlighting both pioneering and recent studies.
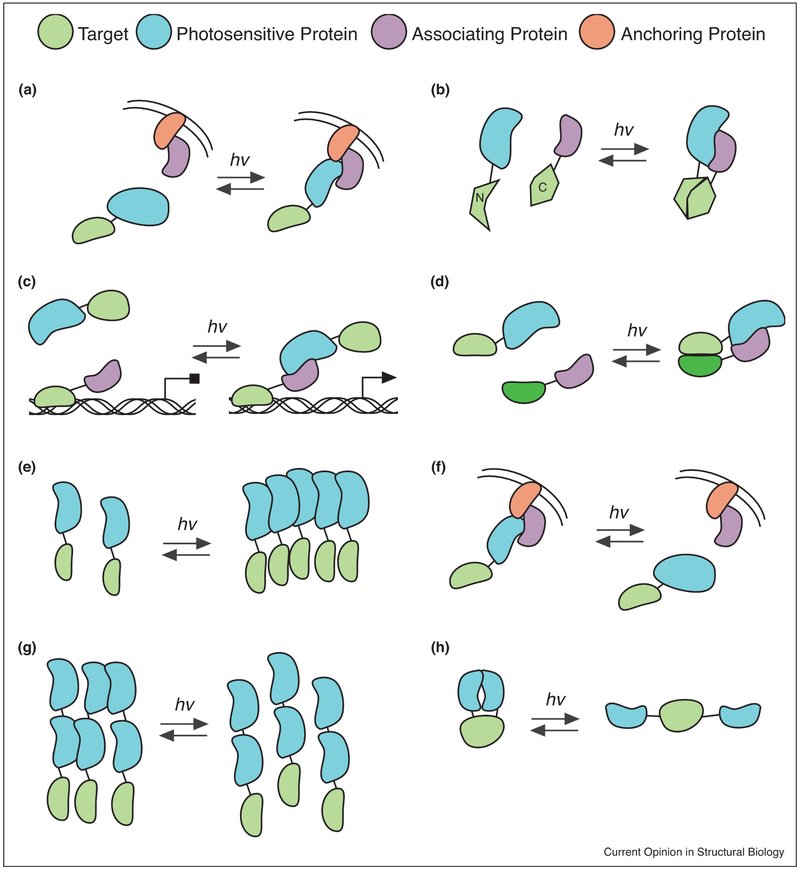
Figure 2. Photodimerizer approaches to regulate protein function.
Schematic showing approaches used to regulate protein activity using photodimerizers. The target protein regulated is colored in green, with photosensory proteins in blue, partner proteins in purple, and anchoring proteins in orange. A) Recruitment to anchored subcellular location. One of the photodimerizer components is fused to a protein or peptide allowing anchored subcellular localization (plasma membrane is shown as an example), while the other is fused to a target protein of interest. Light illumination allows recruitment of the target to the anchored location. B) Reconstitution of a split protein. Activity is achieved by fusing the N-terminus and C-terminus of a split protein to the interacting photodimerizers, allowing functional reconstitution with light. C) Reconstitution of a split transcription factor. Variation of (B), where one of the photodimerizer partners is fused to a DNA binding domain, where it can bind to DNA at a promoter site. The partner photodimerizer is fused to a transcriptional activation domain. Light allows recruitment of the activation domain, resulting in activation of transcription. D) Dimerization of two different proteins. Photodimerizers can be used to bring two different (or the same) target proteins together with light. E) Oligomerization. A target is fused to a photoreceptor (such as CRY2) that undergoes light dependent oligomerization, which can be used to induce or disrupt activity. F) Sequestration/release. A target protein is anchored (sequestered) at an inactive subcellular location, then released with light illumination to allow function. G) Dissociation of protein clusters. Used with light-dissociated dimerizers, protein assembles into clusters that impede trafficking or activity, which are dissociated/dissolved with light. H) Single chain caging. Interacting partners are presented on the same protein chain, along with a target protein or domain. Function of the target protein is sterically or allosterically impeded when photodimerizers are bound, but enabled when partners dissociate.
