Abstract
Approximately 20% of colorectal cancer patients present with metastases at the time of diagnosis, and therapies that specially target these metastases are lacking. We present a novel approach for investigating transcriptomic differences between primary colorectal cancers (CRC) and distant metastases, which may help to identify primary tumors with high risk for future dissemination and to inform the development of metastasis-targeted therapies. To effectively compare the transcriptomes of primary CRC and metastatic lesions at both the gene and pathway levels, we eliminated tissue specificity of the “host” organs where tumors are located and adjusted for confounders such as exposure to chemotherapy and radiation, and identified that metastases were characterized by reduced epithelial-mesenchymal-transition (EMT) but increased MYC target and DNA repair pathway activities. FBN2 and MMP3 were the most differentially expressed genes between primary tumors and metastases. The two subtypes of CRC metastases that were identified, EMT-inflammatory and proliferative, were distinct from the consensus molecular subtype (CMS) 3 suggesting subtype exclusivity. In summary, this study highlights transcriptomic differences between primary tumors and CRC metastases and delineates pathways that are activated in metastases that could be targeted in CRC patients with metastatic disease.
Keywords: metastasis, colorectal adenocarcinoma, comparative transcriptomics
INTRODUCTION
Roughly 20% of individuals with colorectal adenocarcinoma (CRC) present with metastatic disease at the time of diagnosis, and CRC is the primary cause of mortality due to cancer (1,2). In CRC, the liver (70%) is the most common site of disease metastasis followed by the lung (32-47%) (3). Although CRC metastases are aggressively treated with some combination of chemotherapy, curative-intent surgical resection (4), biologics, such as epidermal growth factor (EGFR) inhibitors (5), and immunotherapy (for a sub-group of patients with mismatch repair deficiency) (6), metastasis-targeted therapies are severely lacking. Therefore, understanding the defining features of metastatic tumor cells in distal organs is valuable for the development of targeted drugs and individualized therapies for patients with metastatic disease.
One approach for characterizing the biology of metastatic lesions has been to compare primary tumors and metastatic lesions of the same cancer type (7). However, this is limited by the need for biopsies of normal host organ tissue where metastases are located such that the transcriptomic profiles of metastases can be normalized (7,8). One interesting survey evaluated primary versus metastatic sites and found that expression studies of metastases obtain signatures that partially reflect the host tissue but have additional signatures (9). This finding highlights the need for considering the metastatic site during analyses. Approaches comparing primary tumors and metastatic lesions often fail to address the role of treatment exposure in altering tumor transcriptomic profiles (9,10). This is particularly true for metastases, as biospecimens of metastases obtained in the clinical setting are usually drawn from patients heavily treated with chemotherapy and/or radiation prior to surgical resection (4,11). To identify metastasis-specific features free from potentially confounding signals, we developed a novel approach for comparing primary tumors and metastases which takes both normal host tissue expression, anatomical origin of the tumors, and treatment exposure status of tumors into consideration as all three of these factors can substantially influence the detection of metastasis-specific gene expression patterns. This analytical approach allows for the determination of defining features of lung and liver metastases of CRC, while avoiding the need to always obtain normal host tissues from patients with metastatic disease for the purposes of normalizing tumor gene expression. Last, it allows for the identification of unique subtypes of CRC metastases that are independent of the site of metastasis.
METHODS
Primary and metastatic colorectal cancer samples
Gene expression from human colorectal cancer tissues and corresponding clinical data were analyzed. All participants provided written informed consent for data and tissue collection as part of the following protocols at MCC and Consortium sites: Total Cancer Care (12), Lifetime Cancer Screening, General Banking, Pre-HIPAA Biobanking, or Clinical Collection. All tissue and data analyzed for this project were utilized under the approval of the Advarra Institutional Review Board that ensures research is conducted in accordance with recognized ethical guidelines (MCC# 19066/Pro00023353) under the HHS regulations 45 CFR part 46 for human subjects protections; specifically under Subpart A (US Common Rule) as authorized by 45CFR46.110. All subjects were ≥18 years of age and free of psychiatric incapacity or dementia. Residual tissue collected as part of routine clinical care was assayed using the Rosetta/Merck Human RSTA Custom Affymetrix 2.0 microarray platform and a single standard operating procedure. For the purposes of this study, only tissues collected via surgical resection, as opposed to biopsy, were analyzed. Microsatellite instability status (MSI) was determined for a subset of patients (n=71) using the Bethesda panel (13) genes (BAT25, BAT26, NR21, NR24, and NR27).
We established two distinct datasets from TCC samples. The discovery cohort (MCC dataset), consisting of 517 human colorectal cancer samples from 502 distinct patients, included n=333 primary lesions and n=184 lung and liver metastases of CRC. All samples in the MCC dataset were collected at the MCC hospital location in Tampa, FL. Wherever possible, histology, clinical information, and MSI status for the MCC dataset were verified through examination of electronic medical records. In addition, we examined 618 human colorectal cancer samples from 618 distinct patients including n=545 primary lesions and n=73 lung and liver metastases of CRC in the validation cohort (Consortium dataset). All samples in the Consortium dataset were obtained from non-MCC TCC regional consortium site partner institutions. All transcriptomic and clinical data has been deposited to the Gene Expression Omnibus site curated by the National Center for Bioinformatics (accession number: GSE131418).
Microarray expression normalization and principal component analysis (PCA)
Microarray gene expression data passing multiple internal quality control filters and curated by the MCC Shared Resources Bioinformatics and Biostatistics Core was obtained for all TCC samples. Microarray chips were normalized using iterative rank-order normalization (IRON) (14) and log2 transformation. In addition, we excluded all probes which mapped to multiple genes. Probe set expression was converted to gene level expression by selecting the probe with the maximum expression for a given gene. In addition, we performed PCA analysis within the MCC and the Consortium datasets using all genes captured on the microarray platform. One MCC sample was found to be an outlier (>3 standard deviations away from the mean of PC1) and thus was subsequently removed from the study (Supplementary Fig. S1). Furthermore, we did not note any significant batch effects between the MCC and Consortium datasets (Fig. S2).
Anatomical origin determination for MCC dataset
Anatomical origin for primary tumors was determined as follows: tumors located in the cecum, ascending colon, hepatic flexure, and transverse colon were classified as proximal, while tumors located in the splenic flexure, descending colon, sigmoid colon, and rectum, were classified as distal. Anatomical origin of metastases was classified in the same manner as for primary tumor with the exception of n=7 patients for which anatomical origin was not clearly designated. Specifically, for six of these seven individuals, anatomical origin of the metastatic tumor was obtained from patient-reported history of site of primary tumor resection or site of hemicolectomy (left or right side) noted in the electronic medical record. Anatomical origin could not be determined for n=1 patient in the MCC dataset after medical record examination.
Tumor treatment exposure determination for MCC dataset
Tumor treatment exposure status was determined by assessing history of chemotherapy and/or radiation treatment within two years prior to surgical resection of the tumor sample. If there was no history of chemotherapy or radiation exposure prior to surgical resection of the tumor sample, the sample was considered treatment-naïve (i.e. resection occurred “pre” treatment). If within two years prior to surgical resection of the tumor sample in question any history of chemotherapy or radiation was noted in the medical records, the tumor sample was considered treatment-exposed (i.e. resection occurred “post” treatment).
Anatomical Origin Classifier for Consortium Samples
As clinical data on the Consortium dataset was limited, we imputed missing tumor anatomical origin by developing gene-expression and logistic regression based anatomical origin classifiers which categorize tumors as either originating from the proximal colon or the distal colon/rectum. These classifiers were developed based on naïve differential gene expression analysis comparing proximally and distally originating tumors in the MCC dataset. A gene was determined to be differentially expressed if the fold change was >1.5 and the p-value <0.05. The classifiers were developed separately for primary tumors and metastases. Anatomical origin classification of primary tumors was based on the expression of the PRAC1 and HOXC6 genes (AUCmax =0.93), while the classification of metastases was based on the expression of the PRAC1, HOXC6, OGN, and MUC12 genes (AUCmax =0.85). We imputed missing anatomical origin for n=20 primary tumors and n=13 metastases of CRC in the Consortium dataset. Gene expression differences between proximally and distally originating primary CRC tumors were used for classifying the anatomical origin of metastases. PRAC1 and MUC12 were found to be differentially expressed between proximally and distally originating primary tumors and metastases (Supplementary Fig. S3, S4). The four genes (OGN, MUC12, PRAC1, HOXC6) used to classify anatomical origin in the Consortium dataset were eliminated from all subsequent Consortium analyses.
Treatment classification for Consortium samples
Similar to the development of an anatomical origin classifier, we developed gene expression logistic regression-based classifiers to determine the treatment exposure status of primary tumors and metastases. The classifiers categorize tumors as either treatment exposed or treatment naïve. The treatment classifier for primary Consortium samples was developed based on naïve differential gene expression analysis comparing primary pre- and post-treatment tumors in the MCC dataset. A gene was determined to be differentially expressed if the fold change was >1.5 and the p-value <0.05. The MCC dataset was split into a training and a test set, and the optimal combination of differentially expressed genes which maximized the AUC (AUCmax = 0.815) in the test set were incorporated into the logistic regression model and served as the basis for the primary tumor treatment classifier. The eight genes used to classify primary tumors are: SCRG1, HBB, GREM2, SCN7A, CHRDL2, HSPB6, CXCL12, and PLP1.
