Abstract
ME-344 is a second generation isoflavone with unusual cytotoxic properties that is in clinical testing in cancer. To identify targets that contribute to its anticancer activity and therapeutic index, we used lung cancer cell lines that are naturally sensitive or resistant to ME-344. Drug-induced apoptosis was linked with enhanced levels of reactive oxygen species (ROS) and this initiated an Nrf2 (Nuclear erythroid factor 2-like 2) signaling response, downstream of which, heme oxygenase 1 (HO-1) was also found to be time-dependently inhibited by ME-344. ME-344 specifically bound to, and altered, HO-1 structure and increased HO-1 translocation from the rough endoplasmic reticulum to mitochondria, but only in drug-sensitive cells. These effects did not occur in either drug-resistant or primary lung fibroblasts, with lower HO-1 basal levels. HO-1 was confirmed as a drug target by using surface plasmon resonance (SPR) technology and through interaction with a clickable ME-344 compound (M2F) and subsequent proteomic analyses, showing direct binding of ME-344 with HO-1. Proteomic analysis showed that clusters of mitochondrial proteins, including voltage-dependent anion-selective channels (VDACs), were also impacted by ME-344. Human lung cancer biopsies expressed higher levels of Nrf2 and HO-1 compared to normal tissues. Overall, our data show that ME-344 inhibits HO-1 and impacts its mitochondrial translocation. Other mitochondrial proteins are also affected resulting in interference in tumor cell redox homeostasis and mitochondrial function. These factors contribute to a beneficial therapeutic index and support continued clinical development of ME-344.
Keywords: ME-344, human lung cancer cells, heme oxygenase 1, Nrf2 signaling response, redox cycling, cytotoxicity
Introduction
ME-344, [(3R,4S)-3,4-bis(4-hydroxyphenyl)-8-methyl-3,4-dihydro-2H-chromen-7-ol] (Figure 1A) is a second generation isoflavone related to phenoxodiol that has recently progressed as an anticancer agent into Phase 1 and Phase 1b clinical testing (1). Combinations of ME-344 and topotecan were well tolerated, with manageable dose limiting neuropathies (2), although, response rates in non-selected patients with lung, ovarian and cervical cancers have yet to reach significance (3). Generally, isoflavones have promiscuous interactions with cellular proteins, complicating identification of target(s) that may govern cytotoxicity and that might be used to predict an advantageous clinical therapeutic index. Mitochondria have been shown to be disrupted by ME-344, with complexes I and III particularly sensitive to ME-344, causing altered oxygen consumption rates and decreased ATP production (4,5). The present study was designed to identify target(s) within the mitochondria that might cause these effects. These efforts were facilitated by the fact that cancer cell lines fall into two very discrete categories, expressing either natural (innate or unselected) sensitivity or resistance to the drug. In an analysis of 240 human cell lines, 20 have been shown to be intrinsically resistant to ME-344, while the remainder are sensitive. Using lung cancer as a model system, we compared two sensitive and two resistant cell lines with normal lung fibroblasts, with the goal of identifying differential cellular traits that could be determinant of ME-344 response.
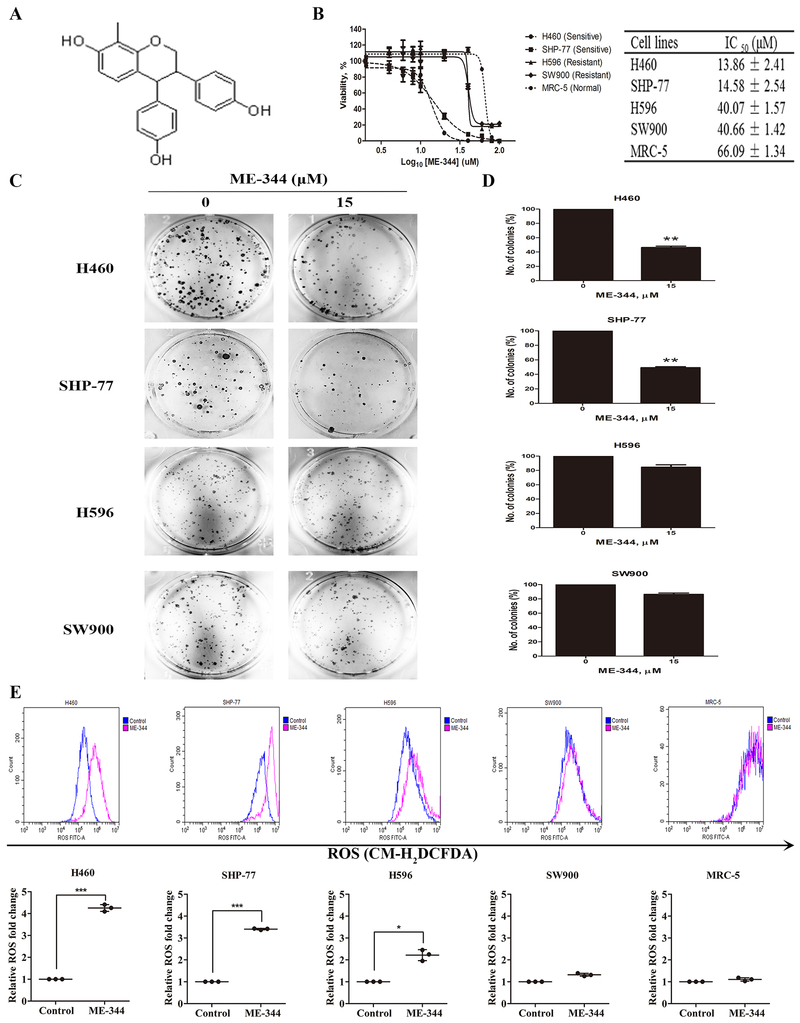
Figure 1. ME-344 effects on cell viabilities, colony formation and generation of reactive oxygen species (ROS) in sensitive (H460 and SHP-77) and resistant (H596 and SW900) human lung cancer and normal cells (MRC-5).
A. Chemical structure of ME-344. B. Cells were treated with ME-344 (0.1–100 μM) for 24 h and viabilities determined by MTT assay. Results (% viability) were calculated relative to untreated control cells. C, D. Cells were seeded into six-well plates in triplicate at an initial density of 500 cells/well and treated with ME-344 for 24 hours. After 10–14 days, colonies containing more than 50 cells were counted by light microscopy. The average number of colonies was determined from three independent experiments. E. Cells were treated with their IC50s of ME-344 for 24 h and intracellular ROS levels determined with CM-H2DCFDA by flow cytometry. Data are presented as the mean ± SEM of three independent experiments. *p < 0.05, ***p < 0.001 vs. the untreated controls by Student’s t-test.
Our initial comparisons revealed differences in resting state levels of reactive oxygen species (ROS) and in activities associated with a major redox transcription factor, nuclear factor, erythroid 2-like 2 (Nrf2), where its interaction with Keap1 can regulate transcription of a battery of antioxidant response genes (6). In addition, the BTB and CNC Homology 1 (Bach1) protein can compete with Nrf2 for binding to antioxidant response elements (ARE), thereby suppressing some target genes, inclusive of heme oxygenase-1 (HO-1) (7,8). Under basal conditions, Bach1 binds to AREs that regulate HO-1 expression (9), but ROS can disrupt this and enable activation of HO-1 by Nrf2. HO-1 is generally regarded as a protective, detoxification enzyme that catalyzes the rate-limiting step in heme degradation, producing equimolar carbon monoxide, iron and biliverdin. In the majority of cases, its antioxidant properties reduce intracellular ROS, although in some instances, oxidant stress can be increased (10). A defining characteristic of cancer is aberrant cellular redox homeostasis and tumor cells general carry high resting state levels of oxidative stress (11). In addition, HO-1 is expressed at high levels in a number of cancers, particularly lung (12,13), a feature linked with cell functions that contribute to, and enhance, tumor promotion (14) and drug resistance (13). Partly as a consequence, selective inhibition of HO-1 has been considered as an attractive target in oncology, but at this time there are no available inhibitors that can be used clinically. Until now, only two approaches to inhibition of HO-1 have been attempted, the use of siRNA or metalloporphyrins (15), but poor solubility and tumor delivery, as well as normal tissue toxicities have limited successful applications of either. Our present results show that ME-344 can target HO-1, causing subsequent impact on mitochondria and energy metabolism. Using a novel click chemistry approach, proteomic analyses confirm that targets in the mitochondria are preferentially impacted by ME-344. These results may help in designing precision medicine trials in optimizing the clinical utility of ME-344 in combination with other drugs (16).
