Abstract
The correct interpretation of hemoglobin (Hb) to identify anemia requires adjusting for altitude and smoking. Current adjustments were derived using data collected before 1985, from low-income preschool-aged children (PSC) in the United States and indigenous men in Peru for altitude, and from White women of reproductive age (WRA) in the United States for smoking. Given the oldness and limited representativeness of these data, we reexamined associations between Hb and altitude and/or smoking using 13 population-based surveys and 1 cohort study each conducted after 2000. All WHO regions except South-East Asia were represented. The dataset included 68,193 observations among PSC (6−59 months) and nonpregnant WRA (15−49 years) with data on Hb and altitude (−28 to 4000 m), and 19,826 observations among WRA with data on Hb and smoking (status or daily cigarette quantity). Generalized linear models were used to assess the robustness of associations under varying conditions, including controlling for inflammation-corrected iron and vitamin A deficiency. Our study confirms that Hb should be adjusted for altitude and/or smoking; these adjustments are additive. However, recommendations for Hb adjustment likely need updating. Notably, current recommendations may underadjust Hb for light smokers and for those residing at lower altitudes and overadjust Hb for those residing at higher altitudes.
Keywords: hemoglobin, altitude, smoking, anemia
Introduction
Hemoglobin (Hb) is the red, iron-containing chromoprotein within a circulating mature erythrocyte that has the primary physiologic function of transporting oxygen to tissues and plays a role in carbon dioxide transport. Anemia, a common global health problem,1 is defined by having an Hb concentration lower than normal. It has been well established that normal Hb concentrations vary with age, sex, and at different stages of pregnancy. Hb cutoffs to define anemia by age, sex, and trimester of pregnancy have remained relatively unchanged since 1968.2
Exposure to chronic hypoxia is also known to increase erythropoiesis.3 The partial pressure of oxygen in the atmosphere decreases with altitude (height above mean sea level, 0 m), thus Hb concentration increases with altitude as an adaptive response to lower oxygen saturation of the blood. Cigarette smoking is also known to cause an increase in Hb concentration, likely mediated by exposure to carbon monoxide, which markedly reduces oxygen-carrying capacity.4,5 To compensate for decreased oxygen delivery, smokers maintain higher Hb concentrations relative to nonsmokers. The increase in Hb is positively associated with the number of cigarettes smoked per day.6 Because of these adaptive increases in Hb, the correct interpretation of Hb concentration to identify anemia requires adjusting for these factors. In 2001, WHO UNICEF UNU established Hb concentration cutoffs to define anemia, which also included adjustments needed to take into account altitude and smoking.7
Adjustments for altitude were published in 1989.8 Based on data from the Centers for Disease Control and Prevention (CDC), Pediatric Nutrition Surveillance System (PedNSS), and data from Hurtado et al.,9 the adjustment for altitude is based on the equation:
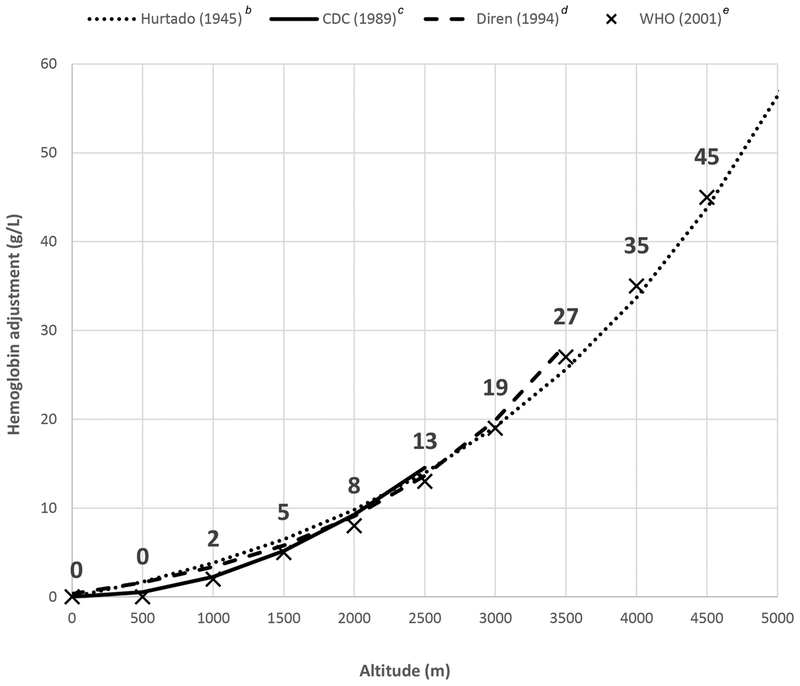
where the adjustment is the value added to the Hb cutoff defining anemia, or subtracted from an individual’s observed Hb concentration using standard cutoffs to define anemia.10,11 PedNSS data were from low-income children, aged 2−4 years (predominately White people), with little to no iron deficiency (ID), enrolled in public health programs from 1974 to the mid-1980s. Children resided at altitudes ranging from <900 to 2150 m above sea level.8 Higher altitude adjustments were derived from Hurtado’s 1945 publication that assessed healthy, indigenous men, 19−48 years of age, living at 3730–4540 m in Peru and men living at sea level in Peru. Hurtado also included data from previous publications in the 1930s from Denver (1500 m), Chile (5335 m), and the United Kingdom (sea level).9 A study of 469 children aged 6−59 months with no evidence of iron or zinc deficiency, residing at 0−3400 m in Ecuador, confirmed similar adjustments.12 Refer to Figure 1 for a summary of equations for Hb adjustment for altitude based on these publications and the current WHO recommended adjustments.
Figure 1.
Summary of previously published equations to adjust hemoglobin for altitude and current WHO adjustment valuesa.
aAdjustment is the amount added to the hemoglobin (Hb) cutoff defining anemia or subtracted from an individual’s observed hemoglobin level.
bBased on the equation Hb_adjustment (g/L) = 6.83 × EXP(0.000445 × altitude) +113.3 and reported as the difference between hemoglobin at a given altitude and hemoglobin at sea level (0 m). Hemoglobin was assessed up to 4540 m.9,12
cBased on the equation Hb_adjustment (g/L) = [(−0.032 × (altitude × 0.0032808) + 0.022 ×(altitude × 0.0032808)2) × 10]. Hemoglobin was assessed up to approximately 2150 m.8
dBased on the equation Hb_adjustment (g/L) = 3.44 × EXP(0.000633 × altitude) + 116.9 and reported as the difference between hemoglobin at a given altitude and hemoglobin at sea level (0 m). Hb was assessed up to 3400 m.12
eWHO cutoffs shown with an X at each (altitude, Hb_adjustment) coordinate with the recommended Hb adjustment value shown above each X.7
Smoking adjustments, published in 1990, were derived from data among White women aged 18−44 years in the United States participating in the Second National Health and Nutrition Examination Survey (NHANES II, 1976−1980).6 Smokers, defined as having ever smoked more than 100 cigarettes and being a current cigarette smoker at the time of the interview, were compared with never smokers and adjustments categorized according to standard cigarette pack size found in the United States (i.e., 20 cigarettes/pack). Smoking adjustments are the same for all population groups (e.g., sex, age, and race). Note, adjustments for altitude and smoking are additive such that a smoker living at a high altitude would have Hb adjusted for both altitude and smoking.