We were unable to identify a set of genes meeting the differential expression threshold criteria when comparing pre- and post-treated metastases in the MCC dataset, and thus used gene expression differences between pre- and post-treatment primary tumors to develop the treatment classifier for metastases (Supplementary Fig. S5–S6). The treatment classifier for metastases (AUCmax = 0.722) was based on the expression of 11 genes: SYNPO2, GREM2, ADH1B, HBB, C7, PLN, SFRP1, AGTR1, CHRDL2, MAMDC2, MYH11. For all subsequent Consortium analyses, we excluded the 16 genes that were used to develop the treatment classifiers.
Tissue specific gene-level and pathway-level weights
Based on the bioinformatics approach developed by Frost, 2018 (15) to address tissue-specificity of genes and gene sets, we computed lung, liver, colon, and rectum tissue-specific weights for individual genes and gene sets (pathways) in the Molecular Signature Database (MSigDB Version 6.0) (16). Tissue sensitive analyses were performed by eliminating genes and pathways exhibiting tissue specificity for lung, liver, colon, or rectum tissues. The filtering criteria for gene-level and pathway-level tissue specificity are as follows: all genes with >2-fold increase in tissue-specific expression and all pathways with a tissue-specific weight >10 are labeled as tissue specific. Additional information on the development of gene and pathway-level tissue weights has been described previously (15).
Linear models for microarray data analysis (LIMMA) and CAMERA application
CAMERA (17) application considered pathways from the MSigDB Hallmark (18) and C2 (CPG and CP), collections. Tissue agnostic LIMMA (19) and CAMERA application to determine differentially expressed genes and gene sets between primary tumors and metastases did not consider tissue-specific gene or pathway expression. All tissue agnostic analyses adjust for age, sex, tumor treatment exposure status, and anatomical origin. Tissue sensitive LIMMA and CAMERA application to determine differential expression of genes and pathways between primary tumors and metastases account for tissue-specific expression by eliminating all genes and pathways exhibiting tissue specificity based on the filtering criteria highlighted above. Tissue sensitive analyses also adjust for age, sex, anatomical origin, and treatment exposure status (unless explicitly indicated otherwise), when comparing primary tumors and metastases. In addition, previous studies have highlighted genetic and transcriptomic differences between CRC tumors arising from the proximal colon and the distal colon/rectum (20). Therefore, if tumor anatomical origin and treatment exposure status were missing for samples in the Consortium dataset, the imputed missing data from gene-expression based classifiers were used as inputs for LIMMA and CAMERA (Supplementary Fig. S4, S6). MSI status for a subset of MCC tumor samples (n=71) was available. Microsatellite instable tumors were typically MSI-high and were classified as such, while tumors labeled MSI-low and microsatellite stable (MSS) were classified as MSS. For this small cohort, LIMMA and CAMERA analyses adjusted for MSI status. Lastly, we also used CAMERA to determine pathways differentially expressed between treatment naïve and treatment exposed tumors while adjusting for age, sex, anatomical origin and tumor type (primary tumor or metastasis of CRC).
Determination of the M1 and M2 clusters in the MCC and Consortium datasets
To discover subtypes of metastases, we performed unsupervised hierarchical clustering using the top 500 differentially expressed genes between primary tumors and metastases in each dataset. Based on our clustering analysis, two main clusters of metastases were identified in each dataset, and differential pathway expression between these two clusters of metastases was determined using CAMERA. To ensure that the M1 and M2 clusters in each dataset exhibit similar underlying biology, we developed a M1,M2 classifier trained on the MCC dataset and tested on the Consortium dataset. Cluster membership was defined by the hierarchical clustering of the top 500 differentially expressed genes between primary tumors and metastases in each dataset. Inputs for the logistic-regression based classifier included the covariates age, sex, tumor anatomical origin, and treatment exposure status as well as single we developed a M1M2 classifier trained on the MCC dataset and tested on the Consortium dataset. Cluster membership was defined by the hierarchical clustering ofsample gene set enrichment scores (21) for pathways found to be differentially expressed between the M1 and M2 clusters in the MCC dataset.
Examining adaptations to distal sites of metastases observed in primary tumors
We examined adaptations to distal sites of metastases in primary tumors of patients who either went on to develop lung (n=18) or liver (n=48) metastases for their initial distal metastasis. Tumors from patients who developed both lung and liver metastases at once or whose initial distal site of metastasis was not the liver or lung were excluded. Using CAMERA, we determined differential pathway expression. Next, we assessed if these differential pathways exhibited high tissue-specificity for normal lung or liver tissues (tissue-weight >10). Enrichment of pathways with high tissue-specific weights was considered to be an adaptation to the lung and liver observed in the primary CRC tumors.
CMS classification and logistic regression
CMS is a transcriptome-based classification of CRCs with prognostic value (22). Tumors were classified into CMS groups 1-4 or CMS_NA using the single sample predictor method as previously described (22). We performed logistic regression to determine the association of CMS with primary tumors or metastases of CRC while adjusting for age, sex, anatomical origin, and tumor treatment exposure status. For both the MCC and Consortium datasets, we excluded CMS3 from the logistic regression models as metastases were never classified as CMS3. Inclusion of CMS3 into the regression model would have resulted in complete separation, thereby eliminating a maximum likelihood estimate and resulting in inflated beta coefficients for the predictor.
Statistical Analysis
All statistical analyses were performed in R (Version 3.5). Difference in group means was determined using the Wilcox rank sum test. Spearman rank correlation was used to determine degree of overlap in pathway enrichment comparisons between datasets. We set the false discovery rate (FDR) to 0.1 to identify associations in expression analyses.
RESULTS
Characteristics of Total Cancer Care Participants
Clinical and gene expression data corresponding to human CRC surgical resection biospecimens were obtained through the Moffitt Cancer Center’s (MCC) Total Cancer Care (TCC) Protocol (12), including participating TCC Consortium sites. Data were separated into a discovery cohort of MCC samples (n=517) and a validation cohort of non-MCC TCC Consortium samples (n=618). Both datasets primarily consist of unmatched tumor samples with n=15 matched primary tumors and metastatic lesion samples from the same individual in the MCC dataset. Therefore, given the small number of paired samples, all statistical analyses were performed ignoring paired status. Clinical characteristics and tumor sample inclusion/exclusion criteria for the discovery cohort, hereon referred to as the MCC dataset, and the validation cohort, hereon referred to as the Consortium dataset, are described in Table 1 and Figure 1. In the MCC dataset, lung and liver metastases of CRC were more likely to originate from the distal colon or rectum (Wilcox-rank sum test; P = 0.00073) and were more likely to have been exposed to chemotherapy and/or radiation treatment prior to surgery (Wilcox-rank sum test; P <0.0001). Interestingly, even within metastases, we observed gene expression differences between metastases originating from the proximal or distal colon/rectum. Therefore, we adjusted for anatomical origin and treatment exposure status in all analyses comparing primary tumors and metastases of CRC (Supplementary Fig. S3–S7).
Table 1.
Baseline Characteristics for Total Cancer Care (TCC) Moffitt Cancer Center (MCC) and Consortium participants.
| MCC (n= 517) | Consortium (n=618) | |||
|---|---|---|---|---|
| Primary (n=333) | Metastatic (n=184) | Primary (n=545) | Metastatic (n=73) | |
| Age at Diagnosis (yrs) | 63.64 | 59.30 | 67.94 | 58.09 |
| Race/Ethnicity (%) | ||||
| White | 303 (90.9%) | 157 (85.3%) | 470 (86.2%) | 62 (85.0%) |
| Black/African American | 15 (4.5%) | 10 (5.4%) | 36 (6.6%) | 8 (10.9%) |
| Other/Unknown | 15 (4.5 %) | 17(9.2%) | 39 (7.2%) | 3 (4.1%) |
| Gender (%) | ||||
| Male | 183 (54.9%) | 106 (57.6%) | 265 (48.6%) | 47 (64.4%) |
| Female | 150 (45.0%) | 78 (42.4%) | 280 (51.4%) | 26 (35.6%) |
| Treatment Status (%) | ||||
| Pre-Treatment | 235 (70.6%) | 56 (30.4%) | 448 (82.2%) * | 10 (13.7%) * |
| Post-Treatment | 98 (29.4%) | 128 (69.6%) | 97 (17.8%) * | 63 (86.3%) * |
| • Chemotherapy Only | 28 (8.4%) | 100 (54.3%) | ||
| • Chemotherapy & Radiation | 63 (18.9%) | 27 (14.7%) | ||
| • Radiation Only | 7 (2.1%) | 1(0.5%) | ||
| Anatomical Origin (%) | ||||
| Proximal Colon | 129 (38.7%) | 44 (23.9%) | 284 (52.1%) * | 19 (26.0%) * |
| Distal Colon/Rectum | 204 (61.3%) | 139 (75.5%) | 261 (47.9%) * | 54 (74.0%) * |
| Microsatellite Instability Status (%) | ||||
| MSI-High | 3 (0.9%) | 0 (0%) | ||
| MSS | ||||
| • MSI-Low | 5 (1.5%) | 3 (1.6%) | ||
| • MSS | 37 (11.1%) | 23 (12.5%) | ||
| Unknown | 288 (86.4%) | 158 (85.9%) | ||
| Primary Tumor Stage (%) | ||||
| Stage 1 | 56 (16.8%) | 0 (0%) | ||
| Stage 2 | 105 (31.5%) | 7 (1.3%) | ||
| Stage 3 | 100 (30.0%) | 21 (3.8%) | ||
| Stage 4 | 72 (21.7%) | 27 (5.0%) | ||
| Unknown | 490 (89.9%) | |||
| Site of Metastasis (%) | ||||
| Liver | 141 (76.6%) | 56 (76.7%) | ||
| Lung | 43 (23.4%) | 17 (23.3%) | ||
Notes:
If anatomical origin and treatment exposure status for tumors in the Consortium dataset was not available, the missing data were imputed using gene expression-based classifiers. Anatomical origin was imputed for n = 20 primary tumors and n= 13 metastases of CRC in the Consortium dataset. Treatment was imputed for n= 336 primary tumors and n = 67 metastases of CRC in the Consortium dataset. Treatment status refers to the exposure of primary tumors and metastases of CRC to chemotherapy and/or radiation treatment prior to surgical resection of the tumor. Microsatellite instability (MSI) status was determined using PCR for five MSI markers (BAT25, BAT26, NR21, NR24, and NR27).