Materials and Methods
Cell lines
H460, SHP-77 and H596 were maintained in RPMI-1640 medium (Corning, Manassas, VA) supplemented with 10% (v/v) fetal bovine serum (Atlas, North Charleston, SC), 100 U/mL penicillin, and 100 mg/mL streptomycin and 2 mM L-glutamine. SW900 was maintained in L-15 (Corning, Manassas, VA) with the same supplement as mentioned above. MRC-5 was maintained in Eagle’s Minimum Essential Medium (EMEM) (ATCC, Manassas, VA) with 10% (v/v) fetal bovine serum (Atlas). SHP-77 small cell lung carcinoma and H460 large cell lung cancer cells were purchased from the National Cancer Institute (Frederick, MD) and cultured according to supplier recommendations. Primary immortalized human lung embryonic fibroblasts (MRC-5), SW900 grade IV squamous cell lung carcinoma, and H596 adenosquamous lung carcinoma were purchased from American Type Culture Collection (Manassas, VA) and cultured according to the supplier recommendations. The cell lines were authenticated by short tandem repeat profiling in 2016 and were tested for mycoplasma contamination with PCR-based method every 3 months. Cell lines used for the experimental study were passaged within 10 to 15 passages after reviving from the frozen vials.
MTT
Cell viabilities were assessed by a MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma-Aldrich, St. Louis, MO) assay after treating the cells with different concentrations (0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50 and 100 μM) of ME-344 for 24 h. Briefly, MTT solution in 1 x PBS was added to each well at the final concentration of 0.5 mg/mL. The plate was incubated for 4 h at 37°C. The MTT medium was aspirated carefully and the dark-blue formazan was solubilized in DMSO (Sigma). Optical density was measured with a spectrometer (BioRad, Hercules, CA) at 550/690 nm. Each experiment was conducted in triplicates and repeated independently 3 times.
Immunoblotting
Total soluble protein was quantitated by bicinchoninic acid protein assay (Pierce, Rockford, IL). Cell lysates were resolved in an SDS-loading buffer (80 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.02% bromophenol blue, 5 mM tris (2-carboxyethyl) phosphine) and heated at 95°C for 5 min. Equal amounts of protein were electrophoretically separated by SDS-PAGE (BioRad) and transferred onto low fluorescent polyvinylidene fluoride membranes (Millipore, Billerica, MA) or nitrocellulose membranes (BioRad) by the Trans-Blot Turbo Transfer System (BioRad). PVDF or nitrocellulose membranes were incubated in the Odyssey blocking buffer (LI-COR, Lincoln, NE) for 1 h to reduce nonspecific binding and then probed with appropriate primary antibodies (listed in Supplementary Materials and Methods, diluted in Odyssey blocking buffer) at 4°C overnight. Immunoblots were then developed with infrared (IR) fluorescence IRDye secondary antibodies (LI-COR) at a dilution of 1:15,000, imaged with a two-channel (red and green) IR fluorescent Odyssey CLx imaging system (LI-COR) and quantified with ImageJ software (FIJI).
RNA isolation and quantitative real-time reverse transcription-PCR
Total RNA was prepared using the Isolate II RNA Mini Kit (Bioline USA, Inc., Taunton, MA) and cDNA was then synthesized with the iScript™ cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s protocols. Subsequently, quantification of gene expression was performed in duplicates using iQ™ SYBR® Green supermix (Bio-Rad, Hercules, CA) with detection on a MyiQ™ Real-Time PCR System (Bio-Rad). The reaction cycles used were 95°C for 5 min, and then 40 cycles at 95°C for 15 s and 58°C for 1 min followed by melt curve analysis. Relative gene expression quantification was based on the comparative threshold cycle (CT) method (2-∆∆Ct) with normalization of the raw data to the included housekeeping gene (18S rRNA). The primer sequences are listed in Supplementary Materials and Methods.
Determination of the HO-1 activity by measuring the formation of bilirubin
Lysates of cultured cells were prepared by mixing with lysis buffer (100 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, pH 7.4, and protease inhibitors (Roche, Basel, Switzerland). Total soluble protein was quantitated by bicinchoninic acid protein assay. The reactions were performed with 2 μg of purified HO-1 protein or 10 μL (20-100 μg total protein) of the cell lysates in 100 mM Tris-HCl with 15 μM hemin, 0.8 mM NADPH, 1 mM MgCl2, 0.8 mM glucose 6-phosphate, 300 μM BSA, 1 U glucose-6-phosphate dehydrogenase (Sigma), and 3 μL of Biliverdin reductase (Sigma). The reaction samples were mixed and 200 μL of the mixture was transferred into quartz micro cuvettes (3 × 3 × 40 mm). The fluorescence was detected in a fluorescence spectrofluorometer (Photon Technology International, New Jersey) at 37°C with excitation at 441 nm and emission at 528 nm, using a gain value of 140. Detection of bilirubin emission was performed in real-time kinetics with resolution of 1 sec. A specific HO-1 inhibitor tin Protoporphoryrin IX dichloride (Santa Cruz Biotechnology, Inc., TX) was used as a positive control. Every measurement of each wavelength and time point was detected five times and the arithmetic mean with standard deviation (SD) was calculated.
ROS Detection
Cells were treated with 1 μM 2’,7’dichlorodihydrofluorescein diacetate (CM-H2DCFDA, Thermo Fisher) for 45 min, followed by 2 times washes with PBS. The reduced CM-H2DCFDA can be oxidized and converted into fluorescent 2’, 7’-dichlorofluorescein (DCF) by intracellular ROS. The fluorescent signals were detected by flow cytometer (FCM, Beckman coulter, Beckman). Totally 10,000 cells were analyzed per sample.
Purification of mitochondria and endoplasmic reticulum
Mitochondria and microsomal/ER fraction were isolated following the Endoplasmic Reticulum Isolation Kit protocol (Sigma-Aldrich, cat# ER0100). Purity of cell fractions was determined by immunoblotting against SERCA2 (ER/microsomal marker) and ATP5A (mitochondrial marker).
Immunohistochemistry
The expression of HO-1 and Nrf2 were evaluated by immunohistochemical staining with 5-μm-thick sections from TMA blocks. The TMAs were purchased from Biorepository and Tissue Analysis Core, Medical University of South Carolina (Charleston, SC, USA). The sections were first deparaffinized in xylene and then rehydrated through graded ethanol. For antigen retrieval, we performed in water bath at 92-98°C for 30 minutes in sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution (Sigma). TMA slides were incubated with primary antibodies at 4°C overnight, and then incubated with biotinylated secondary antibody (Vector, Burlingame, CA) for 30 mins at room temperature. The primary antibodies were rabbit polyclonal anti-HO-1 (Abcam) and rabbit polyclonal anti-Nrf2 (Santa Cruz Biotechnology). TMA slides were incubated with ABC reagent (Vector) for 30 mins at room temperature. Immunocomplexes of horseradish peroxidase were visualized by DAB (Vector) reaction and hematoxylin (Ricca, Arlington, TX) counterstain was applied. Immunoreactivity was scored using a semi-quantitative system combining intensity of staining (0-3) and percentage of positive cells (0-3) (Supplementary Table S1).
Statistical analysis
All measurements were collected from three independent experiments. Statistical analysis was performed using GraphPad Prism 6.0, Microsoft Excel, or Perseus software. Significant differences were determined using two tailed t-tests and one-way analysis of variance (ANOVA) followed by Newman-Keuls as a post-test.
Results
ME-344 cytotoxicity profiles
The comparative cytotoxic effects of ME-344 were determined in a panel of human lung tumor cell lines (H460, SHP-77, H596, SW900) and in human normal lung fibroblasts (MRC-5). ME-344 dose dependently decreased viabilities in H460 and SHP-77 cell lines (Figure 1B) leading us to define these cells as naturally sensitive to the drug. In contrast, H596 and SW900 had IC50 values that were three times higher, representing a naturally (or intrinsically) resistant phenotype. Normal lung fibroblasts (MRC-5) were five-fold more resistant. For the less responsive cell lines, there were apparent shoulders to the survival curves (Figure 1B). Based on these results, respective IC50 values for each cell line were used for subsequent experiments.
