Abstract
Metabolic reprogramming of cancer cells and the tumor microenvironment are emerging as key factors governing tumor growth, metastasis, and response to therapies including immune checkpoint inhibitors. It has been recognized that rapidly proliferating cancer cells, tumor-infiltrating lymphocytes (TIL), and vascular endothelial cells (EC) compete for oxygen and nutrients. Tumor cells and other cell types in the microenvironment not only compete for nutrients, but they also simultaneously produce immunosuppressive metabolites, leading to immune escape. In addition, commensal microbial metabolites can influence regulatory T cells and inflammation in the intestine, thus playing an essential role in cancer prevention or cancer promotion. In this review, we summarize recent advances on metabolic interactions among various cell types in the tumor microenvironment with a focus on how these interactions affect tumor immunity. We also discuss the potential role of blood vessel metabolism in regulating immune cell trafficking and activation.
Introduction
The success of anti-cancer immunotherapy reveals the power of unleashing the host immune system to kill cancer cells. However, many patients are not responsive to such therapy and significant numbers of responding patients eventually relapse. Mechanisms of innate and acquired resistance are poorly understood, but existing evidence points to low immunogenicity of cancer cells and immune suppressive tumor microenvironment. Recent work reveals that immune cells compete with cancer cells and other proliferating cells in the microenvironment for nutrients. Metabolites in the tumor microenvironment, in turn, also influence immune cell differentiation and effector function. This review will cover the most recent literature on metabolic competition between cancer and infiltrating immune cells and how this competition contributes to cancer immune evasion. We will also discuss how metabolites from microbiota influence regulatory T cells and intestinal inflammation, as well as outlining the potential effects of blood vessel metabolism in anti-tumor immunity.
Metabolic competition for nutrients between tumor cells and infiltrating lymphocytes
Tumor infiltrating lymphocytes (TILs) have been linked with good prognosis and responsiveness to therapy (1, 2). Like cancer cells, TILs require nutrients found within the tumor microenvironment (TME) to support proliferation and differentiation (Figure 1A). While naive T cells rely on oxidative phosphorylation, activated T cells require aerobic glycolysis for their activation and effector function (3), as glucose deprivation inhibits calcium signaling, IFN-γ production, and cytotoxicity in T cells (4–7). Aerobic glycolysis is also augmented in cancer cells (8) and vascular endothelial cells (9), raising the possibility of competition among these cell types for glucose consumption in the TME. Several recent studies demonstrated that the glycolytic activities of cancer cells may restrict glucose consumption by tumor infiltrating T cells (TILs) (5, 10, 11), thereby inducing T cell exhaustion and immune escape. Glucose deprivation metabolically restricts T cells, leading to their diminished mTOR activity, glycolytic capacity, and IFN-γ production, resulting in tumor progression (5). Overexpression of the glycolytic enzyme phosphoenolpyruvate carboxykinase 1 (PCK1) in T cells increases glycolysis even when cultured in glucose-poor conditions thereby restoring the T-cell anti-tumor responses (10). Zhao et al demonstrated that ovarian tumors mediate effector T cell dysfunction via glucose restriction to suppress EZH2 methyltransferase expression to epigenetically reduce T cell cytokine production and survival (11). T cells isolated from malignant ascites fluid of ovarian cancer patients activated the IRE1α-XBP1 endoplasmic reticulum (ER) stress response to decrease glucose uptake and suppress mictochondrial activity (12), suggesting an oxidative and glucose-deprived TME microenvironment can contribute to lymphocyte dysfunction in human tumors. Collectively, these studies suggest that tumor cells can outcompete neighboring cells for glucose to sustain their proliferative programs while simultaneously suppressing anti-tumor immune responses.
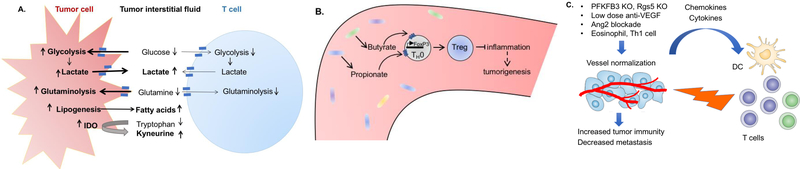
Figure 1. Influence of nutrients and metabolites in the microenvironment on anti-tumor immunity.
(A). Cancer cells outcompete tumor-infiltrating T cells for nutrients, and simultaneously produce metabolites to inhibit T cell function. (B) Microbial metabolites butyrate and propionate induce differentiation of colonic Treg cells, acting to protect against tumor-promoting gut inflammation. (C) Abnormal tumor blood vessels impede leukocyte trafficking. Vessel normalization recruits tumor-infiltrating cytotoxic T cells, leading to enhanced anti-tumor immunity.