Given the oldness and limited representativeness of data (i.e., geographic, age, and race) used to establish existing adjustments as well as the advancement of scientific understanding of biological aspects affecting Hb,13 there is a need to reevaluate guidance on Hb adjustments for altitude and smoking. Our objective was to examine the association between Hb and altitude and/or smoking among diverse population groups using more recent (since 2000), multinational data in an effort to evaluate current WHO recommendations for adjusting Hb for altitude and smoking.
Methods
Data source and study population
Data for this study were primarily drawn from the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project (www.BRINDA-nutrition.org), a multiagency, multi-country collaboration formed in 2012 as an effort to improve micronutrient assessment and anemia characterization. BRINDA includes datasets from nationally and/or regionally representative household nutrition surveys conducted after 2000 with similar sampling and data collection methodologies that have been described in detail elsewhere.14,15 We used 12 out of 25 surveys (representing 11 countries) that reported data on altitude and/or smoking behaviors. Two additional data sources were also included to increase observations at altitudes >2500 m. This included data from the Guatemalan Sistema de Vigilancia Epidemiológicade Salud y Nutrición (SIVESNU, 2013), nationally representative integrated nutrition and maternal child health surveillance system with altitudes up to 3000 m, and the Nutricion, Immunologia, y Diarrea Infantil study (2013−2015) in El Alto, Bolivia (4000 m), a research study where the primary aim was to assess the effect of global nutritional status on rotavirus vaccine response.16 All data sources included a measure of Hb, iron, and/or vitamin A (VA) status as well as biomarkers of inflammation (C-reactive protein (CRP) and/or alpha-1 acid glycoprotein (AGP)). All subjects with data on Hb, age, and either altitude or smoking status were eligible. Analyses were restricted to preschool-aged children (PSC) aged 6−59 months for analysis of altitude, and women of reproductive age (WRA) aged 15−49 years for analysis of altitude and smoking. We did not examine infants <6 months of age because data were scarce in our dataset, and there remains uncertainty around the appropriate cutoff to define anemia in this age group.17 Women known to be pregnant were excluded. A summary of the 15 surveys included in the analysis can be found in Table 1. In total, our multinational dataset included 68,193 observations among PSC and WRA with data on Hb and altitude, and 19,826 observations among WRA with data on Hb and smoking. All data were deidentified and each survey had appropriate prior ethical approval, thus this study was considered nonhuman subjects research and exempt from Institutional l Review Board review.
Table 1.
Data source location, altitude range, and data availability, sorted by maximum altitude
| Altitude | Biomarker data available | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Year | Min | Max | Group | Hb | SF | sTfR | SR | RBP | CRP | AGP | Smoking data available |
| The United Kingdoma | 2008–2014 | 80 | 80 | PSC: | NA | - | ||||||
| WRA: | X | X | X | X | X | Yes/No # cigarettes/day | ||||||
| Laosa | 2006 | 999 | 1251 | PSC: | X | X | X | X | X | - | ||
| WRA: | X | X | X | X | X | NA | ||||||
| Malawi | 2016 | 52 | 1626 | PSC: | X | X | X | X | X | X | X | - |
| WRA: | X | X | X | X | X | X | X | NA | ||||
| Azerbaijan | 2013 | −27 | 1662 | PSC: | X | X | X | X | X | - | ||
| WRA: | X | X | X | X | X | X | NA | |||||
| Georgiaa | 2009 | 3 | 1944 | PSC: | X | X | X | - | ||||
| WRA: | X | X | X | Yes/No # cigarettes/day | ||||||||
| Papua New Guinea | 2005 | 10 | 2400 | PSC: | X | X | X | X | X | - | ||
| WRA: | X | X | X | X | X | Yes/No | ||||||
| Mexico | 2012 | 0 | 2985 | PSC: | X | X | X | - | ||||
| WRA: | X | X | X | Yes/No # cigarettes/day | ||||||||
| Guatemala | 2013 | 0 | 3034 | PSC: | X | X | X | X | X | X | - | |
| WRA: | X | X | X | X | X | X | Yes/No # cigarettes/day | |||||
| Mexico | 2006 | 1 | 3110 | PSC: | X | X | X | X | - | |||
| WRA: | X | X | X | X | Yes/No # cigarettes/day | |||||||
| Colombia | 2010 | −15 | 3204 | PSC: | X | X | X | X | - | |||
| WRA: | X | X | X | Yes/No | ||||||||
| Afghanistan | 2014 | 325 | 3360 | PSC: | X | X | X | X | X | - | ||
| WRA: | X | X | X | X | X | NA | ||||||
| Ecuador | 2012 | 0 | 3931 | PSC: | X | X | X | X | - | |||
| WRA: | X | X | X | X | Yes/No | |||||||
| Bolivia | 2013–2015 | 4000 | 4000 | PSC: | X | X | X | X | X | X | X | - |
| WRA: | X | X | X | X | X | X | X | NAb | ||||
| The United States | 2003–2006 | NAc | NA | PSC: | - | |||||||
| WRA: | X | X | X | X | X | Yes/No # cigarettes/day | ||||||
Altitude reported as categories (the United Kingdom, all assumed to be 80 m based on average altitude of the 10 most populous areas; Georgia, altitude recoded based on an average altitude within the cluster. Laos reported altitude in three categories).
Smoking data not included in the analysis because only two women reported smoking.
Altitude data collected, but not available in the public use dataset.
AGP, alpha-1 acid glycoprotein; CRP, C-reactive protein; NA, data not available; PSC, preschool-aged children; RBP, retinol binding protein; SF, serum ferritin; SR, serum retinol; sTfR, serum transferrin receptor; WRA, women of reproductive age.
Variables
Hb concentrations were measured at the time of blood collection using point-of-care photometers (HemoCue® all surveys, except in Georgia, which used HumaMeter) or by Beckman Coulter MAXM hematology flow cytometer in the United States. Anemia was considered present when Hb <110 g/L for PSC and Hb <120 g/L for WRA after adjusting for altitude and smoking (subtracting indicated adjustment from measured Hb). Altitude, in meters (m), was reported as a continuous variable except for Laos (altitude coded as 999, 1125, and 1251 m), the United Kingdom (altitude coded as 80 m for all subjects based on the average altitude of the 10 most populous areas18), and Georgia (altitude reported as <1000, 1000−1249, 1250−1749, and 1750−2249 m, but recoded based on average altitude in the cluster19). Method for determining altitude was typically at the cluster level using topographic maps, GIS data, or handheld GIS devices (more common in surveys after 2010). Smoking status was reported as smoker or nonsmoker. A number of cigarettes smoked per day were coded as a continuous variable. Cigarettes represent combustible tobacco products, not chewing tobacco or vaping products. Duration of smoking was not available in any survey. Age was a continuous variable coded in months for PSC, and years for WRA. The survey was a categorical variable.