Only n=15 samples in the MCC dataset were paired samples with primary tumors and metastases originating from the same patient.
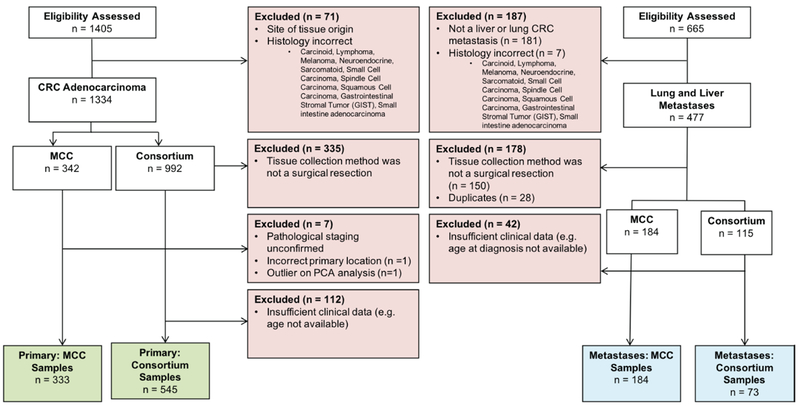
Figure 1. CONSORT flow diagram detailing inclusion and exclusion criteria for primary and metastatic colorectal adenocarcinoma samples (CRC).
All possible CRC samples with available gene expression data originating from the large bowel, rectum, or anus were considered for this study. CRC metastases were restricted to those found in the liver or lung. All tumor samples were restricted to one sample per patient with the exception of 15 patients with matching CRC primary and lung or liver metastases.
Elimination of host tissue-specific gene expression
Lung and liver resection of CRC metastases improves long-term survival (23–25). Surgical resection margins should be tumor-free to ensure removal of the entire tumor mass. Therefore, some remnants of normal tissue will inevitably be found in resected biospecimens. This makes effective comparative transcriptomic analysis of primary tumors and metastatic lesions challenging as expression differences between the normal primary and metastasis host tissue sites can overshadow the true differences between primary tumors and metastases. To address this issue, we generated tissue-specific gene and pathway-level weights, as previously described (15), for all normal host tissues of interest (colon, rectum, lung, and liver). The use of discretized tissue-specific weights eliminates the need to profile each normal host tissue sample adjacent to the metastatic CRC lesions for the purposes of normalizing the tumor transcriptomic data. Using these weights, we performed tissue-sensitive analyses which only included genes where the expression levels in the normal tissue were below a specific threshold (described further in the methods). This resulted in the elimination of genes exhibiting high-tissue specificity. In addition, we performed tissue-agnostic analyses, which ignore the tissue-specificity of genes and pathways. For the tissue-sensitive analysis, we eliminated all genes and pathways exhibiting tissue-specific activity in lung, liver, colon, or rectum (Supplementary Table S1); the tissue agnostic analysis included all genes and pathways irrespective of tissue-specificity.
As a visual confirmation of this approach, we applied t-distributed stochastic neighbor embedding (tSNE) (26) on the MCC and Consortium gene expression datasets in the tissue agnostic (i.e. without elimination of tissue-specific genes) and tissue sensitive (i.e. with elimination of tissue-specific genes) settings (Fig. 2). In the tissue agnostic setting, samples clustered based on the site (colon/rectum, lung or liver) of tumor resection (Hotelling T2 test comparing tSNE clusters of liver and lung metastases; MCC: P < 1*10−16). Conversely, in the tissue sensitive setting, we observed separation of primary tumors and metastases. However, we no longer observed sample clustering based on site of metastatic tumor resection (Hotelling T2 test comparing tSNE clusters of liver and lung metastases; MCC: P = 0.0491), such that lung and liver metastases are integrated across clusters (Fig. 2). To evaluate the potential influence of tumor purity, we inferred tumor purity for all samples in both datasets using the ESTIMATE algorithm (27), which uses gene expression signatures to infer fractions of stromal, immune, and cancer cells from a mixture. We did not find significant differences in tumor purity between samples drawn from primary tumors and metastases of CRC (MCC Wilcox-rank sum test, P = 0.7087, Consortium Wilcox-rank sum test, P = 0.695, Supplementary Fig. S7). This indicated tumor purity is not the main driver of differences between primaries and metastases as both are likely capturing similar quantities of normal host tissue during tumor resection.
Figure 2. t-distributed stochastic neighbor embedding (tSNE) visualizations of Moffitt Cancer Center (MCC) and Consortium primary colorectal adenocarcinoma (CRC) and lung and liver metastases of CRC in the tissue agnostic and tissue sensitive analysis settings.
tSNE visualizations were generated using the first 50 principal components based on tumor gene expression data. In the tissue agnostic setting, all possible genes were considered for principal component analysis (PCA) and subsequent tSNE visualization. In the tissue sensitive setting, genes exhibiting tissue specificity, defined as a 2-fold expression increase of a given gene in normal lung, liver, colon, or rectum tissues, were excluded.
We examined differential expression of genes at the pathway level using pathways in the Hallmark (18) and C2 collections of the Molecular Signature Database (MSigDB Version 6.0) (16). Differential pathway analyses were performed, adjusting for age, sex, treatment exposure status, and anatomical site of origin between CRC primary and lung metastases as well as between CRC primary and liver metastases in the tissue-sensitive and tissue-agnostic settings (Table 2, Supplementary Table S2–S3). The methods and supplementary materials describe additional details about the pathway-level analyses. Spearman rank correlation was used to assess if similar pathways were enriched when comparing CRC primaries with lung metastases and when comparing CRC primaries with liver metastases. In the tissue-agnostic setting, the rank correlations (ρ) observed for the Hallmark and C2 gene set collections in the MCC dataset were ρHallmark = 0.19 and ρC2 = 0.1 respectively, while in the tissue-sensitive setting they were ρHallmark = 0.61 and ρC2 = 0.25. Convergence of pathway enrichment results in the tissue sensitive but not tissue agnostic settings was replicated in the Consortium dataset (Table 2). In the tissue agnostic setting, liver-specific pathways, such as bile acid production (FDRMCC = 1.77*10−08) and xenobiotic metabolism (FDRMCC =2.39*10−13), were enriched in liver metastases compared to primary tumors. However, in the tissue sensitive setting, cancer-related pathways, such as MYC targets (FDRMCC = 4.74*10−04, FDRConsortium = 8.59*10−09) were enriched in both liver and lung metastases as compared to primaries in both datasets (Table 2–3, Supplementary Table S2–S3). These results highlight the role of host organ tissue gene expression when comparing primaries and metastases. Elimination of tissue-specific gene expression of the host organs allowed us to perform a meta-analysis of liver and lung metastases to determine defining features of metastases of CRC after also accounting for tumor anatomical origin and tumor treatment exposure status.
Table 2. Differences between primary tumors and metastases of colorectal adenocarcinomas (CRC) with and without consideration of tissue-specific pathway expression.
Pathway enrichment differences are displayed between primary CRC and liver CRC metastases and primary CRC and lung CRC metastases in the MCC cohort in the tissue agnostic and tissue sensitive settings. Displayed are the top five pathways found in each analysis. Overlapping enrichment results between the analyses of primary CRC vs. liver metastases and primary CRC vs. lung metastases are highlighted in blue.
