Abstract
Rationale:
Evidence indicates drug-paired stimuli can evoke drug-craving leading to drug-seeking and repeated relapse periods can influence drug-seeking behaviors.
Objectives:
The present study examined (1) the effect of an interaction between repeated deprivation cycles and excitatory conditioning stimuli (CS +) on ethanol (EtOH)-seeking; (2) the effects of EtOH-paired cue-exposure in a non-drug paired environment on subsequent conditioning in a drug-paired environment; and (3) the temporal effects of conditioned cues on subsequent EtOH-seeking.
Methods:
Adult female alcohol-preferring (P) rats were exposed to three conditioned odor cues; CS+ associated with EtOH self-administration, CS- associated with the absence of EtOH (extinction training), and a neutral stimulus (CS0) presented in a neutral non drug-paired environment. The rats underwent 4 deprivation cycles or were Non-Deprived, following extinction they were maintained in a home cage for an EtOH-free period, and then exposed to no cue, CS+, CS-, or CS0 to assess the effect of the conditioned cues on EtOH-seeking behavior.
Results:
Repeated deprivations enhanced and prolonged the duration of CS+ effects on EtOH-seeking. Presentation of the CS- in a non-drug paired environment blocked the ability of a CS+ to enhance EtOH-seeking in a drug-paired environment. Presentation of the CS+ or CS- in a non-drug paired environment 2 or 4-hours earlier significantly altered EtOH-seeking.
Conclusion:
Results indicated an interaction between repeated deprivation cycles and CS+ resulted in a potentiation of CS+ evoked EtOH-seeking. In addition, a CS- may have therapeutic potential by providing prophylactic protection against relapse behavior in the presence of cues in the drug-using environment.
Keywords: Drug-seeking, drug-craving, drug-relapse, drug-deprivation, alcohol relapse, alcohol preferring P rats
INTRODUCTION
Alcohol (ethanol: EtOH) addiction is characterized by high rates of compulsive drug-seeking and relapse to drug-taking behavior. It is noteworthy that relapse can occur after weeks or months of abstinence during and after treatment (Ferri et al. 2006). Environmental contexts and associated drug cues can elicit craving and subsequent drug-seeking behaviors after prolonged abstinence periods may contribute to the recurrent chronic relapsing nature of EtOH addiction (O’Brien et al. 1992, 1998). Clinical studies have shown that drug-taking environments play an important role in precipitating drug craving (Conklin et al. 2008, 2009). In particular, ecologically relevant drug-paired environments evoke higher and more persistent levels of EtOH craving compared to general drug-specific environments (Fatseas et al. 2015; McClernon et al. 2016).
Alcoholics go through repeated periods of high EtOH drinking and periods of abstinence (Burish et al. 1981; Hilbrom 1990; McMillen 1997). Similar to animal studies (c.f., Bell et al. 2012, 2016, 2017; Hauser et al. 2016; Rodd et al. 2003, 2004), clinical research has reported that exposure to repeated cycles of EtOH consumption and deprivation has deleterious consequences, including increased withdrawal seizure severity (c.f., Bell et al. 2012; Brown et al. 1988; Duka et al. 2004; LeMoal and Koob 2007; Wright et al. 2007). There is a positive correlation with the number of EtOH abstinence periods, increased craving, reduced latency to relapse, and incidents of binge drinking in an outpatient population (Malcolm 2000a, b).
Preclinical research indicated that exposure to repeated cycles of EtOH access and deprivation results in a step-wise increase in EtOH consumption resulting in excessive blood EtOH concentrations (> 200 mg%; Hauser et al. 2016; Oster et al. 2006; Rodd et al. 2003). In addition, exposure to repeated cycles of EtOH access and deprivation increases its reward saliency (e.g., oral operant progressive ratio; Oster et al. 2006; Rodd et al. 2003) and enhances the reinforcing properties of EtOH infused directly into subregions of the mesocorticolimbic reward pathway (e.g., posterior VTA; Rodd et al. 2005a, b). Furthermore, the expression of context-induced EtOH-seeking (PSR) is enhanced in rats exposed to repeated cycles of EtOH access-deprivations compared to rats given equivalent periods of EtOH exposure but were Non-Deprived, Once-Deprived, or were on a higher FR schedule of reinforcement (FR10) to equate responding differences observed in the repeatedly deprived rats in alcohol-preferring (P) male rats (Hauser et al. 2016).
Preclinical data have also indicated that conditioned cues influence EtOH-seeking behavior (Ciccocioppo et al. 2001, 2002, 2003; Liu and Weiss 2002; Sciascia et al. 2014; Knight et al. 2016). Cues associated with the availability of alcohol (excitatory conditioned cues – CS+) can augment context- induced EtOH-seeking (Rodd-Henricks et al. 2002b). The efficacy of a CS+ to enhance EtOH-seeking is observed during Pavlovian spontaneous recovery (PSR; c.f., Rodd et al. 2004; Knight et al. 2016) and the renewal model (e.g., Janak and Chaudhri 2010) of context-induced EtOH-seeking. Countering excitatory conditioned cues (i.e., CS+), inhibitory conditioned stimuli (i.e., CS-) signal the unavailability of a reinforcer. Studies with unpaired CS- cues have shown that rats can discriminate between CS+ and CS- cues and respond higher in the presence of a CS+ cue during EtOH-seeking (Chaudhri et al. 2013; Sciascia et al. 2014). CS- cues paired with extinction (extinction cues: E-cue) can reduce EtOH-seeking in unselected rats given access to beer (Willcocks and McNally 2014; McNally 2014) as well as selectively bred P rats exposed to EtOH-associated taste cues (Knight et al. 2016).
Preclinical studies, examining the effects of conditioned cues on EtOH-seeking behavior have typically exposed subjects to cues in the drug-paired environment and subsequently measure behaviors previously associated with EtOH delivery (e.g., operant lever presses) (Ciccocioppo et al. 2001, 2002, 2003; Liu and Weiss 2002; Sciascia et al. 2014; Knight et al. 2016). Context-induced EtOH-seeking models include the Pavlovian Spontaneous Recovery (PSR) and the renewal model (e.g., Bouton 2011; Rodd et al. 2004). The expression of PSR is positively correlated with (a) reward saliency (e.g., Honig and Staddon 1977), (b) contextual cues associated with first-learned signals, and the (c) magnitude of first- and second-learned associations (e.g., Brooks 2000). The PSR model has been used to study environmental context-induced EtOH-seeking (Rodd-Henricks et al. 2002a, b; Hauser et al. 2011; Hauser et al. 2016) as well as olfactory (Rodd-Henricks et al. 2002b) and taste cues (Knight et al. 2016) previously associated with EtOH access.