Presence of ID was determined preferentially by serum ferritin (SF; <12 μg/L for PSC; <15 μg/L for WRA).20 If SF was unavailable, the ID was determined using soluble transferrin receptor (sTfR; >8.3 mg/L based on the Ramco method cutoff,21 for both PSC and WRA) of those categorized with ID, <5% were based on sTfR. VA deficiency (VAD) was considered present if either serum retinol (SR) or retinol binding protein (RBP) was low (SR or RBP <0.70 μmol/L for PSC; SR or RBP <1.05 μmol/L for WRA).22 Indicators of iron and VA status were assessed with and without correcting for inflammation as recommended23 and described in detail elsewhere.24 Briefly, the corrected biomarker value was calculated by subtracting out (e.g., SF) or adding back (e.g., RBP) the inflammation “influence” of CRP and/or AGP using a linear regression approach. Specifically, we corrected SF for CRP and/or AGP (depending on indicator availability) among PSC and WRA; sTfR was corrected for AGP among PSC and WRA; and SR and RBP were corrected for CRP and/or AGP for PSC only. BRINDA regression corrections for inflammation for each of the nutritional biomarkers were conducted separately for each survey. Assay methods used to measure SF, sTfR, SR, RBP, CRP, and AGP have been reported elsewhere.14,16,25
Statistical analysis
We began by examining the association between Hb and altitude with a series of generalized linear models (GLMs) to assess robustness of the association under varying conditions. First, we assessed whether altitude (continuous) had a nonlinear association with Hb as well as effect modification by population group and sex (PSC only). Second, because Hb (uncorrected) and altitude were correlated with ID (PSC: r = −0.16, P < 0.0001 and r = 0.05, P < 0.0001; WRA: r = −0.26, P < 0.001 and r = −0.02, P < 0.001, respectively) and VAD (PSC: r = −0.06, P < 0.0001 and r = −0.04, P < 0.0001; WRA: r = −0.07, P < 0.0001 and r = 0.02, P = 0.03, respectively), we examined the independent association between Hb and altitude by adjusting models for ID and VAD (biomarkers examined both uncorrected or corrected for inflammation). Third, we restricted the study sample only to those with no ID and no VAD (biomarkers corrected for inflammation). We refer to this group as “healthy” and assumed that this group may be less likely to have another micronutrient deficiencies that are correlated with ID or VAD. We considered other indicators of health status, including wasting (weight-for-height Z-score <−2) among PSC or presence of malaria, but after excluding those with ID or VAD, we found few additional cases of wasting (<100) and only one survey (Malawi) with data on ID and VAD included data on malaria based on rapid test kit. Finally, we conducted several sensitivity analyses to assess the robustness of results. We examined associations where (1) we excluded from the “healthy” group any observations with evidence of wasting (PSC) or malaria; (2) we excluded surveys where altitude was approximated (i.e., United Kingdom, Laos, and Georgia); (3) ID was defined using SF only; and (4) we excluded Bolivia data as this was not a population-based survey. All models included (1) age (in months for PSC and years for WRA) because Hb was correlated with age (PSC: r = 0.29, P < 0.001; WRA: r = −0.05, P < 0.0001) and altitude (PSC: r = −0.06, P < 0.001; WRA: r = −0.02, P < 0.0001) in these data due to the population selection criteria (Bolivia at 4000 m, in particular, includes younger PSC and WRA) and(2) survey (i.e., country) to account for potential unmeasured confounding associated with each location and to account for the underlying difference in mean Hb associated with each location. For each model, we report the adjustment equation and proportion of Hb variance explained (R2). The model adjusting for ID and VAD (biomarkers corrected for inflammation) was considered the best model to estimate the independent association between altitude and Hb and used as the proposed equation for estimating cutoffs, which were calculated for each 500-m increase in altitude. We additionally examined survey-specific adjustments among surveys that covered a range of altitude by analyzing the model stratified by the survey.
Among surveys that included a marker of erythropoiesis (i.e., sTfR), we assessed whether those identified as having anemia after applying the new proposed equations also had evidence of increased erythropoiesis. We categorized Hb into five groups—severe, moderate and mild anemia and two groups of normal Hb split to be approximately equal in sample size. We determined whether mean sTfr was significantly elevated among those identified as with anemia compared with the normal Hb groups. sTfR was examined both uncorrected and corrected for inflammation.
Mean Hb concentration for smokers and non-smokers was estimated for each survey. We used GLM to estimate the overall means and adjusted mean difference in Hb between smokers and non-smokers controlling for the survey, and without and with adjusting Hb for altitude using the new equation for WRA. We examined the association between smoking and Hb with smoking categorized as smoker or nonsmoker or with smoking assessed as the number of cigarettes smoked per day. Similar to altitude, we used GLM to assess nonlinearity (number of cigarettes smoked per day), effect modification by altitude (continuous), and the association between smoking and Hb, adjusted for ID. Hb (uncorrected) and smoking (number of cigarettes per day) were correlated with ID (WRA: r = −0.19, P < 0.001 and r = 0.03, P = 0.006, respectively). We did not include VAD because the availability of VAD data was limited among surveys that also included data on smoking; furthermore, the prevalence of VAD was low (<2%) and was not associated with smoking (P = 0.7). We additionally examined associations restricted to WRA considered “healthy” (no ID). For sensitivity analysis, we examined associations where ID was defined using SF only. All models included age, which was also correlated with cigarette number (r = 0.13, P < 0.0001) and survey to account for potential unmeasured confounding associated with each location and to account for the underlying difference in mean Hb associated with each location. For each model, we report the adjustment for smoking and the proportion of Hb variance explained (R2). The model adjusting for ID (biomarkers corrected for inflammation) was considered the best model to estimate the independent association between smoking and Hb and used as the proposed adjustments for smoking. Among surveys that included a marker of erythropoiesis (i.e., sTfR), we assessed whether those identified as having anemia, after applying the new proposed equations for smoking and altitude, also had evidence of increased erythropoiesis.
In a post-hoc analysis, we examined models that included weight-for-length/height Z-score (WFH-Z) among children or body mass index (BMI) among women. While these measures were weakly correlated with Hb (PSC, r = 0.07; WRA, r = 0.03; P < 0.0001 for both), altitude (PSC, P = 0.74; WRA, r = −0.02, P < 0.0001), and cigarette number (WRA, r = 0.05, P < 0.0001), their inclusion had no change on total R2 or the Hb adjustments for altitude or smoking. To maximize analytic sample size and model parsimony, we did not include WFH-Z or BMI in final models.
