Abstract
Purpose:
To develop a prognostic model and cytogenetic risk classification for previously treated patients with chronic lymphocytic leukemia (CLL) undergoing reduced intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (HCT).
Patients and Methods:
We performed a retrospective analysis of outcomes of 606 CLL patients who underwent RIC allogeneic HCT between 2008 and 2014 reported to the Center for International Blood and Marrow Transplant Research.
Results:
Based on multivariable models, disease status, comorbidity index, lymphocyte count and white blood cell count at HCT were selected for the development of prognostic model. Using the prognostic score, we stratified patients into low, intermediate, high, and very high risk (4-year progression-free survival (PFS) 58%, 42%, 33%, and 25%, respectively, p<0.0001; 4-year overall survival (OS) 70%, 57%, 54%, and 38%, respectively, p<0.0001). We also evaluated karyotypic abnormalities together with del(17p) and found that del(17p) or ≥5 abnormalities showed inferior PFS. Using a multivariable model, we classified cytogenetic risk into low, intermediate, and high (p<0.0001). When the prognostic score and cytogenetic risk were combined, patients with low prognostic score and low cytogenetic risk had prolonged PFS (61% at 4-year) and OS (75% at 4-year).
Conclusions:
In this large cohort of previously treated CLL patients who underwent RIC HCT, we developed a robust prognostic scoring system of HCT outcomes and a novel cytogenetic based risk stratification system. These prognostic models can be used for counseling patients, comparing data across studies, and providing a benchmark for future interventions. For future study, we will further validate these models for patients receiving targeted therapies prior to HCT.
Keywords: prognostic scoring system, cytogenetic risk classification, chronic lymphocytic leukemia, allogeneic hematopoietic cell transplantation
INTRODUCTION
In recent years, we have seen a rapid development of targeted therapies against pathways that are constitutively activated in chronic lymphocytic leukemia (CLL). However, despite the clinical effectiveness of these therapies, durability of response to these agents is limited and discontinuation is high due to toxicity or progressive disease in high risk patients (1). Allogeneic hematopoietic cell transplantation (HCT) thus remains the only potentially curative therapeutic modality for patients who fail targeted therapies.
The Dana-Farber Cancer Institute (DFCI) transplant group previously reported an excellent five year progression-free survival (PFS) and overall survival (OS) after reduced intensity conditioning (RIC) HCT for CLL (2). In that study, a prognostic model for PFS was proposed using remission status, HCT comorbidity index (HCT-CI), lymphocyte count and lactate dehydrogenase (LDH) at HCT. Although this model was very predictive of PFS as well as OS, the model needed to be validated in a much larger and multicenter cohort.
Cytogenetic risk is critical to stratifying CLL patients and has been extensively studied in the non-transplant setting. However, controversy remains about the importance of high risk cytogenetics in predicting outcome after HCT. In the non-transplant setting, patients with TP53 or ATM gene mutation, or 17p or 11q deletion by cytogenetics, have markedly poorer survivals (3–5). However, a number of transplant studies have suggested that CLL patients with del(17p) can still achieve long-term remission after transplant, but the sample size of these studies was limited and thus the long-term remission rate for these patients would need to be confirmed in a larger study (2, 6–8). Furthermore, a recent study has suggested that transplant outcome is significantly worse for those CLL patients with complex karyotype, defined as five or more abnormalities rather than the traditional three or more abnormalities (9). However, the sample size of this study was also small. The Center for International Blood and Marrow Transplant Research (CIBMTR) patient population provides the best opportunity to determine the optimal definition of complex karyotype for CLL patients who undergo HCT and to truly assess whether HCT overcomes the adverse prognosis of high risk cytogenetics, including del(17p), del(11q) and complex karyotype, which often includes the respective cytogenetic aberrations.
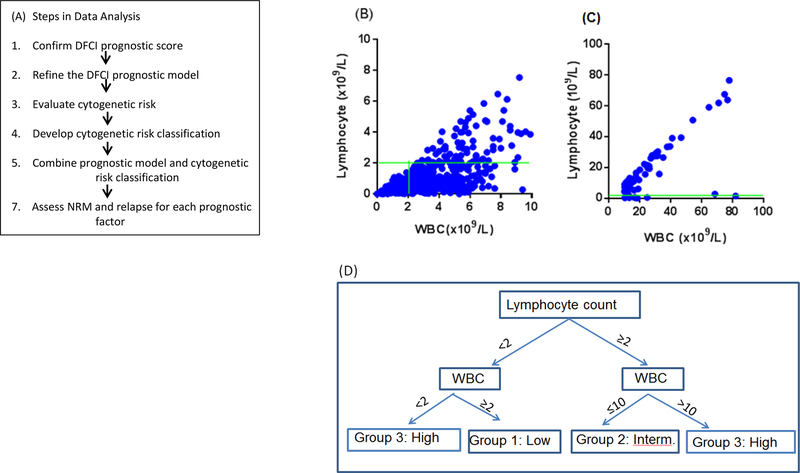
The goals of this study are therefore i) to validate and/or modify the prognostic score developed by the DFCI transplant group (2) using a large independent cohort, ii) to assess whether HCT overcomes the adverse prognosis of high risk cytogenetics, and iii) to develop a cytogenetic risk classification for previously treated CLL patients who underwent RIC HCT. Steps in data analysis are shown in Figure 1A.
Figure 1.
(A) Steps in data analysis. (B) WBC versus lymphocyte count for WBC<10×109/L). (C) WBC versus lymphocyte count for WBC≥10×109/L). Green horizontal line represents lymphocyte count 2×109/L. Green vertical line represents WBC 2×109/L. (D) Classification of lymphocyte count and WBC.
MATERIALS AND METHODS
Data Source
CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a centralized Statistical Center. All CIBMTR research studies are conducted in compliance with U.S. Office of Human Research Protection (OHRP) common rule regulations (45 CFR Part 46) and the Federal Drug Administration (FDA) regulations (21 Code of Federal Regulations (CFR) Part 50 and 21 CFR Part 56). Studies utilizing the CIBMTR database are conducted under its Research Database Protocol. The National Marrow Donor Program/Be the Match central Institutional Review Board (IRB) is fully accredited by the Association for Accreditation of Human Research Protection Programs and has oversight responsibility for all research conducted under the CIBMTR Research Database Protocol. In addition, CIBMTR requires all reporting centers to maintain local IRB approval for the Research Database Protocol and seek consent from patients for use of their data in CIBMTR research. Only patients data that is consented for use in research are included in CIBMTR studies. Additional details regarding the data source are described elsewhere (10).