Previous work in our lab has indicated that exposure to repeated EtOH deprivations enhances context- induced EtOH-seeking (PSR) (Hauser et al. 2016). The present study examined, in part, the effects that repeated EtOH deprivations will have on conditioned cues within the PSR EtOH-seeking model. The objectives of the current study are to (1) determine the impact of repeated deprivation cycles on excitatory CS + effects on EtOH-seeking in the PSR test; (2) determine the effects that pretreatment exposure in the non-drug paired environment will have on CS+ or CS- in the drug-paired environment; and (3) determine the temporal effects of CS pre-exposure on EtOH-seeking in the PSR test. The hypotheses to be tested were whether (a) repeated cycles of deprivation would enhance and/or prolong cue-induced EtOH-seeking behavior in the PSR model; (b) exposure to the CS+ outside the drug-paired environment would enhance the effects of CS+ exposure inside the drug-paired environment, while CS- exposure regardless of pre-conditioning environment would attenuate CS+ effects; and (c) the effects of conditioning on cue-induced EtOH-seeking are time-dependent (i.e., the role of time between cue- exposure and EtOH self-administration).
MATERIALS AND METHODS
Animals.
Adult female P rats from the 68th-71st generations weighing 250–325g at the start of the experiment were used. Previous research indicated that EtOH intake of female P rats was not affected by the estrus cycle (McKinzie et al. 1998). Rats were maintained on a 12-hours reversed light-dark cycle (lights off at 0900 hours). Food and water were available ad libitum throughout the experiment, except during operant conditioning testing. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Research Institute for Laboratory Animal Research 2011).
Operant Conditioning Apparatus.
EtOH self-administration procedures were conducted in standard two-lever experimental chambers (Coulbourn Instruments, Allentown, PA) contained within ventilated, sound-attenuated enclosures. Two operant levers, located on the same wall, were 15 cm above a grid floor and 13 cm apart. A trough was directly beneath each lever, from which a dipper cup could raise to present fluid. Upon a reinforced response on the respective lever, a small light cue was illuminated in the drinking trough and 4 seconds of dipper cup (0.1 ml) access was presented. A personal computer controlled all operant conditioning chamber functions while recording lever responses and dipper presentations.
Operant Conditioning Training.
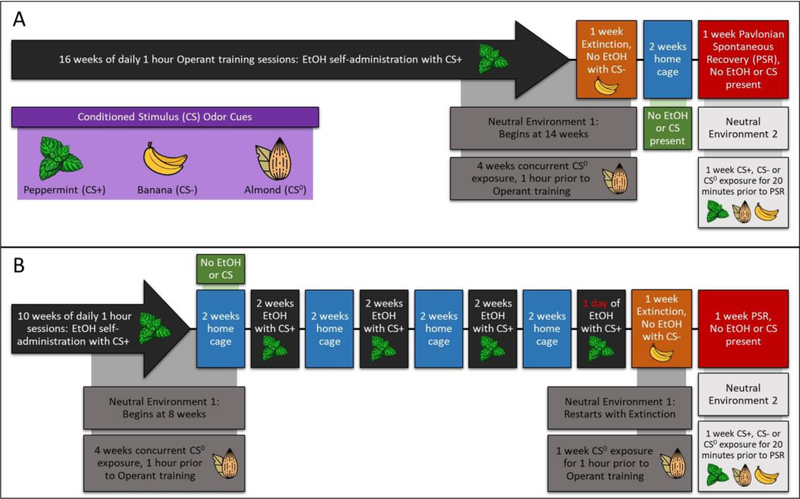
Without prior training, naïve P rats were placed into the operant conditioning chamber. Operant conditioning sessions were 60 min in duration and occurred daily (including weekends) for a total 16 weeks + 1 day (experiments 1 and 2; Figs. 1A-B, Figs. 2A-2B) or 10 weeks (experiment 3; Fig. 3) (Hauser et al. 2016). The EtOH concentration used for operant administration was 15% (vol/vol). Ethyl alcohol (190 proof; McCormick Distilling Co., Weston, MO) was diluted to 15% with distilled water for operant oral self-administration sessions. During the initial 4 weeks of daily operant access rats were reinforced on an FR-1 schedule for the EtOH lever and the water lever. At the end of this time, the response requirement for EtOH was increased to an FR-3 schedule for 3 weeks, and then to FR-5 schedule for 3 weeks. The Non-Deprived group was exposed to EtOH training for an additional 6 weeks +1 day and then given a week of extinction training (Fig. 1). The repeated deprivation (Rep-Dep) group was initially given 10 weeks of daily access to EtOH, followed by 2 weeks of EtOH deprivation, 2 weeks of EtOH access, 2 weeks of EtOH deprivation, 2 weeks of EtOH access, 2 weeks of EtOH deprivation, 2 weeks of EtOH access, 2 weeks of EtOH deprivation, 1 day reinstatement, and then given 1 week of extinction training (Figs. 1B & 2B). All rats in the Non-Deprived and Rep-Dep groups received a total of 16 weeks +1 day of operant EtOH access (Figs. 1 & 2). PSR testing (no EtOH or water present) occurred 2 weeks after completion of extinction training and was conducted over 7 consecutive sessions (Hauser et al. 2016). For the time delay experiment, rats underwent 10 weeks of daily access to EtOH, 1 week of extinction training, 2 weeks of deprivation, and then were tested for PSR for 4 days (Fig. 3).
Figure 1:
Depicts the experimental timelines for the Non-Deprived and Repeated Deprivation.
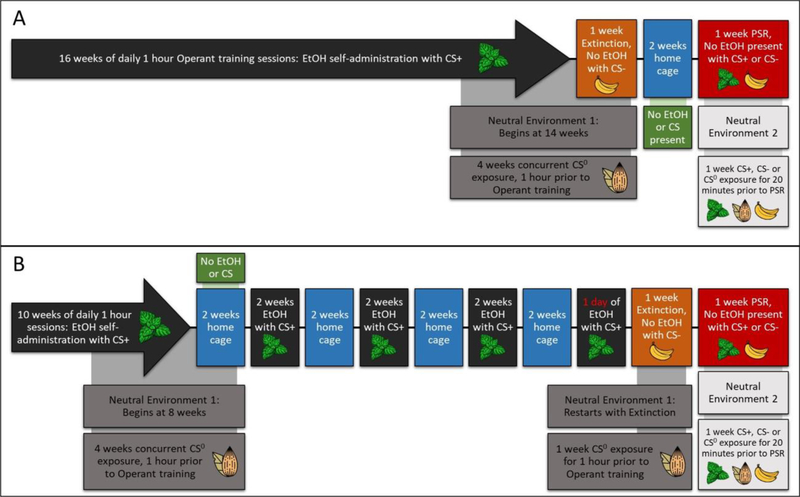
Figure 2:
Depicts the experimental timelines for the Non-Deprived and Repeated Deprivation cycles with conditioned cues prior (pretreatment) to PSR EtOH-seeking and during PSR EtOH-seeking.
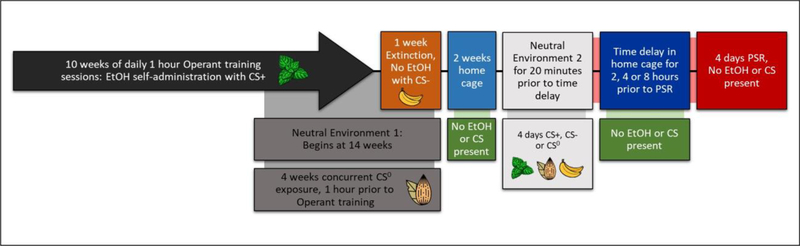
Figure 3:
Depicts the experimental timelines for temporal experiments with conditioned cues.
