Abstract
Purpose
Diabetic neuropathy is a common and disabling disorder and there are currently no proven effective disease modifying treatments. Physical activity and dietary interventions in patients with diabetes and diabetic neuropathy have multiple beneficial effects and are generally low risk, which makes lifestyle interventions an attractive treatment option. We reviewed the literature on the effects of physical activity and dietary interventions on length dependent peripheral neuropathy and cardiac autonomic neuropathy in diabetes.
Methods
The electronic database Pubmed was systematically searched for original human and mouse model studies examining the effect of either dietary or physical activity interventions in subjects with diabetes, prediabetes, or metabolic syndrome.
Results
Twenty studies are included in this review. Fourteen studies were human studies and six were in mice. Studies were generally small with few controlled trials and there are no widely agreed upon outcome measures.
Conclusions
Recent research indicates that dietary interventions are effective in modifying diabetic neuropathy in animal models and there is promising data that they may also ameliorate diabetic neuropathy in humans. It has been known for some time that lifestyle interventions can prevent the development of diabetic neuropathy in type 2 diabetes mellitus subjects. However, there is emerging evidence that lifestyle interventions are effective in individuals with established diabetic neuropathy. In addition to the observed clinical value of lifestyle interventions, there is emerging evidence of effects on biochemical pathways that improve muscle function and effect other organ systems including the peripheral nerve. However, data from randomized control trials is needed.
Keywords: diabetic neuropathy, exercise, diet, dysautonomia, sirtuins
Introduction
An estimated 451 million adults were living with diabetes mellitus worldwide in 2017. This number is predicted to rise to 693 million by 2045. In addition, 374 million people are estimated to have impaired glucose tolerance or prediabetes [14]. Neuropathy is the most common complication of diabetes and it affects approximately half of all patients with type 1 or type 2 diabetes mellitus [22, 23]. In fact, nerve conduction studies performed at the time of diagnosis of diabetes demonstrate that subclinical neuropathy is already present [3]. While nerve conduction studies measure large diameter nerve fiber function, the neuropathy that is present in early diabetes typically involves small nerve fibers. These small diameter, unmyelinated fibers carry pain and temperature sensation. Patients with a small fiber neuropathy can present with neuropathic pain and frequently have autonomic symptoms due to involvement of postganglionic autonomic fibers [78]. Typical, length dependent diabetic neuropathy progresses over time to eventually involve large diameter, myelinated nerve fibers. This leads to the loss of protective sensation, which can lead to foot ulcers and amputations, as well as imbalance. Due to the presence of neuropathic pain, autonomic symptoms and sensorimotor deficits, patients with diabetic neuropathy often report a reduced quality of life, poor sleep and depression.
Metabolic syndrome is a collection of abnormalities that include hypertension, hyperlipidemia, central obesity, and insulin resistance that increase an individual’s risk for heart disease, stroke and diabetes. It has been linked to risk of neuropathy in patients with diabetes. In patients with type 2 diabetes mellitus, those with metabolic syndrome were found to be twice as likely as those with type 2 diabetes mellitus alone to have neuropathy [20]. In nondiabetic patients, metabolic syndrome was found to be associated with a small fiber neuropathy that is similar to the neuropathy seen in early diabetes [62]. In particular, the associated metabolic, endocrine and inflammatory effects of obesity and dyslipidemia are thought to play a critical role in the development and progression of diabetic neuropathy [64]. The relationship between lipotoxicity and small fiber neuropathy is also supported by data that obesity is associated with autonomic nervous system dysfunction [9, 24, 51].
Currently there are no disease modifying treatments for diabetic neuropathy that have been proven to be effective in randomized, blinded (masked) clinical trials. Previous clinical trials that have focused on outcomes such as nerve conduction studies, which measure length dependent large fiber function, have generally been ineffective. As previously mentioned, in diabetic neuropathy small fiber damage precedes large fiber dysfunction. This is thought to occur because small unmyelinated fibers are vulnerable to effects from metabolic derangements [67]. However, they also have a greater regenerative capacity and are more responsive to therapy than large myelinated fibers [52]. Therefore, reproducible, sensitive measurements of small fiber neuropathy such as skin biopsy with measurement of intraepidermal nerve fiber density (IENFD) and cardiac autonomic testing may be more suitable as clinical trial outcome measures. Skin biopsy samples must be carefully processed and measured but with rigorous standardized techniques, IENFD has been shown to be highly reproducible [61]. The European Federation of Neurological Societies and the Peripheral Nerve Society have published a guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy and specified that new laboratories should undergo adequate training in a well-established laboratory and that quality control of the procedure is mandatory [41].