Patients
Between 2008 and 2014, 1505 patients with a diagnosis of CLL underwent allogeneic HCT with 7/8 or 8/8 matched related or unrelated donors, peripheral blood or bone marrow transplants reported to CIBMTR. Of these, 758 patients had disease specific data available from the CIBMTR comprehensive report form or provided by centers. To ensure this cohort of 758 patients was a representative subset of the larger cohort of 1505 patients, OS and PFS were examined. Both OS and PFS were super-imposable between patients with and without available information (Figure S1) and baseline characteristics were similar (data not shown), suggesting the study cohort is representative of the entire cohort. Of 758 patients, 606 (80%) patients underwent RIC and 152 (20%) underwent myeloablative conditioning (MAC) HCT according to the CIBMTR definition of conditioning intensity (26). In this report, we focus on the 606 patients who underwent RIC during the study period and data on MAC HCT recipients will be reported in a subsequent paper.
Cytogenetic Analysis
We performed expert clinician review of all available primary cytogenetics reports submitted to CIBMTR (J.R.B., M.S.D, V.O.V.). Patients with a standard metaphase karyotype analysis with at least five cells, although the majority had the usual 20, were considered evaluable and categorized as to normal karyotype or by the total number of abnormalities, with complex karyotype defined as either three or more, or five or more, for purposes of further evaluation. Patients with a fluorescence in situ hybridization (FISH) analysis were separately categorized as to presence or absence of del13q, del11q, del17p and trisomy 12.
Statistical Analysis
The primary endpoint for development of the prognostic score and cytogenetic risk classification was progression-free survival (PFS). Other endpoints of interest included overall survival (OS), non-relapse mortality (NRM) and relapse. PFS was defined as the time from stem cell infusion to disease relapse, progression or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the time last seen alive and relapse or progression-free. OS was defined as the time from stem cell infusion to death from any cause. Patients who were alive or lost to follow-up were censored at the time last seen alive. The 1- and 4-year follow-up completeness indices were 99% and 96%, respectively. The Kaplan-Meier method was used to estimate PFS and OS whereas cumulative incidence of NRM and relapse was estimated in the context of a competing risks framework. NRM and relapse were treated as competing events. The log-rank and Gray tests11 were used for comparing estimates of PFS and OS and estimates of cumulative incidence of NRM and relapse, respectively. In addition, univariable Cox regression analysis for PFS was performed (Table S1A); multivariable Cox regression analysis for PFS and OS and multivariable competing risks regression analysis12 for NRM and relapse were performed. Center effect was tested using a frailty model and the effect was not significant (p=0.52). Since adding Center to the model did not affect the final models, the center effect was not further adjusted.
To modify the prognostic model, we performed multivariable Cox regression analysis using a bootstrap validation method with 10000 resamples of size 606 with replacement (13). The bootstrap method was used to adjust the model in order to decrease the impact of overfitting to the original dataset and reducing the influence of unusual or outlying values. Although the split-sample method is commonly used for validation, it greatly reduces the sample size for both the training and validation sets. Furthermore, if the process is repeated with a different split, different regression coefficients may be obtained from the validation set. This is a concern particularly when the sample size is not large (14). The bootstrap validation method overcomes these drawbacks and obtains nearly unbiased estimates without sacrificing sample (14). Using 10000 resamples, the bootstrap sampling distribution of each estimator was established and hazard ratio (HR) and respective 95% confidence intervals were taken from the 50th, 2.5th and 97.5th percentiles of the distribution, respectively.
It is known that many factors in CLL are correlated and collinear (2). Collinearity can cause predictors to compete with each other and standard errors of the regression coefficient estimates can be inflated. As a result, some predictors arbitrarily become non-significant (14). However, collinearity does not affect the joint influence of correlated variables. i.e., some variables are correlated and partially redundant but not identical (e.g., lymphocyte count and white blood cell count (WBC)). We therefore performed unsupervised hierarchical clustering analysis to assess potential collinearity among variables and found that no CR/PR, high WBC and high lymphocyte count were clustered (expected predictors of relapse) and HCT-CI and low WBC were clustered (expected predictors of NRM) (Figure S2A). Since collinearity makes it difficult to estimate regression coefficients while holding (highly correlated) variables constant, we performed multivariable analysis including each of these prognostic variables separately but adjusting for all other variables (Table S1B). Prior to performing Cox regression analysis, the proportional hazards assumption was examined and two-way interaction terms were assessed. The linearity assumption for all continuous variables was examined using the methods of restricted cubic spline function on relative hazard (14) and classification and regression tree for survival data (15,16). From this analysis, WBC was found to be non-linear, indicating both low and high WBC were associated with shorter PFS (Figure S2B). Based on this analysis, WBC was categorized as low (<2), normal (2–10), or high (>10 ×109/L), lymphocyte count was dichotomized as low (<2) or high (≥2×109/L) and HCT-comorbidity index (HCT-CI) was categorized as 0–1 vs. ≥2. The cutoff values for WBC are consistent with our previous study of WBC after HCT (17). We also utilized the Akaike information index (AIC) for the assessment of model fit and C-index (14) for predictive ability of models. All P-values were two-sided at a significance level of 0.05. All calculations were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC) and R version 3.3.2.
RESULTS
Patient characteristics
The median age was 58 (range 26, 73) and 72% were male. At the time of HCT, the median number of prior treatments was 3 (range 1, 10), with 13% in complete remission (CR) and 51% in partial remission (PR). Thirty seven percent received a HLA identical sibling donor HCT and 53% received a 8/8 matched unrelated donor HCT. Thirty two percent of patients had del(17p) and 27% had complex karyotype defined as ≥3 abnormalities (Table 1). For the entire cohort, the median survival time among survivors was 49 months (range 4, 99); the 4-year PFS was 41% (95% confidence interval (CI): 37%, 45%) and the 4-year OS was 56% (95% CI: 52%, 60%).