All analyses were conducted with SAS 9.4 with statistical significance considered at P < 0.05 for main effects and P < 0.15 for interactions. Bolivia included some subjects with repeated measures of Hb (185 children and 231 WRA) collected at visits about 7 months apart. Among the PSC, 78 also had indicators of both ID and VAD measured at the second visit (no repeated ID or VAD measures among WRA). We retained repeated measures in our analyses without controlling for correlated data because these subjects accounted for <0.7% of all observations for any model. We did examine crude models using generalized estimating equations, specifying an exchangeable working correlation matrix to account for correlated data, and found no meaningful differences in effect estimates or statistical significance. Survey weights were not applied to analyses examining associations between Hb and altitude or smoking since the intent was to assess the biological association among the study sample. Once the regression coefficients were calculated, we adjusted for complex survey design in later applications of the data. We assessed differences in the population prevalence of anemia among the BRINDA surveys using the new proposed adjustments for altitude (based on equations) and smoking (based on equation for number of cigarettes, if number unknown then adjustment for a smoker, amount unknown applied) compared with the use of existing adjustments (WHO cut-points and/or 1989 MMWR equation). When estimating the population prevalence of anemia for each survey (except Bolivia, which was not a population-based survey), we accounted for complex survey design by using the Taylor linearization method to obtain an unbiased estimate that incorporates the original sampling weights, strata, and cluster (as applicable).
Results
Hb and altitude
There were 31,967 observations (from 12 countries) for PSC and 36,226 observations (from 13 countries) for WRA with data to assess Hb and altitude. However, data were relatively sparse at altitudes below sea level (0 m) and above 3000 m (Table S1, online only). We tested and confirmed effect modification by population group (P < 0.0001) and a statistically significant quadratic term for altitude among each population group (PSC, P = 0.0004; WRA, P = 0.01). We found no effect modification by sex among PSC (P > 0.75). All subsequent analyses included a quadratic term for altitude and examined PSC and WRA separately.
We report variation in sample size and in age for PSC and WRA and anemia prevalence for each survey across select models assessing altitude in Table S2 (online only). Not all surveys contribute data to every model. Mean age tended to be slightly older among PSC and WRA with data on ID and VAD or who were categorized as healthy. Among PSC, 52% were males (data not shown). Prevalence of anemia was generally lower among PSC with data on ID and VAD or who were categorized as healthy but varied among WRA with data on ID and VAD or who were categorized as healthy.
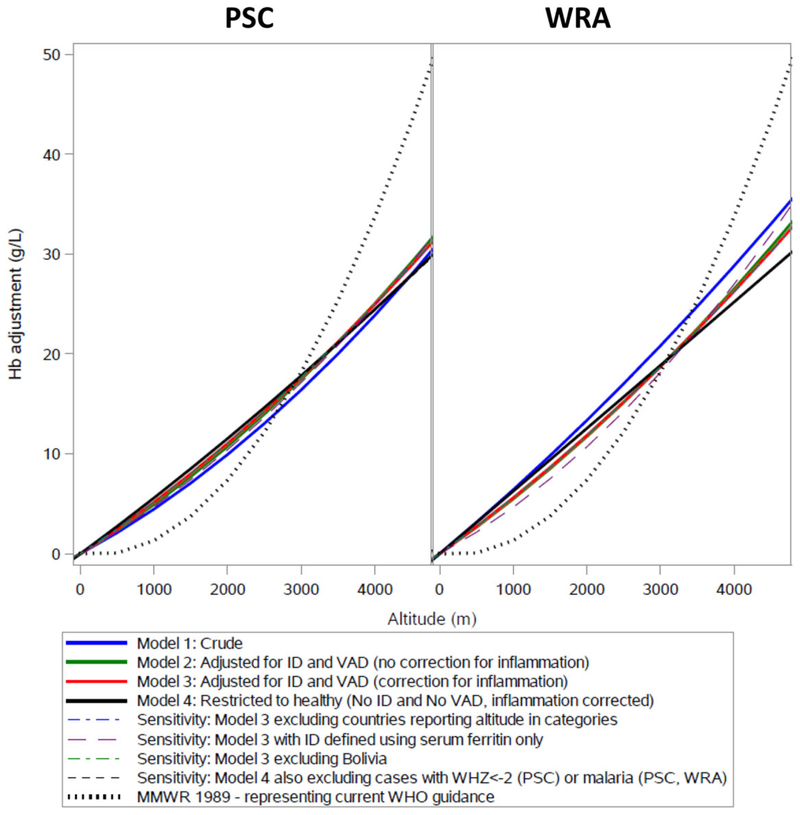
Adjustment equations and R2 for each model are reported in Table S3 (online only) and visualized in Figure 2. Across all models, the proportion of Hb variance explained by models was higher among WRA. All models, including sensitivity analyses, had a similar fit with slightly more variation among WRA. Results were similar when models 1−3 were analyzed with the same subjects (n = 12,887 PSC; n = 13,889 WRA; data not shown). Hb adjustments for each 500-m increase in altitude are reported in Table 2. Adjustment criteria according to WHO cutoffs and the 1989 CDC equation are also provided for comparison. Survey-specific associations are visualized in Figure S1 (online only) and adjustments are provided in Table S4 (online only). Using the new proposed equations, we confirmed that mean sTfR was highest among those categorized as having anemia (Fig. S2, online only).
Figure 2.
Hemoglobin adjustment for altitude for preschool-age children (PSC) and women of reproductive age (WRA) by model. Note: All models adjusted for age and the survey. ID and VAD biomarkers corrected for inflammation using BRINDA method.23,24
Table 2.
Proposed adjustmentsa to hemoglobin (g/L) by 500-m increments of altitude by population group
| Altitude (m) | WHO cutoffsb |
MMWR 1989 equationc |
PSCd | WRAe |
|---|---|---|---|---|
| −50 to −1 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 |
| 1–499 | 0 | 0 | 1 | 1 |
| 500–999 | 0 | 1 | 4 | 4 |
| 1000–1499 | 2 | 2 | 7 | 7 |
| 1500–1999 | 5 | 5 | 10 | 10 |
| 2000–2499 | 8 | 10 | 13 | 13 |
| 2500–2999 | 13 | 15 | 16 | 17 |
| 3000–3499 | 19 | 22 | 20 | 20 |
| 3500–3999 | 27 | 29 | 24 | 24 |
| 4000–4499 | 35 | 38 | 28 | 28 |
Adjustment is amount (g/L) added to the hemoglobin cutoff defining anemia, or subtracted from an individual’s observed hemoglobin level.
WHO cutoffs published as the lowest altitude value in range (e.g., 1500 m for the 1500−1999 range).2
MMWR cutoffs calculated using Hb_adjustment (g/L) = [(−0.032 × (altitude × 0.0032808) + 0.022 × (altitude × 0.0032808)2) × 10], where altitude is based on the midpoint of altitude range (e.g., 1750 m for the 1500−1999 range).8,10
Proposed cutoffs calculated using Hb_adjust_altitude (g/L) = (0.0048108 × altitude) + (0.0000004 × altitude2), where altitude is based on the midpoint of altitude range (e.g., 250 m for the 1−499 range).