Similar to effector lymphocytes, immunosuppressive cells, such as T regulatory (Tregs) and myeloid-derived suppressor cells (MDSCs), are also impacted by glucose deprivation conditions present within the TME. Within murine breast tumors, reduced glucose inhibits expression of granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF), critical cytokines involved in MDSC development, an effect that was abrogated by genetically targeting tumor cell-specific lactate dehydrogenase A (LDHA) (13). Similar to tumor cells, human naturally occurring Tregs and tumor-associated Tregs utilize glycolysis to a greater degree than other effector T cell populations, leading to cell senescence in responder T cells via glucose competition (14). Toll-like receptor 8 (TLR8) signaling blocks glycolysis to reverse the immunosuppressive nature of Tregs (14), suggesting this pathway may be targeted to enhance anti-tumor immunity. However, Tregs are less sensitive to glucose deprivation than oxidative stress, triggering apoptosis and ATP release (15). Live and apoptotic Tregs readily convert ATP to adenosine via CD39 and CD79, leading to activation of A2A signaling and immunosuppression of effector T cells (15).
Apart from glucose, both tumor cells and T lymphocytes utilize glutamine for their proliferative programs. Glutaminolysis in tumor cells is critical to replenish metabolites through anaplerotic reactions and for synthesis of nucleotides, amino acids, and fatty acids, as well as the anti-oxidant glutathione (GSH) (16, 17). Glutamine-derived α-ketoglutarate is also a required co-substrate for JHDMs and TET methylcytosine dioxygenases, which are involved in epigenetic regulation via histone and DNA de-methylation (18). In T cells, glutamine controls mTOR activation and is a key substrate for protein O-GlcNAcylation and synthesis of S-2HG that regulates effector T cell function and differentiation (19–21). Given that evidence supports glycolytic competition within the TME, tumor cells and anti-tumor immune cells may also compete for glutamine within the TME. Not surprisingly, Klysz and colleagues demonstrated that when glutamine uptake is blocked in naïve CD4 T cells, the generation of T effector cells is inhibited without affecting T regulatory cells (Tregs) (22). Wang et al also showed that glutamine deprivation compromised activation-induced T cell proliferation, implicating glutamine as an important source for biosynthetic precursors in active T cells (22). Together, these studies suggest that decreased glutamine within the microenvironment may negatively impact the function of immune cells within the tumor; but this hypothesis remains to be tested directly.
The unique metabolic demands of cancer cells provide opportunities for drug development. The glutaminase (GLS) inhibitor CB-839 is now being tested in multiple solid and blood cancers. However, because both cancer cells and tumor infiltrating lymphocytes consume glutamine, small molecule compounds such as CB-839 may inhibit both cell populations. To investigate this, Johnson et al genetically and pharmacologically targeted GLS in T cells. Interestingly, GLS deficiency promotes CD4 Th1 cells and CD8 CTLs at the expense of CD4 Th17 (23). Furthermore, inhibition of the glutamine transporter ASCT2/SLC1A5 by the potent small molecule inhibitor V-9302 did not appear to significantly affect CD8+ T lymphocyte proliferation and function in vitro (24), suggesting a compensatory mechanism in T cells. These studies raise the exciting possibility of targeting tumor cells with small molecules while enhancing anti-tumor immune response. However, further investigations are needed to test this hypothesis in the tumor setting both in preclinical models and in future human studies.
Effect of tumor cell-generated metabolites on immune cells in the microenvironment
In addition to nutrient consumption, metabolites produced by cancer cells can have a profound effect on immune cells in the microenvironment. For example, lactate, a byproduct from elevated aerobic glycolysis in cancer cells, induces apoptosis via reduced expression of the authophagy factor FIP200 in naïve T cells in both ovarian cancer patients and mouse models (25), while also impairing activation of the transcription factor NFAT and therefore production of IFN-γ in T cells and NK cells (26). Lactic acid also suppresses T cell motility and inhibits T cell cytotoxic and effector functions (27). Genetic targeting of lactate dehydrogenase A (LDHA) in tumors restores T cell infiltration and effector function (26). Furthermore, lactic acid can influence macrophage differentiation towards M2-like phenotype via stabilizing HIF-1α to drive M2-polarization (28).