ME-344 on cell clonogenicity
Colony formation assays were performed in sensitive (H460 and SHP-77) and resistant (H596 and SW900) cell lines in response to ME-344. While H596 and SW900 cells retained high clonogenicity after ME-344, H460 and SHP-77 cells were greatly reduced under the same conditions (Figure 1C and D). These results indicated that the ME-344-mediated inhibitory effects on clonogenicity were greater in sensitive compared to resistant cell lines.
ME-344 generation of ROS
The cytotoxicity profiles correlated with the drug’s capacity to induce intracellular ROS. Figure 1E illustrates the impact of IC50 values of ME-344 on ROS as measured with flow cytometry using CM-H2DCFDA as a fluorescent indicator. The induction of intracellular ROS levels by ME-344 were higher in drug sensitive cells, H460 (4.3 fold) and SHP-77 (3.4 fold), compared to the resistant cells H596 (2.2 fold) and SW900 (1.3 fold) and the normal fibroblasts (1.1 fold) mimicked the resistant cells (Supplementary Table S2). These results indicated that there was either a cause, or effect, association between the extent of ME-344 cytotoxicity and ROS production.
ME-344 and Nrf2
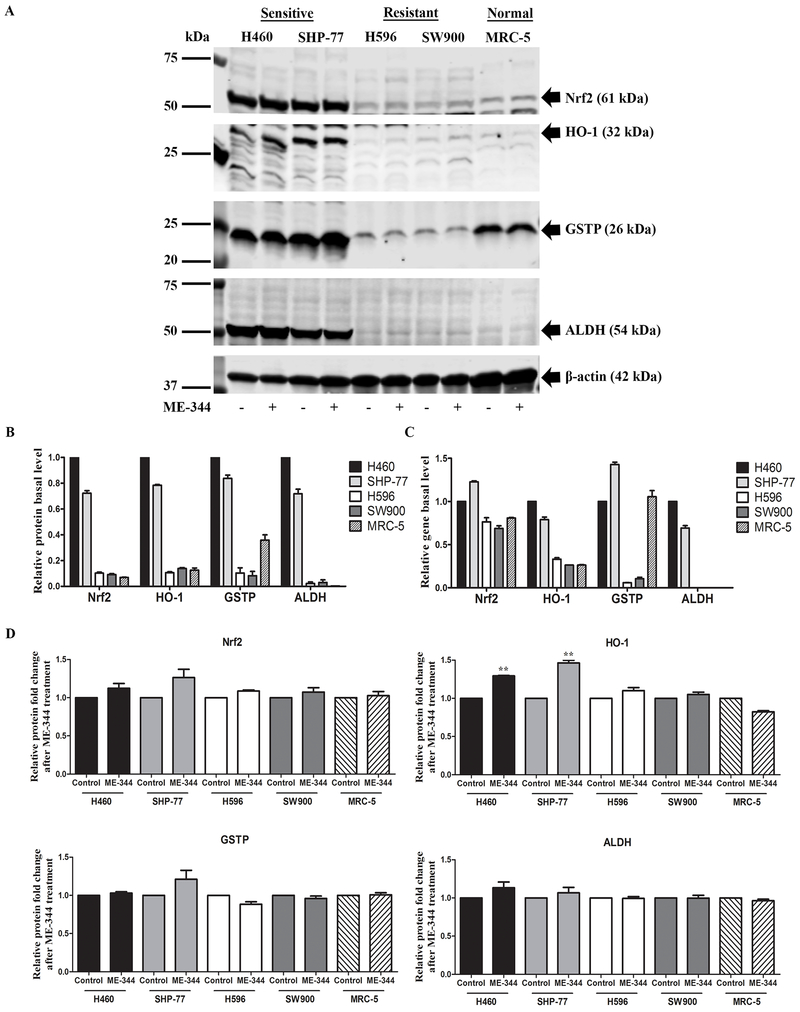
Because of this observed differential in ROS production, the impact of the drug on expression of the primary oxidant response transcriptional regulator Nrf2 and its associated genes was measured. As shown in Figure 2A-C; Supplementary Table S3 and S4, basal levels for both protein and mRNA for Nrf2 and downstream regulated oxidative stress genes, HO-1, GSTP and ALDH, were highest in the sensitive cell lines. In addition, when challenged with ME-344, induction of each was also more pronounced in the sensitive cells than the resistant (Figure 2A, 2D and 3A; Supplementary Table S5 and S6). Expression of Nrf2 and the downstream genes in normal lung fibroblasts was more closely aligned to the resistant cells than sensitive. Once again, in agreement with the comparative levels of ROS and cytotoxicity generated by the drug.
Figure 2. ME-344-induced changes in protein expression of Nrf2, HO-1, GSTP and ALDH in sensitive (H460 and SHP-77) and resistant (H596 and SW900) human lung cancer and normal cells (MRC-5).
Cells were treated with their IC50s for 24 h. A. Proteins were separated by SDS-PAGE and evaluated by immunoblots. B. Basal protein expression in untreated control cells was quantified by Image J software. Results are normalized to β-actin protein expression and relative to H460 cell line with mean values set at 1. C. Basal gene expression was analyzed by real-time PCR in untreated control cells. Relative gene expression quantification was based on the comparative threshold cycle (CT) method (2−ΔΔCt) with normalization of the raw data to the included housekeeping gene (18S rRNA) and relative to H460 cell line with mean values set at 1. D. The protein fold-changes after ME-344 treatment relative to untreated control cells were quantified by Image J software. Results are normalized to β-actin protein expression. Data are derived from three independent experiments and presented as means ± SEM in the bar graphs. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the untreated control by Student’s t-test.
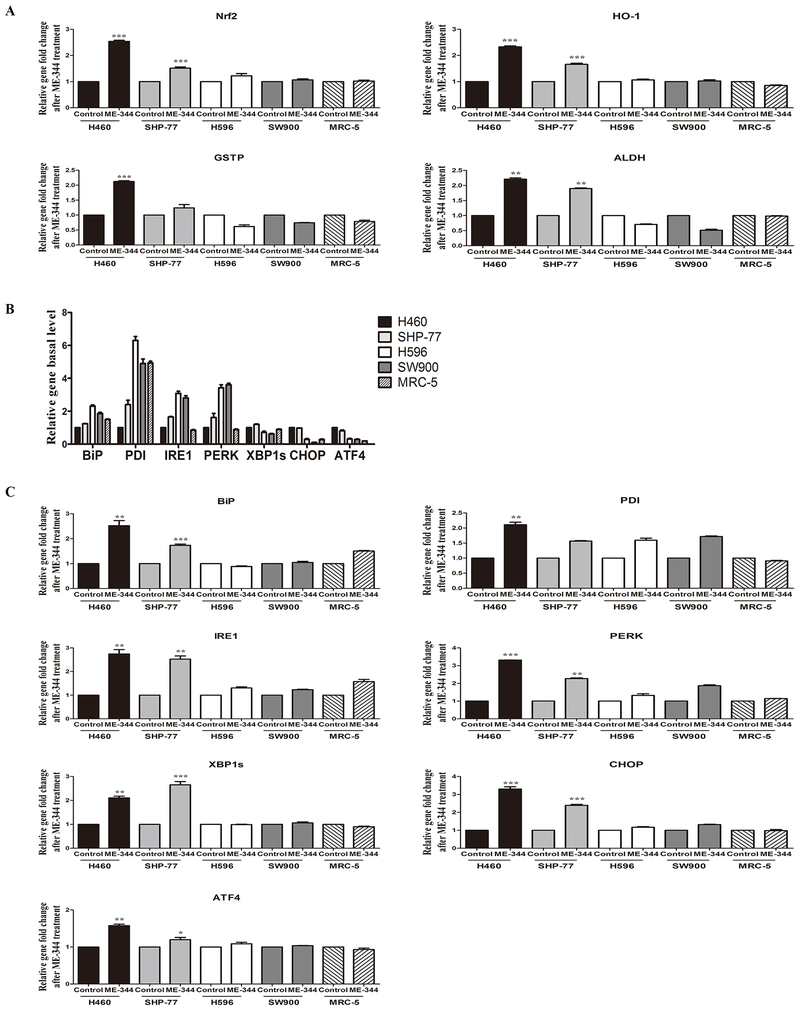
Figure 3. ME-344-induced changes in gene expression of Nrf2, HO-1, GSTP, ALDH and UPR markers in sensitive (H460 and SHP-77) and resistant (H596 and SW900) human lung cancer and normal cells (MRC-5).