| MCC Primary CRC vs. Liver Metastases: Tissue Agnostic | MCC Primary CRC vs. Lung Metastases: Tissue Agnostic | |||||
|---|---|---|---|---|---|---|
| C | Pathway | FDR | Direction | Pathway | FDR | Direction |
| H | HALLMARK_XENOBIOTIC_METABOLISM | 2.39*10−13 | Up | HALLMARK_MYC_TARGETS_V2 | 1.40*10−03 | Up |
| HALLMARK_COAGULATION | 1.77*10−08 | Up | HALLMARK_MYC_TARGETS_V1 | 4.76*10−03 | Up | |
| HALLMARK_BILE_ACID_METABOLISM | 2.66*10−05 | Up | HALLMARK_DNA_REPAIR | 2.82*10−02 | Up | |
| HALLMARK_MYC_TARGETS_V2 | 1.69*10−04 | Up | HALLMARK_E2F_TARGETS | 9.31*10−02 | Up | |
| HALLMARK_MYC_TARGETS_V1 | 4.83*10−03 | Up | HALLMARK_TNFA_SIGNALING_VIA_NFKB | 1.98*10−10 | Down | |
| HALLMARK_FATTY_ACID_METABOLISM | 1.45*10−02 | Up | HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 9.12 *10−18 | Down | |
| C2 | LIVER_SPECIFIC_GENES | 4.83*10−84 | Up | LUNG_CANCER_DIFFERENTIATION_MARKERS | 1.23*10−22 | Up |
| LIVER | 1.48*10−67 | Up | COLON_AND_RECTAL_CANCER_UP | 7.91*10−07 | Up | |
| LIVER_CANCER_SUBCLASS_PROLIFERATION_DN | 7.29*10−42 | Up | BREAST_CANCER_20Q11_AMPLICON | 1.18*10−06 | Up | |
| LIVER_CANCER_SURVIVAL_UP | 2.19*10−26 | Up | REACTOME_INFLUENZA_VIRAL_RNA_TRANSCRIPTION_AND_REPLICATION | 1.43*10−06 | Up | |
| BIOCARTA_INTRINSIC_PATHWAY | 2.7710−26 | Up | RICKMAN_HEAD_AND_NECK_CANCER_D | 1.67*10−06 | Up | |
| KEGG_COMPLEMENT_AND_COAGULATION_CASCADES | 2.4310−24 | Up | REACTOME_PEPTIDE_CHAIN_ELONGATION | 2.32*10−06 | Up | |
| MCC Primary CRC vs. Liver Metastases: Tissue Sensitive | MCC Primary CRC vs. Lung Metastases: Tissue Sensitive | |||||
| C | Pathway | FDR | Direction | Pathway | FDR | Direction |
| H | HALLMARK_MYC_TARGETS_V2 | 4.74*10−04 | Up | HALLMARK_MYC_TARGETS_V2 | 9.25*10−04 | Up |
| HALLMARK_MTORC1_SIGNALING | 7.7*10−02 | Up | HALLMARK_DNA_REPAIR | 7.79*10−02 | Up | |
| HALLMARK_DNA_REPAIR | 8.44*10−02 | Up | HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 1.28*10−07 | Down | |
| HALLMARK_GLYCOLYSIS | 9.32*10−02 | Up | HALLMARK_UV_RESPONSE_DN | 4.61*10−04 | Down | |
| HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 5.38*10−04 | Down | HALLMARK_MYOGENESIS | 2.61*10−03 | Down | |
| HALLMARK_UV_RESPONSE_DN | 4.14*10−03 | Down | HALLMARK_PANCREAS_BETA_CELLS | 1.50*10−02 | Down | |
| C2 | SEMENZA_HIF1_TARGETS | 1.01*10−04 | Up | BREAST_CANCER_20Q11_AMPLICON | 1.84*10−06 | Up |
| REACTOME_INFLUENZA_VIRAL_RNA_TRANSCRIPTION_AND_REPLICATION | 8.90*10−04 | Up | REACTOME_INFLUENZA_VIRAL_RNA_TRANSCRIPTION_AND_REPLICATION | 2.00*10−06 | Up | |
| REACTOME_NONSENSE_MEDIATED_DECAY_ENHANCED_BY_THE_EXON_JUNCTION_COMPLEX | 1.27*10−03 | Up | REACTOME_PEPTIDE_CHAIN_ELONGATION | 3.09*10−06 | Up | |
| BREAST_CANCER_20Q11_AMPLICON | 1.28*10−03 | Up | KEGG_RIBOSOME | 4.51*10−06 | Up | |
| KEGG_RIBOSOME | 1.72*10−03 | Up | REACTOME_3_UTR_MEDIATED_TRANSLATIONAL_REGULATION | 5.21*10−06 | Up | |
| REACTOME_PEPTIDE_CHAIN_ELONGATION | 1.89*10−03 | Up | REACTOME_NONSENSE_MEDIATED_DECAY_ENHANCED_BY_THE_EXON_JUNCTION_COMPLEX | 5.21*10−06 | Up | |
Note: Only filtered pathways enriched with an FDR <0.1 are shown. All pathways examined are from the MSigDB database. C = collection in the MSigDB database, and H = Hallmark collection, C2 = curated (CGP and CP) collection. Direction is chosen in reference to pathways enriched in CRC metastases, such that Up = enriched in CRC metastases, while Down = enriched in primary CRC.
Filtered analyses were performed after removal of pathways exhibiting tissue specificity for liver, lung, colon, and rectum tissues in each collection examined.
All analyses adjust for age, sex, treatment status, and anatomical origin. For the Consortium dataset, missing treatment status and anatomical origin were determined using gene expression-based classifiers.
Table 4. Differences in clusters of metastases in the MCC and Consortium datasets.
The top 500 differentially expressed genes between primary colorectal adenocarcinomas (CRC) and CRC metastases in both datasets were used to perform hierarchal clustering of primary tumors and metastases. Two main clusters of CRC metastases were found in each dataset hereon referred to as M1 and M2. The M1 and M2 clusters in each dataset were compared to each other to determine differences in pathway activity. Shown are the top pathways enriched in the M1 and M2 clusters from both datasets (FDR <0.1). Spearman rank correlation shows similar overlap in pathway enrichment between the M1 and M2 clusters of the two datasets (Hρ = 0.66, C2ρ = 0.56).
| Hallmark & C2.CP.Reactome Pathways | MCC Dataset | Consortium Dataset | ||||
|---|---|---|---|---|---|---|
| C | Pathway | Rank | FDR | Rank | FDR | Direction |
| H | HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 1 | 1.16*10−22 | 1 | 9.47*10−22 | M1 |
| HALLMARK_ALLOGRAFT_REJECTION | 2 | 1.78*10−19 | 2 | 1.05*10−19 | M1 | |
| HALLMARK_MYOGENESIS | 3 | 9.41*10−16 | 7 | 4.93*10−03 | M1 | |
| HALLMARK_E2F_TARGETS | 1 | 1.16*10−22 | 4 | 9.54*10−05 | M2 | |
| HALLMARK_MYC_TARGETS_V1 | 2 | 1.78*10−19 | 3 | 4.69*10−05 | M2 | |
| HALLMARK_MYC_TARGETS_V2 | 3 | 9.41*10−16 | 1 | 5.61*10−15 | M2 | |
| C2.CP.Reactome | REACTOME_IMMUNOREGULATORY_INTERACTIONS_BETWEEN_A_LYMPHOID_AND_A_NON_LYMPHOID_CELL | 1 | 1.03*10−11 | 3 | 5.40*10−12 | M1 |
| REACTOME_COLLAGEN_FORMATION | 2 | 1.83*10−10 | 1 | 8.19*10−13 | M1 | |
| REACTOME_EXTRACELLULAR_MATRIX_ORGANIZATION | 3 | 1.83*10−10 | 2 | 8.56*10−13 | M1 | |
| REACTOME_CHONDROITIN_SULFATE_DERMATAN_SULFATE_METABOLISM | 4 | 1.96*10−09 | 25 | 9.86*10−04 | M1 | |
| REACTOME_GLYCOSAMINOGLYCAN_METABOLISM | 5 | 4.85*10−09 | 18 | 3.18*10−04 | M1 | |
| REACTOME_INTEGRIN_CELL_SURFACE_INTERACTIONS | 6 | 7.08*10−09 | 7 | 5.60*10−07 | M1 | |
| REACTOME_GENERATION_OF_SECOND_MESSENGER_MOLECULES | 7 | 1.76*10−08 | 4 | 4.68*10−09 | M1 | |
| REACTOME_PHOSPHORYLATION_OF_CD3_AND_TCR_ZETA_CHAINS | 8 | 5.95*10−08 | 9 | 3.03*10−06 | M1 | |
| REACTOME_PD1_SIGNALING | 9 | 6.04*10−08 | 5 | 5.99 *10−08 | M1 | |
| REACTOME_TRANSLOCATION_OF_ZAP_70_TO_IMMUNOLOGICAL_SYNAPSE | 10 | 6.10*10−08 | 8 | 6.00*10−07 | M1 | |
| REACTOME_PLATELET_ACTIVATION_SIGNALING_AND_AGGREGATION | 11 | 1.68*10−07 | 31 | 1.26*10−03 | M1 | |
| REACTOME_CHONDROITIN_SULFATE_BIOSYNTHESIS | 12 | 2.50*10−07 | 38 | 2.45*10−03 | M1 | |
| REACTOME_DNA_REPLICATION | 1 | 1.03*10−11 | 21 | 1.72*10−02 | M2 | |
| REACTOME_MITOTIC_M_M_G1_PHASES | 2 | 3.59*10−11 | 25 | 2.03*10−02 | M2 | |
| REACTOME_G2_M_CHECKPOINTS | 3 | 2.48*10−10 | 11 | 1.01*10−02 | M2 | |
| REACTOME_ACTIVATION_OF_THE_PRE_REPLICATIVE_COMPLEX | 4 | 3.94*10−10 | 17 | 1.46*10−02 | M2 | |
| REACTOME_S_PHASE | 5 | 5.51*10−10 | 71 | 6.76*10−02 | M2 | |
| REACTOME_ACTIVATION_OF_ATR_IN_RESPONSE_TO_REPLICATION_STRESS | 6 | 8.18*10−10 | 20 | 1.66*10−02 | M2 | |
| REACTOME_DNA_STRAND_ELONGATION | 7 | 1.39*10−09 | 63 | 5.91*10−02 | M2 | |
| REACTOME_TELOMERE_MAINTENANCE | 8 | 1.39*10−09 | 12 | 1.01 −02 | M2 | |
| REACTOME_DEPOSITION_OF_NEW_CENPA_CONTAINING_NUCLEOSOMES_AT_THE_CENTROMERE | 9 | 3.80*10−09 | 31 | 2.81*10−02 | M2 | |
| REACTOME_SYNTHESIS_OF_DNA | 10 | 1.58*10−09 | 72 | 6.76*10−02 | M2 | |
| REACTOME_CHROMOSOME_MAINTENANCE | 11 | 1.80*10−09 | 13 | 1.04*10−02 | M2 | |
| REACTOME_G1_S_TRANSITION | 12 | 1.96*10−09 | 52 | 4.79*10−02 | M2 | |
Note: All pathways examined are from the MSigDB gene set collections. C = collection in the MSigDB database, and H = Hallmark collection, C2 = Reactome Collection, Pathway enrichment differences were determined after removal of pathways exhibiting tissue specificity for liver, lung, colon, and rectum tissues in each collection examined. In addition, all pathway enrichment analyses adjust for age, sex, treatment status, and anatomical origin. For the Consortium dataset, missing data on treatment exposure status and anatomical origin were imputed using gene expression-based classifiers.
