Abstract
Purpose:
The first-in-human clinical trial with human bolus i.v. infusion IL-15 (rhIL-15) was limited by treatment-associated toxicity. Here, we report toxicity, immunomodulation, and clinical activity of rhIL-15 administered as a 10-day continuous intravenous infusion (CIV) to patients with cancers in a phase I trial.
Patients and Methods:
Patients received treatment for 10 days with CIV rhIL-15 in doses of 0.125, 0.25, 0.5, 1, 2, or 4 mcg/kg/day. Correlative laboratory tests included IL-15 pharmacokinetic (PK) analyses, and assessment of changes in lymphocyte subset numbers.
Results:
Twenty-seven patients were treated with rhIL-15; 2 mcg/kg/day was identified as the maximum tolerated dose (MTD). There were 8 serious adverse events including 2 bleeding events, papilledema, uveitis, pneumonitis, duodenal erosions and 2 deaths (one due to likely drug-related gastrointestinal ischemia). Evidence of antitumor effects were observed in several patients, but stable disease was the best response noted. Patients in 2 mcg/kg/day group had a 5.8-fold increase in number of circulating CD8+ T cells, 38-fold increase in total NK cells, and 358-fold increase in CD56bright NK cells. Serum IL-15 concentrations were markedly lower during the last 3 days of infusion.
Conclusion:
This phase I trial identified the MTD for CIV rhIL-15 and defined a treatment regimen that produced significant expansions of CD8+ T and NK effector cells in circulation and tumor deposits. This regimen has identified several biological features, including dramatic increases in numbers of NK cells, supporting trials of IL-15 with anticancer monoclonal antibodies to increase antibody-dependent cell-mediated cytotoxicity (ADCC) and anticancer efficacy.
Introduction
The goal of immunotherapy is to direct the immune system to attack patients’ cancers. Initial attempts in clinical trials to enhance latent immune responses focused on stimulatory cytokines such as interleukin-2 (IL-2) or interferon alpha (1–6). Results from multiple clinical trials led to FDA approval of high-dose IL-2 (HDIL-2) for treatment of patients with metastatic renal cell carcinoma and metastatic melanoma. The severity of systemic toxicities caused by intensive cytokine regimens, especially with HDIL-2, was a key factor prompting the search for other immunotherapeutic cytokines with the benefits of IL-2 but with fewer negative adverse events (AEs).
IL-2 and IL-15 both stimulate proliferation of T cells, induce generation of cytotoxic lymphocytes and memory phenotype CD8 T-cells, and stimulate prolonged expansion of natural killer (NK) cells (7–30). In contrast to IL-2, IL-15 did not mediate activation-induced cell death (AICD), less consistently activated Tregs, and caused less capillary leak syndrome in mice and nonhuman primates (8). Furthermore, preclinical studies of IL-15 showed expansion and activation of NK cells and CD8 memory T-cells with superior antitumor efficacy in mice compared to IL-2, IL-7, and IL-21 (7).
We performed a first-in-human trial of Escherichia coli-produced recombinant human IL-15 (rhIL-15) where treatment was given as daily 30-minute intravenous bolus (IVB) infusions. Unexpectedly, post-infusion toxicities limited dose escalation and clinical evaluation (31). There was a consistent temporal pattern of post-treatment AEs with fevers, rigors, and hypotension that overlapped with maximum serum concentrations of IL-6 and interferon gamma (IFN-γ) and led to the conclusion that rhIL-15 was too difficult to administer by this schedule. The exceedingly high initial IL-15 Cmax levels were sufficient to contribute to toxicity observed, as well as to allow induction of NK and CD8 T-cells--the biological effects sought. To reduce Cmax we evaluated alternative dosing strategies including subcutaneous (SC) and continuous intravenous infusion (CIV) administrations (32, 33). We report the results of our phase I dose-escalation trial with CIV rhIL-15 in patients with advanced metastatic refractory solid tumors.
Materials and Methods
Recombinant human Interleukin-15 (rhIL-15)
Recombinant human Interleukin-15 (rhIL-15) was produced in Escherichia coli (E. coli) (31, 34).
Patients and Study Design
Patients with advanced metastatic solid tumors for which standard curative or palliative treatments did not exist were enrolled in this phase I, open-label, nonrandomized phase I dose-escalation trial to determine the safety and toxicity of IL-15 in patients with metastatic malignancy. Patients with CIV for 10 consecutive days received IL-15 at a starting dose of 0.1 mcg/kg/day. Dose escalation proceeded in a 3 + 3 standard escalation to dose levels of 0.25, 0.5, 1, 2, and 4 mcg/kg/day. Patients receiving the 10-day treatment schedule without evidence of ongoing response after any 2 consecutive cycles of treatment discontinued rhIL-15. Patients manifesting an ongoing response defined as >15% decrease in sum of marker lesions and/or improvement or disappearance of some non-measurable lesions and/or >10% decrease in tumor markers received additional cycles. Cycles 1 and 2 were 42 days in length but all subsequent cycles were 28 days in length. Toxicities of only the first cycle were considered in selecting the MTD/RP2D. It is possible that cumulative effects may be important in future selection of the dose recommended. The study was approved by the Institutional Review Board (IRB) of the National Cancer Institute, National Institutes of Health. The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki ethical principles of medical research. All patients signed a written informed consent for participation in the clinical trial. rhIL-15 was produced under current good manufacturing practice conditions in the Escherichia coli expression system, as previously described (1).
Supplementary Table 1 summarizes patient demographics and treatment history Investigational Treatment.
The rhIL-15 was delivered intravenously by infusion or ambulatory pump for 10 consecutive days (240 hours). The rhIL-15 was diluted to a concentration of 1 mcg/mL with 0.1% human serum albumin to improve stability and was administered at doses of 0.125, 0.25, 0.5, 1, 2, or 4 mcg/kg per day.
Correlative Laboratory Analyses (see Supplementary material)
PK analysis of serum IL-15 levels was performed during cycle 1 on serum samples obtained; immediately prior to the first dose, at 10 minutes after the first dose, at 1, 2, 4, 8, 12, approximately 24, and approximately 48 hours after the first dose, once daily on days 7, 8, 9, and 10, at the completion of treatment, at 10 and 30 minutes after the completion of treatment, and at 1, 2, 4, and approximately 24 hours after completion of treatment. Patients were assessed for development of anti-IL-15 antibodies using a technique described previously (31).