Cells were treated with their IC50s for 24 h. A. Fold-changes in gene expression (Nrf2, HO-1, GSTP, ALDH) after ME-344 treatment relative to untreated control cells with mean values set at 1. Relative gene expression quantification was based on the comparative threshold cycle (CT) method (2-ΔΔCt) with normalization of the raw data to the included housekeeping gene (18S rRNA). B. Basal gene expression (UPR markers) was analyzed by real-time PCR in untreated control cells. Relative gene expression quantification was based on the comparative threshold cycle (CT) method (2-ΔΔCt) with normalization of the raw data to the included housekeeping gene (18S rRNA) and relative to H460 cell line with mean values set at 1. C. Fold-changes in gene expression (UPR markers) after ME-344 treatment relative to untreated control cells with mean values set at 1. Relative gene expression quantification was based on the comparative threshold cycle (CT) method (2-ΔΔCt) with normalization of the raw data to the included housekeeping gene (18S rRNA). Data are derived from three independent experiments and presented as means ± SEM in the bar graphs. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the untreated control by Student’s t-test.
ME-344 induces the unfolded protein response (UPR)
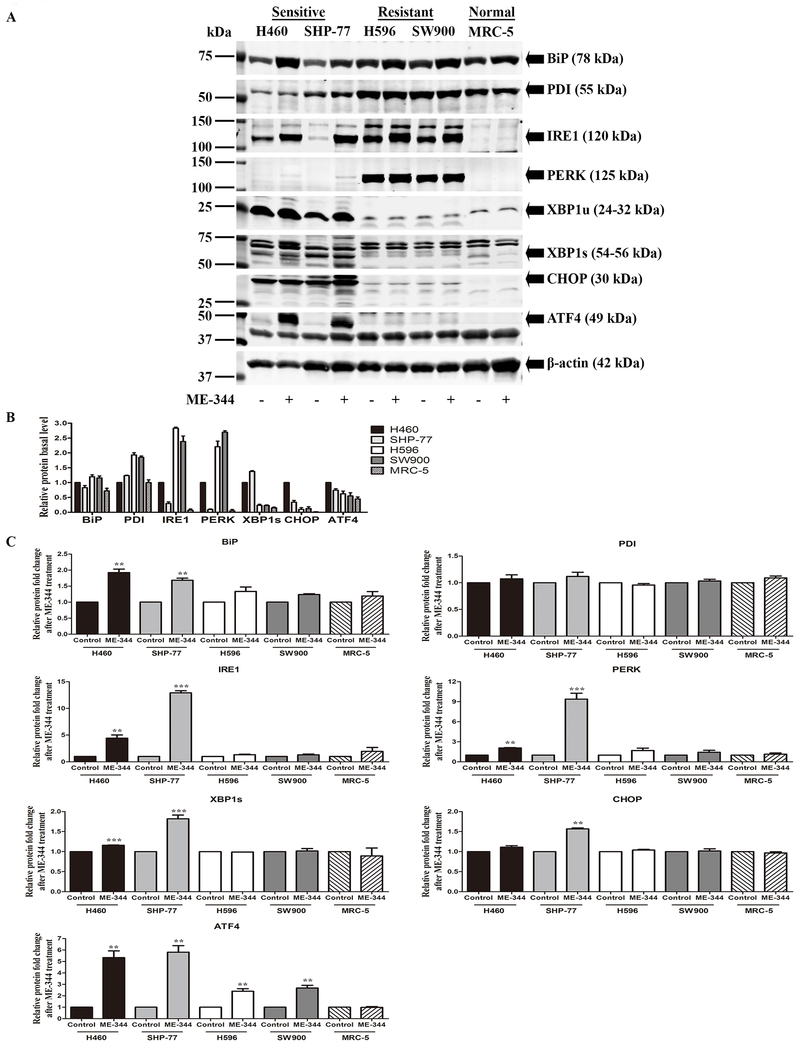
Because the UPR is influenced by ROS, we estimated both basal and induced levels of a battery of genes known to be involved in the control of this process. Basal mRNA expression of BiP, PDI, IRE1 and PERK were higher in resistant than sensitive cells, while for XBP1s, CHOP and ATF4, sensitive cells expressed more. ME-344 induced mRNA expression levels for all of the UPR markers in both sets of cells, but the effects were more pronounced in sensitive cells (Figure 3B and 3C; Supplementary Table S7 and S8). While the basal protein levels of PDI, BiP, IRE1 and PERK were significantly higher in resistant cells, XBP1s, CHOP, and ATF4, were higher in sensitive. ME-344 induced expression of all of these in both sensitive and resistant cells, but similar to mRNA, the effects were more substantial in the sensitive cells (Figure 4A–C; Supplementary Table S9 and S10). Of interest, normal fibroblasts were less susceptible to UPR induction. These results imply that ME-344 induces the UPR at dose levels in sensitive cell lines that do not have equivalent effects in the resistant or normal cells.
Figure 4. ME-344-induced changes in protein expression of UPR markers in sensitive (H460 and SHP-77) and resistant (H596 and SW900) human lung cancer and normal cells (MRC-5).
Cells were treated with their IC50s for 24 h. A. Proteins were separated by SDS-PAGE and evaluated by immunoblot. B. Basal protein expression in untreated control cells was quantified by Image J software. Results are normalized to β-actin protein expression and relative to H460 cell line with mean values set at 1. C. Protein fold-changes after ME-344 relative to untreated controls were quantified by Image J software. Results are normalized to β-actin protein expression. Data are derived from three independent experiments and presented as means ± SEM in the bar graphs. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the untreated control by Student’s t-test.
Effects of ME-344 on HO-1
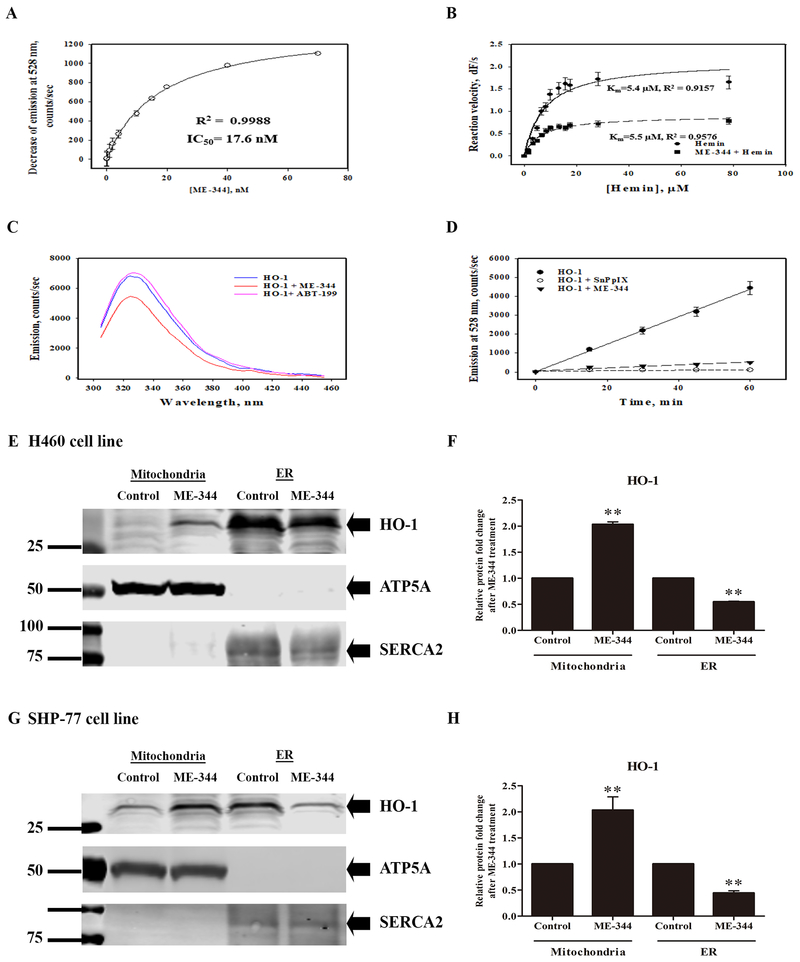
Using the bilirubin emission assay, we measured the capacity of ME-344 to inhibit purified HO-1. Figure 5A shows that the inhibition kinetics were similar to one of the few characterized existing HO-1 inhibitors, SnPPIX (IC50=17.6 nM for ME-344; 11.0 nM for SnPPIX). Simultaneous real-time fluorescence detection of the bilirubin formed was carried out with a range of hemin concentrations (0-80 μM). We observed that activities were diminished 60% by ME-344, with the drug acting as a non-competitive inhibitor of HO-1 with a Km=5.5 μM, similar to the value for SnPPIX (Km=4.6 μM; Figure 5B). Purified HO-1 incubated with approximate equivalent moles of ME-344 decreased the fluorescence of tryptophan residues within HO-1, indicating that the drug induced changes in the tertiary and/or quaternary structure of the enzyme (Figure 5C). Treatment of the cell lysates from H460 sensitive cells with ME-344 significantly reduced the HO-1 activity in a time-dependent manner (Figure 5D). In addition, ME-344 increased the extent of translocation of HO-1 protein from the rough endoplasmic reticulum (ER) to the mitochondria in both H460 and SHP-77 sensitive cells (Figure 5E–H). Expression of HO-1 was very low in the H596 resistant cells and thus translocation was not observed. Manipulation of HO-1 expression levels was achieved through construction of clones of shRNA knockdown cells shown in Supplementary Figure S1. Depletion of HO-1 expression was >60% for each of the 5 clones selected and this was accompanied by increased sensitivity to ME-344 (Supplementary Figure S1A–D). In turn, this correlated with enhanced levels of ROS, implying that HO-1 deficiency carries a phenotype of lowered cellular antioxidant capacity (Supplementary Figure S1E and S1F). Over-expression of HO-1 was achieved by introducing pcDNA3-HO-1 plasmid into the H596 cell line (resistant). As a consequence, these cells became more sensitive to ME-344 (IC50 28.01 ± 0.41 vs 21.14 ± 0.32) with ME-344 treatments resulting in higher levels of ROS when compared to the control vector (Supplementary Figure S1G-J). These results are indicative that HO-1 has a role in determining drug sensitivity.