Manipulating protein localization
Because protein function is intrinsically tied to protein localization, manipulation of protein localization using dimerizers (Fig. 2A) has been a useful approach to regulate function. In initial demonstrations, the PhyB/PIF6 optogenetic system was used to recruit the DHPH catalytic domains of GDP-GTP exchange factors (GEFs) Tiam or intersectin to the plasma membrane (PM), resulting in induction of Rac1 or Cdc42 activity, respectively, with light application [9]. Remarkably, recruitment could be locally controlled, thus light could be steered to specific subcellular locations to regulate activity at these locations. With Tiam DHPH recruitment, the authors demonstrated activation of Rac1 and formation of cellular protrusions extending in the direction of the light [9]. Optogenetic PM recruitment has also been successful for regulating kinase signaling pathways. For example, in yeast, TULIPs and CRY2/CIB1 were used to recruit the MAP kinase scaffold Ste5 to the PM, resulting in activation of the MAP kinase mating pathway [10,11]. Dimerizer tools have also been successfully used to modify lipid signaling networks, through recruiting kinases or phosphatases targeting phosphoinositide species. Idevall-Hagren et al. used CRY2PHR/CIBN to recruit an inositol 5-phosphatase domain or a PI3-kinase domain (inter-SH2 (iSH2) region of the p85α regulatory subunit of PI3-kinase) to the PM, resulting in rapid, local control of phosphoinositide signaling [12]. Recruitment of the inositol 5-phosphatase domain of OCRL resulted in PI(4,5)P2 dephosphorylation and loss of membrane ruffling, while recruitment of PI3-kinase resulted in induction of PI(3,4,5)P3 synthesis and membrane ruffling [12].
As light can be applied at specific times for defined durations, the use of optogenetic tools can allow examination of the responses of signaling networks to different light-entrained inputs. In one study, PhyB/PIF6 dimerizers were used to inducibly recruit the SOS catalytic domain to the PM for different durations and frequencies, leading to activation of the Ras-ERK signaling pathway [13]. In a separate study also examining ERK signaling, CRaf was inducibly recruited to the PM for specific durations using CRY2PHR/CIBN dimerizers [14]. In both studies, the authors demonstrated the powerful ability of such tools to stimulate signaling nodes for precise, user-specified amounts of time, allowing fine-tuned probing of the circuitry and feedback mechanisms inherent to these pathways.
While light focused locally can be used to recruit signaling enzymes to specific regions of the PM (for example as in the PhyB/Pif6 studies noted above [9]), another strategy for local PM recruitment involves tethering one dimerizer to a protein that is differentially localized on the PM, combined with global illumination. Studies by De Renzis et al. used CRY2/CIB1 dimerizers to recruit the catalytic domain of RhoGEF2 to the plasma membrane of Drosophila epithelial cells, resulting in activation of myosin-II in Drosophila embryonic tissue [15,16]. By fusing one of the dimerizers to a protein localized to only the basal PM, they were able to specifically activate myosin-II at the basal side using global light, resulting in disruption of normal cell shape changes that occur during ventral furrow formation in gastrulation [16]. Another approach to increase spatial resolution of PM recruitment involved immobilizing one dimerizer component to both the PM and a secondary anchor, the microtubule cytoskeleton, resulting in slower diffusion of the anchored molecule [17]. Finally, a newly characterized photoreceptor, BcLOV4, undergoes structural changes in light allowing direct recruitment to the PM, showing promise for use in single-component systems for inducible PM recruitment [18].