Non-alcoholic based odors were used for the 3 conditioned stimuli (peppermint, banana, and almond, each odor was used as the CS+, CS- or CS0 in separate groups of rats). The excitatory CS+ was paired continuously with daily EtOH exposure over the EtOH self-administration period in the operant conditioning chambers. The inhibitory CS- odor was paired with the 7 extinction sessions to increase cue- salience, and during extinction session neither water nor EtOH was available. With the exception of no fluid being presented, the delivery system for extinction sessions operated exactly as the preceding EtOH self-administration sessions. The neutral CS (CS0) exposure occurred in a unique, non-drug-paired environment for 60 min, occurred daily 1-hour before the operant conditioning session, and it overlapped both EtOH self-administration and extinction (Figs. 1–3).
Pavlovian Spontaneous Recovery (PSR) testing.
After extinction training, all rats were maintained in the home cages for 14 days without EtOH. Following the abstinence period, rats received additional operant conditioning sessions under the extinction protocol conditions. Lever contingencies and dipper functioning were maintained, but EtOH and water were absent. Rats were given 4 (Hauser et al. 2011) or 7 consecutive PSR test sessions (Knight et al. 2016). Some experiments have 7 consecutive PSR sessions because previous studies have shown that CS cue exposure to taste cues (CS+) (Knight et al. 2016) may enhance PSR responding for 5 or more sessions.
Experiment 1: Effects of Repeated Deprivation on Cue-Induced EtOH-seeking.
In the 1st experiment, Non-Deprived and Rep-Dep adult female P rats were exposed to the 3 conditioned odor cues; CS+ associated with EtOH self-administration, CS- associated with the absence of EtOH (extinction training), and CS0, a neutral stimulus presented in a neutral non drug-paired environment (Figs. 1A-1B). During the 14 day abstinence period, all rats were habituated to a novel environment (a 40 cm diameter glass cylinder). For PSR testing, Non-Deprived and Rep-Dep rats were randomly assigned to be exposed to CS+ in the non-drug paired environment or placed in a control group that did not receive any odors. Before each PSR test session, six drops of the assigned odor were administered into the bedding inside the cylinders; the rats were transferred into the cylinders and exposed to the assigned conditioned cues for 20 min (Non-Deprived n = 4–7/group; Rep-Dep (n = 7–8/group). After this duration, all rats were placed into the drug-paired environment for the 60 min PSR test session. Pretreatment with conditioned stimuli was used to isolate the effects of the cues from the effect of the drug context.
Experiment 2: Determine the Effects of Pretreatment with a Conditioned Cue in a Non-Drug Paired Environment on EtOH-Seeking Influenced by Exposure to Conditioned Cues in the Drug-Paired Environment: Modulation by Exposure to Repeated Deprivations.
A second group of rats were exposed to the Non-Deprived or Rep-Dep protocols (Figs. 2A-2B). After extinction training, all rats were maintained in the home cages for 14 days without EtOH. For PSR Test session, the subjects were randomly assigned to pretreatment groups (CS +, CS0 or CS-) which occurred in a 2nd neutral non-drug- paired environment. Before each PSR test session, six drops of the pretreatment assigned odor (CS +, CS0 or CS-) were administered into the bedding inside the cylinders; the rats were transferred into the cylinders and exposed to the assigned conditioned cues for 20 min (Figs. 2A-2B). Immediately after this duration, all rats were placed into the drug-paired environment in the presence of CS+ or CS- (treatment) for the 60 min PSR test session (Non-Deprived – n = 5–7 pretreatment/treatment; Rep-Dep – n = 8–10 pretreatment/treatment).
In addition, a 13th group was added as an extra control. There is always a concern if cues conditioned in a CS0 protocol remain being a neutral stimulus. The only manner to determine the neutrality of a CS0 is to compare behaviors expressed following the presentation of a CS0 with that observed in animals following the presentation of no stimuli. Therefore, we employed a No Odor pretreatment control group in Non-Deprived rats exposed to the CS+ in the operant conditioning chamber. For statistical analysis, this group was only included in sub-analysis for the Non-Deprived rats exposed to the CS+ in the operant conditioning chamber.
The odors were emitted from a treated cotton ball placed outside the operant conditioning chamber but within the enclosed operant conditioning environment. Therefore, the final group assignments for pretreatment/treatment were the following: CS+/CS+, CS0/CS+, CS-/CS+ or CS+/CS-, CS0/CS-, CS-/CS-. The experiment addressed 3 critical questions; 1) would pretreatment with a CS+ in a non-drug paired environment further augment the increase in EtOH-seeking produced by CS+
presentation in the drug-paired environment (CS+/CS+), 2) would pretreatment with a CS- in the non- drug paired environment reduce/block the increase in EtOH-seeking produced by CS+ presentation in the drug-paired environment (CS-/CS+), 3) would pretreatment with a CS+ in the non-drug paired environment override the inhibitory effect of presentation of a CS- in the drug-paired environment on the expression of EtOH-seeking (CS+/CS-).
Experiment 3: Determine the Persistent Effects of Conditioned Cues on EtOH-seeking.
All rats were exposed to the 3 conditioned odor cues (Fig. 3). After extinction training, all rats were maintained in the home cages for 14 days without EtOH. On each day of PSR testing, the rats were randomly assigned to be exposed to CS +, CS0, or CS- in the non-drug paired environment; the rats were placed into the cylinders and exposed to the assigned conditioned cues for 20 min. After conditioned cue exposure, the rats were returned to the home cage environment and remained in the home cage for 2, 4 or 8-hours (9 groups in total; n = 5–6/conditioned cue/time), before being returned to the drug-paired environment for PSR testing (no EtOH or water present) for 4 consecutive sessions (Hauser et al. 2011; Rodd et al. 2006).
Statistical Analyses.
Overall operant conditioning responding (60-min) data were analyzed with a mixed factorial ANOVA with between subject factors of dose and time point and a repeated measure of ‘session’. For the PSR experiments, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH lever for the last 3 extinction sessions. Operant conditioning responding data were also analyzed in 10-min blocks, which required the additional repeated measure of time. Post-hoc Tukey’s b tests were performed to determine individual differences.
RESULTS
Estimated EtOH intakes for Non-Deprived, Repeated Deprived, and Time Delayed groups.
The estimated EtOH intake for the Non-Deprived groups (total n = 50) and time delayed groups (total n = 59) was between 1.1–1.2 g/kg. The estimated baselines EtOH intake for Rep-Dep groups (total n = 95) was between 1.1–1.2 g/kg and during 3rd re-exposure cycle for sessions 1–3 their EtOH intake was 2 g/kg.
Experiment 1: Effects of Repeated Deprivations on Cue-Induced EtOH-seeking.