The Diabetes Prevention Program demonstrated that a physical activity and dietary intervention can reduce the incidence of type 2 diabetes [39]. Lifestyle changes can also combat some of the negative effects of diabetes and result in weight loss, improvements in glycemic control, dyslipidemia, and blood pressure, and reduce the rate of cardiovascular events and associated mortality. However, the impact of diet and exercise on diabetic neuropathy is less clear and data from large randomized, blinded (masked) control trials is lacking.
Methods
The PubMed database was systematically searched through December 2018 for original studies in either humans with or animal models of diabetes, prediabetes, or metabolic syndrome examining the effect of a dietary and/or physical activity intervention on peripheral neuropathy or autonomic neuropathy. Search terms included: diabetes, prediabetes, impaired glucose tolerance, impaired fasting glucose, metabolic syndrome, neuropathy, autonomic, heart rate variability, diet, lifestyle, exercise, physical activity. Studies were included if they were human studies of adults or studies of mouse models of diabetes, prediabetes, or metabolic syndrome, written in English, examined the effect of a diet and or exercise intervention on peripheral or autonomic neuropathy.
Dietary interventions in diabetic neuropathy
As previously mentioned, oxidative stress is thought to be a major contributor to diabetic neuropathy. Dietary sources of antioxidants may play a role in the successful treatment of diabetic neuropathy. Alpha-lipoic acid (ALA) is a dietary supplement that has antioxidant properties. It has been studied in clinical trials of diabetic neuropathy and has been associated with a symptomatic benefit. In the SYDNEY 1 trial daily intravenous ALA was associated with reduced neuropathic symptoms [5]. Three oral doses of ALA were included in the SYDNEY 2 trial and all three doses were associated with a statistically significant reduction in the Total Symptom Score. Due to side effects seen with higher doses, the optimal dose was 600mg once daily [75]. These were short term studies of only several weeks duration. Further studies are needed to determine the effectiveness of ALA in reducing pain and/or preventing the progression of diabetic neuropathy. Although the phase 3 study did not report benefit based on a priori outcome measures, a meta-analysis of all data does show potential benefit of ALA [76, 77].
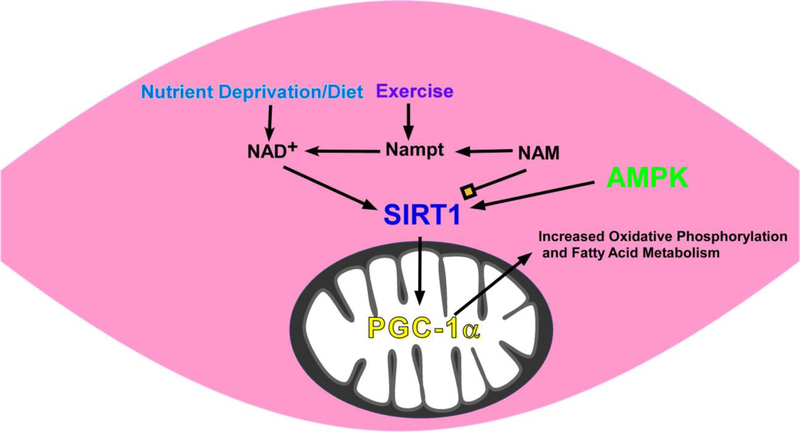
A dietary supplement that may improve or prevent diabetic autonomic neuropathy is nicotinamide riboside, a major generator of nicotinamide adenine dinucleotide (NAD+). NAD+ is a critical metabolite in energy metabolism and improves electron transfer in mitochondria. Levels of NAD+ are reduced in diabetic neurons [13, 69]. High levels of NAD+ can prevent oxidative injury in neurons [44, 45, 66] and increasing tissue levels of NAD+ activates expression of molecular pathways that protect against obesity and dyslipidemia [12]. NAD+ cannot be given directly due to toxic effects but NAD+ precursors such as nicotinamide riboside and nicotinamide mononucleotide may be clinically useful. NAD+ is synthesized from nicotinamide riboside and there is evidence that nicotinamide riboside or intermediates can improve mitochondrial function associated with neuropathy in type 2 diabetes mellitus, energy transfer within neurons, and activate neuronal protection and regeneration pathways [7, 13, 79]. Nicotinamide mononucleotide, which is the direct precursor of nicotinamide riboside, has been shown to reverse neuropathy in animal models of diabetes [56, 69]. Nicotinamide riboside restores NAD+ levels in high fat diet induced type 2 diabetic mice and improves glucose intolerance [74]. Nicotinamide riboside and nicotinamide mononucleotide likely improve muscle function by increasing mitochondrial function and specifically oxidative phosphorylation and beta oxidation (Figure 1). AMP-activated protein kinase and the protein deacetylase, sirtuin 1 (SIRT1), are fuel sensing molecules. During energy deprivation, AMP-activated protein kinase activation restores energy balance by increasing levels of ATP, for example by increasing fatty acid oxidation, and reducing processes that consume ATP. AMP-activated protein kinase is also know to circulate in the blood once released from muscle [40] and this could explain why exercise, which improves muscle function, can also have remote effects on somatic and autonomic nerve fibers.