Table 1:
Baseline characteristics
| N | % | N | % | ||
|---|---|---|---|---|---|
| Total | 606 | 100 | Number of Prior Treatment* | ||
| Age | Median (range) | 3 (1, 10) | |||
| Median (range) | 58 (26, 73) | Donor Type | |||
| < 40 | 12 | 2 | HLA-identical sibling | 225 | 37.1 |
| 40–49 | 71 | 11.7 | 8/8 matched unrelated | 319 | 52.6 |
| 50–59 | 272 | 44.9 | 7/8 matched unrelated | 62 | 10.2 |
| 60–69 | 233 | 38.5 | D-R Sex Match | ||
| ≥ 70 | 18 | 3 | M-M | 273 | 45.1 |
| Patient Sex | M-F | 93 | 15.4 | ||
| Male | 438 | 72.3 | F-M | 131 | 21.6 |
| Female | 168 | 27.7 | F-F | 60 | 9.9 |
| KPS | UNK | 49 | 8.1 | ||
| 90–100 | 415 | 68.5 | D-R CMV Serology | ||
| < 90 | 172 | 28.4 | +/+ | 147 | 24.3 |
| UNK | 19 | 3.1 | +/− | 66 | 10.9 |
| HCT Comorbidity Score | −/+ | 173 | 28.6 | ||
| 0 | 242 | 39.9 | −/− | 168 | 27.7 |
| 1 | 109 | 18 | UNK | 52 | 8.6 |
| 2 | 77 | 12.7 | Graft Source | ||
| ≥3 | 177 | 29.2 | BM | 11 | 1.8 |
| UNK | 1 | 0.2 | PBSC | 594 | 98 |
| LDH (U/L) | UNK | 1 | 0.2 | ||
| Median (range) | 214 (2.4, 2738) | GVHD Prophylaxis | |||
| Normal | 377 | 62.2 | CI + MTX ± other(s) | 280 | 46.2 |
| Elevated | 208 | 34.3 | CI ± other(s) | 313 | 51.7 |
| UNK | 21 | 3.5 | Other | 11 | 1.8 |
| Lymphocyte Count (/μL) | UNK | 2 | 0.3 | ||
| Median (range) | 0.8 (0, 177) | Conditioing Regimen | |||
| ≤ 2000/μL | 449 | 74.1 | RIC | 324 | 53.5 |
| > 2000/μL | 142 | 23.4 | TBI±Other | 35 | |
| UNK | 15 | 2.5 | Flu/Bu±Other | 87 | |
| WBC Count (× 109/L) | Flu/Mel±Other | 88 | |||
| Median (range) | 3.6 (0, 204) | Other | 4 | ||
| < 2 | 84 | 13.9 | NST | 282 | 46.5 |
| 10-Feb | 448 | 73.9 | TBI±Other | 174 | |
| > 10 | 71 | 11.7 | Flu±Other | 52 | |
| UNK | 3 | 0.5 | Other | 56 | |
| Disease Status at HCT | Cytogenetic Abnormality** | ||||
| CR | 78 | 12.9 | Del(13q) | 167 | 35.6 |
| PR | 308 | 50.8 | Trisomy 12 | 66 | 14.1 |
| Nodal PR | 14 | 2.3 | Del(11q) | 108 | 23 |
| SD/PD/Relapse | 197 | 32.5 | Del(17p) | 151 | 32.2 |
| UNK | 9 | 1.5 | Normal | 116 | 24.7 |
| Time from Diagnosis to HCT | Complex 3,4 | 73 | 15.6 | ||
| < 3 yrs | 187 | 30.9 | Complex 5 | 55 | 11.7 |
| 3–6 yrs | 180 | 29.7 | UNK | 137 | 22.6 |
| ≥ 6 yrs | 239 | 39.4 | |||
| UNK | |||||
278 patients have missing information.
some patients fall into multiple categories.
UNK: unknown. KPS: Karnofsky Performance Score. LDH: lactate dehydrogenase. WBC: white blood cell count. CR: complete remission. PR: partial remission. SD: stable disease. PD: progressive disease. D-R: donor-recipient. M-M: male donor & male recipient. M-F: male donor & female recipient. F-M: female donor & male recipient. F-F: female donor & female recipient.BM: bone marrow. PBSC: peripheral blood stem cell. GVHD: graft-versus-host disease. CI: calcineurin inhibitor. MTX: methotrexate. RIC: reduced intensity conditioning. NST:Non-myeloablative conditioning. TBI: total body irradiation. Flu: fludarabine. Mel: Melphalan. TLI: total lymphoid irradiation. Complex 3,4: 3 or 4 cytogenetic abnormalities. Complex 5: 5 or more cytogenetic abnormalities.
Validation of DFCI Prognostic Score
We first examined whether the previously reported prognostic scoring system (2) applied in this cohort. Using this system, PFS for patients with high (score=2) and very high risk (score>=3) are very similar; OS for patients with low (score=0) and intermediate risk (score=1) are similar and OS for patients with high (score=2) and very high risk (score>=3) are also similar. As a result, patients were stratified into three groups in PFS and two groups in OS instead of the initially intended four groups (Figure S3). Because this stratification is not optimal, we examined each of these four factors separately and found that elevated LDH was not associated with PFS (Figure S4A–D).
Lymphocyte count and WBC
It is widely believed that WBC and lymphocyte count are closely related. To investigate if one metric is a surrogate of the other, we plotted lymphocyte count against WBC and found that for WBC ≤10 (x109/L), there was no correlation between these two metrics (Figure 1B). For WBC >10 (x109/L), WBC is nearly linearly associated with lymphocyte count (Figure 1C). To assess the prognostic implication of these metrics, we performed multivariable analysis. Since there is a correlation in high WBC and high lymphocyte count, two multivariable analyses were performed for each metric adjusting for other factors. For high lymphocyte count, HR for PFS was 1.76 (p<0.0001); for low and high WBC, the HR was 1.87 (p<0.0001) and 1.62 (p=0.01), respectively. We then created a joint variable from these two metrics as: low lymphocyte count with WBC≥2×109/L (low risk), 2) high lymphocyte count with normal WBC (intermediate risk), 3) low WBC or high lymphocyte count with high WBC (high risk) (Figure 1D). The hazard ratio for PFS was 1.73 (p=0.0012) for intermediate and 2.05 (p<0.0001) for high risk group compared to low risk group (Table S1B).
Prognostic Risk Classification: Refinement of DFCI score
We then sought to rebuild an improved prognostic scoring system using the CIBMTR cohort. Based on hazard ratios from multivariable models with 10,000 times resampling, one point (HR <1.5) was assigned to no CR/PR and HCT-CI≥2. For WBC and lymphocyte count, 1.5 points were assigned to the intermediate risk group (1.5≤HR≤2) and 2 points (HR>2) were assigned to the high risk group. The summary of score assignment is presented in Table 2A. The sum score, ranging from 0 to 4, was then grouped into 4 categories: 0 (low), 1 (intermediate), 1.5–2.5 (high), ≥3 (very high). Relative to the low risk group, the HR was 1.66 (p=0.0024), 2.36 (p<0.0001), and 2.7 (p<0.0001) for the intermediate, high, and very high risk group, respectively (Table 2B). This prognostic scoring system also stratified patients for OS (HR 1.68, 2.2, 2.76 for intermediate, high and very high risk group, respectively), NRM and relapse (Table 2B).