The role of tumor treatment exposure in comparing CRC primaries and metastases
Given the unbalanced distribution of treatment exposure between CRC primary tumors and metastatic lesion biospecimens that underwent gene expression profiling, we aimed to examine the role of treatment as a potential confounder when comparing the transcriptomic patterns of primaries and metastases. In the tissue-sensitive setting, we found that pathways such as angiogenesis and hypoxia were enriched in metastases of CRC compared to primary tumors when treatment status is ignored (Table 3, Supplementary Table S4). Importantly, angiogenesis and hypoxia were also enriched in treatment-exposed tumors relative to treatment-naïve tumors, indicating that their apparent enrichment in metastases is due to confounding by treatment status (Table 3, Supplementary Table S4–5). Supporting the role of treatment status as a confounder, angiogenesis and hypoxia are no longer enriched in metastases when the pathway analysis adjusts for treatment exposure status. In order to determine features of chemotherapy and/or radiation treatment exposed metastases of CRC, we compared treatment naïve (n= 56) and exposed (n=128) metastases in the MCC dataset to one another. Treatment exposed metastases shared characteristics with treatment exposed primaries, such as increased epithelial mesenchymal transition (EMT) (HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION; FDRMCC = 4.81*10−04), angiogenesis (HALLMARK_ANGIOGENESIS; FDRMCC = 6.21*10−02), and hypoxia (HALLMARK_HYPOXIA; FDRMCC = 6.36*10−02) whereas treatment naïve metastases, exhibited increased MYC_TARGETS_V2 (FDRMCC = 3.59*10−07) and proliferative activity (REACTOME_S_PHASE; FDRMCC = 2.76*10−03). These findings (Table 3, Supplementary Table S6) highlighted not only the role of treatment exposure in altering the transcriptomic landscapes of both CRC primary tumors and metastases, but also demonstrated the importance of considering treatment exposure as a covariate when comparing gene expression patterns of primary tumors and metastatic lesions.
Table 3. The role of chemotherapy and radiation exposure when comparing primary tumors and metastases of colorectal adenocarcinoma (CRC).
We evaluated pathway enrichment differences between primaries and metastases while adjusting for tumor treatment status, and while ignoring tumor treatment status. In addition, we examined differences between treatment naïve and treatment exposed tumors in both datasets. Here we display the top three pathways enriched in metastases and primary CRC tumors in the treatment adjusted and treatment naïve analyses, as well as the top three pathways enriched in naïve and treatment exposed tumors. Pathways overlapping between the MCC and Consortium analyses are highlighted in yellow.
| MCC Dataset: Primary CRC vs. lung and liver CRC metastases—adjusted for treatment status. | MCC Dataset: Primary CRC vs. lung and liver CRC metastases—not adjusted for treatment status | MCC Dataset: Pre vs. post treatment—adjusted for primary vs. metastatic tumor status | |||||
|---|---|---|---|---|---|---|---|
| C | Pathway | FDR | Pathway | FDR | Pathway | FDR | Direction |
| H | HALLMARK_MYC_TARGETS_V2 | 9.25*10−04 | HALLMARK_ANGIOGENESIS | 6.69*10−02 | HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 3.30*10−18 | Up |
| HALLMARK_DNA_REPAIR | 7.78*10−02 | HALLMARK_HYPOXIA | 6.69*10−02 | HALLMARK_MYOGENESIS | 4.28*10−12 | Up | |
| HALLMARK_GLYCOLYSIS | 7.78*10−02 | HALLMARK_GLYCOLYSIS | 1.75*10−01 | HALLMARK_HYPOXIA | 9.15*10−08 | Up | |
| HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 9.25*10−04 | HALLMARK_PANCREAS_BETA_CELLS | 6.69*10−02 | HALLMARK_MYC_TARGETS_V2 | 4.60*10−18 | Down | |
| HALLMARK_UV_RESPONSE_DN | 4.66*10−03 | HALLMARK_UV_RESPONSE_DN | 1.59*10−01 | HALLMARK_DNA_REPAIR | 1.36*10−03 | Down | |
| HALLMARK_PANCREAS_BETA_CELLS | 2.13*10−02 | HALLMARK_PROTEIN_SECRETION | 1.76*10−01 | HALLMARK_MTORC1_SIGNALING | 2.00*10−03 | Down | |
| Consortium Dataset: Primary CRC vs. lung and liver CRC metastases—adjusted for treatment status. | Consortium Dataset: Primary CRC vs. lung and liver CRC metastases—not adjusted for treatment status | Consortium Dataset: Pre vs. post treatment—adjusted for primary vs. metastatic tumor status | |||||
| C | Pathway | FDR | Pathway | FDR | Pathway | FDR | Direction |
| H | HALLMARK_MYC_TARGETS_V2 | 8.59*10−09 | HALLMARK_ANGIOGENESIS | 2.17*10−03 | HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 1.22*10−24 | Up |
| HALLMARK_MTORC1_SIGNALING | 6.38*10−04 | HALLMARK_HYPOXIA | 2.86*10−03 | HALLMARK_MYOGENESIS | 1.45*10−12 | Up | |
| HALLMARK_GLYCOLYSIS | 5.75*10−03 | HALLMARK_PEROXISOME | 5.54*10−02 | HALLMARK_UV_RESPONSE_DN | 7.82*10−10 | Up | |
| HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION | 2.53*10−14 | HALLMARK_PANCREAS_BETA_CELLS | 1.96*10−01 | HALLMARK_MYC_TARGETS_V2 | 8.97*10−19 | Down | |
| HALLMARK_MYOGENESIS | 1.46*10−06 | HALLMARK_ESTROGEN_RESPONSE_LATE | 6.72*10−01 | HALLMARK_MTORC1_SIGNALING | 4.64*10−06 | Down | |
| HALLMARK_UV_RESPONSE_DN | 4.93*10−06 | HALLMARK_SPERMATOGENESIS | 6.72*10−01 | HALLMARK_DNA_REPAIR | 1.68*10−04 | Down | |
Note: All pathways examined are from the MSigDB gene set collections. Top three up-regulated and top three down-regulated pathways from each MSigDB collection are shown. C = collection in the MSigDB database, and H = Hallmark collection. Direction is chosen in reference to pathways enriched in CRC metastases (Up) or in treatment exposed samples (Up). The down direction refers to pathways enriched in either primary CRC tumors or treatment naïve tumors depending on the analysis. All analyses contrasting primary CRC and metastases of CRC were performed after filtering for pathways exhibiting tissue specificity for colon, rectum, lung, or liver tissues, and after adjusting for age, sex, anatomical origin, and treatment status (unless indicated otherwise). When contrasting pathway enrichment between different treatment groups, we adjust for tumor type (primary CRC vs. lung/liver CRC metastases). For Consortium cases, missing data on treatment status and anatomical origin were imputed using gene expression-based classifiers.
Key gene and pathway enrichment differences between CRC primaries and metastases
We aimed to discover transcriptomic signatures of metastatic lesions after consideration of host tissue expression, treatment exposure status, tumor anatomical origin, age, and sex. We examined pathway and gene enrichment differences between CRC metastases in the lung and liver versus the CRC primary tumors (Supplementary Table S7–S8) in the MCC discovery and Consortium validation datasets.
Examination of the Hallmark collection showed that CRC metastases exhibited increased MYC signaling (FDRMCC = 4.74*10−04), DNA repair (FDRMCC = 8.44*10−02), and glycolysis (FDRMCC = 7.78*10−02) activity (Table 3, Supplementary Table S7). Examination of the C2 collection provides additional support for enhanced MYC signaling in metastases of CRC based on the numerous transcription, translation, and ribosomal pathways found to be enriched in metastases (Supplementary Table S7). Metabolic machinery was also altered in metastases showing increased gluconeogenesis (MOOTHA_GLUCONEOGENESIS; FDRMCC = 6.53*10−03 and REACTOME_GLUCONEOGENESIS; FDRMCC = 6.52*10−03) activity, likely as a result of MYC upregulation. In addition, Hypoxia Inducible Factor (HIF) targets (SEMENZA_HIF1_TARGETS; FDRMCC = 9.87*10−05) (28) and hypoxia targets of VHL (WACKER_HYPOXIA_TARGETS_OF_VHL; FDRMCC = 1.18*10−02) (29) were also enriched in metastatic lesions albeit to a lesser degree than MYC and the metabolic changes associated with it.
Microsatellite instability (MSI) status could be a potential confounder when assessing differences between primary tumors and CRC metastases, as MSI-high tumors are typically diagnosed at less advanced stage (30). Therefore, we replicated our findings in a smaller subset of samples in the MCC dataset for which MSI status was available and adjusted for MSI status in the tissue sensitive setting (Supplementary Table S9). We found the PECE_MAMMARY_STEM_CELL_UP (FDRMCC = 6.84*10−03) (31) and BENPORATH_ES_CORE_NINE (FDRMCC =6.69*10−02) (32) gene sets, which are potential cancer stem cell pathways, to be enriched in lung and liver metastases of CRC. We observed almost no overlap between the genes defining these stem cell signatures and genes defining EMT activity (Fig. 3). As such, our findings suggest EMT and cancer stemness are not necessarily coupled as EMT signatures were more prevalent in CRC primaries while cancer stemness activity was enriched in metastases. Furthermore, we showed that metastases are enriched in expression of cancer stem cell genes even after adjusting for treatment. This suggested that cancer stem cells likely exist in all metastases, but that chemotherapy and radiation treatment exposure may select for them. Similarly, hypoxia and angiogenesis activity are likely enriched in all metastases, but treatment exposure again appears to enhance the activation of these pathways. Viral replication and transcription pathways were also found in metastases. However, these pathways highly overlapped with multiple global cellular transcription and translation pathways which are likely due to the enhanced MYC signaling observed in metastases (Supplementary Table S10) and therefore are not indicative of distinct viral activity. Similarly, the hallmark myogenesis pathway was enriched in primary tumors as it shares many mesenchymal phenotype genes with the hallmark EMT pathway (Supplementary Table S11).