In addition to recruitment to the PM, studies have used dimerizers to recruit proteins to many other intracellular structures. TULIPs and CRY2PHR/CIBN were used to recruit cytoskeletal motor proteins to cargos, including peroxisomes and mitochondria, resulting in light control of intracellular organelle positioning [19,20]. Dimerizer tools have also been used to localize proteins to subcellular compartments, such as the nucleus or cytoplasm. One study fused phyB(1-908) and a NES to a target protein, resulting in cytosolic localization of the target in dark but induced nuclear targeting by light-triggered interaction with PIF3 (which contains an endogenous NLS) [21]. CRY2/CIBN have also been used to guide cargo into exosomes, by fusing the cargo to CRY2PHR and anchoring CIBN to an exosome-associated tetraspanin protein, CD9 [22]. A recent study compared three different light-induced dimerizer tools, CRY2/CIB1, iLIDs, and Magnets for efficient local recruitment to a precise subcellular location (for example, a specific site on mitochondria) [23]. While each system was effective in recruitment, systems possessing very fast dark reversion rates showed enhanced local recruitment [23]. Using Magnet dimerizers, the authors also demonstrated recruitment to single organelles, including lysosomes or endosomes.
Reconstituting split proteins
Another approach effective with photodimerizers has been to reconstitute split proteins (Figure 2B-C). Proteins are split into two fragments, with each fragment attached to one half of a dimerizing pair. While the fragments do not have sufficient affinity on their own, light induced-dimerization increases the proximity of the of the split protein fragments, leading to reconstitution of activity. The earliest demonstration of reconstitution of split protein fragments using photodimerizers used phyB and PIF3 to regulate a split Gal4 binding domain and activation domain [24]. In this case, red light-induced dimerization of phyB and PIF3 led to reconstitution of activity, while far-red light dissociated fragments resulting in inactivation. Subsequently, similar approaches have been used with other photodimerizers, allowing for precise control of transcription in space and time [25–34]. In a recent publication, control of a split transcription factor in mammalian cells was induced with a bacterial phytochrome BphP1-PpsR2 interaction, allowing transcriptional control by red/far-red light without the requirement of exogenous cofactor (needed with plant phyB-based systems) [35].
While the above examples involved reconstitution of split protein fragments, they did not attempt to reconstitute a split enzyme active site, which can be more difficult. In one of the first examples of split enzyme reconstitution, the phyB/PIF3 system was used in yeast to reconstitute a split protein splicing enzyme, the yeast vacuolar ATPase intein [36]. Cre DNA recombinase was also reconstituted with light allowing recombination at loxP sites in the genome using CRY2/CIB1 photodimerizers [25]. Further optimization yielded an optimized (paCre2.0) system with higher dynamic range, that can be activated by a single pulse of light [37]. A different split Cre system was also developed using nMag/pMag photodimerizers, which was shown to be functional in vivo [38]. A split version of Cas9 (paCas9), allowing induction of targeted genome modifications in mammalian cells, was also developed using nMag/pMag photodimerizers [39], which was optimized for enhanced transcriptional control through additional coactivator recruitment [40]. Split versions of T7 RNA polymerase were also effectively reconstituted with light, using dimerization of Magnets and/or Vivid [41,42]. In many of these studies generating split protein systems, multiple split sites were systematically tested for efficacy, prior to chosing an optimal location. A recent study reports a new computational method to determine effective sites for splitting proteins for inducible assembly, which has the potential to be highly useful for such studies [43].
Protein homodimerization, heterodimerization, and oligomerization
A common mechanism used in Nature to regulate protein activity is through induced homodimerization, heterodimerization, and oligomerization. Using photodimerizers or photooligomerizers, scientists can utilize the same mechanisms to inducibly regulate cell signaling or activity with light (Fig. 2D-E). In one of the first studies to stimulate protein-protein interactions using light, PhyB and PIF3 were used to inducibly recruit a weak-affinity, GDP-bound form of Cdc42 to its effector, WASP (Wiskott-Aldrich Syndrome Protein), stimulating actin assembly [44]. This study demonstrated that two proteins with low affinity could be inducibly forced to associate upon dimerization of the tethered light-interacting molecules.
Photodimerizers have since been used in many ways to promote protein association. Tyrosine kinase receptors, which are naturally activated by ligand-induced dimerization, can be induced to dimerize and signal using photodimerizers attached to intracellular domains, for example with TrkB receptor [45] and FGFR receptor [46], both using CRY2PHR/CIBN photodimerizers. The LOV domains of aureochrome-1 have also been used to dimerize receptor tyrosine kinases (FGFR1, EGFR, and RET) [47]. Downstream kinases involved in signaling have also been regulated using these approaches, such as CRaf and BRaf homodimerization and heterodimerization using CRY2PHR/CIBN [48].