Table 2:
(A) Score assignment and sum scores for prognostic risk group and combined risk group. (B) Results from multivariable Cox regression models using the bootstrap method with 10000 times of resampling for prognostic scoring system. (C) Results from multivariable Cox regression models using the bootstrap method with 10000 times of resampling for cytogenetic risk classification. (D) Results from multivariable Cox regression models using the bootstrap method with 10000 times of resampling for combined risk classification.
| Score assignment | ||||
|---|---|---|---|---|
| 0 | 1 | 1.5 | 2 | |
| Remission status | CR/PR | No CR/PR | ||
| Lymphocyte count & WBC | <2 & ≥2 | - | ≥2 & <10 | (<2 & <2) or (≥2 & ≥10) |
| HCT-CI | 0–1 | ≥2 | - | - |
| Cytogenetics risk | no del(17p) & no Complex 5 | del(17p) without Complex 3,4 or 5 | - | del(17p) with Complex 3,4 or 5 |
| Sum prognostic score | 0 | 1 | 1.5–2.5 | ≥3 |
| Prognostic risk group | Low | Intermediate | High | Very high |
| Prognostic and cytogenetic risk combined score | 0 | 1–2 | 2.5–3.5 | ≥4 |
| Combined risk group | Low | Intermediate | High | Very high |
| PFS | OS | NRM | Relapse | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Group | N | E | HR (95% CI) | p-value | E | HR (95% CI) | p-value | E | sHR (95% CI) | p-value | E | sHR (95% CI) | p-value |
| Low | 184 | 75 | ref | 55 | ref | 34 | Ref | 41 | ref | ||||
| Intermediate | 143 | 80 | 1.66 (1.18,2.33) | 0.0024 | 59 | 1.68 (1.13, 2.5) | 0.0082 | 43 | 1.89 (1.17, 3.13) | 0.01 | 37 | 1.25 (0.78, 2.01) | 0.35 |
| High | 143 | 97 | 2.36 (1.7, 3.3) | <0.0001 | 75 | 2.2 (1.53, 3.2) | <0.0001 | 41 | 1.78 (1.08, 2.97) | 0.02 | 56 | 2.21 (1.44, 3.49) | <0.0001 |
| Very high | 132 | 97 | 2.7 (1.91, 3.83) | <0.0001 | 81 | 2.76 (1.87, 4.15) | <0.0001 | 57 | 2.68 (1.67, 4.49) | <0.0001 | 40 | 1.65 (1.01, 2.68) | 0.047 |
| PFS | OS | NRM | Relapse | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Group | N | E | HR (95% CI) | p-value | E | HR (95% CI) | p-value | E | sHR (95% CI) | p-value | E | sHR (95% CI) | p-value |
| Low | 298 | 151 | ref | 114 | ref | 79 | ref | 72 | ref | ||||
| Int. | 88 | 55 | 1.56 (1.1, 2.19) | 0.012 | 43 | 1.5 (1.0, 2.22) | 0.048 | 28 | 1.24 (0.73, 2.0) | 0.41 | 27 | 1.52 (0.9, 2.49) | 0.12 |
| High | 83 | 63 | 2.0 (1.4, 2.89) | <0.0001 | 51 | 1.89 (1.27, 2.75) | 0.0016 | 26 | 1.24 (0.73, 2.06) | 0.41 | 37 | 2.11 (1.28, 3.44) | 0.006 |
| PFS | OS | ||||||
|---|---|---|---|---|---|---|---|
| Risk Group | N | E | HR (95% CI) | p-value | E | HR (95% CI) | p-value |
| Low | 116 | 43 | ref | 30 | ref | ||
| Intermediate | 173 | 96 | 1.92 (1.32, 2.89) | 0.0006 | 72 | 1.98 (1.26, 3.32) | 0.004 |
| High | 94 | 63 | 2.62 (1.67, 4.1) | <0.0001 | 50 | 2.63 (1.59, 4.57) | <0.0001 |
| Very high | 86 | 67 | 3.71 (2.44, 5.87) | <0.0001 | 56 | 4.0 (2.43, 6.90) | <0.0001 |
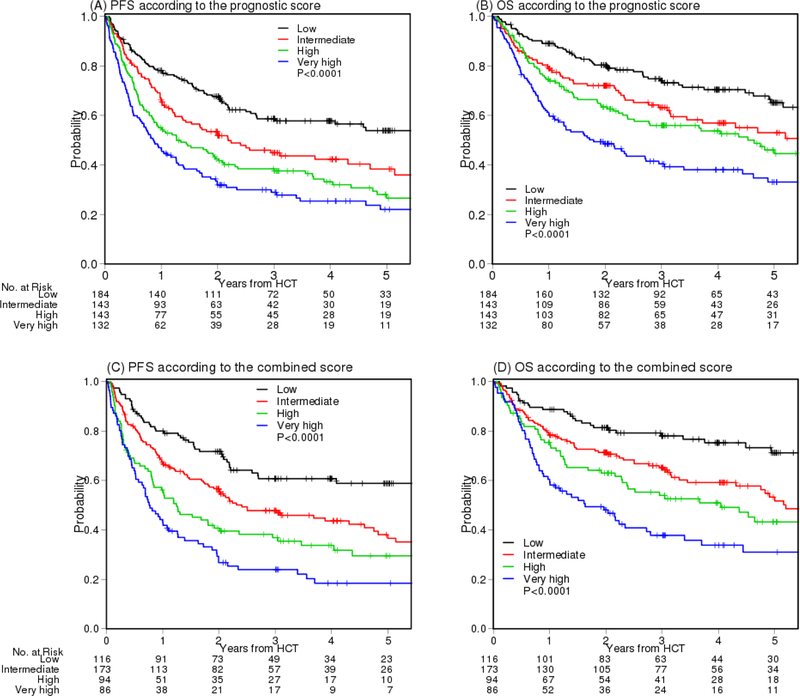
Using this scoring system, the 4-year PFS was 58%, 42%, 33%, and 25%, p<0.0001 and the 4-year OS was 70%, 57%, 54%, and 38%, p<0.0001, for low, intermediate, high and very high risk group, respectively (Figures 2A –2B and Table S2A). The 4-year cumulative incidence of NRM was 19%, 31%, 25%, 44%, p<0.0001, respectively. The 4-year cumulative incidence of relapse was 23%, 27%, 41%, 31%, p=0.007, respectively (Figures S5A –S5B and Table S2A).
Figure 2.