Figure 3. Heatmap visualization and volcano plots showing the top differentially expressed genes between primary tumors and metastases of colorectal adenocarcinomas in the Moffitt Cancer Center (MCC) and Consortium datasets.
A-B) Of the top 500 differentially expressed genes between primary tumors and metastases while adjusting for clinical variables, the top 25 genes are shown. Hierarchal clustering was performed which revealed two main clusters of metastases in both datasets. For Consortium, we excluded 21 genes from the differential expression analysis which were used to develop the anatomical origin and treatment status classifiers. In both the MCC and Consortium datasets, both clusters of metastases, named as MCC-M1 or Consortium-M1 and MCC-M2 and Consortium-M2 include liver and lung metastases. Treatment status of each tumor is also denoted. C-D) Volcano plots displaying the top 500 differentially expressed genes. The x-axis shows the log2 fold change, and the y-axis displays the -log10 of the p-values where all p-values are <0.001. The most differentially expressed genes in primaries (negative log2FC) and metastases (positive log2FC) are highlighted in blue and red respectively. E) A ROC curve showing the performance of the M1/M2 classifier predicting M1 and M2 status in the Consortium dataset based on MCC M1/M2 cluster-membership is shown. F) Overlap between stem cell gene sets and the epithelial mesenchymal transition (EMT) gene set are highlighted.
The most significant differentially expressed genes (Fig. 3, Supplementary Table S8) between primary tumors and metastases of CRC are related to EMT. FBN2 (fold change= 7.6; FDRMCC =1.12*10−99), MMP3 (fold change= 38.2; FDRMCC =1.64*10−91), and FGF10 (fold change= 5.7; FDRMCC =8.74*10−67) are all either known stimulators or markers of EMT (33,34) and were highly elevated in primary tumors compared to metastases (Fig. 3). These findings were supported by our pathway-level results which showed EMT (HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION; FDRMCC = 1.16*10−22) is highly upregulated in primary CRCs. In comparison, genes significantly elevated in metastases (Fig. 3, Supplementary Table S8) include GATA4 (fold change = 3.9; FDRMCC =2.09*10−36), CLND10 (fold change = 3.9; FDRMCC =3.92*10−33), and SYT12 (fold change = 3.4; FDRMCC =1.12*10−54). GATA4 is thought to mark fully differentiated epithelial cells and its expression is often silenced in CRC as forced expression of GATA4 results in impaired CRC cell line proliferation and migration (35). Increased expression of GATA4 in metastases supported our pathway level results which showed decreased EMT activity in metastases. Similarly, CLND10 codes for a claudin protein. Claudin proteins are integral components of tight junctions and their expression has been associated with recurrence of primary hepatocellular carcinoma (36). Last, SYT12 is involved in regulating calcium independent sections in non-neuronal cells, and it has been previously linked with unfavorable prognosis in pancreatic cancer (37).
Based on the top 500 differentially expressed genes between primary tumors and metastases of CRC (Supplementary Table S8), we performed hierarchical clustering of all CRC tumor samples in both datasets in order to determine the degree of transcriptomic similarity between clusters of primaries and metastases. We hypothesized a strong degree of separation between primary tumors and metastases. However, despite having generated five main clusters from the top 500 differentially expressed genes, we observed several primary CRC tumors embedded within the clusters of metastases. Surprisingly, we also noticed that metastases only appeared in two main clusters, hereon called M1 and M2 (Fig. 3, Table 4), in both datasets. We aimed to confirm that the M1 and M2 clusters in the MCC and Consortium datasets were defined by similar underlying biology. M1/M2 cluster membership in each dataset was defined by the top 500 differentially expressed genes between primary tumors and metastases in an adjusted regression analysis in each dataset. Therefore, we developed a classifier trained on the MCC dataset which predicted the cluster membership of the Consortium metastases (Fig. 3E) based on pathway-level expression differences between the MCC M1 and M2 clusters (Table 4). Similar to the differential gene expression analysis comparing primaries and metastases, inputs for the M1/M2 classifier also adjusted for age, sex, anatomical origin, and treatment exposure status. Based on the strong performance of our classifier (AUC = 0.905), we believe the M1 and M2 clusters in both datasets have similar biology. Therefore, we further investigated the pathway-level differences between the M1 and M2 clusters of metastases observed in both datasets.
Subtypes of CRC metastases in the lung and liver
We compared enrichment of pathways in the MSigDB hallmark and C2.CP.REACTOME collections between the M1 and M2 clusters of metastases found in both the MCC and Consortium datasets. We restricted the analysis to these two collections to both assist with interpretation of results and avoid redundant enrichment of functional pathways (results for the complete C2 collection can be found in Supplementary Table S12). Tumors in the M2 cluster primarily exhibited a proliferative phenotype with increased MYC target activity (HALLMARK_MYC_TARGETS_V1; FDRMCC = 1.78*10−19; HALLMARK_MYC_TARGETS_V2; FDRMCC = 9.41*10−16) and E2F target activity (HALLMARK_E2F_TARGETS; FDRMCC = 1.16*10−22). Tumors in the MCC M1 cluster primarily exhibited an inflammatory and immune escape phenotype (Table 4, Supplementary Table S12). Notably, pathway enrichment differences showed not only an innate immune response (REACTOME_INNATE_IMMUNE_SYSTEM; FDRMCC = 4.91*10−04) in the MCC M1 cluster, but also a very strong adaptive immune response (REACTOME_ADAPTIVE_IMMUNITY; FDRMCC = 2.53*10−03), defined by T cell infiltration (Table 4, Supplementary Table S12) in M1 metastases, which is likely blunted by the tumor through expression of immune checkpoint inhibitors such as PD1 (REACTOME_PD1_SIGNALING; FDRMCC = 6.04*10−08). We examined the degree of overlap from pathway enrichment results when comparing M1 and M2 clusters in both the MCC and Consortium datasets using Spearman rank correlation, where ρHallmark = 0.66, and ρC2.CP.Reactome = 0.56. These results further highlighted the robustness of the M1 and M2 metastatic clusters found in each dataset. Of note, the immune-related differences found between the M1 and M2 clusters in the MCC dataset best predicted cluster membership of Consortium metastases (Fig 3E). Overall, our results suggested there are two main types of CRC metastases to the lung and liver—those which can be considered immune “hot” tumors and exhibit an inflammatory phenotype, and those which can be considered immune “cold” tumors which are not characterized by inflammation, but rather canonical MYC and E2F signaling with a proliferative signature.
Consensus Molecular Subtype (CMS) classification of colorectal lung and liver metastases
Due to the observance of canonical oncogenic signaling in CRC metastases, we assessed whether CRC metastases are enriched for a particular consensus molecular subtype (CMS). CMS is one of the most robust gene expression-based CRC classification systems with known prognostic implications (22). There are four main CMS groups, CMS1-CMS4, as well as the CMS_NA group, which consists of tumor samples that cannot be classified as CMS1-CMS4. Samples in the CMS_NA group are thought to contain properties of multiple CMS groups and as such, CMS_NA is not considered to be a distinct CMS (22). We implemented the CMS classifier (22) in order to determine if metastases of CRC exhibit a propensity to be in a specific CMS group. In both the MCC and Consortium datasets, metastases were never classified as CMS3, suggesting subtype exclusivity. In addition, implementation of logistic regression models (Supplementary Fig. S8), which adjust for age, sex, anatomical origin, and treatment exposure status of the tumor, showed the odds of a tumor being a metastasis compared to a primary tumor is 2.3 times higher among CMS2 tumors than among CMS4 tumors (MCC dataset: odds ratio 2.30, 95% confidence interval 1.40-3.82, P <0.001). Furthermore, CMS2 (MCC: 36.4%, Consortium: 38.3%) and CMS4 (MCC: 44.0%, Consortium: 45.2%) appeared to be the dominant subtypes found in CRC metastases compared to primary tumors. In addition, 86.6% and 85.7% of metastases in the M1 clusters were CMS4 and 63.4% and 51.9% of metastases in the M2 clusters were CMS2 in the MCC (Fisher’s Exact Test; P < 2.2*10−16) and Consortium (Fisher’s Exact Test; P = 2.5*10−05) datasets respectively. Logistic regression modeling applied to the MCC dataset further supported the association between M1 metastases and the CMS4 group and M2 metastases and the CMS2 group (Supplementary Table S13).
Adaptations to distal tissue sites observed within primary CRC tumors
As metastases are likely to have adapted to the microenvironments of their sites of metastasis prior to dissemination (38), we aimed to determine if these adaptations could already be detected in primary tumors of patients who later go on to develop lung or liver metastases. We compared primary CRC tumors from patients who developed lung (n= 18) metastases to primary CRC tumors from patients who developed liver metastases (n=48) while adjusting for age, sex, stage 4 disease status, tumor anatomical origin, and treatment exposure status. Specifically, we looked for pathways with high lung or liver-specific tissue weights when comparing primary CRC tumors to one another (Supplementary Table S14). In primary tumors from patients who went on to develop liver metastases, we found lipid digestion (REACTOME_LIPID_DIGESTION_MOBILIZATION_AND_TRANSPORT; FDRMCC = 6.96*10−02), lipid transport (REACTOME_CHYLOMICRON_MEDIATED_LIPID_TRANSPORT; FDRMCC = 7.28*10−02), and adipogenesis (STEGER_ADIPOGENESIS_UP; FDRMCC = 6.28*10−03) pathways to be enriched. In primary tumors from patients who went on to develop lung metastases, we found enrichment of interferon alpha response pathways (HALLMARK_INTERFERON_ALPHA_RESPONSE; FDRMCC = 7.17*10−03 and MOSERLE_IFNA_RESPONSE; FDRMCC = 1.21*10−03), which are known to modulate lung inflammation.