Proposed cutoffs calculated using Hb_adjust_altitude (g/L) = (0.0052792 × altitude) + (0.0000003 × altitude2), where altitude is based on the midpoint of altitude range (e.g., 250 m for the 1−499 range).
Note: Current WHO recommendations and adjustments based on the MMWR 1989 equation provided for comparison.
PSC, preschool-aged children (6−59 months); WRA, women of reproductive age (15−49 years).
Hb and smoking
Among WRA, there were 19,826 observations (from nine surveys) with data to assess Hb and smoking status; 14.3% were smokers. Women who reported being a current smoker were older than nonsmokers (31.8 (SD = 10.4) versus 29.6 (SD = 10.4) years old, P < 0.0001). Eight surveys also included data on the number of cigarettes smoked per day (9724 observations; 22.0% categorized as smokers). Among smokers, women smoked 6.5 (SD = 7.9) cigarettes per day on average. The majority (63.9%) reported smoking 1−5 cigarettes per day. Only 3.7% reported smoking more than 20 cigarettes per day. Detailed smoking characteristics for each survey are reported in Table 3. We report variation in sample size and anemia prevalence for each survey across select models assessing smoking in Table S5 (online only). There were no meaningful differences in mean age. Prevalence of anemia was generally lower among WRA who were categorized as healthy (no ID).
Table 3.
Smoking characteristics among women of reproductive age for each country
| Observations with data on smoking (yes/no) |
Smokera | Observations with data on cigarette quantity |
Among smokers, distribution of the number of cigarettes smoked per day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n) | (% yes) | (n) | Mean (SD) | 1–5 (%) | 6–10 (%) | 11–15 (%) | 16–20 (%) | >20 (%) | |
| Colombia | 9674 | 4.1 | 0 | - | - | - | - | - | - |
| Ecuador | 2225 | 21.5 | 2002 | 1.9 (2.2) | 94.8 | 4.4 | 0.3 | 0.5 | 0.0 |
| Georgia | 1710 | 3.3 | 1710 | 12.9 (6.9) | 12.3 | 43.9 | 10.5 | 29.8 | 3.5 |
| Guatemala | 1616 | 6.8 | 1616 | 2.3 (2.5) | 97.3 | 0.9 | 0.0 | 1.8 | 0.0 |
| Mexico (2006) | 939 | 31.8 | 846 | 3.5 (6.2) | 81.1 | 13.1 | 2.4 | 1.9 | 1.5 |
| Mexico (2012) | 1110 | 42.6 | 1018 | 2.2 (3.4) | 90.6 | 7.1 | 0.8 | 1.6 | 0.0 |
| Papua New Guinea | 759 | 21.5 | 755 | 5.0 (5.9) | 81.1 | 8.8 | 1.3 | 6.9 | 1.9 |
| The United Kingdom | 896 | 25.8 | 886 | 12.9 (7.6) | 16.7 | 30.3 | 21.7 | 19.9 | 11.3 |
| The United States | 897 | 69.8 | 891 | 11.3 (9.3) | 33.9 | 30.2 | 9.8 | 18.6 | 7.6 |
| Total | 19,826 | 14.3 | 9724 | 6.5 (7.9) | 63.9 | 17.1 | 5.9 | 9.4 | 3.7 |
Unweighted prevalence represents the proportion of smokers in the data and not population prevalence.
NA, data not available.
Mean Hb concentration was generally higher among smokers compared with nonsmokers across all surveys although the difference was not always statistically significant (Fig. S3, online only). Overall, mean Hb was 3.3 g/L (95% CI 2.5−4.0) higher among smokers compared with nonsmokers controlling for the survey, and 2.3 g/L (95% CI 1.6−2.9) higher after additionally adjusting Hb for altitude.
We tested and confirmed a statistically significant quadratic term for cigarette quantity (P < 0.001). All subsequent analyses examining cigarette quantity as a continuous variable included a quadratic term for cigarette quantity. There was no evidence of effect modification between altitude and smoking quantity (P value for interaction >0.2). Therefore, we used Hb adjusted for altitude (based on the proposed equation for WRA) so that any adjustments for smoking be considered independent of, and in addition to, altitude.
Smoking adjustments based on mean difference in Hb between smokers and nonsmokers and by cigarette quantity are reported in Table 4. Model R2 ranged from 0.09 to 0.20 with models controlling for ID having the highest R2 (data not shown); however, the calculated adjustments for smoking were the same regardless of model specification. We confirmed that mean sTfR was highest among those categorized with anemia after adjusting for altitude and smoking (Fig. S2, online only).
Table 4.
Proposed adjustmentsa to hemoglobin for smoking status and cigarettes per day
| WHO adjustments (g/L)b | Proposed Hb adjustment (g/L)c | ||
|---|---|---|---|
| Smoker, quantity unknown | 3 | Smoker, quantity unknown | 2 |
| Cigarettes (packs) per day | Cigarettes per day | ||
| 0 | 1–5 | 2 | |
| 6–10 | 4 | ||
| 3 | 11–15 | 5 | |
| 16–20 | 6 | ||
| 20–39 (1–2) | 5 | >20 | 7 |
| ≥40 (>2) | 7 | ||
Adjustment is the amount added to the hemoglobin cutoff defining anemia, or subtracted from an individual’s observed hemoglobin level.
WHO cutoffs published as packs per day.2
Adjustments based on Hb adjustment (g/L) = (0.4565 × cigarette_number) + (−0.0078 × cigarette_number2), solved for the upper end of each interval (e.g., 5 for the 1−5 range). >20 solved using 30 cigarettes/day.
Note: Current WHO recommendations provided for comparison.