Figure 1. Effect of Diet and Exercise on energy signaling pathways that regulate mitochondrial metabolism in a skeletal muscle fiber.
Nutrient deprivation leads to an increase in NAD+ and a decrease in nicotinamide (NAM), which in turn leads to activation of SIRT1 and 35kDa peroxisome proliferator-activated receptor-gamma co-activator-1□ (PGC1-α) in the mitochondrion resulting in an increase in oxidative phosphorylation and fatty acid metabolism. NAM and NADH act as inhibitors of SIRT1. NAM phosphoribosyltransferase (Nampt) catalyzes conversion of NAM to NAD+. Exercise has been shown to increase Nampt activity in human muscle and also circulates to increase insulin sensitivity. In turn, Nampt decreases NAM and increases NAD+ resulting in further activation of the SIRT1- PGC1-α pathway and increasing mitochondrial function. AMP-activated protein kinase (AMPK) likely regulates SIRT1 by acting as an energy sensor and activates Nampt. Thus, the net effect of nutritional restriction and exercise is to increase NAD+ and drive the SIRT1- PGC1-α pathway towards improved mitochondrial function.
There is considerable interest in the effect of lipid metabolism on diabetic neuropathy. Translational research indicates that high fat diets can result in neuropathy in diabetes and that withdrawing or otherwise manipulating a high fat diet can reduce neuropathy [18, 19, 34]. In humans, a nutritional intervention study that was targeted to improve essential fatty acid dysmetabolism in type 1 diabetes mellitus examined supplementation with seal oil omega-3 polyunsaturated fatty acids in individuals with type 1 diabetes mellitus and neuropathy. After 12 months of supplementation they found that patients had an increase in corneal nerve fiber length [43]. Corneal nerve fiber length is being validated as a sensitive test of small fiber neuropathy [42, 55]. They did not observe progression of clinical disease symptoms and there were no declines in small and large fiber sensory and functional measures. However, there was no improvement detected in nerve conduction studies or sensory function.
In summary, dietary supplements may play an important role in the treatment of diabetic neuropathy. Dietary and exercise interventions affect major metabolic pathways that are likely to have a significant long term impact on glucose and lipid metabolism and even axonal regeneration in much the same way that a medication might be expected to act.
Lifestyle interventions in neuropathy associated with metabolic syndrome
Mice fed with a high fat diet provide an animal model of obesity and insulin resistance that can be used to study metabolic syndrome. In a dietary study of the effects of the ketogenic diet, mice fed with a ketogenic diet had increased epidermal axon density compared to mice fed with a high fat diet or mice fed with a high fat diet that exercised. In addition, the mice fed with a ketogenic diet did not develop mechanical allodynia that is seen with peripheral neuropathy. In this same study the ketogenic diet was also used as an intervention. Mice fed with a high fat diet for 8 weeks were switched to a ketogenic diet and were found to have an increased epidermal axon density and significantly reversed mechanical allodynia. In comparison, high fat diet fed mice that were switched to a control diet improved their metabolic parameters but not mechanosensation [18]. These results suggest that in an animal model, a ketogenic diet can prevent certain complications of metabolic syndrome and provide significant benefits to peripheral axons and sensory dysfunction. Despite a high fat content in both the high fat and ketogenic diets, the ketogenic diet appears to affect peripheral nerves differently and it may alter neuronal metabolism and promote axon growth, but this requires further study.