Several groups have exploited light-dependent oligomerization of CRY2 to activate biological processes normally activated by protein oligomerization. CRY2-mediated clustering of TopBP1, a protein that activates ATR kinase, was used to induce the DNA damage response in the absence of DNA damage [49]. Stimulation of β-catenin or Rho GTPase pathways was induced by light-induced clustering of CRY2PHR-LRP6c or CRY2PHR-Rac1/RhoA, respectively [50]. In a follow up study, binding domains fused to CRY2PHR were found to show increased binding avidity upon light-induced clustering, providing a means to target and activate endogenous receptors with light [51]. CRY2olig, a mutant of CRY2PHR with increased oligomerization potential, was used to stimulate actin polymerization by clustering CRY2 fused SH3 domains of Nck [52]. Other mutants of CRY2 have also been found that enhance or even disrupt self-oligomerization properties [53,54]
Using photodimerizers to sequester/release proteins
Dimerization tools have also been used to sequester protein activity, allowing release at a user-specified time (Figure 2F-H). One of the first demonstrations of sequester/release with photodimerizers used UVR8/UVR8 interactions [55]. Tandem dimers of UVR8 were fused to an endoplasmic reticulum-processed protein, VSVG, which led to sequestration and entrapment of fused VSVG in the ER in dark. Light illumination disrupted UVR8 interactions resulting in release of ER sequestration and resumption of trafficking [55]. This work showed the power of using light to inducibly release proteins from a sequestered site.
In a different study, Yang et al. elegantly demonstrated use of phyB/PIF3 to sequester/release proteins [56]. They attached Clb2 to PIF6, allowing recruitment to phyB-tethered cellular locations such as the nucleus or spindle pole body. Sustained recruitment (with red light) resulted in alteration in Clb2-dependent pathways. The group used far-red light to release Clb2 from sequestration at specific times, for specific durations, testing whether recovery of Clb2 function at those times and durations was sufficient for rescue of altered pathways. The same approach was also used to sequester/release Bem1, a protein that regulates activity of Cdc42 in yeast [57]. In the LOVTRAP system, proteins of interest are fused to Zdk, which binds tightly to the dark state of AsLOV2 [58]. Localization of AsLOV2 to a subcellular location (for example, the mitochondrial membrane) allows sequestration of Zdk-fused cargo in dark, which can be released with light. This approach was utilized to regulate Rho GTPase signaling by sequestering constitutively active versions of Vav2, Rac1, or PI3K at the mitochondria in dark and releasing them with light [58].
The CRY2/CIB system has also been used to sequester proteins to block function using the LARIAT (light activated reversible inhibition by assembled trap) system [59]. This approach takes advantage of the propensity of CRY2 to cluster when associated with a multimeric protein, which can also occur through binding to a CIB-multimeric protein fusion. A variety of proteins including Vav2, Tiam1, Rac1, Cdc42, RhoG, and tubulin were clustered and regulated using this approach [59]. CRY2olig has also been used to disrupt function: attachment of CRY2olig to clathrin light chain resulted in disruption of endocytosis with light due to steric effects of clustering [52].
Another approach to sequestering proteins uses internal photodimerizer pair protein-protein interactions to block function (Fig. 2H). For example, one or two monomers of Dronpa were tethered on either side of an enzyme, blocking the catalytic site in dark [60,61]. Upon light-induced monomerization of Dronpa, the enzyme catalytic site was exposed and active. This approach was used on GEF proteins that stimulate Cdc42, the hepatitis C virus NS3-4A protease, Cas9, and various kinases [60–62]. These studies provide powerful examples of the potential of optogenetic tools for acutely modulating protein function by sequester/release approaches.