Clinical Assessment
Patients had regular measurement of vital signs, oral intake and output, physical examination, daily chemistry and hematology laboratories. Patients had restaging CT scans and/or other appropriate radiographic procedures to evaluate their disease after cycle 1, cycle 2, and every two cycles thereafter with antitumor response assessed by RECIST 1.1 criteria (35).
Results
From July 2012 through January 2017, 27 patients with a variety of refractory cancers were treated with CIV rhIL-15. As shown in Supplementary Table 1, the most common diagnoses were head and neck cancer (n=4), sarcoma (n=4), pancreatic cancer (n=3) and renal cell carcinoma (n=3). The median age of patients was 60 years, 8 patients were 65 years or older. The median number of prior therapeutic interventions was 4 (range 2 to 10).
Safety and Toxicity
Clinical Adverse Events
Patients treated with the CIV rhIL-15 regimen experienced typical recombinant cytokine side effects. Patients treated at 1 mcg/kg/day or higher levels frequently demonstrated a mild erythematous, sometimes pruritic rash. Interestingly, two patients treated at 2 mcg/kg/day developed a malar “butterfly rash” (Supplementary Figure 1) classically associated with systemic lupus erythematosus, though neither patient had other findings consistent with this disease, nor developed any autoantibodies.
There were a total of eight serious adverse events (SAEs) possibly, probably, or definitely drug related (Table 1). There were two significant bleeding events (duodenal hemorrhage in a 1 mcg/kg treated patient from erosions caused by a duodenal stent, and bronchial hemorrhage in a 0.25 mcg/kg treated patient from known intrabronchial tumor mass that became necrotic) both definitely related to disease. One patient treated at 0.5 mcg/kg had delayed recurrence of her grade 2 gastritis, with biopsy showing a brisk infiltrate of CD4+ lymphocytes. A patient with melanoma treated at 1 mcg/kg developed isolated grade 3 papilledema without evidence of elevated intracranial pressure with MRI scans used to determine new enhancing lesions. A patient with melanoma treated at 1 mcg/kg dose level developed repeated episodes of blurred vision in the week after discontinuing treatment for disease progression. Because of the concern for new CNS metastases the patient had an MRI which demonstrated no intracranial lesions but did show bilateral focally enhancing retinal abnormalities felt to represent isolated papilledema.
Table 1:
Treatment Related Clinical Adverse Events and Laboratory Abnormalities
| Treatment Related Adverse Events | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.125 mcg n = 4 |
0.25 mcg n = 3 |
0.5 mcg n = 3 |
1 mcg n = 6 |
2 mcg n = 9 |
4 mcg n = 2 |
||||||||||||||||||||
| CTC Grade | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 |
| Chills | 2 | 1 | 1 | 1 | 5 | ||||||||||||||||||||
| Cognitive disturbance | 1 | 1 | 1 | ||||||||||||||||||||||
| Dyspnea | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||
| Edema | 1 | 1 | 2 | 1 | 6 | ||||||||||||||||||||
| Fatigue/malaise | 1 | 1 | 1 | 1 | 5 | 1 | 1 | ||||||||||||||||||
| Fever | 1 | 1 | 2 | 1 | 3 | 2 | 6 | 2 | 1 | 1 | |||||||||||||||
| Hypotension | 1 | 3 | 1 | 1 | |||||||||||||||||||||
| Nasal congestion/rhinitis | 1 | 1 | 1 | ||||||||||||||||||||||
| Nausea | 1 | 1 | 5 | 1 | |||||||||||||||||||||
| Pruritus, Rash, desquamation | 1 | 2 | 6 | ||||||||||||||||||||||
| Vomiting | 2 | 1 | |||||||||||||||||||||||
| Duodenal erosions | 1 | ||||||||||||||||||||||||
| Papilledema | 1 | ||||||||||||||||||||||||
| Bleeding | 1 | 1 | |||||||||||||||||||||||
| Pneumonitis | 1 | ||||||||||||||||||||||||
| Uveitis | 1 | ||||||||||||||||||||||||
| Bowel ischemia | 1 | ||||||||||||||||||||||||
| Laboratory Abnormalities | |||||||||||||||||||||||||
| Anemia | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 3 | 7 | 1 | |||||||||||||
| ALT | 2 | 1 | 3 | 2 | 6 | 1 | 1 | ||||||||||||||||||
| AST | 1 | 1 | 2 | 1 | 1 | 7 | 1 | 1 | |||||||||||||||||
| Alkaline Phosphatase | 2 | 2 | 1 | 3 | 5 | 2 | |||||||||||||||||||
| Bilirubin | 1 | 1 | 1 | 1 | |||||||||||||||||||||
| Hypoalbuminemia | 1 | 1 | 1 | 1 | 3 | ||||||||||||||||||||
| Lymphopenia | 3 | 1 | 2 | 2 | 6 | 2 | 5 | 1 | 1 | ||||||||||||||||
| Neutropenia | 1 | 1 | 4 | 1 | 1 | ||||||||||||||||||||
| Thrombocytopenia | 1 | 2 | 1 | 3 | 1 | 4 | 1 | ||||||||||||||||||
| Leucopenia | 1 | 2 | 1 | 2 | 2 | 3 | 2 | 1 | |||||||||||||||||
These findings were assessed as uveitis (grade 3) related to his rhIL-15 treatment and possibly related to his prior treatment with high-dose IL-2 or ipilimumab or nivolumab, as was recently described (36). The patient’s symptoms and uveitis resolved after treatment with high-dose corticosteroids. A patient with squamous cell head and neck cancer developed worsening laryngeal inflammation from rapid disease progression that required a tracheostomy. A patient with multifocal renal cell carcinoma and pulmonary metastases treated at 1 mcg/kg dose level developed grade 3 hypoxia and presumed pneumonitis that required discontinuation of treatment.