A similar phenomenon has been observed in animal models with an exercise intervention and suggest that measures of metabolic syndrome associated neuropathy may improve with exercise. Mice that were fed a high fat diet developed a neuropathy with cutaneous and visceral hypersensitivity and altered cutaneous innervation. The mice were either sedentary or given a running wheel (exercise group). The high fat diet fed mice in the exercise group improved in terms of hypersensitivity and the epidermal innervation normalized. These improvements were not seen in the sedentary group [31]. These findings not only support the association between metabolic syndrome and neuropathy, but also suggest that exercise might be able to improve measures of neuropathy.
The effects of exercise on unmyelinated epidermal axons in metabolic syndrome have been examined in humans. This subset of neurons was examined because they are thought to be not only more vulnerable to the toxic effects of metabolic derangements, but also have a higher regenerative capacity and be more amenable to potential therapies. A chemical axotomy technique that utilizes topical capsaicin has been developed and epidermal reinnervation can be assessed by skin biopsy and serial measurement of IENFD [52, 57]. Patients with metabolic syndrome or type 2 diabetes mellitus, without signs or symptoms of neuropathy, underwent thigh capsaicin axotomy and their baseline distal leg IENFD and 30-day regeneration rate was assessed. A subset of patients then participated in a 6 month, intensive exercise and lifestyle counseling program and the regeneration rate was reassessed in month 4. At baseline, distal leg IENFD was significantly reduced in both metabolic syndrome and type 2 diabetes mellitus groups and the 30-day regeneration rate was comparable in both groups. After the exercise intervention, participants with metabolic syndrome increased the 30-day reinnervation rate compared to baseline. Participants with greater improvement in metabolic syndrome features had a greater degree of reinnervation [59].
This clinical trial not only reinforced the link between metabolic syndrome and neuropathy but it suggests that at least some of the benefits of a lifestyle intervention program on neuropathy are through an improvement in axon regeneration rate. Furthermore, from the animal studies, dietary interventions and specifically ketogenic diets may be effective.
Lifestyle interventions in diabetic peripheral neuropathy
The pathophysiology of diabetic neuropathy involves persistent hyperglycemia as well as multiple biochemical pathways that ultimately result in oxidative stress and inflammation. As previously mentioned, intensive glucose control has been shown to reduce the risk of neuropathy in type 1 diabetes mellitus. While lifestyle changes have been shown to prevent or delay the diagnosis of type 2 diabetes mellitus and its complications, glucose control alone has not been shown to reduce the risk of neuropathy in type 2 diabetes mellitus. Similarly, clinical trials targeted at various pathologic pathways (glucose entry into the polyol pathway, oxidative stress, advanced glycation end product formation, microvascular ischemia, or adipocyte derived toxicity) have been unsuccessful in human studies of diabetic neuropathy. In contrast to targeted therapies, aerobic exercise results in beneficial effects on many of the pathways that are adversely affected by diabetes. Exercise has a number of beneficial effects on the body. Importantly it can improve three of the biggest risk factors for diabetic neuropathy: insulin sensitivity and glucose control, obesity, and dyslipidemia. In addition, exercise improves hypertension, increases end organ perfusion, reduces lipid and protein oxidation, inhibits adipocyte production of free fatty acids and adipokines and reduces humoral inflammation [59, 60]. Details of exercise studies in diabetic neuropathy are described in Table 1.
Table 1.