Figure 5. ME-344 inhibits HO-1 activity and changes HO-1 structure.
A. Purified HO-1 protein was incubated with different concentrations of ME-344 (1.25–70 nM) and its activity determined by measuring the formation of bilirubin at 441/528 nm Ex/Em. The HO-1 IC50 for ME-344 (17.6 nM) was similar to another known HO-1 inhibitor, SnPPIX (IC50=11.0 nM). B. Purified HO-1 protein was incubated with ME-344 for Michaelis-Menten analysis and the results indicated non-competitive inhibition of HO-1 by ME-344 with Km=5.5 μM. C. The tryptophanyl fluorescence of purified HO-1 was substantially (~27.0%) decreased in the presence of ME-344, indicating that ME-344 induced changes in HO-1 tertiary and/or quaternary structure. ABT-199 was used as a negative control. D. Total proteins from H460 cells were incubated with ME-344 for 15, 30, 45, and 60 min, and inhibition of HO-1 activity determined by measuring the formation of bilirubin. SnPPIX was used as a positive control. E. G. Sensitive cell lines H460 and SHP-77 were treated with IC50s for 24 h and mitochondria and ER were isolated. Proteins were separated by SDS-PAGE and evaluated by immunoblots. F. H. Protein fold-changes in either mitochondria or ER fractions after ME-344 relative to untreated control cells were quantified by Image J software. Data are presented as the mean ± SEM of three independent experiments.
ME-344 binds directly to HO-1
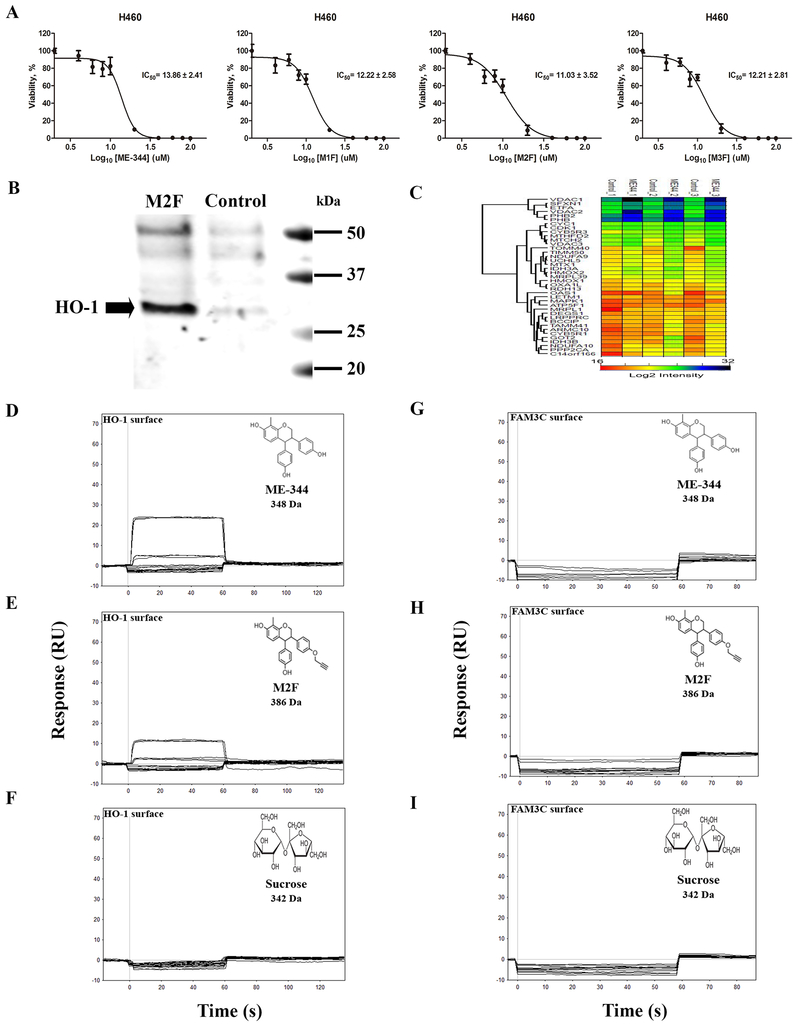
Syntheses of derivatives of ME-344 were carried out as outlined in Methods and summarized in Supplementary Figure S2A. Three fractions M1F, M2F, and M3F were collected (Supplementary Figure S2A) and H460 cells were treated with ME-344, M1F, M2F or M3F (dose range, 0.1-100 μM) for 24 h. Cell survivals are indicated in Figure 6A, with calculated IC50 values of ME-344 (13.86 ± 2.41 μM), M1F (12.22 ± 2.58 μM), M2F (11.03 ± 3.52 μM) and M3F (12.21 ± 2.81 μM), suggesting that the modifications did not dramatically impact drug cytotoxicity.
Figure 6. HO-1 was identified as an ME-344 target by using click-chemistry, mass spectrometry and surface plasmon resonance (SPR).
A. Propargylation of ME-344 with expected multiple products. A total of three fractions (M1F, M2F and M3F) were separated and purified for affinity pulldown chromatography. Cytotoxicities of ME-344, M1F, M2F or M3F were determined by MTT assays. H460 human lung cancer cells were treated with various concentrations (0.1–100 μM) of ME-344, M1F, M2F or M3F for 24 h, and relative cell viabilities (%) were expressed as percentages relative to the untreated control cells. The IC50s for M1F, M2F and M3F were comparable to ME-344, and M2F was utilized further to identify the active targets of ME-344. B. Click chemistry was adapted to pull down ME-344 protein targets using M2F. M2F or solvent control was first conjugated to azide agarose resin and then incubated with proteins from H460 cells. Resin bound proteins were separated into two fractions, one of which was subjected to SDS-PAGE and immunoblotted with anti-HO-1 antibodies. HO-1 was detected in H460 cells treated with M2F, but not in untreated control, indicating that ME-344 binds to HO-1. C. Affinity-enriched and gel-fractionated proteins between 25–50 kDa were analyzed by LC-MS/MS and quantified by label free proteomics. The log2 intensities of mitochondrial and heme oxygenase proteins with >1.5-fold enrichment by M2F beads as compared to control are provided in the heat-map. HO-1 exhibited a 1.6-fold enrichment with M2F-conjugated beads as compared to control. D, E, F. Kinetic curve for ME-344 interacting with a 5000-RU HO-1 surface. G, H, I. Kinetic curve for ME-344 interacting with a 5000-RU FAM3C surface as a negative control. Each compound was tested in duplicate in a two-fold dilution series starting at 100 μM. The compound structure, name, molecular mass are provided on each data set.