This patient’s pulmonary status improved with initiation of high-dose corticosteroids and diuresis but still required supplementary oxygen. The patient died one week after stopping treatment after returning home; autopsy was not performed but death was presumed to be due to disease progression. Both patients treated at the 4 mcg/kg dose level had SAEs requiring early discontinuation of treatment. The first patient had grade 2 diarrhea initially linked to a change in enteral feeding formulation and later antibiotic—related diarrhea from empiric Amoxicillin sulbactam treatment. The CIV rhIL-15 infusion was interrupted for 24 hours until her diarrhea improved after initiation of antimotility treatment, but soon after rhIL-15 infusion was restarted the patient developed abdominal pain, decreased bowel sounds, and tenderness upon palpation, followed soon after by progressively increasing rigidity and rebound pain. The patient was taken to surgery and was seen to have diffuse patchy ischemia that involved her entire enteral tract (stomach, large and small intestines) without evidence of tumor in the peritoneum or serosa, arterial thrombi or perforation. The patient was given appropriate supportive measures (i.v. fluids, vasopressors, and broad-spectrum antibiotic treatment) with the hope that reperfusion would occur after treatment was discontinued but the patient eventually expired from multiorgan failure a few days later. The second 4 mcg/kg patient had appreciable involvement of the liver with metastatic GIST and developed hepatic encephalopathy. Treatment was discontinued on day 5 and the patient’s AEs resolved within a few days of supportive care.
The most common clinical toxicities experienced by patients treated based on treatment level and grade of the adverse event are listed, as well as the serious adverse events and the most common abnormal laboratory measurements in the patients treated in the protocol. N: the number of patients treated in the dosing cohort. CTC, V4: NCI clinical toxicity criteria version 4 (Table 1). In addition to the lymphocytopenia, an expected effect of IL-15 administration, anemia was the most common laboratory abnormality (24 of 27 patients), generally grade 1, except at higher rhIL-15 doses. Leukopenia, neutropenia, and thrombocytopenia were common at higher doses but were not associated with infectious complications or bleeding. Transient elevations of liver functions tests (ALT, AST and alkaline phosphatase) generally beginning the second or third day of treatment, occurred in nearly all patients treated at or above 0.5 mcg/kg dose level and resolved spontaneously to baseline by the end of treatment or the week following completion of treatment (Supplementary Figures 2A and B). Aside from the 4 mcg/kg patient who developed the DLT of hepatic encephalopathy, no other patients had clinical AEs related to this finding.
Clinical Response to Treatment
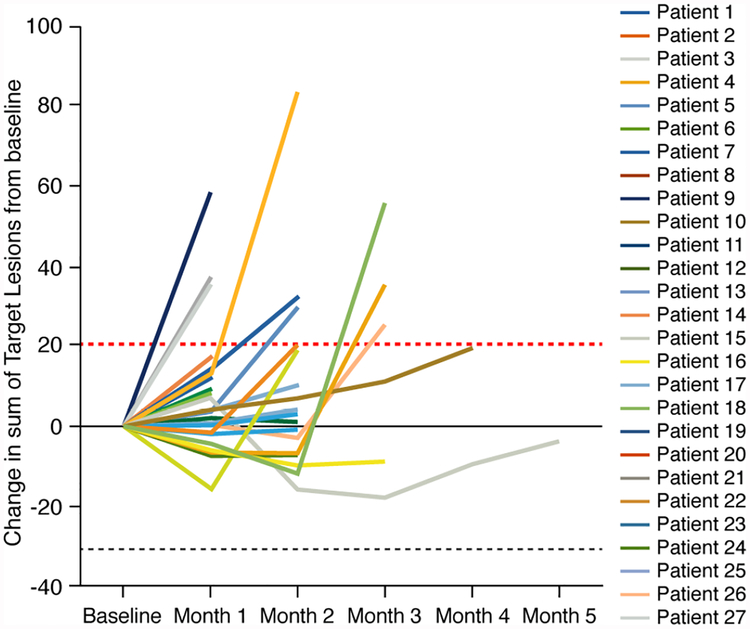
Patients response to therapy was assessed by RECIST 1.1 criteria (35). As seen in Figure 1, at least 6 patients had some evidence of tumor regression. Seventeen of 27 patients had stable disease as the best response, for a disease control rate (DCR) of 63%. Two patients treated at 1 mcg/kg/day dose fulfilled the criteria defined in Supplementary Materials required to receive additional cycles of treatment. In particular, patient #15 had a 16% decrease in his marker lesions, and complete disappearance of a cutaneous tumor nodule that was shown to be necrotic and having lymphocytic infiltration when biopsied. Patient #16 had a 9% decrease in marker lesions, and her CA-19–9 decreased from a baseline of 126 to 47 U/mL. The first of these had pancreatic cancer; in addition to radiographic findings, had substantial decrease in CA 19–9 from 125.7 U/mL baseline to 47.3 U/mL post-cycle 1 and 56.9 U/mL post-cycle 2. The second patient with chondrosarcoma involving the right chest wall developed ipsilateral pleural effusions during each of the 6 cycles, had a decrease in sum of marker lesions measurements, and complete regression of the rarely observed (37–39) cutaneous metastatic lesion during treatment. Biopsy of this lesion showed an infiltration of CD3 (2+), CD4 (2+), CD8(1+) and CD56 (±) positive cells. While no patient had a partial response, almost half demonstrated stabilization of disease that had been progressing prior to initiation of treatment.
Figure 1. Spider Plots of Response to CIVrhIL-15 Treatment.
Spider plots of all treated patients showing changes from baseline in the tumor burden (y-axis), measured as the product of the longest diameters of solid metastatic target lesions > 1 cm on high resolution CT scans (shortest diameter for lymph nodes) assessed at the end of every CIVrhIL-15 cycle (x-axis). Above the dashed red line (>20%) indicates progressive disease by RECIST criteria and below the lower black dashed line (> 30%) indicates partial response. Patients who had stable disease after their first 2 cycles of treatment continued to be restaged at regular intervals even though their treatment had been stopped.