Conclusions
In summary, light regulated dimerization systems are powerful tools for regulating biochemical function with superb spatial and temporal control. A variety of dimerizer systems have been developed, each with diverse properties for different biological applications. While the blue light dimerizer systems are widely available and have been the most extensively used, new applications and optimized versions of these tools are continuing to emerge. Red and far-red responsive dimerizers have been less characterized and utilized, but emerging systems show significant potential and some advantages over blue light approaches. The studies highlighted here demonstrate some of the different approaches for using such tools to acutely manipulate protein function, altering localization, interactions, domain associations, or active site access. As this relatively recent field evolves, new approaches for manipulating protein activity using dimerizers will emerge, further enhancing the versatility of these powerful tools.
Highlights.
Photodimerizer systems are a recently developed technology with similarity to chemical dimerizer approaches, allowing control of protein-protein interactions with light.
A variety of photodimerizers have been developed, which can be used to induce and/or dissociate interactions with light or control oligomeric states.
Dimerizer systems have primarily been used to manipulate protein localization, reconstitute split proteins, and manipulate dimerization or oligomerization states.
These powerful tools can enable spatial, temporal, reversible, and dose-dependent control of biological processes.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [grant numbers R01GM100225, R21GM126253, UF1NS107710].
Footnotes
Declaration of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ni M, Tepperman JM, Quail PH: Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 1999, 400:781–784. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Yu X, Li K, Klejnot J, Yang H, Lisiero D, Lin C: Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322:1535–1539. [DOI] [PubMed] [Google Scholar]
- 3.Moglich A, Yang X, Ayers RA, Moffat K: Structure and function of plant photoreceptors. Annu Rev Plant Biol 2010, 61:21–7. [DOI] [PubMed] [Google Scholar]
- 4.Van der Horst MA, Hellingwerf KJ: Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res 2004, 37:13–20. [DOI] [PubMed] [Google Scholar]
- 5.Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, et al. : Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 2012, 335:1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D, Hu Q, Yan Z, Chen W, Yan C, Huang X, Zhang J, Yang P, Deng H, Wang J, et al. : Structural basis of ultraviolet-B perception by UVR8. Nature 2012, 484:214–9. [DOI] [PubMed] [Google Scholar]
- 7.Wilmann PG, Turcic K, Battad JM, Wilce MCJ, Devenish RJ, Prescott M, Rossjohn J: The 1.7 Å Crystal Structure of Dronpa: A Photoswitchable Green Fluorescent Protein. J Mol Biol 2006, 364:213–224. [DOI] [PubMed] [Google Scholar]
- 8.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR: Controlling signal transduction with synthetic ligands. Science 1993, 262:1019–1024. [DOI] [PubMed] [Google Scholar]
- **9.Levskaya A, Weiner OD, Lim WA, Voigt CA: Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 2009, 461:997–1001.Pioneering study demonstrating potential of photodimerization tools for control of cell signalling, first demonstration of use in mammalian cells and controlling subcellular localization.
- *10.Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, Sosnick TR, Weiss EL, Glotzer M: TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat Methods 2012, 9:379–384.One of the first engineered blue light photodimerization systems showing robust light-dependent control of cell signaling and biological function.
- 11.Pathak GP, Strickland D, Vrana JDJD, Tucker CLCL: Benchmarking of optical dimerizer systems. ACS Synth Biol 2014, 3:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P: Optogenetic control of phosphoinositide metabolism. Proc Natl Acad Sci U S A 2012, 109:E2316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Toettcher JE, Weiner OD, Lim WA: Using optogenetics to interrogate the dynamic control of signal transmission by the ras/erk module. Cell 2013, 155:1422–34.This work nicely demonstrates the utility of optogenetic dimerizers for fast user-defined temporal manipulation of signaling nodes.
- 14.Aoki K, Kumagai Y, Sakurai A, Komatsu N, Fujita Y, Shionyu C, Matsuda M: Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Mol Cell 2013, 52:529–40. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo E, Quinkler T, De Renzis S: Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat Commun 2018, 9:2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger D, Tardivo P, Nguyen C, De Renzis S: Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J 2018, doi: 10.15252/embj.2018100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Geel O, Hartsuiker R, Gadella TWJ: Increasing spatial resolution of photoregulated GTPases through immobilized peripheral membrane proteins. Small GTPases 2018, doi: 10.1080/21541248.2018.1507411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.**.Glantz ST, Berlew EE, Jaber Z, Schuster BS, Gardner KH, Chow BY: Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids. Proc Natl Acad Sci 2018, 115:E7720–E7727.Describes a previously uncharacterized LOV-domain containing photoreceptor, BcLOV4, that translocates from the cytosol to the plasma membrane upon photostimulation, via binding to anionic membrane phospholipids. For membrane recruitment applications, this molecule could provide a single-component substitute for photodimerizer tools.