(A) PFS and (B) OS according to the prognostic scoring system. (C) PFS and (D) OS according to the prognostic scoring system and cytogenetic risk classification combined score
Cytogenetic risk classification
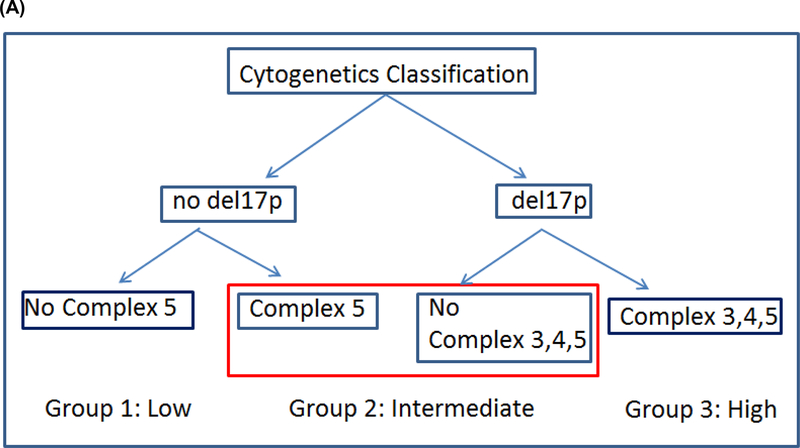
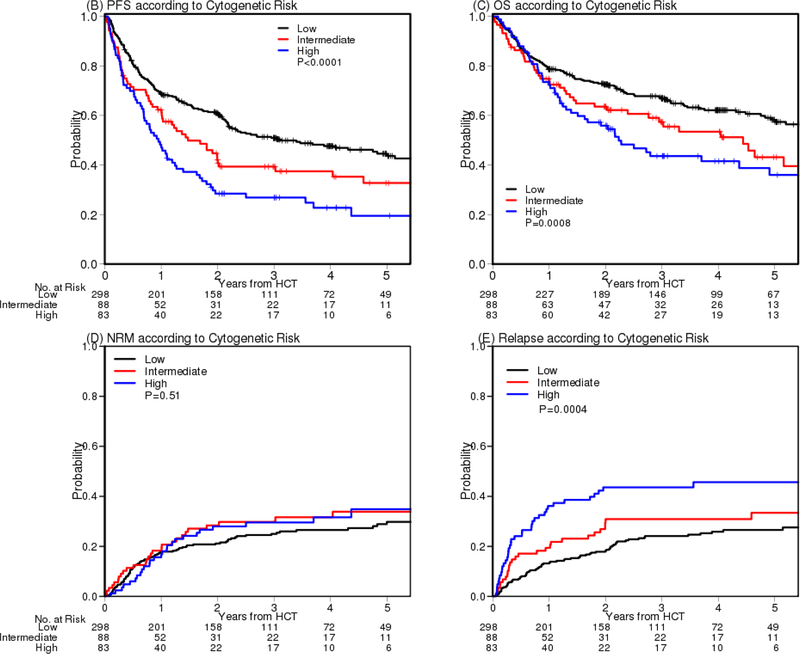
Of 606 patients with RIC, 469 patients had cytogenetics information, which led us to analyze this information separately and then combine with the prognostic score later. To ensure that patients with cytogenetics information are representative of the cohort of 606, we examined PFS and OS as before and the curves are super-imposable between patients with and without this information (Figures S6A–S6B). We then assessed each individual cytogenetic abnormality and found that patients with del(17p) or complex karyotype with ≥5 abnormalities (Complex 5) had significantly worse PFS (Figure S7). We also found that having complex karyotype with 3 or 4 abnormalities (Complex 3–4) was not associated with poor PFS. Using both classification and regression tree (15, 16) for survival data and a multivariable Cox model with 10,000 times resampling, we built a cytogenetics risk classification tree (Figure 3A) and stratified patients into 3 groups: 1) no del(17p) and no Complex 5 (low risk), 2) del(17p) without Complex 3–4, or 5; or Complex 5 without del(17p) (intermediate risk), and 3) del(17p) with Complex 3–4, or 5 (high risk). Relative to the low risk group, HR for PFS was 1.56 (p=0.012) and 2.0 (p<0.0001) for the intermediate and high risk group, respectively. For OS, HR was 1.56 (p=0.048) and 1.89 (p=0.0016) for the intermediate and high risk group, respectively. For relapse, the subdistribution hazard ratio (12) (sHR) was 1.52 (p=0.12) and 2.11 (p=0.006) for the intermediate and high risk group, respectively (Table 2C). Using this classification, the 4-year PFS was 48%, 37%, 23% (p<0.0001), the 4-year OS was 62%, 53%, 42% (p=0.0008) and the 4-year cumulative incidence of relapse was 26%, 31%, 46% for the low, intermediate and high cytogenetic risk groups, respectively (p=0.0004) (Table S2B, Figure 3B–3E).
Figure 3.
Cytogenetics. (A) Classification tree of cytogenetics risk, (B) PFS, (C) OS, (D) cumulative incidence of NRM and (E) cumulative incidence of relapse according to cytogenetic risk classification
Combined Risk Classification
We then examined cytogenetic risk within each prognostic score and found that a small proportion of patients with poor cytogenetic risk within each prognostic score group did poorly, particularly within the low and intermediate risk group (Figure S8). We therefore attempted to combine the prognostic score and cytogenetic risk classification by adding one and two points for patients with intermediate and high cytogenetic risk, respectively, to the prognostic score (Table 2A). The sum score was again grouped into 4 categories based on hazard ratios: low, intermediate, high, and very high. The low risk group now includes patients with low prognostic score and low cytogenetic risk. Relative to the low risk group, the HR for PFS was 1.92 (p=0.0006), 2.62 (p<0.0001), and 3.71 (p<0.0001) for the intermediate, high, and very high risk group, respectively (Table 2D). The result for OS is similar (Table 2D). Using this stratification, the 4-year PFS was 61%, 44%, 34% and 18% (p<0.0001) and 4-year OS was 75%, 59%, 51%, and 34% (p<0.0001) for the low, intermediate, high, and very high risk group, respectively (Figure 2C–2D, Table S2C). Because patients with high cytogenetic risk had poor outcome irrespective of the prognostic risk, if the cytogenetics information is also available, both sets of information should be utilized to risk stratify patients.