Correlative Laboratory Assessment
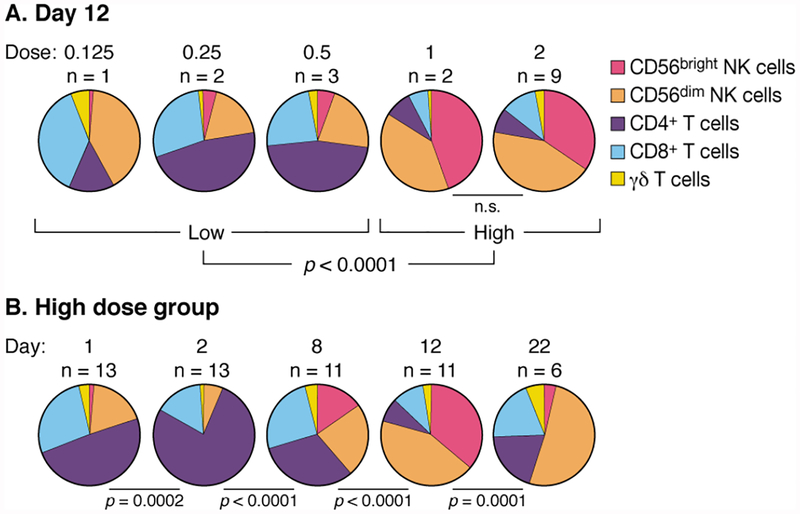
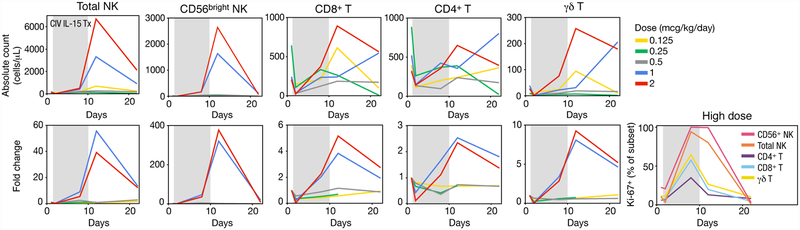
Previously we demonstrated that IV bolus treatment with IL-15 resulted in acute lymphopenia followed by hyperproliferation of IL-15-responsive cells with an increase in NK and CD8+ T cells sustained for more than 3 weeks after first treatment. Post-treatment, cells were hypoproliferative until homeostatic baseline proliferative rates were restored (31). Here, we show that administration of rhIL-15 by continuous IV infusion had an even greater impact on CD8+ T and NK - particularly CD56bright NK-cell expansions. A significant threshold dose effect was seen between patients receiving low (0.125, 0.25, 0.5 mcg/kg/day) and high (1.0, 2.0 mcg/kg/day) doses of rhIL-15 (Figure 2A). Changes were maximal two days following treatment cessation but were still dramatic 10 days later (Figure 2B). As expected, all patients experienced generalized acute lymphopenia at initiation of treatment (Supplementary Figure 3). Following this, blood lymphocytes returned with evidence of high levels of proliferation, lasting through the end of treatment (Figure 3). At the cessation of treatment, high-dose patients had a dramatic 358-fold increase of CD56bright NK cells, a 38-fold increase of total NK cells, and a 5.8-fold increase of CD8+ T cells (Figure 3). γδ and CD4 + T cells also responded to rhIL-15 treatment, thoughto a lesser degree than NK cells (Figure 3, Supplementary Figure 3). There was also evidence of CD8+ T cell activation, evidenced by increased frequency of CD38+ and HLA-DR+ cells without a corresponding increase in CD25+ cells including Tregs (Supplementary Figure 4).
Figure 2. Altered composition of lymphocyte subsets in response to CIV IL-15 administration.
A. Representation of lymphocyte subsets at low and high dose groups after continuous infusion of rhIL-15. On day 12, two days after treatment cessation, lymphocyte representations in patients receiving low doses of IL-15 (0.125, 0.25, and 0.5 mcg/kg/day) were not different from each other but were significantly different from patients receiving 1.0 and 2.0 mcg/kg/day (p = 0.003 and p = 0.0002, respectively). Based on this, we grouped patients receiving 0.125, 0.25, and 0.5 mcg/kg/day as “low dose” and patients receiving 1.0 and 2.0 mcg/kg/day as “high dose”. These groups differed significantly at p < 0.0001.
B. Kinetics of lymphocyte representations before, during, and after administration of CIV IL-15 at high dose. The relative proportion of CD56+bright NK cells was increased by day 8 of the treatment. This proportion was further increased on day 12, two days after cessation of the IL-15 infusion. By day 22, the proportions were normalizing. All statistics performed by permutation test.
Figure 3. Treatment-related changes in lymphocyte subsets.
Data are shown as the mean for each dose group of CIV rhIL-15, unless otherwise stated. Fold change values were computed by individual relative to their pre-infusion baseline. Shaded gray areas indicate continuous rhIL-15 infusion. It should be noted that the vertical axes numbers differ to facilitate the analyses of the different lymphocyte populations.
Absolute count and fold changes for total NK cells, CD56bright NK cells, CD8+ T cells, CD4+ T cells, and ɣδ T cells. Data for all patients are shown in Supplemental Figure S3.
The frequency of cells expressing Ki-67, a marker for recent cell division, of cell subsets in the high dose group.
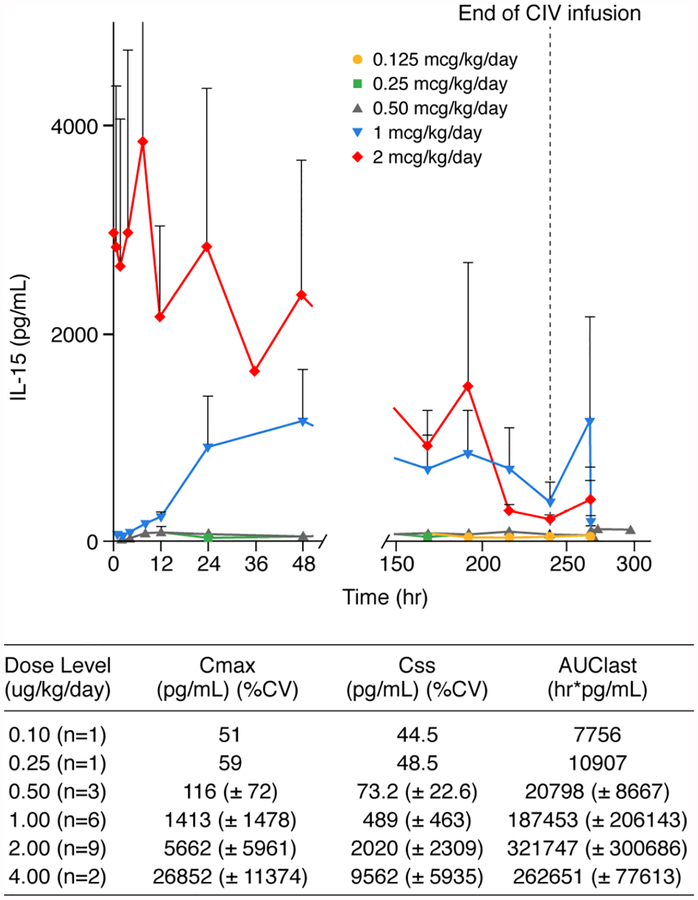
IL-15 Pharmacokinetics
Twenty-seven patients had pharmacokinetic (PK) samples collected, but only 22/27 had sufficient data above the lower level of quantitation (LLOQ) for analysis (Figure 4). IL-15 was below LLOQ for patients receiving the 0.125 and 0.25 mcg/kg/day dose levels during the first 48 hours, and modest levels of IL-15 (30 to 40 pg/mL range) were detected later (Figure 4). IL-15 levels were relatively constant throughout the 10-day continuous intravenous (CIV) infusion for patients in the 0.5 and 1 mcg/kg/day dose groups. Serum rhIL-15 levels for the patients treated at the 2 mcg/kg expansion dose were markedly lower during days 7 through 11 of the treatment cycle compared to earlier time points. There was a modest increase in the expression of IL-15R alpha per cell. In addition, there was a marked increase in the number of cells expressing high levels of IL-2/IL-15R beta (CD122) which with gamma-c (CD132) binds IL-15 to yield 10−9 M.