Apart from lactic acid production, cancer cells produce massive amounts of fatty acids via de novo lipogenesis (29, 30). A fatty acid-enriched microenvironment can inhibit effector T cell function and M1-polarization in macrophages while favoring the differentiation of Tregs and M2-like macrophages (31, 32). In tumor-associated dendritic cells, the oxidative stress-generated lipid peroxidation byproduct 4-hydroxy-trans-2-noneanal (4-HNE) induces ER stress and XBP1 activation, suppressing antigen presentation and thus anti-tumor immunity (33).
Tumors are also often contain necrotic regions due to cancer cells outgrowing their nutrient supply, and a recent study found a much higher concentration of postassium ions (K+) in tumor interstitial fluid as compared to serum, which could inhibit CD8 T effector function through suppression of AKT-mTOR signaling (34). CD8 T effector function could be restored by decreasing CD8 T cell intracellular [K+] either pharmacologically or through overexpression of a K+ Efflux channel (34).
Additionally, cancer cells and cancer-associated fibroblasts impose immunosuppression by metabolizing the essential amino acid tryptophan into kynurenine (35, 36). Pro-inflammatory cytokines, including IL-1, IFN-γ, and TNFα, increase indoleamine 2,3-dioxygenase (IDO) IDO expression, resulting in increased kynurenine production within the tumor (35–37). Kynurenine acts as an endogenous ligand of the aryl hydrocarbon receptor (AhR) expressed on T cells, resulting in the induction of FoxP3-expressing Tregs (37). Kynurenine also directly suppresses effector T cell function through blockade of IL-2 signaling, leading to fewer CD4 memory T cells (38). Together, these studies illustrate that cancer cells can not only deprive immune cells of nutrients in the local environment but also produce metabolites that suppress immune defense.
Role of microbial metabolites in suppressing intestinal inflammation and colorectal cancer and on systemic immunity
While anti-tumor immune response has been harnessed as an effective form of immunotherapy, it has long been recognized that abnormally active immune cells in various inflammation states can lead to tumorigenesis. For example, two inflammatory bowel conditions, ulcerative colitis and Crohn’s disease, are known precursors for colorectal cancer (39). The delicate balance between the pro- and anti-inflammatory state that is essential for gut immune homeostasis is regulated by intestinal commensal microbes (40–43). Recent analyses of gut microbiota revealed that the microbial fermentation products butyrate and propionate induced differentiation of colonic Tregs in mice, acting to protect against tumor-promoting gut inflammation (44–46). These short-chain fatty acids maintain the regulatory T cell pool by binding to G protein coupled receptors which leads to enhanced histone H3 acetylation in the promoter/enhancer regions of Foxp3 locus (44–46). As such, butyrate ameliorated the development of colitis induced by adoptive transfer of CD4+ CD45RBhi T cells in Rag1-deficient mice (45). These studies suggest that bacterial metabolites can influence the host immune system to suppress the emergence of an inflammatory state, thereby playing a role in tumor prevention (Figure 1B).
In addition to the role of microbiota on colorectal cancer development, several studies have found a link between microbiota composition and immune checkpoint blockade efficacy in melanoma patients (47, 48). The mechanism by which changes in the gut microbiota alters systemic immunity is under investigation, but a recently published study from Kenya Honda’s group showed that colonization of mice with a consortium of 11 bacterial strains from healthy human donors could improve CD8 T cell-mediated immunity in several mouse models, and they demonstrated that the 11-strain consortium was associated with caecal and systemic elevation in metabolites that could potentially augment immune function (49).
Potential role of vascular endothelial cell metabolism in TIL recruitment and function
In addition to cancer cells and infiltrating immune cells, angiogenic sprouts from blood vessels are also rapidly proliferating cells that consume glucose and glutamine (9, 50). Abnormal tumor blood vessels impair perfusion, obstruct blood flow, and lead to poor leukocyte trafficking and drug delivery (9, 51, 52). Furthermore, the elevated permeability of the tumor vasculature increases interstitial pressure in tumors, increasing tumor cell motility (53, 54). Together, these tumor vessel aberrations drive cancer progression, metastasis, and treatment resistance. Anti-VEGF antibodies and other angiogenesis inhibitors were originally designed to block vessel growth in tumors; however, in some cases, the hypoxic tumor milieu enhanced tumor metastasis through multiple mechanisms (55, 56). An alternative approach that is rapidly gaining interest is “normalization” of tumor vessels, with the goal to increase pericyte coverage, tissue perfusion, and drug delivery while decreasing hypoxia and metastasis (57–59).