Comparison of proposed adjustments to current adjustments
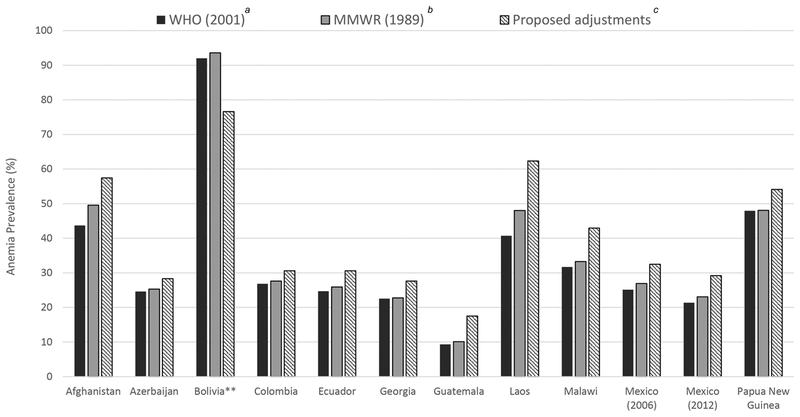
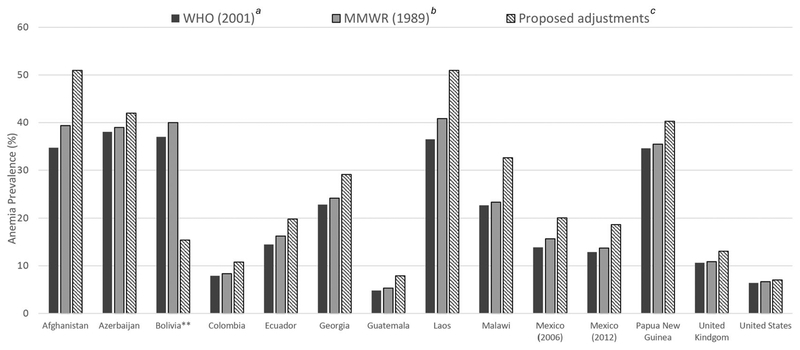
We estimated the population prevalence of anemia for each survey applying the new proposed adjustments for altitude and smoking and compared prevalence with that estimated when applying either current WHO cut-points or 1989 MMWR equation for altitude or the WHO cut-points for smoking (PSC, Fig. 3; WRA, Fig. 4). Relative to current WHO cutoffs and 1989 MMWR equation, the population prevalence of anemia was generally higher using the proposed adjustments across all surveys for both PSC and WRA. Among population-based surveys only adjusting for altitude (i.e., all PSC and WRA in Afghanistan, Azerbaijan, Laos, and Malawi), the increase in anemia prevalence across adjustment criteria ranged from 3 to 22 (Laos) percentage points. Among population-based surveys only adjusting for smoking (i.e., WRA in the United Kingdom and the United States), the increase in anemia prevalence across adjustment criteria ranged from 1 to 2 (the United Kingdom) percentage points. In Bolivia, a research study where participants all resided at 4000 m, prevalence of anemia decreased with application of proposed adjustments by 15–25 percentage points. Application of the WHO cutoffs consistently resulted in lower prevalence of anemia relative to the 1989 MMWR equation because the WHO cutoff is equivalent to the adjustment at lowest altitude in the given altitude category range (e.g., an adjustment of 2 for 1000−1499 m, an adjustment of 5 for 1500−1999 m, etc.), whereas the equation adjusts for the exact altitude reported.
Figure 3.
Prevalence of anemia among preschool-aged children (6−59 months) based on different criteria to adjust for hemoglobin for altitude.* Note: *All adjustments subtracted from individual’s Hb concentration. Anemia defined as Hb < 110 g/L. **Prevalence weighted to be nationally representative, except for Bolivia, as it was not sampled to be a representative survey.
aWHO (2001) based on WHO recommended cutoffs.2
bMMWR (1989) based on the equation Hb_adjust_altitude (g/L) = [(−0.032 ×(altitude × 0.0032808) + 0.022 (altitude × 0.0032808)2) ×10].8
cProposed adjustment, Hb_adjust_altitude (g/L) = (0.0048108 × altitude) + (0.0000004 × altitude2).
Figure 4.
Prevalence of anemia among women of reproductive age (15−49 years) based on different criteria to adjust for hemoglobin for altitude and smoking.* Note: *The United Kingdom and the United States adjusted for smoking only; Afghanistan, Azerbaijan, Bolivia, Laos, and Malawi adjusted for altitude only. All adjustments subtracted from the individual’s Hb concentration. Anemia defined as Hb < 120 g/L. **Prevalence weighted to be nationally representative, except for Bolivia, as it was not sampled to be a representative survey.
aWHO (2001) based on WHO recommended cutoffs for altitude and smoking.2
bMMWR (1989) based on the equation Hb_adjust_altitude (g/L) = [(−0.032 (altitude ×0.0032808) + 0.022 × (altitude × 0.0032808)2) × 10] and the WHO recommended cutoffs for smoking.2,10
cProposed adjustments, Hb_adjust_altitude (g/L) (0. 0052792 × altitude2); Smoker, cigarette quantity known Hb_adjust_smoking (g/L) = (0.4459 ×cigarette_number)+(−0.007× cigarette_number2); Smoker, cigarette quantity unknown Hb_adjust_smoking (g/L) = 2.
Discussion
It has been well established that Hb concentrations increase as an adaptive response to hypoxic conditions, such as residing at high altitudes and smoking cigarettes. The results of this multinational study among approximately 32,000 PSC and 35,000 nonpregnant WRA confirm the need to adjust Hb concentration for altitude and/or smoking for the correct interpretation of Hb concentration to identify anemia. Further, these adjustments are additive. However, our findings suggest that current WHO recommendations for the adjustment of Hb for altitude and/or smoking likely need updating. Notably, current recommendations may underadjust Hb for light smokers (<10 cigarettes per day) and for those residing at lower altitudes (<2000 m) and overadjust Hb for those residing at higher altitudes (>3000 m).
Our findings also demonstrate that the association between Hb and altitude is modified by population group (young children versus WRA), suggesting that adjustments to Hb may need to be tailored to different population groups. Population-specific adjustments increase the complexity of adjustments, particularly in the clinical context. The difference in the adjustment made to Hb at a given altitude between PSC and WRA in our study was no more than 3 g/L suggesting that common criteria for all population groups may be reasonable and more practical, but this needs confirmation. It should be noted that population surveys often sample participants from the same cluster and households, and some of the WRA could have been mothers or related to the PSC. Thus, our population groups are not entirely independent of each other. For Bolivia, women and children were enrolled as dyads. Additional data confirming associations between Hb and altitude among other population groups, including school-age children, pregnant women, men, and the elderly, as well as additional modeling determining the degree of misclassification in anemia status using a combined population-group criteria would assist with determining the appropriateness of one common criterion across all population groups.
We estimated the population prevalence of anemia using different criteria for adjusting Hb and found differences in anemia prevalence between the currently recommended WHO cutoffs and the direct equation (1989 MMWR). Thus, better guidance on the preferred adjustment method is warranted. Guidance should specify the utility of using cutoffs versus equations for clinical practice and for population-based surveys. Direct use of prediction equations gives a more exact adjustment of Hb concentration for altitude and/or smoking, but one has to balance this with ease of practical application and the technical capacity of the setting. Further, the current WHO recommendation describes the cutoff for altitude at a given altitude (e.g., 5 g/L at 1500 m).2 Clearer guidance is needed as to whether the adjustment is applied to all altitudes between cutoffs (e.g., Fig. 1: the 5 g/L adjustment is applied to 1500−1999 m) or whether the adjustment represents the midpoint and is applied to the surrounding altitudes (e.g., Fig. 1: the 5 g/L adjustment is applied to 1250−1749 m); the latter approach more closely resembles the adjustment based on the 1989 MMWR prediction equation.