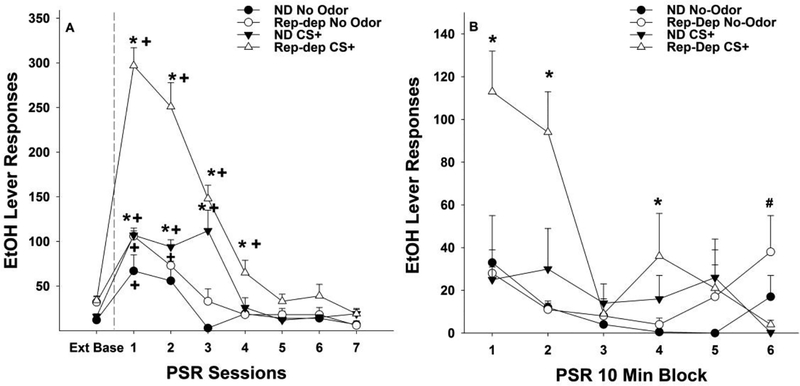
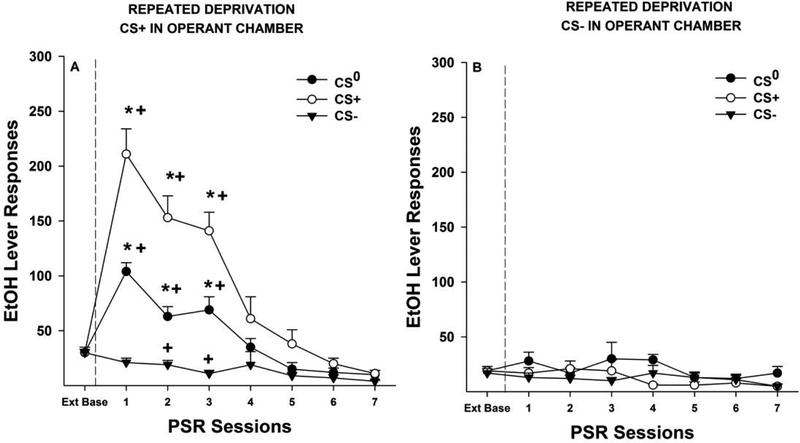
The overall analysis consisted of a mixed factor ANOVA with between subject factors of ‘Drinking History’ and ‘Conditioned Cue’ and a within subject factor of ‘Session’ (Fig. 4A). The overall analysis indicated a significant ‘Drinking History’ by ‘Conditioned Cue’ by ‘Session’ interaction (F7, 147 = 6.07; p > 0.001). This significant interaction term was decomposed by holding ‘Session’ constant (examining the effects of the between subject factors on each individual session). The results indicated a significant ‘Drinking History’ by ‘Conditioned Cue’ interaction term for the PSR test sessions 1–4 (F1,21 values > 7.3; p values < 0.013). These significant interactions terms were reduced for analysis by examining the effect of ‘Drinking History’ separated by ‘Conditioned Cue’. The results indicated that in rats that were in the No Odor group, repeatedly deprived rats responded more in the 1st PSR test session (F1,8 = 5.67; p = 0.044). In rats exposed to the CS+ in the operant conditioning chamber responding was enhanced following repeated deprivations during the 1st and 2nd PSR test session (F1,12 values > 15.2;p values < 0.002). Analyses also examined the effects of ‘Conditioned Cue’ separated by ‘Drinking History’ on responding on the lever previously paired with the delivery of EtOH. In Non-Deprived rats, animals exposed to the CS+ in the operant conditioning chamber responded more than No Odor controls during PSR test sessions 1–3 (F1,8 values > 5.8; p values< 0.039) (Fig. 4A). In rats exposed to repeated cycles of EtOH deprivation responding on the EtOH lever was significantly greater in the CS+ group compared to the No Odor group during PSR test sessions 1–4 (F1,12 values > 16.97; p values < 0.001). EtOH-seeking is determined by examining responding during PSR testing compared to baseline responding. Therefore, in each separate group a repeated measure ANOVA (within subject factor of Session) was performed followed by pair t- tests. These analyses revealed that there was a significant effect of ‘Session’ in each group and in the Non-Deprived No Odor group responding during the 1st PSR test sessions was significantly greater than that observed during extinction baseline (p = 0.027). In Non-Deprived rats exposed to the CS+ in the operant conditioning chamber responding was elevated compared to baseline during PSR test sessions 1–3 (Fig. 4A). Exposure to repeated cycles of EtOH deprivations enhanced this effect; No Odor group significantly expressed increases in PSR test sessions 1–2 and CS+ group expressing an increase in responding during PSR test sessions 1–4 (Fig. 4A).
Figure 4:
Depicts the mean (± SEM) responses on the lever previously associated with the delivery of EtOH for the Repeated Deprivation (Rep-Dep) and Non-Deprived (ND) groups. Fig. 4A; Asterisk (*) indicates that rats in the CS+ groups responded significantly more than the No Odor groups for Rep-dep group during PSR sessions 1–4 and for ND group during session 1–3 (p <0.05). Plus (+) indicates that rats in the ND No Odor (PSR session 1), ND CS+ (PSR session 1–3) , Rep-Dep No Odor (PSR session 1 −2), and Rep-Dep CS+ (PSR sessions 1–4) groups responded significantly more on the lever previously associated with EtOH during PSR testing than extinction baseline (Ext Base) (p <0.05). Fig. 4B; Depicts the mean (±S.E.M.) responses per 10-min blocks on the lever previously associated with the delivery of EtOH in the ND No Odor group, ND CS+ group, Rep-Dep No Odor group, and Rep-Dep CS+ group during the first PSR session. Asterisk (*) indicates rats in the Rep-Dep CS+ group responded significantly (p < 0.05) more on the EtOH lever during the 1st, 2nd and 4th 10-min blocks of PSR testing compared to all other groups. Pound (#) indicates that the ND No Odor and Rep-Dep No Odor groups responded significantly (p < 0.05) more on the EtOH lever during the 6th 10-min block compared to the ND CS+ and the Rep-Dep CS+ groups.
Examining the time course effects (in 10-min bins) of CS (Fig. 4B) indicated that, during the 1st PSR session, EtOH lever responding temporal profile was influenced by both ‘group’ and ‘session’. An analysis of the EtOH lever responding separated into 10-min blocks indicated a significant ‘group” x ‘session’ interaction (F5, 36 = 2.8; p = 0.012). The Rep-Dep CS+ group was significantly higher than all the other groups during the 1st and 2nd 10-min blocks (p ≤ 0.001). Rep-Dep CS+ group was significantly higher than both No Odor groups during the 4th 10-min block (p < 0.033). Both No Odor groups were significantly higher during 6th 10-min block than both CS+ groups (p = 0.028).
Experiment 2: Determine the Effects of Pretreatment with a Conditioned Cue in a Non-Drug Paired Environment on EtOH-Seeking Influenced by Exposure to Conditioned Cues in the Drug-Paired Environment: Modulation by Exposure to Repeated Deprivations.