- 19.Van Bergeijk P, Adrian M, Hoogenraad CC, Kapitein LC: Optogenetic control of organelle transport and positioning. Nature 2015, 518:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan L, Che D, Zhang K, Ong Q, Guo S, Cui B: Optogenetic Control of Molecular Motors and Organelle Distributions in Cells. Chem Biol 2015, 22:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyer HM, Juillot S, Herbst K, Samodelov SL, Müller K, Schamel WW, Römer W, Schäfer E, Nagy F, Strähle U, et al. : Red Light-Regulated Reversible Nuclear Localization of Proteins in Mammalian Cells and Zebrafish. ACS Synth Biol 2015, 4:951–8. [DOI] [PubMed] [Google Scholar]
- 22.Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park J-H, et al. : Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 2016, 7:12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Benedetti L, Barentine AES, Messa M, Wheeler H, Bewersdorf J, De Camilli P: Light-activated protein interaction with high spatial subcellular confinement. Proc Natl Acad Sci 2018, 115:E2238–E2245.This comprehensive study compares three different photodimerizer tools, iLIDs, Magnets, and CRY2/CIB1 for ability to allow recruitment to a precise subcellular location with high spatial resolution.
- **24.Shimizu-Sato S, Huq E, Tepperman JM, Quail PH: A light-switchable gene promoter system. Nat Biotechnol 2002, 20:1041–1044.Pioneering first demonstration of use of light-induced protein-protein interactions for synthetic control of a biological process (transcription).
- *25.Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL: Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods 2010, 7:973–975.First description of robust blue light photodimerizer system widely adopted for controlling enzymatic activity, cell signalling, and other biological processes.
- 26.Pathak GP, Spiltoir Jl, Höglund C, Polstein LR, Heine-Koskinen S, Gersbach CA, Rossi J, Tucker CL: Bidirectional approaches for optogenetic regulation of gene expression in mammalian cells using Arabidopsis cryptochrome 2. Nucleic Acids Res 2017, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE: Induction of protein-protein interactions in live cells using light. Nat Biotechnol 2009, 27:941–945. [DOI] [PubMed] [Google Scholar]
- 28.Motta-Mena LB, Reade A, Mallory MJ, Glantz S, Weiner OD, Lynch KW, Gardner KH: An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat Chem Biol 2014, 10:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Chen X, Yang Y: Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods 2012, 9:266–9. [DOI] [PubMed] [Google Scholar]
- 30.Polstein LR, Gersbach CA: Light-inducible spatiotemporal control of gene activation by customizable zinc finger transcription factors. J Am Chem Soc 2012, 134:16480–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polstein LR, Gersbach CA: A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol 2015, 11:198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada M, Suzuki Y, Nagasaki SC, Okuno H, Imayoshi I: Light Control of the Tet Gene Expression System in Mammalian Cells. Cell Rep 2018, 25:487–500.e6. [DOI] [PubMed] [Google Scholar]
- 33.Quejada JR, Park S-HE, Awari DW, Shi F, Yamamoto HE, Kawano F, Jung JC, Yazawa M: Optimized light-inducible transcription in mammalian cells using Flavin Kelch-repeat F-box1/GIGANTEA and CRY2/CIB1. Nucleic Acids Res 2017, 45:e172–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nihongaki Y, Yamamoto S, Kawano F, Suzuki H, Sato M: CRISPR-Cas9-based Photoactivatable Transcription System. Chem Biol 2015, 22:169–74. [DOI] [PubMed] [Google Scholar]
- *35.Kaberniuk AA, Shemetov AA, Verkhusha VV: A bacterial phytochrome-based optogenetic system controllable with near-infrared light. Nat Methods 2016, 13:1–15.Promising red/far-red light-controlled optogenetic dimerization system. Advantages of this system include no requirement for addition of exogenous chromophore, ability of red and far-red light to penetrate much farther within cells, and reduced toxicity of red/far-red light.