Our study had several strengths. We were able to examine associations with Hb using population-based data (with the exception of Bolivia) from multiple countries; all WHO Regions, except South East Asia, were represented. This enhanced our ability to examine Hb across a wide range of altitudes with data encompassing multiple racial and ethnic groups and levels of socioeconomic status. Similarly, with multicountry data, we were able to examine the additive effect of smoking across a wide range of altitudes and the associations with smoking account for heterogeneity in the smoke delivery (e.g., carbon monoxide) of local tobacco products.26 In addition, we were able to control for individual iron and VA deficiency. Micronutrient deficiencies are associated with Hb and may vary by altitude due to the context of location, including diet quality and composition. Controlling for micronutrient status permits the assessment of the independent contribution of altitude and smoking on Hb concentration. Our findings were robust across multiple model specifications and sensitivity analyses. Furthermore, erythropoietic activity is known to increase in response to low oxygen concentration due to low Hb and functional ID.27 We found that classification of anemia independent of altitude and/or smoking (i.e., adjusted using the new proposed equations) was associated with significantly higher levels of sTfR (a marker of erythropoiesis), indicating that those with anemia were correctly identified.
Our study has limitations. First, we were unable to explore whether the association between Hb and altitude or smoking is modified by race/ethnicity or other population groups (e.g., adolescents, men, and pregnancy) as these data are not currently harmonized across datasets. Second, data among individuals residing at altitudes above 3000 m were limited in our study. However, our finding that current WHO recommendations may overadjust Hb for those who reside at high altitudes has also been reported in a recent study of children 6−59 months residing in Peru.28 In this paper, the regression equation resulted in altitude adjustments about 2−4 g/L lower than we report. Studies also report genetic or epigenetic adaptions to high-altitude hypoxia that may differ among indigenous populations residing in high-altitude Andean, Tibetan, and Ethiopian regions.29–31 The increase in Hb concentrations due to altitude has been shown to be higher in high-altitude Andean compared with high-altitude Tibetan and Ethiopian populations.30 Because ongoing debate remains whether adaptation to high altitude hypoxia varies by genetic descent and duration of residence, and our high-altitude data predominantly came from populations in the Andean region (Bolivia and Ecuador), our findings warrant confirmation in other high-altitude regions. In general, we observed the association between altitude and Hb to be similar across surveys among the two population groups examined. There also remains debate as to whether the threshold to define anemia (or polycythemia) based on health outcomes differs between those who reside at low and high altitudes.32,33 Our study was not designed to examine differences in Hb concentration and health outcomes by altitude. Third, smoking status and cigarette quantity were self-reported and there was no information on the duration of smoking. Self-reported smoking is known to be underreported,34,35 which may lead in an overestimate of adjustment at lower smoking levels of smoking. Less than 4% of women reported being heavy smokers (>20 cigarettes per day), thus additional research is likely needed to confirm the adjustment for this group. We also had no data on e-cigarette use. Fourth, some study subjects were missing data on key variables, such as inflammation-corrected ID and VAD reducing sample size for models. Subjects with complete data tended to be older, and fewer children and more women were categorized with anemia, thus missing data may have introduced selection bias. However, associations and adjustments were similar across models indicating the impact of selection bias may be minimal. Finally, our models accounted for less than half of the variation in Hb. It is possible that we have not controlled for all sources of confounding, including obstructive pulmonary diseases, exposure to air pollution, or the status of other micronutrients. Folate and B12, in addition to iron, are required for hematopoiesis and may be correlated with altitude due to diet quality and composition.
Anemia is prevalent worldwide. Findings from this analysis will inform the current review of global guidelines on the use of altitude and smoking−adjusted Hb concentrations for the assessment of anemia.
Supplementary Material
Figure S1. Association between altitude and hemoglobin fit with a penalized B-spline with three knots by survey for preschool-aged children (PSC) and women of reproductive age (WRA).
Figure S2. Mean (95% CI) inflammation-corrected soluble transferrin receptor (sTfR) concentration by hemoglobin adjusted for altitude using the proposed equations to adjust hemoglobin (Hb) for altitude and smoking.
Figure S3. Mean (95% confidence interval) hemoglobin concentrations by smoking status for each country and for all countries combined (Panel A: Crude difference; Panel B, Hb adjusted for altitude).
Table S1. Maximum sample size of hemoglobin observations by altitude.
Table S2. Variation in sample size and in study population characteristics for each country across select models assessing hemoglobin and altitude.
Table S3. Equations for adjustment to hemoglobin (g/L) for altitude by model. Highlighted model indicated the model used for proposed adjustments for each population group.
Table S4. Proposed adjustmentsa to hemoglobin (g/L) by 500-m increments of altitude by survey for each population group where data were available for analysis of Model 3 (adjusted for inflammation-corrected ID and VAD). Adjustments shown only for the range of altitude assessed within each survey. Current WHO recommendations2 and proposed adjustments based on the analysis of all surveys combined provided for comparison.
Table S5. Variation in sample size and in study population characteristics for each country across select models assessing hemoglobin and smoking status (smoker, nonsmoker) or number of cigarettes smoked per day.
Statement.
This manuscript was presented at the World Health Organization (WHO) technical consultation “Use and Interpretation of Haemoglobin Concentrations for Assessing Anaemia Status in Individuals and Populations,” held in Geneva, Switzerland on November 29−30 and December 1, 2017. This paper is being published individually but will be consolidated with other manuscripts as a special issue of Annals of the New York Academy of Sciences, the coordinators of which were Drs. Maria Nieves Garcia-Casal and Sant-Rayn Pasricha. The special issue is the responsibility of the editorial staff of Annals of the New York Academy of Sciences, who delegated to the coordinators preliminary supervision of both technical conformity to the publishing requirements of Annals of the New York Academy of Sciences and general oversight of the scientific merit of each article. The workshop was supported by WHO, the Centers for Disease Control and Prevention (CDC); the United States Agency for International Development (USAID); and the Bill & Melinda Gates Foundation. The authors alone are responsible for the views expressed in this paper; they do not necessarily represent the views, decisions, or policies of the WHO. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors, publisher, or editorial staff of Annals of the New York Academy of Sciences.
Acknowledgments
This work was commissioned by the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development of the World Health Organization (WHO), Geneva, Switzerland.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Competing interests
As part of their routine work duties, the authors provide technical assistance to countries in the design, training, implementation, data management, analysis, and dissemination of public health population-based surveys, including collection of Hb and anemia.
Supporting information
Additional supporting information may be found in the online version of this article.