The overall analysis indicated a significant Pretreatment Odor x Treatment Odor x Drinking History x Session interaction (F14,497 = 2.68; p ≤ 0.01; Fig. 5). The significant interaction term was decomposed by reducing the analysis by Drinking History. In rats exposed to repeated cycles of EtOH deprivations there was a significant Pretreatment Odor x Treatment Odor x Session interaction (F14,301 =8.6; p < 0.001). This interaction was further reduced by holding Treatment Odor constant. In rats exposed to repeated cycles of EtOH deprivation, there was a significant Pretreatment Odor x Session interaction (F14,175 = 14.6; p < 0.001) when the CS+ was present in the operant conditioning chamber during PSR testing. Individual ANOVAs performed on each responding during each session indicated significant effects of Pretreatment Odor during PSR test sessions 1–3. Post-hoc comparisons (Tukey’s B) indicated that in Rep-Dep rats exposed to the CS+ in the operant conditioning chamber responding in rats pretreated with the CS+ was significantly higher than rats pretreated with the CS0, and these rats were significantly higher than rats pretreated with the CS- (CS+ > CS0 > CS-) (Fig. 5A). In contrast, in rats exposed to repeated cycles of EtOH deprivation there was no significant Pretreatment Odor x Session interaction (F14, 126 = 0.74; p = 0.43) thus indicating that there was no evidence of EtOH-seeking when the CS- was presented in the operant conditioning chamber in all Pretreatment Odor groups. Within group analyses for rats exposed to repeated cycles of EtOH deprivation that were exposed to the CS+ in the operant conditioning chamber during PSR testing indicated significant effects of Session for all groups (F7,63 values > 3.0; p values < 0.01). Pretreatment with the CS- resulted in a reduction in responding compared to baseline during PSR test sessions 2 and 3 despite having the CS+ present in the operant conditioning chamber (Fig. 5A). EtOH-seeking (responding significantly greater than extinction baseline) was evident in rats administered the Pretreatment Odor of CS0 or CS+ for PSR test sessions 1–3 when the CS+ was present in the operant conditioning chamber during testing (Fig. 5A).
Figure 5:
Depicts the mean (±S.E.M.) responses on the lever previously associated with the delivery of EtOH in Repeatedly Deprived (Rep-Dep) P rats that were exposed to CS+, CS0, and CS- in the non-drug paired environment then tested for PSR expression in the presence of CS+ (Fig. 5A) or CS- (Fig. 5B) in drug-paired environment. Fig. 5A; Asterisk (*) indicates that Rep-Dep rats, exposed to CS+ in the non-drug paired environment, responded significantly more than the CS0 and CS- during sessions 1–3, and the CS0 group responses were significantly higher than CS- during sessions 1–3 (p <0.05). Plus (+) indicates that Rep-Dep rats lever responding was significantly greater than extinction baseline (p <0.05) for CS+ and CS0 groups during PSR sessions 1–3 and CS- rats lever responding was significantly lower than extinction baseline (Ext Base) (p <0.05) during PSR session 2–3. Fig. 5B; There were no significant differences found for any of the groups.
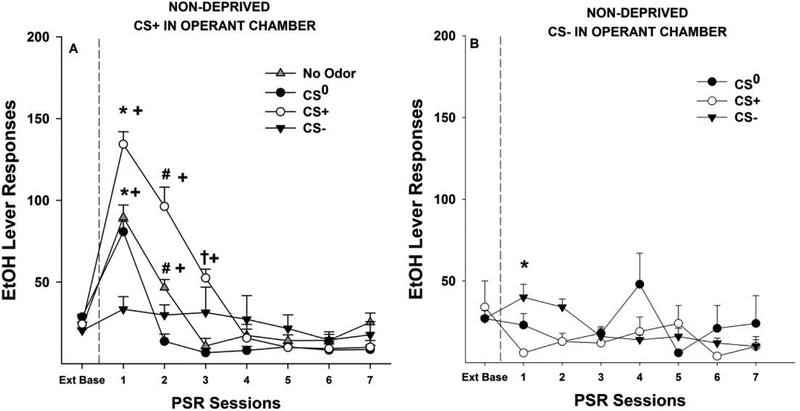
Examining the statistics in Non-Deprived rats revealed a significant Pretreatment Odor by Treatment Odor by Session (F14,196 = 7.4; p < 0.001; Fig. 6B). Reducing this 3-way interaction term by holding Treatment Odor constant revealed that for Non-Deprived rats exposed to the CS- in the operant conditioning chamber during PSR testing there was a significant Pretreatment Odor by Session interaction (F14,70 = 2.34; p = 0.01). Individual ANOVAs performed on each PSR test session revealed a significant effect of Pretreatment Odor during the 1st PSR test session (F2,10 = 6.424; p = 0.016). Post-hoc comparisons indicated that in Non-Deprived rats pretreated with the CS+ but presented with the CS- in the operant conditioning chamber during PSR testing responded significantly less than Non-Deprived rats pretreated with the CS0 or CS- (Fig. 6B).
Figure 6:
Depicts the mean (±S.E.M.) responses per session on the lever previously associated with the delivery of EtOH in Non–Deprived (ND) P rats that were exposed to CS+, CS0 and CS- in the non-drug paired environment then tested for PSR expression in the presence of CS+ (Fig. 6A) or CS- (Fig. 6B) in the drug-paired environment. Fig. 6A; Asterisk (*) indicates CS+ group was responding more than the CS0 and No Odor groups during PSR session 1 (p < 0.05). The CS0 and No Odor responded more than the CS- group during PSR session 1 (p < 0.05). Pound (#) indicates that during the 2nd PSR test session responding in rats pretreated with the CS+ responded more than the No Odor group which responded more than the CS0 and CS- groups (p < 0.05). Dagger (†) indicates that during the 3rd PSR test session, rats pretreated with the CS+ responded more than all other groups (p < 0.05). Plus (+) indicates that rats lever responding was significantly greater than extinction baseline (Ext Base) (p <0.05) for CS+ during PSR sessions 1–3, CS0 during PSR session 1, and for No Odor group during PSR session 1–2 (p <0.05). Fig. 6B; Asterisk (*) indicates that CS+ responding was less than the CS0 and CS- during PSR session 1 (p < 0.05).
The analysis examining the effects of conditioned cues in Non-Deprived rats exposed to the CS+ in the operant conditioning chamber during PSR testing included the additional control group of No Odor during pretreatment exposure. The subgroup analysis revealed a significant Pretreatment Odor by Session interaction (F21,154 = 12.1; p < 0.001). Individual ANOVAs performed on each individual PSR test session revealed significant effects of Pretreatment Odor in Non-Deprived rats presented with the CS+ in the operant conditioning chamber during PSR test sessions 1–3 (F3,22 values > 5.7;p values < 0.005). Post- hoc comparisons (Tukey’s b) indicated that for the 1st PSR test session revealed that rats pretreated with the CS+ responding more than the CS0 and No Odor groups which responded more than the CS- group (CS+ > CS0/No Odor > CS-) (Fig. 6A). During the 2nd PSR test session responding in rats pretreated with
the CS+ responded more than the No Odor group which responded more than the CS0 and CS- groups (CS+ > No Odor > CS0/CS-) (Fig. 6A). During the 3rd PSR test session, rats pretreated with the CS+ responded more than all other groups (Fig. 6A). Examining the evidence for EtOH-seeking in each individual group indicated that pretreatment with the CS- blocked the expression of EtOH-seeking when the CS+ was presented in the operant conditioning chamber. In contrast, there were significant effects of session in the No Odor, CS+, and CS0 pretreatment groups (F values > 16.23; p values < 0.001). Two- sided paired t-tests indicated that each group displayed an increase in responding over extinction baseline responding for 1 (CS0), 2 (No Odor), or 3 (CS+) PSR test session (Fig. 6A).