The fractions were collected and their spectra analyzed using proton NMR, with spectra showing that all were mono-propargylated ME-344. The NMR spectrum for M2F is shown as an example in Supplementary Figure S2B. Based on the proton NMR of M2F, only two OH hydrogens were observed. Since ME-344 has three hydroxyl (-OH) hydrogens this suggests that M2F is a mono-propargylated derivative of ME-344. 1H NMR (acetone-d6, ppm) 2.15 (s, 3 H, CH3), 3.08 (t, 1 H, J = 2.4 Hz, CC-H), 3.47-3.52 (m, 1 H, ring C-H), 4.19 (d, 1 H, J = 5.2 Hz, ring C-H), 4.23-4.27 (m, 1 H, ring C-H), 4.40-4.45 (m, 1 H, ring C-H), 4.75 (d, 2 H, J = 2.4 Hz, CH2), 6.40-6.46 (m, 3 H, Ar-H), 6.54-6.58 (m, 3 H, Ar-H), 6.71-6.83 (m, 4 H, Ar-H), 8.03 (s, 1 H, OH), 8.08 (s, 1 H, OH).
Subsequently, M2F was conjugated to azide agarose resin using click chemistry to verify binding to HO-1 and identify other putative targets of ME-344 via affinity enrichment-mass spectrometry (AE-MS; Supplementary Figure S2C). An aliquot of the proteins affinity purified with M2F conjugated resin was analyzed by SDS-PAGE and immunoblotting with anti-HO-1 antibodies. HO-1 was detectable in the H460 cells treated with M2F, but not in those without drug treatment. Such a result is consistent with direct binding of ME-344 to HO-1 (Figure 6B). The remaining fraction was used for affinity enrichment mass spectrometry (17). This approach enables less stringent washing, retention of transient drug-protein interactions and discrimination of drug targets by quantitative label free proteomics. The relative abundance of proteins enriched by M2F-conjugated beads as compared to the control resin was measured by LC-MS/MS. Out of 1,070 proteins identified, 413 were quantified by at least 2 peptides in the replicate M2F affinity enrichments (Supplementary Table S11 and S12). Seventeen proteins were enriched 4-fold and 21 were identified only in the M2F enriched samples (Supplementary Table S13 and S14; Supplementary Figure S2D). HO-1 was enriched by 1.6-fold with M2F beads as compared to control beads (Supplementary Figure S2E). Identification of differentially abundant proteins using MaxLFQ relies on the assumption that the control and experimental data are very similar with Pearson correlation coefficients > 0.75 (Supplementary Figure S2F). The normalized log2 protein intensities of HO-1, HO-2, and mitochondrial proteins observed with 1.5-fold change are shown in the heat-map (Figure 6C) and can be compared to Supplementary Figure S2D.
Additionally, Biacore 3000 instrumentation was used to test direct binding of ME-344 to HO-1. Both ME-344 (348 g/mol) and the clickable ME-344 (386 g/mol) showed increased signal (RU) with increasing concentrations of the compounds when the drug was “flowed over” immobilized purified HO-1 protein (Figure 6D and 6E). As a negative control, sucrose (342 g/mol; similar molecular mass) displayed no binding to HO-1 (Figure 6F). Further, FAM3C protein was immobilized as a negative control to show that neither ME-344 nor clickable ME-344, or sucrose bound to this comparably sized, yet unrelated, protein (Figure 6G–6I). Calculation of a precise KD value was hindered by limitations in drug solubility, but the binding of ME-344 to HO-1 was both specific and direct compared to controls.
Nrf2 and HO-1 expression in human biopsy samples
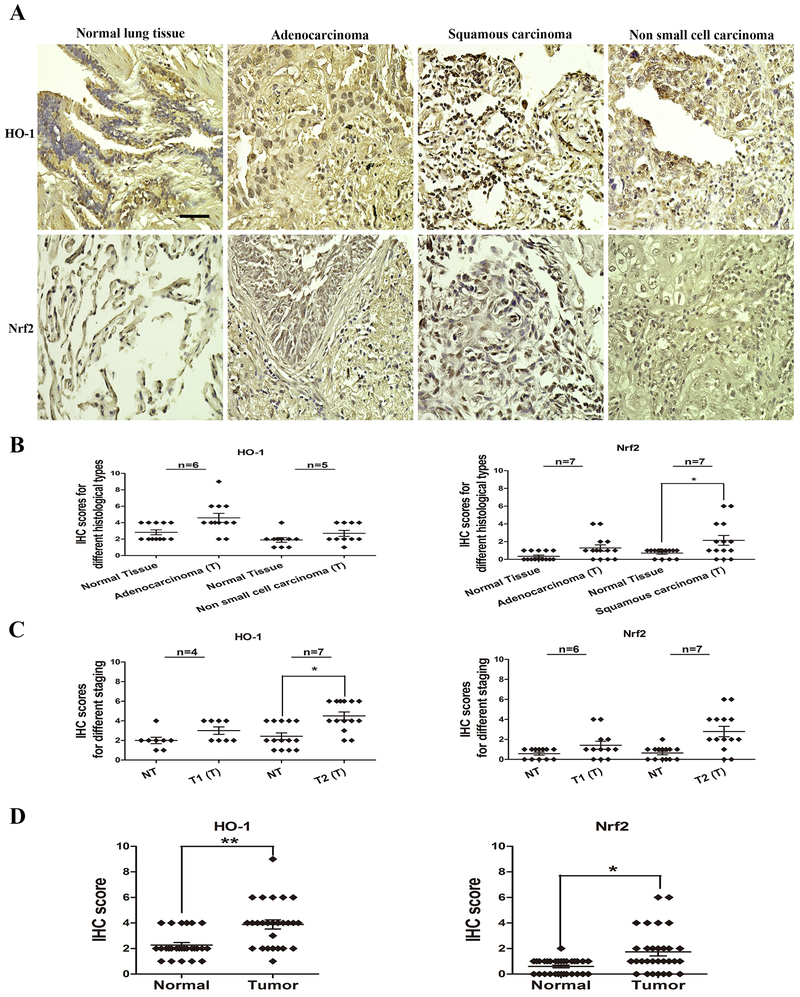
To provide a translational setting for our results, comparative expression of HO-1 and Nrf2 levels were determined in human lung cancer tissues and adjacent normal tissue using microarray (TMA) samples. A total of 13 patients (TMA1) were investigated where the cohort contained 6 adenocarcinomas, 5 non-small cell carcinomas and 2 NA (not assigned). A total of 4 tumors were in TNM stage I, 7 in TNM stage II and 2 tumors NA. A total of 14 patients were involved on TMA5, and the cohort contained 7 adenocarcinomas and 7 squamous cell carcinomas. A total of 6 tumors were in TNM stage I, 7 tumors in TNM stage II and 1 tumor NA. TMA1 was used for HO-1 expression and TMA5 for Nrf2 expression analyses. Representative IHC images of HO-1 and Nrf2 expression in normal lung tissues and lung tumor tissues are shown in Figure 7A. The average immunoreactivity scores of HO-1 and Nrf2 in different histological types are shown in Figure 7B and Supplementary Table S15 and S16. The average immunoreactivity scores for Nrf2 and HO-1 for different tumor stages are shown in Figure 7C and Supplementary Table S17. Comparative expression patterns for Nrf2 and HO-1 indicate significant increased expression in lung tumors compared to those in matched adjacent normal tissues (Figure 7D).
Figure 7. HO-1 and Nrf2 expression in normal and tumor human lung tissues by immunohistochemistry.
A. Representative IHC images of normal or tumor lung tissues. Paraffin sections from tissue microarrays were immunostained with anti-HO-1 and anti-Nrf2 antibodies (brown) and counterstained with hematoxylin (blue). Tumor cores of 13 and 14 different patients were present on the TMA1 (HO-1) and TMA5 (Nrf2), as well as adjacent normal lung tissue cores. There was strong brown staining in tumor lung tissues, but significantly lighter staining in normal tissues (magnification, x400; Bar = 50 μm). Immunohistochemistry analysis of HO-1 and Nrf2 protein levels in normal and tumor lung specimens of different histological types and different stages are shown in B and C. Immunoreactivity is scored as the percentage of positive cells (0–3) multiplied by the intensity of cellular staining (0–3). HO-1 and Nrf2 expression scores in all tissues are shown in D and E. HO-1 and Nrf2 expression patterns are significantly increased in lung tumor tissues. Data are derived from two independent experiments and presented as means ± SEM in the scatter graphs. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the human lung normal tissue by Student’s t-test.