Prognostic Factors for NRM and Relapse
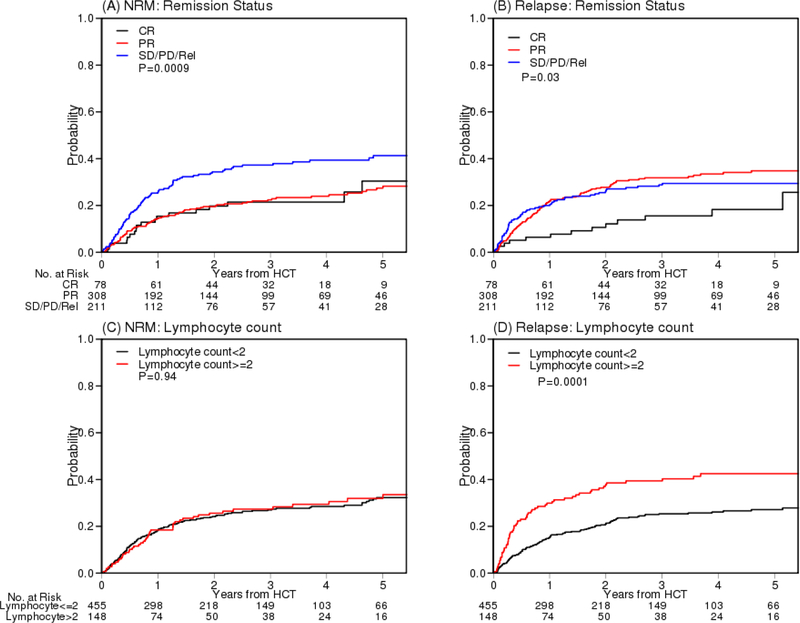
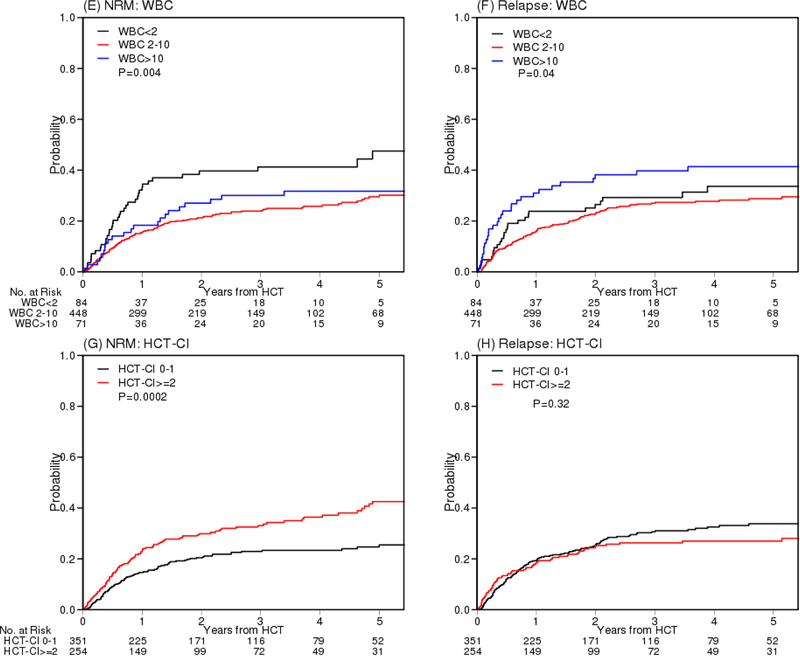
To further identify which factors contribute to NRM and relapse, we performed multivariable competing risks regression analysis using the bootstrap method. Less than PR (sHR 1.89, 95% CI 1.13–3.52, p=0.013), low WBC (sHR 2.05, p=0.002), and high HCT-CI (sHR 1.59, p=0.005) were significant factors for NRM whereas less than CR (sHR 2.02, p=0.0046), high lymphocyte count (sHR 2.0, ,p=0.0002) and high WBC (sHR 1.71, p=0.034) were significant factors for relapse (Table S3). The cumulative incidences of NRM and relapse of these factors are presented in Figure 4. PFS and OS according to remission status and WBC group are presented in Figure S9.
Figure 4.
Cumulative incidence of NRM and relapse according to according to remission status (A, B), lymphocyte count (C, D), WBC (E, F) and HCT-CI (G, H). WBC: white blood cell count. HCT-CI: hematopoietic cell transplantation comorbidity index.
Assessment of Other Factors
We examined the impact of the conditioning intensity (non-myeloablative vs reduced intensity conditioning) in a multivariable model and found it not significant. In fact, the PFS and OS curves are superimposable (Figure S6C–S6D). We therefore did not consider this factor further.
We also explored other potential prognostic factors that had incomplete data. The sample size for these factors ranged from 180 to 328 out of 606. These included bulky adenopathy, prior spleen involvement, prior chemotherapy, refractoriness to fludarabine, refractoriness to chemotherapy, prior rituximab and number of lines of prior treatment. Of these factors, refractoriness to fludarabine and refractoriness to chemotherapy were significantly associated with PFS (HR 1.54, p=0.023; HR 1.53, p=0.017, respectively). In addition, these variables were highly correlated with CR/PR (r=0.79 p<0.0001 with refractoriness to chemotherapy, r=0.53, p<0.0001, with refractoriness to fludarabine) and were therefore well represented by disease status in the model. For example, 89% of patients who were not chemo-refractory were responders, whereas chemo-refractory patients were all non-responders. In addition, the number of prior treatments was significantly associated with PFS (HR 2.48 for >=2 vs <2, p=0.0007). The number of prior treatments was also strongly associated with disease status as 74% of patients without CR/PR had 3 or more prior treatments (p=0.005).
DISCUSSION
In this large cohort of previously treated high risk CLL patients who underwent RIC HCT in a multicenter setting, we first assessed the previously developed DFCI model. We then further modified the model and developed a more robust prognostic scoring system that effectively risk-stratifies CLL patients at the time of HCT. We also developed a novel cytogenetic based risk stratification system which has been lacking in the HCT setting. When the prognostic score was combined with the cytogenetic risk, patients in the low risk group had a prolonged survival (4-year OS 75%) compared to patients in the very high risk group (4-year OS 34%). This result shows that despite the fact that these patients had already failed multiple other therapies and thus transplant was offered as the last resort, transplant outcome is promising, particularly for patients with low risk. By analyzing for the first time the impact of complex karyotype with or without del(17p) in the context of HCT, we were able to establish the prognostic impact of both complex karyotype, and del(17p), particularly in the context of complex karyotype.