Since leukocytes circulate through the hematogenous vasculature, abnormal tumor blood vessels often impair tumor infiltration by lymphocytes, which may contribute to tumor immune evasion. Increasing evidence suggests that tumor vessel normalization may enhance the efficacy of immunotherapy, not only by improving delivery of immune checkpoint inhibitors (ICIs) to tumors, but also by promoting new lymphocyte infiltration (60–62) (Figure 1C). Therefore, therapeutic strategies that improve vessel normalization may synergize with ICIs to improve treatment response and patient outcome (63).
The mechanism of tumor blood vessel normalization, however, is poorly understood [reviewed in (60)]. Like cancer cells, vascular endothelial cells (ECs) also reprogram their metabolism for rapid proliferation. Rapidly proliferating ECs rely heavily on glycolysis, with a glycolytic rate similar to what is seen in highly glycolytic tumor cells (64). Endothelial cell-specific knockout of one allele of PFKFB3 or inhibition of PFKFB3, a key enzyme in regulating glycolysis, were reported to restore tumor perfusion and oxygenation via vessel normalization and improved drug delivery (50, 64). Given that normalized vessels have been shown to promote lymphocyte infiltration (60–62), it is possible that inhibition of PFKFB3 would also increase anti-cancer immunity by attracting immune cells to the tumor parenchyma. Aside from glucose, a growing body of evidence shows that glutamine metabolism also contributes to EC proliferation, providing carbons for biomass production that is required for EC proliferation (9). Vascular endothelial-specific deletion of glutaminase (GLS) in vivo suppressed retinal angiogenesis, and negatively affected tricarboxylic acid (TCA) cycle anaplerosis, macromolecule production, and redox homeostasis in ECs (65, 66). It remains to be determined whether inhibition of glutamine consumption can normalize tumor blood vessels and enhance tumor immunity.
Concluding remarks
It is now clear that metabolic communication between cancer cells and other cell types in the tumor microenvironment contributes to immune evasion. It is, therefore, critical to develop new strategies for immune cells to acquire sufficient nutrients to maintain their anti-tumor activities. Intuitively, a combination of cancer immunotherapies with metabolic inhibitors may sustain metabolic fitness of tumor-reactive infiltrating lymphocytes. However, we need to be mindful of potential treatment toxicities as both tumor cells and immune cells often utilize the same metabolic pathways for proliferation. Identification of metabolic targets that are selectively utilized in tumor cells may greatly improve anti-tumor immunity by avoiding detrimental effects on the immune cells. For example, the receptor tyrosine kinase (RTK) EphA2 promotes glutamine metabolism in HER2 positive and triple negative breast cancer cells (67, 68), but it is not expressed in the T cells (69). Targeting EphA2 may selectively inhibit glutaminolysis in tumor cells while simultaneously avoiding similar effects on T cells. It is also important to consider that tumor cells and lymphocytes may also differentially express amino acid and nutrient transporters, especially given the plethora of these carriers involved in metabolism. Identifying these differences may permit selective inhibition of metabolic pathways in cancer cells. Furthermore, existing anti-cancer metabolic therapies should be re-evaluated to determine their effects on the tumor microenvironment.
Conversely, it may be possible to develop immune therapies that improve the metabolic fitness of certain T cell subsets or metabolically synergize with adoptive T cell therapy. Indeed, overexpression of phosphoenolpyruvate carboxykinase (PCK1) in T cells or administering exogenous pyruvate can increase TIL activation and function (10, 70). In addition, immune cell subsets display a reliance on distinctive metabolic pathways (3). For example, memory CD8 T cells do not rely on glycolysis or amino acid metabolism, which may help them better adapt to the tumor microenvironment (71, 72). Thus, tumor-reactive memory T cells could be investigated for adoptive T cell therapy, either alone or in combination with inhibitors of glycolysis or glutaminolysis.
Apart from cancer cells and immune cells, growing evidence demonstrates that tumor blood vessels also have critical roles in determining the effectiveness of cancer immunotherapies. While blood vessels are known to regulate leukocyte trafficking, normalization of tumor vessels has only recently gained attention for its role in anti-tumor immunity via TIL recruitment. However, the mechanisms of tumor vessel normalization, including the role of metabolism, are only beginning to be understood. Therefore, it will be critical to further characterize different metabolic pathways in tumor-associated endothelial cells. In addition, endothelial cells from different vascular beds are heterogenous (73); therefore, it will be important to investigate how organ-specific EC metabolism impacts vessel normalization and tumor immunity at a variety of anatomical sites. Overall, greater understanding of metabolic communications among tumor cells, immune cell populations, and tumor blood vessel ECs will open new avenues for identifying strategies to further boost anti-immune responses.