Figure 4. Pharmacokinetic Analysis of Mean Serum IL-15 Levels During CIV rhIL-15 Treatment.
PK samples were obtained at multiple timepoints during day 1 at the end of CIV rhIL-15 (decline PK analysis) on day 11. After the 3–4 day timepoint no samples were obtained for the subsequent 3 days and single daily “steady state” samples were obtained on days 7 through 10.
The expanded expression of such IL-15 binding NK and CD8 T-cells evident in Figure 3 may act as a sink for the administered IL-15 thereby providing a partial explanation for the decline of levels of serum IL-15 during the last 3 days of the infusion.
Assessment of Anti-rhIL-15 Antibodies
Patients were assessed for the development of anti-IL-15 antibodies 3 times during each treatment cycle. None of the samples obtained for the 27 patients treated in this trial showed evidence of antibodies against IL-15.
Inflammatory Cytokine Production
Increases in serum IL-1β levels were first noted within 60 minutes after the start of rhIL-15 infusions (Supplementary Figure 5A). Dose dependent increases in IL-6 were seen by 8 hours after treatment initiation (Supplementary Figure 5B). Serum tumor necrosis factor-alpha (TNFα) levels increased gradually over the first 48 hours and remained elevated throughout the course of treatment (Supplementary Figure 5C). Serum IFNγ was not measurable for most dose levels until 24 or 48 hours of treatment (Supplementary Figure 5D) and generally showed a late-cycle dose-dependent increase paralleling the TNFα trend. The dose-proportional increase in inflammatory cytokines seen in the final days of the infusion coincided with the increased number of activated, cytokine-producing CD8+ T and NK cells. Prior to infusion serum PD-1 level (normal range nondetectable to 50 pg/mL) was below the lower limit of detection, 20 pg/mL in 8 of 9 patients. With rhIL-15 infusions at 2 mcg/kg/day the arithmetic mean concentrations rose to 23.7 pg/mL at 24 hours, 27.8 pg/mL at 48 hours, 43.9 pg/mL at day 8 and 45.0 pg/mL at day 12 following initiation of infusions. This is in accord with our previous observations that IL-15 induces PD-1 on CD8 T-cells. The serum PD-L1 concentration range 44.5 to 106 pg/mL remained below the lower limit of detection, 200 pg/mL. The arithmetic mean of the serum PD-L2 concentration pre-infusion was 7453 pg/mL (normal range 1425–10678 pg/mL) and only rose to a maximum of 8419 pg/mL on day 8 of the 2 mcg/day infusions.
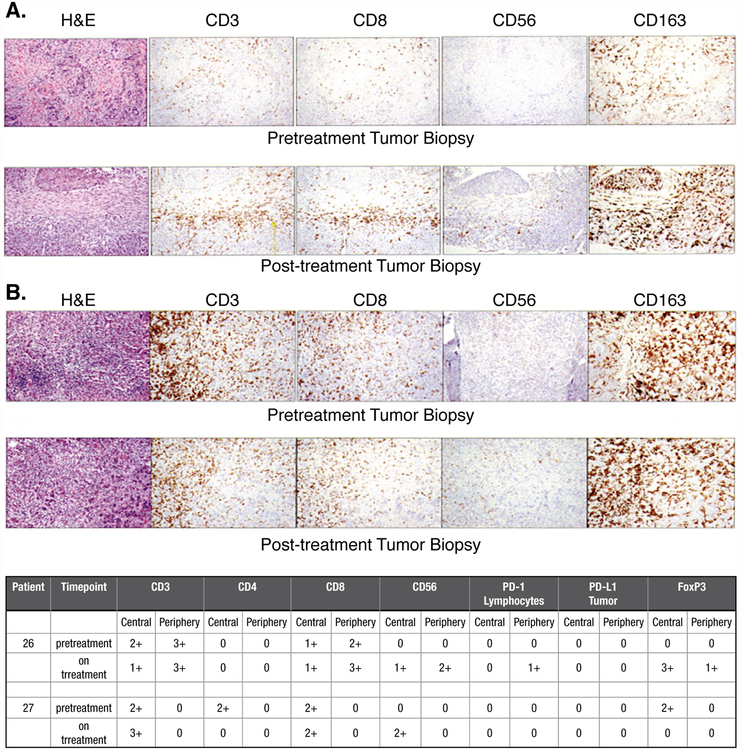
Effector Cell Infiltration of Tumor Deposits
Analysis of effector cell infiltration and checkpoint inhibitor expression was performed on preand post-treatment tumor biopsies from two patients treated at higher dose levels (Figure 5). The patient shown in Figure 5B and upper portion of the Table had a slight increase of CD8+ T cells in the periphery and a noticeable increase in CD56+bright NK cells both peripherally and centrally in the colorectal tumor deposit. A patient with squamous cell carcinoma of the head and neck shown in Figure 5A and in lower portion of the Table had a modestly increased infiltrate of CD3+ cells and an appreciably increased number of CD56+bright NK cells in the central portion of the tumor.