DISCUSSION
A tissue sensitive approach for determining features of metastases
Our tissue sensitive approach allows the comparison of metastases located in different host tissue sites without the need for sampling and transcriptomic profiling of normal host tissues. For the comparative analyses of primary tumors and metastases, a tissue sensitive approach supports pooling of metastatic cancers from multiple tissue sites, which both improves statistical power and helps elucidate the common phenotype of metastases from a single primary cancer type (Table 2). In addition, the use of gene-based classifiers to impute tumor anatomical origin and treatment exposure status allows future researchers to adjust for these variables in their analyses when clinical data are limited for retrospective studies.
Role of treatment exposure in comparing CRC primary and metastatic tumors
We highlighted the role of chemotherapy and/or radiation exposure prior to tumor surgical resection as a confounder when comparing transcriptomic profiles of primary CRCs and metastatic lesions (Table 3). As a key example of the confounding effect of treatment status, hypoxia and angiogenesis activity were found to be enriched in treatment-exposed tumors and in metastases when treatment exposure status was not appropriately considered (Supplementary Table 4–5). With appropriate adjustments for treatment exposure, hypoxia and angiogenesis genes were no longer differentially expressed between metastases and primaries. Similarly, we showed that treatment exposed tumors were enriched in EMT, and once treatment exposure status is taken into consideration, we found that primary tumors are more likely to exhibit EMT enrichment than metastases of CRC. Our findings, especially on GATA4 enrichment in metastases, align with previous studies which suggest that metastases undergo mesenchymal epithelial transition (MET) to establish themselves at distal sites and that metastatic tumor cells with a MET phenotype are more likely to exhibit rapid proliferation compared to cells with an EMT phenotype (39,40), which typically divide slowly. In addition, neoadjuvant chemotherapy has been strongly associated with a mesenchymal phenotype in both primary tumors and metastases and is therefore known to affect CRC CMS classification (10,41), which corroborate our findings on the role of treatment when comparing primary tumors and metastases of CRC.
Characteristic features of metastases of CRC
Compared to CRC primary tumors, lung and liver metastases tended to be more differentiated with reduced EMT activity. Metastases exhibited a shift towards glycolysis and were also enriched in MYC target pathways and the downstream effects of MYC such as increased proliferation and global upregulation of transcription and translational cellular machinery (42). HIF targets were also found to be enriched; indeed, oncogenic MYC is known to collaborate with HIF to induce metabolic alterations such as increased glycolysis (Warburg effect) (43,44). In particular, HIF1α expression is required for MYC-induced proliferation and anchorage-independent growth.
Previous studies in melanoma suggest genetic stability appears to be necessary for the development of metastases (45). The observance of activated DNA repair pathways in metastases suggests that a similar metastatic program may be at play in CRC. These results are corroborated by our CMS analysis, where genetically unstable subtypes such as CMS1 and CMS3 are almost non-existent among metastases of CRC (22). CMS classification results show metastases are more likely to be CMS2 in reference to CMS4 than primary tumors. Although CMS4 has previously been associated with advanced stages (III, IV) of disease, CMS2 was not previously associated with advanced disease. CMS2 is characterized by epithelial differentiation and strong upregulation of MYC and WNT signaling (22). This is supported by our gene and pathway enrichment results, which showed metastases exhibit lower EMT activity, were more likely to be differentiated compared to primary tumors and were enriched in MYC signaling and its downstream proliferative pathways. Furthermore, the lack of CMS3 metastases in both the MCC and Consortium datasets was particularly intriguing and raises the question of whether the metabolic and genomic features of CMS3 are incompatible with metastases. Future studies using paired primaries and metastases are warranted to address this question.
Many metastases are also thought to contain cancer stem cells, which can drive drug resistance and are often associated with poor prognosis and survival. We found potential cancer stem cell activity was upregulated in metastases of CRC, suggesting that cancer stem cell features exist in metastases independent of treatment exposure and EMT activity. Our results also suggest that cancer stem cell like features are not exclusively found in EMT-high tumors as primary CRC tumors exhibited higher EMT activity compared to metastases, but cancer stem cell gene sets were found to be enriched in metastases, which exhibited lower EMT activity. This agrees with our gene-level results showing increased GATA4 expression in metastases, which are thought to undergo MET at distal organs, and therefore should exhibit epithelial features compared to primary tumors.
Identification of two main phenotypes of CRC metastases
We identified two main groups of metastases based on transcriptomic features. When comparing the two groups of metastases to one another, we found the first group (M1) was characterized primarily by inflammation featuring adaptive immune system responses, immune evasion pathways (e.g. PD1 signaling) (46,47) and lymphocytic cell-mediated immunity (Table 4, Supplementary Table S12). The second group (M2) was characterized by cell proliferation and MYC signaling (Table 4, Supplementary Table S12). Moreover, the enrichment of EMT activity found in both the M1 cluster and post-treatment metastases and the enrichment of MYC activity in pre-treatment metastases and the M2 cluster (Table 4) suggests these metastatic phenotypes may be influenced by treatment exposure. Nevertheless, the M1 cluster exhibits very strong activation of inflammatory and immune response pathways and this immune-phenotype appears to be the defining feature of the M1 clusters in both datasets. This immune-phenotype was not observed in post treatment metastases. Therefore, it is not clear if treatment exposure can help drive metastases to specific phenotypes. However, our results are consistent with previous research in humans and mouse models, which have suggested metastases fall into two main subtypes—those characterized by EMT and inflammation signatures and those characterized by proliferation (48,49). Recent work in melanoma (50) has shown “cold” metastases which do not respond to immunotherapy and which are enriched in a T cell exclusion program are characterized by MYC signaling and E2F targets. As our work has potentially characterized two phenotypes of metastases, one of which is also characterized by MYC and E2F proliferation signaling, we believe these metastatic phenotypes can inform immunotherapy treatment decisions for CRC as well.
Limitations and future directions
Though we describe a novel method for transcriptomic comparative analyses between primary tumors and metastases of CRC, this study should be considered within the context of its limitations. While this study compares primary CRC tumors to liver and lung CRC metastases, it does so with a limited set of matched (n=15) primary and metastatic tumor samples from the same individuals. Moreover, our analysis comparing primary tumors from patients which go on to develop lung or liver metastases suggests some adaptations to the distal site of metastasis can already be observed in the primary tumor. These adaptations may be lost when implementing our tissue-sensitive adjustments for comparing primary tumors and metastases, and this could potentially produce false negative results. Future studies should examine larger cohorts of matched samples, where available, to explore features of metastatic progression and drug resistance. In addition, although this study captured and appropriately adjusted for chemotherapy and radiation exposure, due to data limitations, it did not capture specific features of treatment, such as radiation dose, duration of treatment, and drug class of the chemotherapeutic agents. The use of classifiers to impute anatomical origin and treatment status, though imperfect and requiring significant methodological improvement, allows future research on metastases with limited clinical information to be performed while appropriately adjusting for potential confounders. With regards to the M1/M2 clusters, we show replicability across datasets at the pathway level but acknowledge the limitations of M1/M2 cluster replicability across datasets at the gene-level due to underlying differences between the clinical characteristics of each dataset as these differences inform gene-level M1/M2 cluster membership. Lastly, the prognostic differences between subtypes of metastases should be carefully explored. In particular, the EMT inflammatory group of metastases can be better characterized to understand immune escape mechanisms employed by metastases.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Findings identify a CRC metastasis-specific gene expression signature that is free from potentially confounding background signals coming from treatment exposure and the normal host tissue that the metastasis is now situated within.
ACKNOWLEDGEMENTS
The authors are grateful for the financial support from research grants, (1) 5T32LM012204-03 NIH-NLM, (2) 1K01LM012426 NIH-NLM. This work was supported in part by Moffitt’s Total Cancer Care Initiative, the Collaborative Data Services Core and the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center, under grant number P30-CA076292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the H. Lee Moffitt Cancer Center & Research Institute. We would also like to thank Drs. Eric A. Welsh and Michael J. Schell for their assistance in the acquisition, curation, and cleaning of the datasets used in this manuscript. Partial support for this research was provided to support Dr. Amos efforts through Cancer Prevention Research Institute of Texas grant RR170048 and NIH/NCI grant U01CA196386.