- 36.Tyszkiewicz AB, Muir TW: Activation of protein splicing with light in yeast. Nat Methods 2008, 5:303–305. [DOI] [PubMed] [Google Scholar]
- 37.Taslimi A, Zoltowski B, Miranda JG, Pathak GP, Hughes RM, Tucker CL: Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat Chem Biol 2016, 12:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawano F, Okazaki R, Yazawa M, Sato M: A photoactivatable Cre–loxP recombination system for optogenetic genome engineering. Nat Chem Biol 2016, doi: 10.1038/nchembio.2205. [DOI] [PubMed] [Google Scholar]
- 39.Nihongaki Y, Kawano F, Nakajima T, Sato M: Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nat Biotechnol 2015, 33:755–760. [DOI] [PubMed] [Google Scholar]
- 40.Nihongaki Y, Furuhata Y, Otabe T, Hasegawa S, Yoshimoto K, Sato M: CRISPR–Cas9-based photoactivatable transcription systems to induce neuronal differentiation. Nat Methods 2017, 14:963–966. [DOI] [PubMed] [Google Scholar]
- 41.Han T, Chen Q, Liu H: Engineered Photoactivatable Genetic Switches Based on the Bacterium Phage T7 RNA Polymerase. ACS Synth Biol 2017, 6:357–366. [DOI] [PubMed] [Google Scholar]
- 42.Baumschlager A, Aoki SK, Khammash M: Dynamic Blue Light-Inducible T7 RNA Polymerases (Opto-T7RNAPs) for Precise Spatiotemporal Gene Expression Control. ACS Synth Biol 2017, 6:2157–2167. [DOI] [PubMed] [Google Scholar]
- *43.Dagliyan O, Krokhotin A, Ozkan-Dagliyan I, Deiters A, Der CJ, Hahn KM, Dokholyan NV: Computational design of chemogenetic and optogenetic split proteins. Nat Commun 2018, 9:4042.First study to set out to define computational parameters for selecting split protein fragments for induced dimerization applications. Authors developed an automated approach, as well as a server and web interface that predicts potential split sites for proteins.
- 44.Leung DW, Otomo C, Chory J, Rosen MK: Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway. Proc Natl Acad Sci U S A 2008, 105:12797–12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang K-Y, Woo D, Jung H, Lee S, Kim S, Won J, Kyung T, Park H, Kim N, Yang HW, et al. : Light-inducible receptor tyrosine kinases that regulate neurotrophin signalling. Nat Commun 2014, 5:4057. [DOI] [PubMed] [Google Scholar]
- 46.Kim N, Kim JM, Lee M, Kim CY, Chang K-Y, Heo W Do: Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem Biol 2014, 21:903–12. [DOI] [PubMed] [Google Scholar]
- 47.Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, Inglés-Prieto Á, Janovjak H: Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J 2014, 33:1713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wend S, Wagner HJ, Müller K, Zurbriggen MD, Weber W, Radziwill G: Optogenetic Control of Protein Kinase Activity in Mammalian Cells. ACS Synth Biol 2014, 3:280–285. [DOI] [PubMed] [Google Scholar]
- 49.Ozkan-Dagliyan I, Chiou Y-Y, Ye R, Hassan BH, Ozturk N, Sancar A: Formation of Arabidopsis Cryptochrome 2 photobodies in mammalian nuclei: application as an optogenetic DNA damage checkpoint switch. J Biol Chem 2013, 288:23244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV, et al. : Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods 2013, 10:249–52. [DOI] [PubMed] [Google Scholar]