Acknowledgments
This work was supported by a VA Merit Award 5101BX000134 (J.C.), NIH grants R01 CA177681 (J.C.), R01 CA95004 (J.C.), T32 CA009592 (V.M.N & D.N.E.), and Susan G. Komen Award PDF #17480733 (D.N.E.).
Footnotes
These authors declare no potential conflicts of interest
References
- 1.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer research. 2011;71:5601–5. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nature reviews Immunology. 2016;16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell metabolism. 2014;20:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. European journal of immunology. 2008;38:2438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Endothelial Cell Metabolism in Health and Disease. Trends in cell biology. 2018. [DOI] [PubMed] [Google Scholar]
- 10.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nature immunology. 2016;17:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, et al. IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell metabolism. 2018;28:87–103.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, et al. TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell metabolism. 2019;29:103–23.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nature immunology. 2017;18:1332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nature reviews Cancer. 2016;16:749. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. The EMBO journal. 2017;36:1302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinnaird A, Zhao S, Wellen KE, Michelakis ED. Metabolic control of epigenetics in cancer. Nature reviews Cancer. 2016;16:694–707. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nature immunology. 2013;14:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nature immunology. 2016;17:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, et al. S-2-hydroxyglutarate regulates CD8(+) T-lymphocyte fate. Nature. 2016;540:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, et al. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Science signaling. 2015;8:ra97. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell. 2018;175:1780–95.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nature medicine. 2018;24:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia H, Wang W, Crespo J, Kryczek I, Li W, Wei S, et al. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naive T cell apoptosis and affects tumor immunity. Science immunology. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell metabolism. 2016;24:657–71. [DOI] [PubMed] [Google Scholar]
- 27.Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS biology. 2015;13:e1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell metabolism. 2013;18:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annual review of immunology. 2013;31:259–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell metabolism. 2006;4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:5427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of immunology. 2010;185:3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagenais-Lussier X, Aounallah M, Mehraj V, El-Far M, Tremblay C, Sekaly RP, et al. Kynurenine Reduces Memory CD4 T-Cell Survival by Interfering with Interleukin-2 Signaling Early during HIV-1 Infection. Journal of virology. 2016;90:7967–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer prevention research (Philadelphia, Pa). 2016;9:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science (New York, NY). 2011;331:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. [DOI] [PubMed] [Google Scholar]
- 43.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- 46.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (New York, NY). 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (New York, NY). 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nature medicine. 2018;24:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–5. [DOI] [PubMed] [Google Scholar]
- 50.Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer cell. 2016;30:968–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganss R, Arnold B, Hammerling GJ. Mini-review: overcoming tumor-intrinsic resistance to immune effector function. European journal of immunology. 2004;34:2635–41. [DOI] [PubMed] [Google Scholar]
- 52.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nature medicine. 2008;14:28–36. [DOI] [PubMed] [Google Scholar]
- 53.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer research. 2007;67:2729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nature reviews Cancer. 2004;4:806–13. [DOI] [PubMed] [Google Scholar]
- 55.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nature reviews Drug discovery. 2011;10:417–27. [DOI] [PubMed] [Google Scholar]
- 58.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer cell. 2014;26:605–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin JD, Seano G, Jain RK. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annual review of physiology. 2019;81:505–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nature reviews Immunology. 2018;18:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassara A, Wyser Rmili C, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Science translational medicine. 2017;9. [DOI] [PubMed] [Google Scholar]
- 63.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nature reviews Clinical oncology. 2018;15:325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–63. [DOI] [PubMed] [Google Scholar]
- 65.Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Bruning U, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. The EMBO journal. 2017;36:2334–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim B, Li J, Jang C, Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. The EMBO journal. 2017;36:2321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Youngblood VM, Kim LC, Edwards DN, Hwang Y, Santapuram PR, Stirdivant SM, et al. The Ephrin-A1/EPHA2 Signaling Axis Regulates Glutamine Metabolism in HER2-Positive Breast Cancer. Cancer research. 2016;76:1825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards DN, Ngwa VM, Wang S, Shiuan E, Brantley-Sieders DM, Kim LC, et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Science signaling. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shiuan E, Chen J. Eph Receptor Tyrosine Kinases in Tumor Immunity. Cancer research. 2016;76:6452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siska PJ, Beckermann KE, Mason FM, Andrejeva G, Greenplate AR, Sendor AB, et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Windt GJ, O’Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. The Journal of clinical investigation. 2013;123:4479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcu R, Choi YJ, Xue J, Fortin CL, Wang Y, Nagao RJ, et al. Human Organ-Specific Endothelial Cell Heterogeneity. iScience. 2018;4:20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]