Experiment 3: Determine the Persisting Effects of Conditioned Cues on EtOH-seeking.
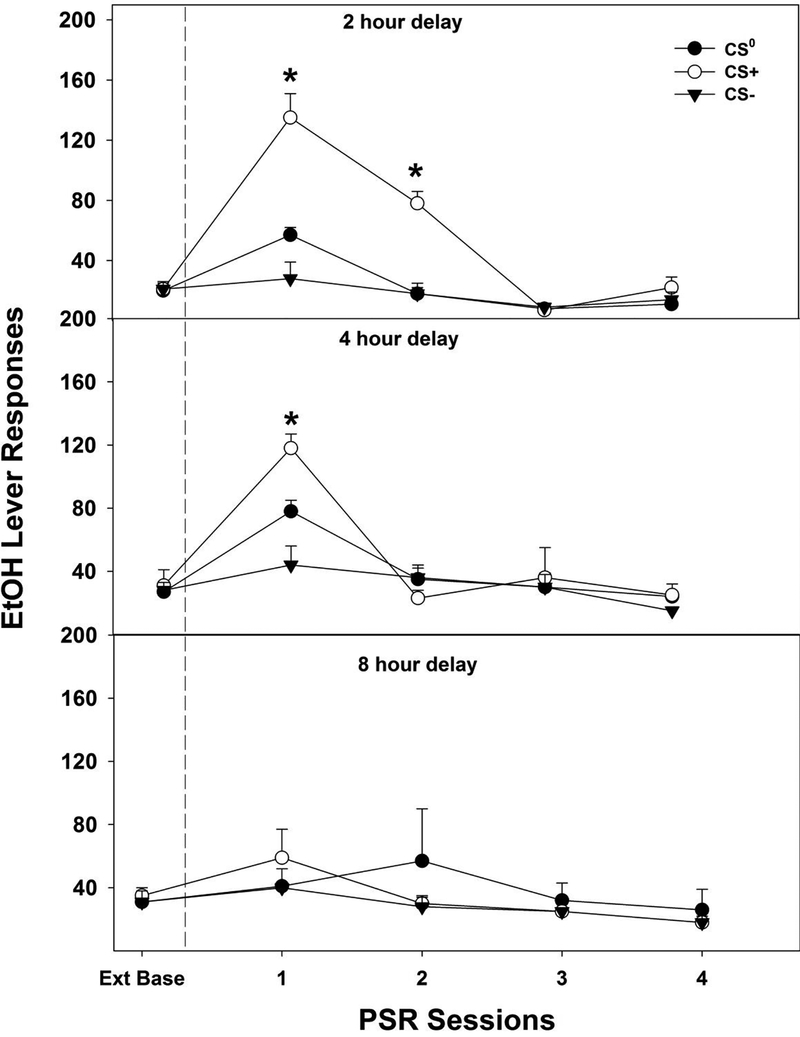
Examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 6) indicated a significant effect of ‘session’ by ‘CS’ by ‘time’ interaction (F16, 200 = 3.8; p ≤ 0.001. Decomposing the significant interaction term by holding session constant, allowed for the examination of the effects of ‘CS’ and ‘time’ for each individual operant conditioning session. There were no significant ‘CS’ by ‘time’ interaction terms for the average baseline level of responding or during the 3rd and 4th PSR test session (F4,50 values < 0.65; p values > 0.63). In contrast, there were significant ‘CS’ by ‘time’ interactions terms for the 1st and 2nd PSR test sessions (F4,50 values > 3.69; p values < 0.01). The two-way interaction term was decomposed by holding ‘time’ constant first. Individual ANOVAs performed for each ‘time’ group revealed that in rats exposed to the conditioned cues 2-hour before PSR testing there was a significant effect of CS during the 1st and 2nd PSR test session (F2,19 values > 23.39; p values < 0.0001). Post-hoc comparisons Tukey’s b indicated that CS+ > CS0 > CS- for both the 1st and 2nd PSR test sessions (Fig. 7, upper panel). For rats exposed to conditioned cues 4-hour prior to PSR testing, there was only a significant effect of ‘CS’ during the 1st PSR test session (F2,18 = 13.25; p < 0.001) (Fig. 7, middle panel). Post-hoc comparisons indicated that CS+ > CS0 > CS-. In contrast, there was no significant effect of CS for any sessions in rats presented with conditioned cues 8-hour prior to PSR testing (F2, 15 values < 0.69; p values > 0.52) (Fig. 7, lower panel).
Figure 7.
Depicts the mean (±S.E.M.) responses on the lever previously associated with the delivery of EtOH in P rats that were exposed to CS+ CS0 and CS- in the non-drug paired environment then tested for PSR expression in the absence of CS in the drug-paired environment following a 2, 4 and 8- hour delay. Upper Panel: The asterisks (*) indicates that during the 1st and 2nd PSR sessions, the 2-hour delay CS+ group responding on the lever previously associated with EtOH was significantly higher than the 2-hour CS0 and 2-hour CS- groups (p < 0.05). The 2-hour CS0 responding on the lever previously associated with EtOH was significantly higher than CS- group (p < 0.05). In addition, the 2-hour CS+ group lever responding was greater for the 1st and 2nd PSR sessions compared to extinction baseline (Ext Base), while the CS0 2-hour group lever responding was only significant for the PSR session 1 compared to extinction baseline (p <0.05). Middle Panel: The asterisks (*) indicates that, during the 1st PSR session, the 4-hour delay CS+ group responded significantly more than the 4-hour CS0 and 4-hour CS- groups (p < 0.05). The 4-hour CS0 responding on the lever previously associated with EtOH was significantly higher CS- group (p < 0.05). In addition, the 4-hour CS+ and CS0 group lever responding was greater for the 1st PSR sessions compared to extinction baseline (Ext Base) (p <0.05). Lower Panel: There were no significant differences found for any of the groups following the 8-hour delay.