In this study, we refined cutoff values of lymphocyte count and HCT-CI. In the previous DFCI study (2), the cutoff value for lymphocyte count was 1×109/L. However, this cutoff value was based on a small number of patients (N=76). With a much larger sample size, we were able to determine the prognostic cutoff value for lymphocyte count to be 2×109/L and HCT-CI ≥2. In many clinical studies, WBC is typically assumed to be linear in relation with outcome. That is, the higher the value, the worse the outcome. Because 14% of our patients had low WBC at baseline and these patients had poor outcomes, WBC loses its prognostic significance if WBC is simply dichotomized into >10 vs ≤10. More importantly, because low WBC may reflect bone marrow dysfunction or a defective marrow microenvironment, whereas high WBC reflects disease burden, both low and high WBC are prognostic factors and must be analyzed separately. Indeed, consistent with this result, in our previous study of WBC, low WBC was associated with an increased risk of NRM whereas high WBC was associated with an increased risk of relapse (17), as also demonstrated in the current data. Furthermore, we found that high lymphocyte count is correlated with high WBC. However, for WBC<10, there is no clear correlation between these two metrics and neither predicts the other. We also found that low WBC is a significant prognostic factor in CLL patients.
Patients with CR at the time of HCT had lower relapse and thus better PFS compared to those with PR (Figures 4B and S9A). However, the OS is similar (Figure S9B), indicating that repeat HCT, DLI or other salvage therapies, likely provided benefit to patients with PR who experienced relapse. When those patients with CR in the low risk prognostic score group (N=38) were compared to those with PR (N=146), the hazard ratio for PFS was not significantly improved (HR 0.84, p=0.57). i.e., if patients were in remission without any other risk factors, the outcome is similar between CR and PR. We therefore collapsed CR and PR in the prognostic model but this warrants further investigation in future studies.
Controversy remains about the importance of high risk cytogenetics in predicting outcome after HCT. In a non-transplant setting, due to the difficulty of obtaining metaphase karyotypes from non-dividing CLL cells, the Dohner hierarchical model (3), which utilizes FISH and not standard karyotype analysis, came into widespread use. As a result, complex karyotype is not well defined in CLL and has typically borrowed the definition from acute leukemias (i.e., ≥3 or ≥4 abnormalities) (18, 19). Recent studies have evaluated the impact of complex karyotype in the context of standard therapy and found it to be adverse (20–22). In the transplant setting, a small study (N=51) performed by Jaglowski et al (9) reported that outcome is significantly worse for those CLL patients with ≥5 abnormalities (HR = 4.75, 95% CI: 2.12–10.6, P=0.0001). Consistent with this finding, our study found that HCT outcome is significantly worse for patients with ≥5 abnormalities. Our study also defined a novel interaction between complex karyotype and del(17p); HCT overcomes any adverse effect of 3 or 4 abnormalities unless these include del(17p). When we assessed the interaction between high risk FISH and complex karyotype, patients without del(17p) and with fewer than 5 abnormalities had low cytogenetic risk whereas patients with del(17p) with ≥3 abnormalities had high cytogenetic risk. Furthermore, patients with del(11q) alone or del(11q) with ≥3 abnormalities did not have a poor outcome after HCT.
We acknowledge limitations of this study including its retrospective design. In this particular setting, however, this approach can also be a strength that improves upon our prior single center study (2) since the study cohort represents all patients from a comprehensive data set derived from a diverse spectrum of transplant programs worldwide and thus avoids potential selection bias, inflated efficacy and underestimated real world toxicity. Although data on CLL karyotype is a great strength of our study, a potential limitation is that other data were not available. For example, CIBMTR did not collect information on prior targeted therapy during the study period, although the majority of this study period pre-dates the era of approved targeted therapy in CLL. Also, CIBMTR does not capture certain disease-specific prognostic information, such as IGHV mutation status, TP53 mutation status and beta 2 microglobulin (B2m). Again, neither IGHV mutation status nor TP53 mutation status were commonly performed in clinical practice at the time that most of these patients were diagnosed or undergoing transplant. Furthermore, at time of HCT, the prognostic value of these markers is not well established, nor do most patients have sufficient circulating disease to measure these even if desired. In our previous study of del(17p) in the non-transplant setting (23), 88% of patients with TP53 mutation had del(17p) in parallel. Due to this overlap, we can assume that TP53 mutation information has likely been well represented through del(17p). In our previous study (2), B2m was not included in the prognostic model due to missing data. However, we noticed that B2m was highly correlated with disease status and may therefore be well represented, as many prognostic factors in CLL are highly correlated. A final limitation is that we excluded patients receiving less than 7/8 matched donor transplants. Therefore, caution is required before extrapolating these results to heavily mismatched transplants such as umbilical cord blood or haploidentical transplant recipients.
Some known prognostic factors in HCT are not included in the prognostic model. These include age, HLA matching and patient and donor sex mismatch. Our hazard ratio for patients age 70 or higher was 1.8 (p=0.07). Although this is not significant at the 0.05 level because of the small number of patients in this age group (N=18), the importance of advanced age should not be discounted. For patients age 60–69, the hazard ratio was 1.17 and 1.1 for age 50–59 compared to patients age <50. The hazard ratio for the 7/8 matched unrelated cohort here was 1.04 (p=0.82) compared to HLA identical sibling donors. In a recent retrospective study of CLL patients by the European Society for Blood and Marrow Transplantation (EBMT) (24), risk factors for PFS included age and patient donor sex mismatch (HR=1.4 for male patients with female donors (F→M) compared to male patients with male donors (M→M), 95% CI 1.1, 1.8, p=0.01). We examined sex mismatch in a multivariable model and the hazard ratio for PFS was 1.09 (95% CI 0.82, 1.43) for F→M compared to M→M. This is consistent with our previous report for patients with all hematologic malignancies using the CIBMTR data (25). In that study, we reported that patient sex and not sex mismatch was detrimental. An important difference between the EBMT and our current study is that the EBMT study included patients with myeloablative conditioning and largely relied on HCT risks rather than on CLL risks. Furthermore, the EBMT study did not assess the impact of complex karyotype as the cytogenetic abnormalities were classified according to Dohner et al (3) and thus cytogenetic study results between these two studies are not directly comparable. Nonetheless, patients with del(17p) in the EBMT study had a poor prognosis compared to patients with other abnormalities (24). We did not investigate NRM specific or relapse specific prognostic factors. Since PFS is a composite endpoint of NRM and relapse, factors associated with increased risk of NRM but decreased risk of relapse (or vice versa) were therefore not selected since the net effect was negated due to the opposing effects.