- 51.Bugaj LJ, Spelke DP, Mesuda CK, Varedi M, Kane RS, Schaffer DV: Regulation of endogenous transmembrane receptors through optogenetic Cry2 clustering. Nat Commun 2015, 6:6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, Tucker CL: An optimized optogenetic clustering tool for probing protein interaction and function. Nat Commun 2014, 5:4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan L, Hope J, Ong Q, Lou H-Y, Kim N, McCarthy C, Acero V, Lin MZ, Cui B: Understanding CRY2 interactions for optical control of intracellular signaling. Nat Commun 2017, 8:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park H, Kim NY, Lee S, Kim N, Kim J, Heo W Do: Optogenetic protein clustering through fluorescent protein tagging and extension of CRY2. Nat Commun 2017, 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen D, Gibson ES, Kennedy MJ: A light-triggered protein secretion system. J Cell Biol 2013, 201:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Jost AP-T, Weiner OD, Tang C: A light-inducible organelle-targeting system for dynamically activating and inactivating signaling in budding yeast. Mol Biol Cell 2013, 24:2419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jost AP-T, Weiner OD: Probing Yeast Polarity with Acute, Reversible, Optogenetic Inhibition of Protein Function. ACS Synth Biol 2015, 4:1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Vilela M, Winkler A, Tarnawski M, Schlichting I, Yumerefendi H, Kuhlman B, Liu R, Danuser G, Hahn KM: LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat Methods 2016, 13:755–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S, Park H, Kyung T, Kim NY, Kim S, Kim J, Do Heo W: Reversible protein inactivation by optogenetic trapping in cells. Nat Methods 2014, 11:633–6. [DOI] [PubMed] [Google Scholar]
- 60.Zhou XX, Chung HK, Lam AJ, Lin MZ: Optical control of protein activity by fluorescent protein domains. Science 2012, 338:810–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Zhou XX, Fan LZ, Li P, Shen K, Lin MZ: Optical control of cell signaling by single-chain photoswitchable kinases. Science 2017, 355:836–842.This study demonstrated that multiple engineered versions of Dronpa could be used in a single amino acid chain to regulate enzymatic activity through light-dependent intramolecular interactions.
- 62.Zhou XX, Zou X, Chung HK, Gao Y, Liu Y, Qi LS, Lin MZ: A Single-Chain Photoswitchable CRISPR-Cas9 Architecture for Light-Inducible Gene Editing and Transcription. ACS Chem Biol 2018, 13:443–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Guntas G, Hallett RA, Zimmerman SP, Williams T, Yumerefendi H, Bear JE, Kuhlman B: Engineering an improved light-induced dimer (ILID) for controlling the localization and activity of signaling proteins. Proc Natl Acad Sci U S A 2015, 112:112–7.Describes widely-used improved iLID photodimerizer systems for control of protein-protein interactions with blue light. This manuscript and the Zimmerman et. al. manuscript (Ref. 64) describe tunable versions of iLID with lower and higher affinities.
- 64.Zimmerman SP, Hallett RA, Bourke AM, Bear JE, Kennedy MJ, Kuhlman B: Tuning the Binding Affinities and Reversion Kinetics of a Light Inducible Dimer Allows Control of Transmembrane Protein Localization. Biochemistry 2016, 55:5264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *65.Kawano F, Suzuki H, Furuya A, Sato M: Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat Commun 2015, 6:6256.Describes engineering of widely-used Magnet system for control of protein-protein interactions with blue light. To generate Magnets, the authors used screening and site-directed mutagenesis to identify variants of Vvd that cannot homodimerize with each other, but can heterodimerize.
- 66.Dine E, Gil AA, Uribe G, Brangwynne CP, Toettcher JE: Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Syst 2018, 6:655–663.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masuda S, Nakatani Y, Ren S, Tanaka M: Blue Light-Mediated Manipulation of Transcription Factor Activity In Vivo. 2013, doi: 10.1021/cb400174d. [DOI] [PubMed] [Google Scholar]
- 68.Crefcoeur RP, Yin R, Ulm R, Halazonetis TD: Ultraviolet-B-mediated induction of protein-protein interactions in mammalian cells. Nat Commun 2013, 4:1779. [DOI] [PubMed] [Google Scholar]