DISCUSSION
The major findings of this study are that (1) Rep-Dep increased the magnitude and prolonged the duration of the stimulatory CS+ effects on EtOH-seeking compared to the Non-Deprived group (Fig. 4); (2) pretreatment with a CS+ in the non-drug paired environment can enhance the actions of a CS+ presented in a drug-paired environment (Figs. 5A & 6A); (3) pretreatment CS- in a non-drug paired environment blocked the stimulatory actions of CS+ on EtOH-seeking (Figs. 5A & 6A); (4) exposure to a CS- in a drug-paired environment also blocked the stimulatory actions of CS+ cue presented in the non- drug paired environment on EtOH-seeking (Figs. 5B & 6B); and (5) CS+ and CS0 induced EtOH seeking following 2 and 4-hour time delay (Fig. 7). Previously, Hauser et al. (2016) demonstrated that multiple cycles of deprivation can enhance and prolonged the effects of context-induced EtOH-seeking in the PSR test compared to Non-Deprived rats (Hauser et al. 2016). Those findings suggested that neuronal alterations associated with EtOH-seeking behavior may have been strengthened with repeated cycles of deprivation. The current study extends Hauser et al. (2016) findings showing that repeated cycles of deprivation can potentiate the excitatory effects of CS+ on EtOH-seeking. In contrast, Ciccocioppo et al. (2003) did not observe an increase in EtOH-seeking behavior following multiple withdrawals, but their findings suggest that neuroadaptations did occur following multiple withdrawals because the efficacy of naltrexone to attenuate EtOH-seeking was reduced in dependent rats with repeated prior intoxication experiences. The discrepancy between the previous and current studies may be due to strain, EtOH exposure procedure, and deprivation procedure.
In order to obtain a better understanding of CS + effects on EtOH-seeking, we examined 10- min bins of the 1st PSR session (Fig. 4B). The Rep-Dep CS+ group’s responding was greater in the 1st and 2nd 10-min block compared to all other groups, suggesting Rep-Dep CS+ increases the initial motivation of the animals to obtain EtOH at the beginning of the PSR session, even though EtOH was unavailable. In contrast to Hauser et al. (2016), the excitatory CS+ induced PSR expression for 4 days (Fig. 4A) compared to 2 days for context-induced EtOH-seeking, and this is with the CS being presented in the non-drug paired environment. These findings are in line with Knight et al. (2016), which indicated that taste CS+ cues could prolong EtOH–seeking behavior for 6 days compared to the other CS cues or context cue. CS drug associations can develop quickly and they share common mechanisms with associative learning and memory consolidation, therefore CS association with learning may be stronger than contextual cues (Fuchs et al. 2008; c.f. Knight et al. 2016).
Previous studies have demonstrated that S+/CS+ (EtOH) presented in the drug-paired environment induced EtOH-seeking, while the S-/CS- (non-reward) did not alter EtOH-seeking behavior in P rats (Ciccocioppo et al. 2001). The current data set extends our knowledge about the ability of the CS+ to enhance drug-seeking behaviors in P rats. One novel finding is that exposure to a CS+ in a non- drug paired environment can augment EtOH-seeking enhanced by the presentation of the same CS+ in the drug-paired environment (Figs. 5A & 6A). The data indicate that exposure to a pro-drug cue (CS+) in a non-drug paired environment can be considered a facilitating factor to the deleterious effects of exposure to a CS+ in a drug-paired environment on drug-seeking. The cumulative effect of the CS+/CS+ cue exposure was enhanced and prolonged in rats exposed to repeated cycles of alcohol access and abstinence (Fig. 5A).
Another significant finding of the current study is the efficacy of the presentation of the CS- to block EtOH-seeking. In general, the data suggest that exposure to the CS- can override subsequent exposure to the CS+ with the end result being the failure to express EtOH-seeking behaviors. The ability of the CS- (exposed in the non-drug paired environment) to prophylactically inhibit the ability of the CS+ to evoke EtOH-seeking behaviors in the drug-paired environment was evident in Rep-Dep rats which express heightened context- and cue-induced EtOH-seeking (Fig. 5A). Pretreating rats with a CS+ in a non-drug paired environment prior to PSR testing did not alter the ability of the CS- to inhibit EtOH- seeking (Figs. 5B and 6B). All data indicate that the CS- remained effective at blocking EtOH-seeking under all test conditions. These findings extend previous research that demonstrated extinction cues paired with extinction context can attenuate alcohol-seeking behavior (Willcocks and McNally, 2014). In addition, the current findings support Kearns et al. (2005) findings that reported adding a CS- to a drug- paired environment reduced the ability of the CS+ to evoke cocaine-seeking (Kearns et al. 2005).
The context in which the cues are presented mediates the saliency (associative strength) of the cue (c.f., Drummond 2000). Extinction cues are thought to attenuate spontaneous recovery because they facilitate the retrieval of the extinction memory (Brooks and Bouton 1993) and the retrieval of extinction memory depends on the presence of contextual cues associated with extinction training (Bouton 2004). In this study, the extinction training and exposure to the inhibitory CS- were conducted in the same context as EtOH self-administration, suggesting that the CS- paired with drug-associated environmental context may play an important role in increasing the saliency of inhibitory CS-. The interaction between the drug- paired environment and CS- training may influence the prophylactic effects of the CS- on EtOH-seeking regardless of whether the animals were exposed to CS- inside or outside the drug-paired environment. This consideration could be relevant for cue exposure therapy (CET) treatment with CS- for addiction. Rosenthal and Kutlu (2014) suggested that using mobile phones, automated dialing, and data collection software could be used as extinction reminders in clinical settings and serve to remind high-risk users of their therapeutic experiences inside the clinic, which may help decrease the probability of substance use.
The results also provide novel data since this is the first published report examining the temporal persistence of conditioned cues to influence EtOH-seeking. The data indicate that the efficacy of the CS+ and CS- is apparent up to 4-hours after exposure to the cues (Fig. 7, top and middle panel). The clinical implications of these findings are two-fold. First, exposure to a CS+ may have lingering effects that promote EtOH-seeking, which could lead to relapse. The persistent heightened level of EtOH-seeking caused by exposure to the CS+ may create a mental state that gradually wears down the resistance of the individual to abstain from EtOH. In contrast, the temporal ability of the CS- to persistently inhibit EtOH- seeking increases the potential therapeutic value of CS- therapy/treatment. The persistent (Fig. 7) and prophylactic (Figs. 5 & 6) effects of the CS- on EtOH-seeking could result in possible treatment for addiction.
The biological basis for the ability of conditioned cues to influence drug-seeking is not yet known. However, research has indicated that the ventral tegmental area-basolateral amygdala- nucleus accumbens shell (VTA-BLA-AcbSh) neurocircuitry mediates a number of excitatory alcohol conditioned cue effects (Millan et al. 2017) including heroin-conditioned immunomodulation (Szczytkowski et al. 2011). Multiple data sets indicate that there are distinct neurocircuitries mediating the effects of the CS+ and CS-. To date, the effects of Rep-Dep on these neurocircuits has not been examined, but it is possible that Rep-Dep produces neuroadaptations in one or more of these neurocircuits.
Overall, the findings in the current study indicated that excitatory CS+ cue salience is increased with multiple cycles of deprivation and that stimulatory effects of conditioned cues can be long-lasting. In addition, the current study suggests that utilization of the inhibitory conditioned CS- can generalize across contexts and may be a potential therapeutic tool for the treatment of addiction. The development of potential pharmacotherapeutics for the treatment of drug addiction should include two targets; a treatment that reduces the CS+ associated neurocircuit (reduce desire) and treatment that enhances the CS- associated neurocircuit (increase behavioral control).