Figure 5. Immunochemical Analysis of Cellular Infiltrates and Tumor Deposits.
Pre-and-post-treatment analysis of the core biopsy specimen subjected to hematoxylin and eosin staining as well as immunochemical analysis for CD3 (OKT-3), CD4 (S3.5), CD8 (3B5), CD56 (56CO4), CD163 (ED2), PD-1 (MIH4) and PD-L1 (29E.2A3) was performed as previously reported (31).
A. Histology from a patient with squamous cell head and neck cancer
B. Histology from a patient with metastatic colorectal cancer.
Discussion:
Preclinical data demonstrated IL-15’s considerable potential as an immunotherap eutic from the cytokine’s ability to generate prolonged proli feration and activation of effector lymphocytes without AICD (7–31, 40), and low potential to activate Tregs compared to IL-2. In addition to defining safety profiles and MTD for new agents, phase I trials should also establish an optimal schedule and route of administration. As predicted by the preclinical NHP toxicology studies, the CIV regimen sustained serum rhIL-15 in the 1,000 to 5,000 pg/mL range and generated greater expansion of NK and CD8 T effector cells than the 3 mcg/kg dose in the IV bolus regimen (Cmax 43,900 pg/mL and t 1/2 of 2.37 hours) or the subcutaneous (SC) regimen (Cmax 1,638 pg/mL and t 1/2 = 10 hours) (31, 41, 42) (Supplementary Table 2). We suspect that rhIL-15 infusion causes lymphocytes and especially NK cells to marginate or efflux from the circulation, where they undergo proliferation throughout the treatment course. At the termination of treatment, there is a redistribution of lymphocytes back into the circulation, resulting in dramatic increases in the numbers of circulating cells (Supplementary Figure 6).
Continuous intravenous infusion of rhIL-15 resulted in the greatest increase in circulating effector cells compared with administration by IVB or SC regimens or with IL-15 superagonist complex ALT-803 (Supplementary Table 2) (31, 41–43). The CIV regimen at 2 mcg/kg/day resulted in a 38-fold increase in the number of circulating NK cells and 358-fold increase in the number of CD56bright NK cells. The IVB regimen at the MTD of 0.3 mcg/kg/day resulted in only an ~2–3-fold increase of NK cells. Even the highest dose tested by IVB (3.0 mcg/kg/day) resulted in only a 10-fold increase in NK cells while causing dose-limiting toxicities (31). IL-15 administered subcutaneously at the expansion dose of 2 mcg/kg per day was well-tolerated but only produced a 10.8-fold increase in NK cells and 29.7-fold increase in CD56bright NK cells (42). ALT-803 (IL-15/IL-15Rα-IgFc) treatment elicited cytokine release-related AEs with IV treatment and skin toxicity with SC dosing limiting the dose that could be used (43). However, when administered by IVB or SC, ALT-803 at 5–10 times the dose (10–20 mcg/kg/day) containing 2.6 mg/kg/day of IL-15 induced a smaller expansion (8 times) of NK cells than the 38-fold increase with CIV rhIL-15 at 2 mcg/kg/day in the present study. Since IL-15 is only 26 percent of the ALT-803 complex, 10 mcg of ALT-803 contain 2.6 mcg of IL-15.
At the termination of the 2 mcg/kg per day 10-day CIV infusions the NK cells including the CD56bright NK cells were not exhausted but were vigorously proliferating. Ki-67+ was present in over 95% of CD56bright NK cells on both days 8 and 12 with the 10-day CIV. Furthermore, as reported previously (41), evaluated on day 12 of the 10-day CIV infusions IL-15 augmented the cytotoxic activity of all NK cell subsets against three target cell lines, the killing of which involved different receptors: CD20 rituximab antibody-coated RAJI, K562 and C1R-MICA. These cell lines were recognized by the functionally active NK cells, with CD16 mediating ADCC, NKp30 and NKp46 mediating natural cytotoxicity for K562, and NKG2D mediating it for CIR-MICA. In our previous publication (41) we discussed functional and phenotypic analysis of the NK cells prior to infusion and on day 12 of a 10-day infusion, that is, 2 days following termination of the infusion during the influx of NK cells at their maximal number. CD56dim NK cells treated with IL-15 acquired features normally observed with CD56bright NK cells, that is, increased expression of NKG2D, Trail and IL-15R alpha. Furthermore, after IL-15 infusions CD56bright NK cells expressed increased amounts of CD16, NKp30 and NKp46. In addition, IL-15 infusions increased perforin in CD56bright NK cells. These data show that 10-day continuous IL-15 infusions enable all subsets of NK cells to function with increased cytotoxicity at the termination of the cultures. Thus, among available agents and dosing regimens, rhIL-15 administered CIV provided the greatest augmentation of circulating functional NK cells.
Even in phase I safety trials of new agents, there is some expectation of clinical activity. Though no patients achieved even a partial response, the data in Figure 1 demonstrate a flattening or negative deflection in tumor burden over time for a number of patients with a 63% DCR in a heavily pretreated population. One patient had a cutaneous chondrosarcoma nodule—a rare metastasis (36–39) that disappeared during the course of treatment. Complete regression of this nodule in the chondrosarcoma patient, minor radiographic improvements, and the 3-fold decrease in CA 19–9 and disease stabilization for nearly 6 months in one of the pancreatic cancer patients also implies antitumor activity. The extensive prior treatment history of most patients and, the small number of classically immunosensitive tumor patients only 2 of which were treated at the more biologically active doses of rhIL-15 must be considered when weighing lack of sustained significant responses.