ME-344 induced apoptosis in sensitive cell lines H460 and SHP-77 through caspase 3 activation
As shown in Supplementary Figure S3A, ME-344 induced a markedly high cleavage of caspase 8, caspase 9 in H460 and SHP-77 cell lines. Consequently, caspase 3 was activated, PARP was cleaved and apoptosis induced. Significantly, ME-344 resulted in higher levels of caspase 8 cleavage in H460 cell line than that in SHP-77 cell line. Moreover, in SHP-77 cell line, cleaved caspase 9 levels were higher than that in H460 cell line (Supplementary Figure S3B). PARP cleavage, which was measured to verify the apoptotic pathway, was confirmed in both H460 and SHP-77 cell lines. Overall, these results demonstrate that ME-344 induced cell apoptosis by the activation of caspase 3 that led to the cleavage of PARP.
NAC attenuated ME-344-induced cell death and the increase in ROS level in H460 and H596 cell lines
NAC was used as a thiol scavenger for ROS in both the H460 (sensitive) and H596 cell lines (resistant). In both H460 and H596 cell lines, pretreatment with 5 mM NAC for 4h was associated with a significant increase in GSH generation (Supplementary Table S18), cell viability (Supplementary Table S19), decreased cell resting state ROS and attenuated ROS increase caused by subsequent ME-344 treatments (Supplementary Figure S4A-D). These findings lend support to the observations that ME-344 induced cytotoxicity is linked with ROS production.
Discussion
The goal of the present study was to use complementary techniques to identify direct drug binding/intracellular targeting of ME-344 that might account for the drug’s unusual cytotoxicity through impedance of complexes I and III mitochondrial energy production (18). Initially, our data showed that the essential “redox phenotype” of normal lung fibroblasts paralleled those characteristics found primarily in the intrinsically drug resistant cell lines, while in the parameters measured, intrinsically sensitive cells possessed profiles that were distinct. This was encouraging in so far as suggesting that we could use these cell lines as predictors of ME-344 targets. Following ME-344 treatments, drug sensitive cells had consistently higher levels of ROS (primarily H2O2 and hydroxyl radicals) suggesting that the drug impacted cell target(s) linked with production of these species, presumably mitochondria. For this reason, we focused attention on those pathways that may regulate ROS levels and directly impact redox homeostasis. The increased levels of ROS found in the sensitive cells is consistent with our results that showed accompanying higher expression levels of Nrf2 and downstream gene products regulated by this oxidative stress transcription factor. Moreover, when exposed to ME-344, the induction of these stress-related proteins (including HO-1) was significantly more robust in the sensitive cells. Reaction products of HO-1 include biliverdin and subsequent bilirubin, each of which can protect against ROS. Ferrous iron is also produced and can stimulate ferritin synthesis, also with cytoprotective outcomes. However, potential pro-oxidant consequences of HO-1 can accrue from released iron, perhaps leading to Fenton chemistry production of highly reactive hydroxyl radicals. Thus, contingent upon the localized cellular milieu and redox homeostasis, HO-1 can have both pro- and antioxidant sequelae (10). We used a variety of methods to demonstrate specific ME-344 effects on HO-1. In spite of the fact that HO-1 is considered an attractive and high priority anticancer target, there are presently no clinically useful inhibitors of the enzyme (13,19). From this perspective, our present studies used the complementary surface plasmon resonance (SPR; Biacore) and click chemistry mass spectal techniques to show that while ME-344 may have other effects in cells, the drug does directly bind to, and inhibit, HO-1. For example, from kinetic analyses the inhibition constant (IC50) value for ME-344 was 17.6 nM, similar to that of SnPPIX (11.0 nM), one of the few known and characterized HO-1 inhibitors, albeit without druggable characteristics. With hemin as substrate, the KM value was 5.5 μM and as evidenced by changes in hypsochromic shift and Trp fluorescence patterns, non-competitive inhibition altered the tertiary/quaternary structure of the HO-1 protein.
We generated substantial data relevant to direct drug interaction from the click chemistry experiments, where HO-1 was identified as a target of the modified clickable drug species. While there was a possibility that substituents at the ring hydroxyls (Supplementary Figure S2) might influence the pharmacology of the drug, we determined that the four compounds had essentially equivalent IC50 values, implying that the molecules were comparable. Identification (through immunoblotting) of the alkylated product as HO-1 confirmed that the previously determined ME-344 inhibition of HO-1 activity was concomitant with direct binding to the protein. This principle was confirmed by the SPR results, where the binding had lower avidity than SnPPIX, but appeared direct (Figure 6D). An overall proteomic analysis was used to highlight differences between drug treated and untreated samples. LFQ intensity measurements identified a number of mitochondrial proteins in the drug treated samples, a result in concordance with the reported drug effects on energy production (5). We used two mechanisms to express these data. Both the heat-map (Figure 6C) and the volcano plot (Supplementary Figure S2D) showed protein target enrichment patterns, particularly for mitochondrial proteins. These included HO-1 as well as highlighting voltage dependent anion-selective channels 1 and 2 (VDAC1 and 2). Both channels are critical to functional aspects of the outer mitochondrial membrane, operating to facilitate ATP diffusion into the cytoplasm (20). Also, there is an existing literature detailing numerous proteins that interact with VDAC channels and some are enzymes involved in redox metabolism (21). VDAC-1 and −2 were shown to be bound by the clickable derivative of ME-344 (Supplementary Figure S5A) and it is conceivable that this, in of itself, may have cytotoxic significance. However, we did determine that HO-1 does not co-immunoprecipitate with VDAC 1 or 2 (Supplementary Figure S5B), implying that drug effects on these proteins may not be co-dependent. In light of the involvement of VDAC’s in control of apoptosis (22,23), the observed ME-344 effects on these proteins may be mechanistically important (Supplementary Figure S3, S5C, S5D, and S5E; Supplementary Table S20 and S21), but unlike HO-1, are less likely to explain the altered ROS effects. Taken together, this series of proteomic results following click chemistry drug binding confirmed the mitochondrial selectivity of ME-344 and supported the conclusion that HO-1 is a direct target of the drug. The overall consequences of HO-1 inhibition are likely contributory to the data showing drug-induced increases in ROS and the pattern of mitochondrial protein targeting in line with the observed impact on energy metabolism (4,5,18).
To support these pharmacological observations, we exogenously manipulated either HO-1 expression or GSH levels. It seems that it is not basal ROS levels, but rather the ability of the cells to handle the oxidative stress that determines the sensitivity of the cells to ME-344. Indeed, resistant cell lines (H596 and SW900) have greater capacities to handle the ROS compared to the sensitive (H460 and SHP-77), with consistently lower ROS stimulation (fold-change) and induction of the oxidative stress-related proteins (fold-change) (Supplementary Table S22). Basal levels of ROS are lower in H460 cells (sensitive) than H596 cells (resistant). We generated the H460 HO-1 knockdown cells to mimic the resistant cells’ phenotype (Supplementary Table S23). This resulted in increased basal ROS levels, however, the cells’ ability to deal with the ROS was compromised, with higher ME-344-induced increase in ROS (fold-change). In this case, the cells actually become more sensitive to ME-344. We also generated H596 HO-1 overexpressing cells to mimic the sensitive cells’ phenotype (Supplementary Table S23). As expected, the basal ROS levels decreased, ME-344 caused a greater fold-increase of ROS, meaning that H596 HO-1 overexpressing cells became more sensitive to ME-344 with a lower IC50 value (21 μM compared to 28 μM; Supplementary Figure S1). These results suggest that HO-1 plays a role in drug sensitivity and in fact, similar to our present observations, knockdown of HO-1 has previously been shown to increase ROS generation (24), accounting for increased cytotoxicity. By supplementing the culture conditions with exogenous NAC, cysteine is no longer a rate limiting amino acid and thus GSH biosynthesis is stimulated. Here, we have employed NAC as both a GSH precursor and a scavenger of ROS (reducing H2O2 and hydroxyl radicals). Interpretation of such data is slightly complicated by the fact that under certain circumstances NAC can exert pro-oxidant effects (25). Nevertheless, under the conditions used here, NAC significantly reduced ROS generation and increased IC50 in both H460 and H596 cell lines. These results demonstrate that ROS are part of the cytotoxic properties of ME-344.