References
- 1.Kassebaum NJ 2016. The global burden of anemia. Hematol. Oncol. Clin. North Am 30: 247–308. [DOI] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization). 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: WHO: Accessed March 17, 2019. http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 3.Haase VH 2010. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Renal Physiol 299: F1–F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leifert JA 2008. Anaemia and cigarette smoking. Int. J. Lab. Hematol 30: 177–184. [DOI] [PubMed] [Google Scholar]
- 5.Northrop-Clewes CA & Thurnham DI. 2007. Monitoring micronutrients in cigarette smokers. Clin. Chim. Acta 377: 14–38. [DOI] [PubMed] [Google Scholar]
- 6.Nordenberg D, Yip R & Binkin NJ. 1990. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA 264: 1556–1559. [PubMed] [Google Scholar]
- 7.WHO, UNICEF & UNU (World Health Organization). 2001. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers. Geneva: WHO: Accessed March 17, 2019. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.html. [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1989. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb. Mortal. Wkly. Rep 38: 400–404. [PubMed] [Google Scholar]
- 9.Hurtado A, Merino C & Delgado E. 1945. Influence of anoxemia on the hemopoietic activity. Arch. Intern. Med 75: 284–323. [Google Scholar]
- 10.Sullivan KM, Mei Z, Grummer-Strawn L, et al. 2008. Haemoglobin adjustments to define anaemia. Trop. Med. Int. Health 13: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 11.Yip R 1993. Altitude and hemoglobin elevation: implications for anemia screening and health risk of polycythemia In Hypoxia and the Brain: Proceedings of the 8th International Hypoxia Symposium, Burlington, VT. [Google Scholar]
- 12.Dirren H, Logman MH, Barclay DV, et al. 1994. Altitude correction for hemoglobin. Eur. J. Clin. Nutr 48: 625–632. [PubMed] [Google Scholar]
- 13.Sankaran VG & Weiss MJ. 2015. Anemia: progress in molecular mechanisms and therapies. Nat. Med 21: 221– 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namaste SM, Aaron GJ, Varadhan R, et al. 2017. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr 106: 333S–347S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchdev PS, Namaste SM, Aaron GJ, et al. 2016. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv. Nutr 7: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke RM, Rebolledo PA, Fabiszewski de Aceituno AM, et al. 2017. Early deterioration of iron status among a cohort of Bolivian infants. Matern. Child Nutr 10.1111/mcn.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domellof M, Dewey KG, Lonnerdal B, et al. 2002. The diagnostic criteria for iron deficiency in infants should be reevaluated. J. Nutr 132: 3680–3686. [DOI] [PubMed] [Google Scholar]
- 18.2014. Elevation and elevation maps of cities/towns/villages in UK. Accessed October 13, 2017. http://www.floodmap.net/Elevation/CountryElevationMap/?ct=GB.
- 19.2014. Elevation and elevation maps of cities/towns/villages in Georgia. Accessed October 13, 2017. http://www.floodmap.net/Elevation/CountryElevationMap/?ct=GB.
- 20.WHO (World Health Organization). 2011. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. V.a.M.N.I. System. Geneva: WHO. Accessed March 17, 2019. https://www.who.int/vmnis/indicators/serum_ferritin.pdf.
- 21.Erhardt JG, Estes JE, Pfeiffer CM, et al. 2004. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosor-bent assay technique. J. Nutr 134: 3127–3132. [DOI] [PubMed] [Google Scholar]
- 22.WHO (World Health Organization). 2011. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: WHO: Accessed March 17, 2019. http://www.who.int/vmnis/indicators/retinol.pdf. [Google Scholar]
- 23.Stoltzfus RJ & Klemm R. 2017. Research, policy, and programmatic considerations from the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr 106: 428s–434s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BRINDA. 2017. Special supplement: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA). Am. J. Clin. Nutr 106 10.3945/ajcn.117.999. [DOI] [PubMed] [Google Scholar]
- 25.Instituto de Nutrición de Centro América y Panamá (INCAP). 2015. Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición-SIVESNU—2013. Documento de trabajo. Guatemala: INCAP. [Google Scholar]
- 26.Calafat AM, Polzin GM, Saylor J, et al. 2004. Determination of tar, nicotine, and carbon monoxide yields in the mainstream smoke of selected international cigarettes. Tobacco Control 13: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beguin Y 2003. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin. Chim. Acta 329: 9–22. [DOI] [PubMed] [Google Scholar]
- 28.Ocas-Cordova S, Tapia V & Gonzales GF. 2018. Hemoglobin concentration in children at different altitudes in Peru: proposal for [Hb] correction for altitude to diagnose anemia and polycythemia. High Alt. Med. Biol 19: 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beall CM 2000. Tibetan and Andean patterns of adaptation to high-altitude hypoxia. Hum. Biol 72: 201–228. [PubMed] [Google Scholar]
- 30.Beall CM, Decker MJ, Brittenham GM, et al. 2002. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc. Natl. Acad. Sci. USA 99: 17215–17218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore LG 2001. Human genetic adaptation to high altitude. High Alt. Med. Biol 2: 257–279. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales GF, Tapia V & Gasco M. 2014. Correcting haemoglobin cutoffs to define anaemia in high-altitude pregnant women in Peru reduces adverse perinatal outcomes. Arch. Gynecol. Obstet 290: 65–74. [DOI] [PubMed] [Google Scholar]
- 33.Gonzales GF, Steenland K & Tapia V. 2009. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am. J. Physiol. Regul. Integr. Comp. Physiol 297: R1477–R1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liber AC & Warner KE. 2018. Has underreporting of cigarette consumption changed over time? Estimates derived from US National Health Surveillance Systems between 1965 and 2015. Am. J. Epidemiol 187: 113–119. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Stable EJ, Marin BV, Marin G, et al. 1990. Apparent underreporting of cigarette consumption among Mexican American smokers. Am. J. Public Health 80: 1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Association between altitude and hemoglobin fit with a penalized B-spline with three knots by survey for preschool-aged children (PSC) and women of reproductive age (WRA).
Figure S2. Mean (95% CI) inflammation-corrected soluble transferrin receptor (sTfR) concentration by hemoglobin adjusted for altitude using the proposed equations to adjust hemoglobin (Hb) for altitude and smoking.
Figure S3. Mean (95% confidence interval) hemoglobin concentrations by smoking status for each country and for all countries combined (Panel A: Crude difference; Panel B, Hb adjusted for altitude).
Table S1. Maximum sample size of hemoglobin observations by altitude.
Table S2. Variation in sample size and in study population characteristics for each country across select models assessing hemoglobin and altitude.
Table S3. Equations for adjustment to hemoglobin (g/L) for altitude by model. Highlighted model indicated the model used for proposed adjustments for each population group.
Table S4. Proposed adjustmentsa to hemoglobin (g/L) by 500-m increments of altitude by survey for each population group where data were available for analysis of Model 3 (adjusted for inflammation-corrected ID and VAD). Adjustments shown only for the range of altitude assessed within each survey. Current WHO recommendations2 and proposed adjustments based on the analysis of all surveys combined provided for comparison.
Table S5. Variation in sample size and in study population characteristics for each country across select models assessing hemoglobin and smoking status (smoker, nonsmoker) or number of cigarettes smoked per day.