This modest impact on tumors with IL-15 monotherapy led to our conclusion that to have a major clinical contribution to cancer therapy, IL-15 will have to be used in combination therapy. In particular, IL-15 could be used in combination with agents such as monoclonal antibodies that take advantage of the increase in NK numbers and that impart tumor specificity (44). In modern immunotherapy there has been a great contrast between the relative lack of efficacy of NK cells infused as monotherapy for cancer and its critical link in the chain of effectors in combination therapy involving NK cells with those anticancer monoclonal antibodies (e.g. rituximab) that function predominantly by ADCC (45–50). Nevertheless, in the clinical trials cytokine (interleukin-12, IL-15 and IL-18)-induced memory-like natural killer cells exhibited enhanced responses against myeloid leukemia. Clinical responses were observed in 5 of 9 evaluable AML patients including 4 complete remissions (46). Furthermore, there has been efficacy in bone marrow transplant studies in this leukemia (46, 48–50). In addition, there has been a positive association in some studies of NK infiltrate not T-cell infiltrate in renal cancer (45, 47). Renal cell carcinoma infiltrating natural killer cells but not T cells have been shown to have the inherent ability to recognize transformed cells but require cytokine activation and are sensitive to inhibition by inhibitory receptor ligands (45, 47).
The dramatic increases in NK cells alone in the present study were not sufficient to produce antitumor activity, likely because most tumors express self-MHC Class I molecules that inhibit NK-effector functions. However, it should be noted that when NK cells in combination therapy are considered they are a pivotal link in the chain of effectors required for the efficacy of certain (e.g. rituximab) antitumor monoclonal antibodies that function by ADCC alone. We investigated combination therapy of IL-15 with rituximab in a syngeneic mouse model of lymphoma transfected with human CD20 and with alemtuzumab (CAMPATH-1H) and in a xenograft model of human adult T-cell leukemia (ATL) (51). IL-15 greatly enhanced the therapeutic efficacy of both rituximab and alemtuzumab in these tumor models. The additivity/synergy was shown to be associated with augmented ADCC. Both NK cells and macrophages were critical elements in the chain of interacting effectors involved in optimal therapeutic responses mediated by rituximab and IL-15. We provided evidence supporting the hypothesis that NK cells interact with macrophages to augment the NK-cell activation and expression of Fc gamma receptors and the capacity of these cells to become effectors of ADCC. Therefore, a very efficacy. Recent publications describe results from two clinical trials that demonstrated appreciable clinical activity from the combination of ALT-803 and rituximab or nivolumab to patients with relapsed refractory non-Hodgkin’s lymphoma or patients with relapsed refractory non-small cell lung cancer respectively (52, 53).
These observations and the results of our preclinical animal model testing of the combination of IL-15 with monoclonal antibodies support combination clinical trials involving IL-15 (54, 55). Preclinical studies of IL-15 with anti-CTLA-4 and anti-PD-L1 supported our initiation of a clinical trial with this triple combination () involving IL-15 (54, 55). Furthermore, they provided the scientific basis for our initiation of a phase I trial of IL-15 combined with alemtuzumab (anti-CD52) that has been opened for patients with refractory and relapsed adult T-cell leukemia (). Additional trials are being initiated in patients with refractory and relapsed chronic lymphocytic leukemia where rhIL-15 will be administered in combination with obinutuzumab (anti-CD20, ), and in patients with renal cancer where IL-15 and avelumab (anti-PD-L1) will be co-administered.
Supplementary Material
Translational Relevance.
IL-15 administered by continuous infusion at 2 micrograms/kilogram per day to patients with cancer induced a massive 38-fold increase in total circulating NK cells and 358-fold increase in CD56bright NK cells. This continuous infusion of IL-15 treatment regimen resulted in the greatest increase in functional effector cells involved in antibody ADCC compared with IV bolus, subcutaneous or ALT-803 IL-15. These observations support trials of IL-15 with anticancer monoclonal antibodies to increase their antibody-dependent cell-mediated cytotoxicity (ADCC) and anticancer efficacy. In particular to translate the observations in the present trial and preclinical studies of IL-15 in combination therapy a phase I trial of IL-15 combined with alemtuzumab has been opened in patients with adult T-cell leukemia (ATL) , along with a phase I trial of IL-15 and obinutuzumab for patients with chronic lymphocytic leukemia (CLL) , both in the relapsed/refractory setting.
Acknowledgment of Research Support
Supported by the Intramural Research Programs of the Center for Cancer Research, National Cancer Institute and the Vaccine Research Center, (National Institute of Allergy and Infectious Diseases), National Institutes of Health (NIH, Bethesda, MD). Leidos Biomedical Research, Inc. (Reston, VA) and the Biopharmaceutical Development Program, National Cancer Institute Frederick (Frederick, MD) provided the recombinant human interleukin-15 for this study. The clinical trial registration number is .
Footnotes
Study Presented in Part Elsewhere: The study was presented in part as a meeting abstract Cancer Research 77, supplement 13, Meeting Abstract 1596, 2017 at the Annual Meeting of the American-Association-for-Cancer Research (AACR).
Conflict of interest: The authors declare no potential conflicts of interest.