In summary, using readily available data, we have developed a prognostic scoring system and cytogenetic risk classification that risk-stratifies CLL patients who undergo RIC HCT. We demonstrate in a large well characterized cohort that patient factors as well as del(17p) and complex karyotype with more than 5 abnormalities are both associated with poorer outcomes, consistent with recent observations also with novel agents. These can be used for counseling patients, comparing data across studies, and providing a benchmark for the evaluation of future interventions. For future study, we plan to validate all three systems using an independent dataset for patients receiving targeted therapies prior to HCT, although we suspect that the genetic and/or risk factors used for our risk scoring systems will still be significant as disease burden (represented by remission status, lymphocyte count, and WBC), patient fitness (HCT-CI) and cytogenetic risk are the main drivers of clinical outcomes in CLL. Because the durability of response to targeted therapy in a high risk patient population is relatively low, ultimately an integrative approach of transplant and targeted therapy, that is, inducing remission in high risk patients with targeted therapy, offering HCT during remission, and potentially reinstating targeted therapy for consolidation post HCT, might enhance the clinical outcome of these patients. Alternative approaches, such as CAR T therapy, are also under investigation.
Supplementary Material
Statement of Significance:
Based on pretransplant factors from multivariable models, patients with previously treated CLL undergoing RIC HCT were risk stratified into low, intermediate, high and very high risk groups (p<0.0001). Complex karyotype is optimally defined and high risk cytogenetics found to include del(17p) and ≥5 karyotypic abnormalities. Using a multivariable model, cytogenetic risk was classified as low, intermediate, and high (p<0.0001).
TRANSLATIONAL RELEVANCE.
In this large retrospective analysis, patients with previously treated CLL undergoing RIC HCT were risk stratified into low, intermediate, high and very high risk groups (p<0.0001) based on readily available pretransplant factors using multivariable models. Furthermore, complex karyotype is determined to be best defined as 5 or more karyotypic abnormalities; high risk cytogenetics include del(17p) and complex karyotype. Using a multivariable model, cytogenetic risk was classified as low, intermediate, and high (p<0.0001). This is the first large study to define complex karyotype and to be adequately powered to evaluate its risk in relation to allogeneic HCT outcome as well as in relation to del(17p). Both prognostic and cytogenetic risk models can be used, either separately or combined, for counseling patients undergoing RIC HCT. For future study, these models will be validated using an independent dataset for patients receiving targeted therapies prior to HCT.
Financial Support
This work was supported by NIH grants R01CA183559–02. JRB acknowledges support from NCCN, from NCI (R01CA213442; P01CA206978) and from the Susan and Gary Rosenbach Fund for Lymphoma Research and the Melton Family Fund for CLL Research.
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
* Corporate Members
Footnotes
Conflicts of Interest: JRB has served as a consultant for Abbvie, Acerta, Astellas, Beigene, Genentech/Roche, Gilead, Juno/Celgene, Kite, Loxo, Novartis, Pfizer, Pharmacyclics, Redx, Sun, Sunesis, TG Therapeutics, Verastem; received honoraria from Janssen and Teva; received research funding from Gilead, Loxo, Sun and Verastem; and served on data safety monitoring committees for Morphosys and Invectys. The other authors declare no potential conflicts of interest
REFERENCES
- 1.O’Brien S, Furman RR, Coutre S, Single-Agent Ibrutinib in Treatment-Naïve and Relapsed/Refractory Chronic Lymphocytic Leukemia: A 5-Year Experience. Blood. 2018. February 2 pii: blood-2017-10-810044. doi: 10.1182/blood-2017-10-810044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR, Kim HT, Armand P, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia 2013;27:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine 2000;343:1910–6. [DOI] [PubMed] [Google Scholar]
- 4.Oscier DG, Gardiner AC, Mould SJ, et al. Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood 2002;100:1177–84. [PubMed] [Google Scholar]
- 5.Van Dyke DL, Werner L, Rassenti LZ, et al. The Dohner fluorescence in situ hybridization prognostic classification of chronic lymphocytic leukaemia (CLL): the CLL Research Consortium experience. Br J Haematol. 2016. April;173(1):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JR, Kim HT, Li S, et al. Predictors of improved progression-free survival after nonmyeloablative allogeneic stem cell transplantation for advanced chronic lymphocytic leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2006;12:1056–64. [DOI] [PubMed] [Google Scholar]
- 7.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 2010;116:2438–47. [DOI] [PubMed] [Google Scholar]
- 8.Krämer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017. September 21;130(12):1477–1480. [DOI] [PubMed] [Google Scholar]
- 9.Jaglowski SM, Ruppert AS, Heerema NA, et al. Complex karyotype predicts for inferior outcomes following reduced-intensity conditioning allogeneic transplant for chronic lymphocytic leukaemia. British journal of haematology 2012;159:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz M The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42 Suppl 1:S1–S2. [DOI] [PubMed] [Google Scholar]
- 11.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics 1988;16:1141–54. [Google Scholar]
- 12.Fine JP, Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 13.Efron and Tibshirani (1993). An Introduction to the Bootstrap, Chapman &Hall/CRC [Google Scholar]
- 14.Harrell FE (2001). Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer. [Google Scholar]
- 15.Breiman L, Friedman JH, Olshen RA, and Stone CJ (1984) Classification and Regression Trees. Wadsworth [Google Scholar]
- 16.Therneau TM and Atkinson EJ. An introduction to recursive partitioning using the rpart routines Divsion of Biostatistics 61, Mayo Clinic, 1997. [Google Scholar]
- 17.Kim HT, Frederick D, Andler E, et al. Prognostic Significance of White Blood Cell Counts on Clinical Outcome After Allogeneic Hematopoietic Cell Transplantation. Am J Hematol. 2014. May;89(6):591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000. December 15;96(13):4075–83. [PubMed] [Google Scholar]
- 19.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010. July 22;116(3):354–65. [DOI] [PubMed] [Google Scholar]
- 20.Byrd JC, et al. (2015). Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones J, et al. (2016). Evaluation of 243 Patients with Deletion 17P Chronic Lymphocytic Leukemia Treated with Ibrutinib: A Cross-Study Analysis of Treatment Outcomes. Haematologica 101(S1): 150. [Google Scholar]
- 22.Herling CD, Klaumünzer M, Rocha CK, et al. Complex karyotypes and KRAS and POT1 mutations impact outcome in CLL after chlorambucil-based chemotherapy or chemoimmunotherapy Blood 2016. July 21;128(3):395–404. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Kim HT, Kasar SN, et al. Survival of del17p CLL depends on genomic complexity and somatic mutation. Clin Cancer Res. 2017. February 1;23(3):735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schetelig J, de Wreede LC, van Gelder M, et al. Risk factors for treatment failure after allogeneic transplantation of patients with CLL: a report from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2017. April;52(4):552–560 [DOI] [PubMed] [Google Scholar]
- 25.Kim HT, Zhang MJ, Woolfrey AE, et al. Donor and Recipient Sex in Allogeneic Stem Cell Transplantation: What Really Matters. Haematologica 2016. October;101(10):1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacigalupo A, Ballen K, Rizzo D, et al. : Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15:1628–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.