ACKNOWLEDGMENTS
The work was supported in part by NIAAA grants AA07611, AA07462, AA020908, AA022287, and AA024612. None of the authors has a conflict of interest associated with this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA (2017) Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 122:201–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser S, Rodd ZA, Liang T, Sari Y, McClintick J, Rahman S, Engleman EA (2016) A Genetic Animal Model of Alcoholism for Screening Medications to Treat Addiction. Int Rev Neurobiol 126:179–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L (2012) Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav 103:119–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, Winterbauer NE (2011) Renewal after the extinction of free operant behavior. Learn Behav 39:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem 11:485–94 [DOI] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME (1993) A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Process 19:77–89 [DOI] [PubMed] [Google Scholar]
- Brooks DC (2000). Recent and remote extinction cues reduce spontaneous recovery. Q J Exp Psychol B 53:25–58 [DOI] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC (1988) Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry 23:507–14 [DOI] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB (1981) Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol 42: 1013–1020 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH (2013) Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci 38:2751–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F (2003) Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 168:208–215 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F (2002) Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27:391–9 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F (2001) Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res 25:1414–9 [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP (2009) Bringing the real world into the laboratory: personal smoking and non-smoking environments. Drug Alcohol Depend 111:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ (2008) Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol 16: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC (2000) What does cue-reactivity have to offer clinical research? Addiction Suppl 2: S129–44 [DOI] [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, Veatch LM, Becker HC, Crews FT (2004) Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res 28:233–46 [DOI] [PubMed] [Google Scholar]
- Fatseas M, Serre F, Alexandre JM, Debrabant R, Auriacombe M, Swendsen J (2015) Craving and substance use among patients with alcohol, tobacco, cannabis or heroin addiction: a comparison of substance- and person-specific cues. Addiction 110:1035–42 [DOI] [PubMed] [Google Scholar]
- Ferri M, Amato L, Davoli M (2006) Alcoholics Anonymous and other 12-step programmes for alcohol dependence.Cochrane Database Syst Rev 3: CD005032. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X (2008) Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models 5:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA Jr, Knight CP, Toalston JE, McBride WJ, Rodd ZA (2016). Parameters of Context-Induced Ethanol (EtOH)-Seeking in Alcohol-Preferring (P) Rats: Temporal Analysis, Effects of Repeated Deprivation, and EtOH Priming Injections. Alcohol Clin Exp Res 40: 2229–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA (2011) The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther 336: 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbrom ME (1990) Alcohol withdrawal seizures and binge versus chronic drinking In Alcohol and Seizures: Basic Mechanisms and Clinical Concepts (Port RJ, Mattson RH, Cramer JA, Diamond I, eds) pp 206–215, FA Davis, Philadelphia. [Google Scholar]
- Honig WK, Staddon JER (1977) Handbook of operant behavior. Englewood Cliffs, N.J.: Prentice-Hall. Janak PH, Chaudhri N (2010) The potent effect of environmental context on relapse to alcohol-seeking after extinction. Open Addict J 3: 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ, Schindler CW, Panlilio LV (2005) Conditioned inhibition of cocaine seeking in rats. J Exp Psychol Anim Behav Process 31:247–53 [DOI] [PubMed] [Google Scholar]
- Knight CP, Hauser SR, Deehan GA Jr, Toalston JE, McBride WJ, Rodd ZA (2016) Oral Conditioned Cues Can Enhance or Inhibit Ethanol (EtOH)-Seeking and EtOH Relapse Drinking by Alcohol- Preferring (P) Rats. Alcohol Clin Exp Res 40:906–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Koob GF (2007) Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol 17:377–93 [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F (2002) Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther 300:882–9 [DOI] [PubMed] [Google Scholar]
- Malcolm R, Herron JE, Anton RF, Roberts J, Moore J (2000a) Recurrent detoxification may elevate alcohol craving as measured by the Obsessive Compulsive Drinking Scale. Alcohol 20:181–5 [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF (2000b) Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol 22: 159–164 [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, Addicott MA, Chou YH, Chen NK, Hallyburton MB, DeVito AM (2016) Hippocampal and Insular Response to Smoking-Related Environments: Neuroimaging Evidence for Drug-Context Effects in Nicotine Dependence. Neuropsychopharmacology 41:877–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK (1998) The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res 22:1170–6 [PubMed] [Google Scholar]
- McMillen BA (1997) Toward a definition of a valid animal model of alcoholism: Multiple animal models for multiple diseases. Alcohol 14: 409–419 [DOI] [PubMed] [Google Scholar]
- McNally GP (2014) Extinction of drug seeking: Neural circuits and approaches to augmentation. Neuropharmacology 76 Pt B: 528–32 [DOI] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, Janak PH (2017) Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience 360:106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Alterman A, Childress AR, McLellan AT (1992) Developing and evaluating new treatments for alcoholism and cocaine dependence. Recent Dev Alcohol 10:303–25 [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R (1998) Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav 15: 355–365 [DOI] [PubMed] [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA (2006) Effects of multiple alcohol deprivations on operant ethanol self-administration by high- alcohol-drinking replicate rat lines. Alcohol 38:155–64 [DOI] [PubMed] [Google Scholar]
- Research Institute for Laboratory Animal Research (2011) Guide for the Care and Use of Laboratory Animals. 8th ed. The National Academies Press, Washington, D.C. [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ (2005a) Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther 315: 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ (2005b) Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res 29:358–66 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ (2004) Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79:439–50 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li T-K, McBride WJ (2003) Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology 28: 1614–1621 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ (2006) The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res 171: 207–215 [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K (2002a) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res 26: 1632–1641 [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K (2002b) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res 26: 1642–1652 [DOI] [PubMed] [Google Scholar]
- Rosenthal MZ, Kutlu MG (2014) Translation of associative learning models into extinction reminders delivered via mobile phones during cue exposure interventions for substance use. Psychol Addict Behav 28:863–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia JM, Mendoza J, Chaudhri N (2014) Blocking dopamine d1-like receptors attenuates context- induced renewal of pavlovian-conditioned alcohol-seeking in rats. Alcohol Clin Exp Res 38:418–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Fuchs RA, Lysle DT (2011) Ventral tegmental area-basolateral amygdala-nucleus accumbens shell neurocircuitry controls the expression of heroin-conditioned immunomodulation. J Neuroimmunol 237:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP (2014) An extinction retrieval cue attenuates renewal but not reacquisition of alcohol seeking. Behav Neurosci 128:83–91 [DOI] [PubMed] [Google Scholar]
- Wright TM, Myrick H, Malcolm R, Randall P, Boyle E, Henderson S, Anton R (2007) Impact of lifetime alcohol quit attempts and medicated detoxifications on time to relapse during an index alcohol detoxification. J Addict Med 1:15–20 [DOI] [PubMed] [Google Scholar]