Bibliography
- 1.Choudhury M, Efros M, Mittelman A. Interferons and interleukins in metastatic renal cell carcinoma. Urology 1993; 41:67–72. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman HL, Kirkwood JM, Hodi FS, Agarwala S, Amatruda T, Bines SD, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol 2013; 10:588–598. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192:5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linehan WM, Walther MM, Alexander RB, Rosenberg SA. Adoptive immunotherapy of renal cell carcinoma: studies from the Surgery Branch, National Cancer Institute. Semin Urol 1993; 11:41–43. [PubMed] [Google Scholar]
- 5.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17:2105–2116. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann TA: Cytokines in cancer immunotherapy: In cytokines from basic mechanisms of cellular control to new therapeutics. Edited by Leonard WJ and Schreiber RD. Cold Spring Harbor Laboratory Press, Spring Harbor, NY, 2018, pp 431–453. [Google Scholar]
- 7.Rosenthal R, Groeper C, Bracci L, Adamina M, Feder-Mengus C, Zajac P, et al. Differential responsiveness to IL-2, IL-7, and IL-15 common receptor gamma chain cytokines by antigen-specific peripheral blood naive or memory cytotoxic CD8+ T cells from healthy donors and melanoma patients. J Immunother 2009; 32:252–261. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006; 6(8): 595–601. [DOI] [PubMed] [Google Scholar]
- 9.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15R alpha recycles and presents IL-15 in trans to neighboring cells. Immunity 2002; 17(5): 537–547. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Patel HJ, Waldmann TA. Tagaya Y. The IL-15/IL-15R alpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci USA 2007; 104: 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Giuntoli RL 2nd, Omiya R, Kobayashi H, Kennedy R, Celis E Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res 2002; 8:3877–3884. [PubMed] [Google Scholar]
- 12.Benito-Miguel M, García-Carmona Y, Balsa A, Perez de Ayala C, Cobo-Ibanez T, Martin-Mola E, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25− responder T cells. J Immunol 2009; 183:8268–8279. [DOI] [PubMed] [Google Scholar]
- 13.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol 2008; 181(10): 6738–6746. [DOI] [PubMed] [Google Scholar]
- 14.Zeng R, Spolski R, Finkelstein SE, Oh SK, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 2005; 201:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads toenhanced tumor immunosurveillance. Blood 2005; 105(2):721–727. [DOI] [PubMed] [Google Scholar]
- 16.Munger W, Dejoy SQ, Jeyaseelan R Sr, Torley LW, Grabstein KH, Eisenmann J, et al. Studies evaluating the antitumor-activity and toxicity of interleukin-15, a new T-cell growth-factor: comparison with interleukin-2. Cell Immunol 1995; 165(2):289–293. [DOI] [PubMed] [Google Scholar]
- 17.Evans R, Fuller JA, Christianson G, Krupke DM, Troutt AB. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: The potential role of NK cell subpopulations. Cell Immunol 1997; 179(1):66–73. [DOI] [PubMed] [Google Scholar]
- 18.Kudo-Saito C, Wansley EK, Gruys ME, Wiltrout R, Scholm J, Hodge JW. Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res 2007; 13(6):1936–1946. [DOI] [PubMed] [Google Scholar]
- 19.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA 2003; 100(6):3392–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller YM, Petrovas C, Bojczuk PM, Dimitriou LD, Beer B, Silvera P, et al. Interleukin-15 increases effector memory CD8(+) T cells and NK cells in simian immunodeficiency virus-infected macaques. J Virol 2005; 79(8):4877–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine 2004; 22(25–26): 3510–3521. [DOI] [PubMed] [Google Scholar]
- 22.NCI Immunotherapy Agent Workshop. https://dcb.nci.nih.gov/Reports/Pages/immunotherapyagentworkshop.aspx.
- 23.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. : Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 2000; 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XH, Sun SQ, Hwang IK, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8(+) T cells in vivo by IL-15. Immunity 1998; 8:591–599. [DOI] [PubMed] [Google Scholar]
- 25.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood 2001; 97:14–32. [DOI] [PubMed] [Google Scholar]
- 26.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol 2006; 24:657–679. [DOI] [PubMed] [Google Scholar]
- 27.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56(+) natural killer cells from CD34(+) hematopoietic progenitor cells. Blood 1996; 87:2632–2640. [PubMed] [Google Scholar]
- 28.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, et al. Potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest 1997; 99:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 2009; 206:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15R alpha (+) bone marrow-derived cells. Blood 2004; 103:988–994. [DOI] [PubMed] [Google Scholar]
- 31.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015; 33:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood 2011; 118:6845–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood 2011; 117:4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soman G, Yang X, Jiang H, Giardina S, Vyas V, Mitra G, et al. MTS dye based colorimetric CTLL-2 cell proliferation assay for product release and stability monitoring of interleukin-15: Assay qualification, standardization and statistical analysis. J Immunol Methods 2009; 348:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 36.Karlin J, Gentzler R, Golen J. Bilateral anterior uveitis associated with Nivolumab therapy. Ocul Immnol Inflamm 2018; 26(2):283–285. [DOI] [PubMed] [Google Scholar]
- 37.Leal-Khouri SM, Barnhill RL, Baden HP. An unusual cutaneous metastasis of a chondrosarcoma. J Cutan Pathol 1990; 17:274–277. [DOI] [PubMed] [Google Scholar]
- 38.Arce FP, Pinto J, Portero I, Echevarria S, Val-Bernal JF. Cutaneous metastases as initial manifestation of dedifferentiated chondrosarcoma of bone. An autopsy case with review of the literature. J Cutan Pathol 2000; 27:262–267. [DOI] [PubMed] [Google Scholar]
- 39.Tseng YA, Youn T, Eisenkraft B, Drexler S. A case report: primary cutaneous chondrosarcoma metastatic to the brain. Am J Clin Pathol 2012; 138:A126, issue suppl 2, doi:org/10.1093/ajcp/138.suppl2.7. [Google Scholar]
- 40.Lugli E, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood 2010; 116:3238–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubois S, Conlon KC, Muller JR, Hsu-Albert J, Beltran N, Bryant BR, et al. IL15 infusion of cancer patients expands the subpopulation of cytotoxic CD56(bright) NK cells and increases NK-cell cytokine release capabilities. Cancer Immunol Res 2017; 5:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, et al. A first-in human Phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res 2018; 24(7):1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018; 131(23): 2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res 2015; 3(3):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer 2003; 106(6):905–912. [DOI] [PubMed] [Google Scholar]
- 46.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016; 8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Grone EF, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res 2006; doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 48.Parisi S, Lecciso M, Ocadlikova D, Salvestrini V, Ciciarello M, Forte D, et al. The more, the better: “Do the right thing” for natural killer immunotherapy in acute myeloid leukemia. Front Immunol. 8:1330, 2017; doi: 10.3389/fimmu.2017.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachanova V and Miller JS. NK cells in therapy of cancer. Crit Rev Oncog. 2014; 19(0):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He L, Zhu HY, Qin SC, Li Y, Miao Y, Liang JH, et al. Low natural killer (NK) cell counts in peripheral blood adversely affect clinical outcome of patients with follicular lymphoma. Blood Cancer J. 6(8):e457 2016; doi: 10.1038/bcj.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Wen B, Anton OM, Yao Z, Dubois SP, Ju W, et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci USA 2018: 115(46): E10915–E10924. doi: 10.1073/pnas.1811615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase ib trial. Lancet Oncol 2018; 19(5):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fehniger TA, Hess BT, Bachanova V, Becker-Hapak M, McClain E, et al. : First-in-human phase I combination of the IL-15 receptor super agonist complex ALT-803 with a therapeutic (anti-CD20) monoclonal antibody (mAb) for patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL). Proc Amer Assoc Can Research 2018. (abstr CT146). [Google Scholar]
- 54.Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci USA 2012; 109:6187–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010; 16:6019–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.