Our comparative studies identified basal expression levels of Nrf2 and downstream gene products are higher in cells intrinsically sensitive to ME-344. ME-344 treatments caused greater fold-increases in ROS, reflecting a stoichiometric association with higher ROS stimulating a more robust, if ultimately futile (in terms of cytotoxicity) drug response. These studies were extended into a translational setting by measuring coordinate expression of Nrf2 and HO-1 in human biopsy samples of normal and malignant lung. Increased levels of each have been reported for a broad range of human tumors (13) and this has been associated with poor prognosis and survival and/or resistance to drugs like cisplatin (26), doxorubicin (27) or bortezomib (28). In the present study, we showed increased expression levels of both Nrf2 and HO-1 in lung cancer (compared to normal) biopsy samples, implying that malignancy is accompanied by parallel activation of each, perhaps linked with the increased levels of ROS associated with tumor cell high energy production. Generally, cancer cells exist with a resting state of increased oxidative stress (particularly those in the lung) and the additional load applied by ME-344 and its impact on enzymes such as HO-1 that regulate redox homoeostasis appear to be sufficient to enact mitochondrial based cell killing. Such results provide a framework for interpretation of existing clinical results and provide a rationale for combination studies where HO-1 may be a critical cellular target in diseases such as lung cancer.
Supplementary Material
Statement of Significance:
A novel cytotoxic isoflavone is shown to inhibit heme oxygenase, a desirable yet elusive target that disrupts redox homeostasis causing cell death.
Acknowledgments
This work was supported by grants from the National Institutes of Health (5P20GM103542, COBRE in Oxidants, Redox Balance and Stress Signaling), support from the South Carolina Centers of Excellence program and was conducted in a facility constructed with the support from the National Institutes of Health, Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. Proteomic analysis was performed at the Mass Spectrometry Facility, a University Shared Research Resource at the Medical University of South Carolina, using instrumentation acquired through the NIH shared instrumentation grant program (S10 OD010731-Orbitrap Elite Mass Spectrometer). Financial support from MEI Pharma is also acknowledged.
Abbreviations:
- AE-MS
Affinity enrichment-mass spectrometry
- ALDH
aldehyde dehydrogenases
- ARE
antioxidant response elements
- ATF4
activating transcription factor 6
- Bach1
BTB and CNC Homology 1
- ER
endoplasmic reticulum
- GSH
glutathione
- GSTP
glutathione S-transferase P
- HO-1
heme oxygenase 1
- IRE1
inositol-requiring enzyme 1
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LFQ
label free quantification
- NAC
N-acetyl-L-cysteine
- Nrf2
Nuclear erythroid factor 2-like 2
- PDI
protein disulfide isomerase
- PERK
PKR-like ER kinase
- ROS
reactive oxygen species
- SnPPIX
tin protoporphyrin IX
- SPR
surface plasmon resonance
- UPR
unfolded protein response
- VDAC
voltage-dependent anion-selective channels
- XBP-1
X-box binding protein 1
Footnotes
Disclosure: All authors have no potential conflicts of interest.
Supplementary Information: Supplementary Figures and Tables, Supplementary Materials and Methods
References
- 1.Miguel Quintela-Fandino JVA, Salgado Alfonso Cortes, Mouron Silvana Andrea, Guerra Juan Antonio, Cortes Mana Gion, Morente Manuel, Manso Luis. Abrogation of resistance against bevacizumab (Bev) by mitochondrial inhibition: A phase 0 randomized trial of Bev plus ME344 or placebo in early HER2-negative breast cancer (HERNEBC). J Clinical Oncology 2018;36:abstract 2552. [Google Scholar]
- 2.Bendell JC, Patel MR, Infante JR, Kurkjian CD, Jones SF, Pant S, et al. Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors. Cancer 2015;121(7):1056–63 doi 10.1002/cncr.29155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond JR, Goff B, Forster MD, Bendell JC, Britten CD, Gordon MS, et al. Phase Ib study of the mitochondrial inhibitor ME-344 plus topotecan in patients with previously treated, locally advanced or metastatic small cell lung, ovarian and cervical cancers. Invest New Drugs 2017. doi 10.1007/s10637-017-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SC, Carey KT, McKenzie M. Anti-cancer analogues ME-143 and ME-344 exert toxicity by directly inhibiting mitochondrial NADH: ubiquinone oxidoreductase (Complex I). American journal of cancer research 2015;5(2):689–701. [PMC free article] [PubMed] [Google Scholar]
- 5.Manevich Y, Reyes L, Britten CD, Townsend DM, Tew KD. Redox Signaling and Bioenergetics Influence Lung Cancer Cell Line Sensitivity to the Isoflavone ME-344. J Pharmacol Exp Ther 2016;358(2):199–208 doi 10.1124/jpet.115.229344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med 2015;88(Pt B):108–46 doi 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J 2006;20(14):2651–3 doi 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 8.Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res 2007;35(21):7074–86 doi 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 2002;21(19):5216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 2000;28(2):289–309. [DOI] [PubMed] [Google Scholar]
- 11.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res 1994;54(16):4313–20. [PubMed] [Google Scholar]
- 12.Bahmani P, Hassanshahi G, Halabian R, Roushandeh AM, Jahanian-Najafabadi A, Roudkenar MH. The expression of heme oxygenase-1 in human-derived cancer cell lines. Iran J Med Sci 2011;36(4):260–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Furfaro AL, Traverso N, Domenicotti C, Piras S, Moretta L, Marinari UM, et al. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid Med Cell Longev 2016;2016:1958174 doi 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauber S, Jais A, Jeitler M, Haider S, Husa J, Lindroos J, et al. Transcriptome analysis of human cancer reveals a functional role of heme oxygenase-1 in tumor cell adhesion. Mol Cancer 2010;9:200 doi 10.1186/1476-4598-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinobe RT, Dercho RA, Nakatsu K. Inhibitors of the heme oxygenase - carbon monoxide system: on the doorstep of the clinic? Can J Physiol Pharmacol 2008;86(9):577–99 doi 10.1139/y08-066. [DOI] [PubMed] [Google Scholar]
- 16.Berberat PO, Dambrauskas Z, Gulbinas A, Giese T, Giese N, Kunzli B, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res 2005;11(10):3790–8 doi 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 17.Keilhauer EC, Hein MY, Mann M. Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE-MS) rather than affinity purification mass spectrometry (AP-MS). Mol Cell Proteomics 2015;14(1):120–35 doi 10.1074/mcp.M114.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urra FA, Weiss-Lopez B, Araya-Maturana R. Determinants of Anti-Cancer Effect of Mitochondrial Electron Transport Chain Inhibitors: Bioenergetic Profile and Metabolic Flexibility of Cancer Cells. Curr Pharm Des 2016;22(39):5998–6008. [DOI] [PubMed] [Google Scholar]
- 19.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacological reviews 2008;60(1):79–127 doi 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 20.Rostovtseva TK, Bezrukov SM. ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys J 1998;74(5):2365–73 doi 10.1016/S0006-3495(98)77945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvira CM, Umesh A, Husted C, Ying L, Hou Y, Lyu SC, et al. Voltage-dependent anion channel-2 interaction with nitric oxide synthase enhances pulmonary artery endothelial cell nitric oxide production. Am J Respir Cell Mol Biol 2012;47(5):669–78 doi 10.1165/rcmb.2011-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 2003;301(5632):513–7 doi 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 23.McCommis KS, Baines CP. The role of VDAC in cell death: friend or foe? Biochim Biophys Acta 2012;1818(6):1444–50 doi 10.1016/j.bbamem.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabunia K, Ellison SP, Singh H, Datta P, Kelemen SE, Rizzo V, et al. Interleukin-19 (IL-19) induces heme oxygenase-1 (HO-1) expression and decreases reactive oxygen species in human vascular smooth muscle cells. J Biol Chem 2012;287(4):2477–84 doi 10.1074/jbc.M111.312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagrista ML, Garcia AE, Africa De Madariaga M, Mora M. Antioxidant and pro-oxidant effect of the thiolic compounds N-acetyl-L-cysteine and glutathione against free radical-induced lipid peroxidation. Free Radic Res 2002;36(3):329–40. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Bao L, Zhang Z, Yi X. Nrf2 induces cisplatin resistance via suppressing the iron export related gene SLC40A1 in ovarian cancer cells. Oncotarget 2017;8(55):93502–15 doi 10.18632/oncotarget.19548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, et al. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol Carcinog 2013;52(10):824–34 doi 10.1002/mc.21921. [DOI] [PubMed] [Google Scholar]
- 28.Furfaro AL, Piras S, Domenicotti C, Fenoglio D, De Luigi A, Salmona M, et al. Role of Nrf2, HO-1 and GSH in Neuroblastoma Cell Resistance to Bortezomib. PLoS One 2016;11(3):e0152465 doi 10.1371/journal.pone.0152465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.