Footnotes
Conflict of Interest: There are no conflicts of interest for all authors
These authors contributed equally to this work
REFERENCES
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer [Internet] 2002. [cited 2018 Oct 16];2:563–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12154349 [DOI] [PubMed] [Google Scholar]
- 2.van der Geest LGM, Lam-Boer J, Koopman M, Verhoef C, Elferink MAG, de Wilt JHW. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis [Internet]. 2015. [cited 2018 Oct 16];32:457–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25899064 [DOI] [PubMed] [Google Scholar]
- 3.Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Nat Publ Gr [Internet]. 2016. [cited 2018 Aug 21];6 Available from: www.nature.com/scientificreports [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol [Internet]. 2017. [cited 2018 Oct 16];28:iv22–iv40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28881920 [DOI] [PubMed] [Google Scholar]
- 5.Chan DLH, Segelov E, Wong RS, Smith A, Herbertson RA, Li BT, et al. Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst Rev [Internet]. 2017. [cited 2018 Nov 19];6:CD007047 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28654140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med [Internet]. 2015. [cited 2016 Oct 31];372:2509–20. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignot S, Lefebvre C, Frampton GM, Meurice G, Yelensky R, Palmer G, et al. Comparative analysis of primary tumour and matched metastases in colorectal cancer patients: Evaluation of concordance between genomic and transcriptional profiles. Eur J Cancer [Internet]. 2015. [cited 2018 Oct 16];51:791–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25797355 [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Zhang C, Zhang Z, Qian W, Sun Y, Ji B, et al. Transcriptome analysis in primary colorectal cancer tissues from patients with and without liver metastases using next-generation sequencing. Cancer Med [Internet]. Wiley-Blackwell; 2017. [cited 2018 Aug 31];6:1976–87. Available from: http://doi.wiley.com/10.1002/cam4.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartung F, Wang Y, Aronow B, Weber GF. A core program of gene expression characterizes cancer metastases. Oncotarget [Internet]. Impact Journals, LLC; 2017. [cited 2018 Oct 12];8:102161–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29254233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trumpi K, Ubink I, Trinh A, Djafarihamedani M, Jongen JM, Govaert KM, et al. Neoadjuvant chemotherapy affects molecular classification of colorectal tumors. Oncogenesis [Internet]. Nature Publishing Group; 2017. [cited 2018 Jul 23];6:e357 Available from: http://www.nature.com/doifinder/10.1038/oncsis.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet]. 2014. [cited 2018 Oct 16];25:iii1–iii9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25190710 [DOI] [PubMed] [Google Scholar]
- 12.Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer J [Internet]. NIH Public Access; 2011. [cited 2018 Oct 16];17:528–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22157297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst [Internet] 1997. [cited 2018 Oct 23];89:1758–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9392616 [DOI] [PubMed] [Google Scholar]
- 14.Welsh EA, Eschrich SA, Berglund AE, Fenstermacher DA. Iterative rank-order normalization of gene expression microarray data. BMC Bioinformatics [Internet]. 2013. [cited 2018 Oct 16];14:153 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23647742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost HR. Computation and application of tissue-specific gene set weights Stegle O, editor. Bioinformatics [Internet]. 2018. [cited 2018 Oct 16];34:2957–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29659714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics [Internet]. 2011. [cited 2018 Jun 28];27:1739–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21546393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Smyth GK. Camera: a competitive gene set test accounting for inter-gene correlation Nucleic Acids Res [Internet]. Oxford University Press; 2012. [cited 2018 Oct 16];40:e133 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22638577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberzon A, Birger C, Thorvaldsdó H, Ghandi M, Mesirov JP, Tamayo P, et al. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst [Internet]. 2015. [cited 2017 Oct 12];1:417–25. Available from: 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies Nucleic Acids Res [Internet]. Oxford University Press; 2015. [cited 2018 Oct 16];43:e47 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25605792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med [Internet]. 1990. [cited 2018 Oct 16];113:779–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2240880 [DOI] [PubMed] [Google Scholar]
- 21.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature [Internet]. 2009. [cited 2018 Jun 28];462:108–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19847166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med [Internet]. 2015. [cited 2018 Oct 16];21:1350–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26457759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valderrama-Treviño AI, Barrera-Mera B, Ceballos-Villalva JC, Montalvo-Javé EE. Hepatic Metastasis from Colorectal Cancer. Euroasian J hepato-gastroenterology [Internet]. Jaypee Brothers Medical Publishing (P) Ltd.; 2017. [cited 2018 Oct 11];7:166–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29201802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg [Internet]. 1992. [cited 2018 Oct 16];127:1403–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1365684 [DOI] [PubMed] [Google Scholar]
- 25.Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, et al. Surgical Resection of Hepatic and Pulmonary Metastases from Colorectal Carcinoma. J Am Coll Surg [Internet]. 2006. [cited 2018 Oct 8];202:468–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16500252 [DOI] [PubMed] [Google Scholar]
- 26.van der Maaten L, Hinton G. Visualizing Data using t-SNE. J Mach Learn Res [Internet]. 2008. [cited 2018 Oct 16];9:2579–605. Available from: http://www.jmlr.org/papers/v9/vandermaaten08a.html [Google Scholar]
- 27.Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun [Internet]. 2013. [cited 2017 Nov 10];4 Available from: https://www.nature.com/articles/ncomms3612.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med [Internet]. 2001. [cited 2018 Oct 16];7:345–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11516994 [DOI] [PubMed] [Google Scholar]
- 29.Wacker I, Sachs M, Knaup K, Wiesener M, Weiske J, Huber O, et al. Key role for activin B in cellular transformation after loss of the von Hippel-Lindau tumor suppressor. Mol Cell Biol [Internet]. 2009. [cited 2018 Oct 16];29:1707–18. Available from: http://mcb.asm.org/cgi/doi/10.1128/MCB.01184-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benatti P, Gafà R, Barana D, Marino M, Scarselli A, Pedroni M, et al. Microsatellite Instability and Colorectal Cancer Prognosis. Clin Cancer Res [Internet]. 2005. [cited 2018 Dec 3];11:8332–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16322293 [DOI] [PubMed] [Google Scholar]
- 31.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell [Internet]. 2010. [cited 2018 Oct 23];140:62–73. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867409015542 [DOI] [PubMed] [Google Scholar]
- 32.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet [Internet]. 2008. [cited 2018 Oct 23];40:499–507. Available from: http://www.nature.com/articles/ng.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abolhassani A, Riazi GH, Azizi E, Amanpour S, Muhammadnejad S, Haddadi M, et al. FGF10: Type III Epithelial Mesenchymal Transition and Invasion in Breast Cancer Cell Lines. J Cancer [Internet]. 2014. [cited 2018 Nov 29];5:537–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25057305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen QK, Lee K, Radisky DC, Nelson CM. Extracellular matrix proteins regulate epithelial–mesenchymal transition in mammary epithelial cells. Differentiation [Internet]. 2013. [cited 2018 Nov 29];86:126–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23660532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng R, Blobel GA. GATA Transcription Factors and Cancer. Genes Cancer [Internet]. Impact Journals, LLC; 2010. [cited 2018 Nov 29];1:1178–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21779441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung ST, Leung KL, Ip YC, Chen X, Fong DY, Ng IO, et al. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res [Internet]. 2005. [cited 2018 Nov 29];11:551–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15701840 [PubMed] [Google Scholar]
- 37.Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. [cited 2017 Oct 12]; Available from: http://science.sciencemag.org/content/sci/357/6352/eaan2507.full.pdf [DOI] [PubMed]
- 38.Cunningham JJ, Brown JS, Vincent TL, Gatenby RA. Divergent and convergent evolution in metastases suggest treatment strategies based on specific metastatic sites. Evol Med Public Heal [Internet]. 2015. [cited 2019 Feb 20];2015:76–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25794501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal Regulation of Epithelial-Mesenchymal Transition Is Essential for Squamous Cell Carcinoma Metastasis. Cancer Cell [Internet]. 2012. [cited 2018 Oct 16];22:725–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23201165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Pozo Martin Y, Park D, Ramachandran A, Ombrato L, Calvo F, Chakravarty P, et al. Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep [Internet]. 2015. [cited 2018 Oct 16];13:2456–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26670048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HH, Bellat V, Law B. Chemotherapy induces adaptive drug resistance and metastatic potentials via phenotypic CXCR4-expressing cell state transition in ovarian cancer Ahmad A, editor. PLoS One [Internet]. Public Library of Science; 2017. [cited 2018 Oct 16];12:e0171044 Available from: http://dx.plos.org/10.1371/journal.pone.0171044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, Metabolism, and Cancer. Cancer Discov [Internet]. 2015. [cited 2018 Sep 30];5:1024–39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26382145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doe MR, Ascano JM, Kaur M, Cole MD. Myc posttranscriptionally induces HIF1 protein and target gene expression in normal and cancer cells. Cancer Res [Internet]. NIH Public Access; 2012. [cited 2018 Oct 10];72:949–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22186139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Podar K, Anderson KC. A therapeutic role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell Cycle [Internet]. NIH Public Access; 2010. [cited 2018 Oct 10];9:1722–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20404562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kauffmann A, Rosselli F, Lazar V, Winnepenninckx V, Mansuet-Lupo A, Dessen P, et al. High expression of DNA repair pathways is associated with metastasis in melanoma patients. Oncogene [Internet]. 2008. [cited 2018 Oct 10];27:565–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17891185 [DOI] [PubMed] [Google Scholar]
- 46.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and Its Ligands in Tolerance and Immunity. Annu Rev Immunol [Internet]. 2008. [cited 2018 Oct 16];26:677–704. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18173375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev [Internet] 2008. [cited 2018 Oct 16];224:166–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18759926 [DOI] [PubMed] [Google Scholar]
- 48.Robinson R, Wu Y-M, Lonigro J, Vats P, Cobain R, Everett J, et al. Integrative clinical genomics of metastatic cancer. Nature [Internet]. 2017. [cited 2017 Aug 3]; Available from: https://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature23306.pdf [DOI] [PMC free article] [PubMed]
- 49.Bakhoum SF, Ngo B, Laughney AM, Cavallo J-A, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature [Internet]. NIH Public Access; 2018. [cited 2019 Feb 20];553:467–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29342134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su M-J, Melms JC, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell [Internet] 2018. [cited 2018 Nov 5];175:984–997. e24 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30388455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.