A summary of clinical trials utilizing an exercise intervention in diabetic neuropathy
| Study | Population | Intervention | Endpoint measure | Results |
|---|---|---|---|---|
| Smith 2006 (IGTN study) [63] | 32 subjects with IGT and neuropathy | 1-year standard of care diet and exercise counseling | Change in IENFD measured at the distal leg and proximal thigh | 0.3 ± 1.1-fiber/mm improvement in distal IENFD and a 1.4 ± 2.3-fiber/mm improvement in proximal IENFD (P < 0.004) Significant improvements in BMI, 2-hour post load glucose and total serum cholesterol Non-significant improvement in pain scores |
| Kluding 2012 [38] | 17 subjects with diabetic neuropathy | 10 week aerobic and strengthening exercise program | Visual analog scale, MNSI questionnaire of neuropathic symptoms, NCS and QST, and IENFD and branching in distal and proximal lower extremity skin biopsies | Significant reductions in pain, neuropathic symptoms on the MNSI, and increased intraepidermal nerve fiber branching (+0.11±0.15 branch nodes/fiber, P=.008) from a proximal skin biopsy No change in BMI or waist circumference HbA1c significantly improved No significant changes in NCS or QST |
| Singleton 2014 [58] | 100 subjects with type 2 diabetes without neuropathy | 1 year of lifestyle counseling (n=40) or weekly exercise program and dietary counseling (n=60) | Change in IENFD measured at the distal leg and proximal thigh | Distal leg IENFD significantly increased in the exercise group and was unchanged in the counseling cohort (1.5 ± 3.6 vs. −0.1 ± 3.2 fibers/mm, P = 0.03). IENFD at the proximal thigh was significantly greater after 1 year in the exercise group compared to the control group (12.1 ± 4.8 vs. 9.6 ± 4.7, P = 0.02) No significant change in NCS or clinical neuropathy scales (UENS) Significant improvement in HDL in the exercise group |
IGTN Impaired glucose tolerance causes neuropathy
IGT Impaired glucose tolerance
IENFD Intraepidermal nerve fiber density
BMI Body mass index
MNSI Michigan Neuropathy Screening Instrument
NCS Nerve Conduction Study
QST Quantitative Sensory Testing
UENS Utah Early Neuropathy Scale
The Diabetes Prevention Program showed that exercise and diet counseling can normalize features of metabolic syndrome and reduce the incidence of type 2 diabetes mellitus [39, 49]. There is also evidence that exercise can prevent and treat neuropathy due to prediabetes and diabetes. A 4 year-long Italian supervised treadmill study randomized patients with diabetes but without neuropathy to either an intense aerobic exercise program vs. control with no intervention. The exercise group did not significantly change their BMI, waist circumference, or lipid profile. However, they did significantly improve their exercise capacity and there was improvement in the nerve conduction velocity. Assessments of small fiber neuropathy were not included in this study, but the exercise group was significantly less likely than the control group to develop signs or symptoms of neuropathy over the 4-year duration of the study [8].
The effect of exercise on small fiber injury, measured by the change in IENFD, in type 2 diabetes mellitus was examined by a University of Utah study that randomized patients with type 2 diabetes mellitus and no signs or symptoms of neuropathy to a weekly resistance and aerobic exercise program or general health counseling. After the 1 year intervention, participants in the exercise group had a significantly increased IENFD at the distal leg compared to control subjects, who received general health counseling) despite no significant changes in their metabolic parameters, other than an improvement in HDL [58]. The results from these two trials suggest that exercise exerts a direct benefit on peripheral nerves that is independent on changes in standard measures of metabolism. They also support the potential role of a lifestyle intervention in the prevention of diabetic neuropathy.
Studies of patients with existing diabetic neuropathy have also shown that diet and exercise interventions can improve measures of neuropathy. The Impaired Glucose Tolerance Neuropathy Trial study was a 12-month natural history trial to examine the effects of a lifestyle modification on measures of diabetic neuropathy [63]. Subjects with impaired glucose tolerance and existing neuropathy received diet and exercise counseling that was similar to that used in the Diabetes Prevention Program. After 1 year, there were significant improvements the proximal IENFD and foot sweat volume measured by quantitative sudomotor axon reflex as well as patient reported scores on the visual analog pain scale. These improvements were associated with significant improvements in weight, glucose tolerance, and lipid profile[63]. However, while there was an improvement in the Visual Analogue Scale and the Gracely Pain Scale these changes in pain scales were not statistically significant.
While the Impaired Glucose Tolerance Neuropathy Trial examined patients with impaired glucose tolerance and neuropathy, a study at the University of Kansas examined the effect of an exercise program on patients with type 2 diabetes mellitus and neuropathy. This was a pilot study of a small group of patients with type 2 diabetes mellitus and neuropathy who participated in a 10-week aerobic and resistance exercise program. At the end of the program the participants significantly improved their HbA1c as well as neuropathic symptoms including the Michigan Neuropathy Symptom Inventory and pain levels on a visual analog scale. Significant reductions in pain on a 100 mm scale were seen P=.05. There was also a significant improvement seen on the epidermal nerve fiber branching on proximal skin biopsy [38]. There was a nonsignificant improvement in IENFD, but given that the study was only 10 weeks long this was consistent with greater improvements seen over 1 year in the Impaired Glucose Tolerance Neuropathy Trial [63].
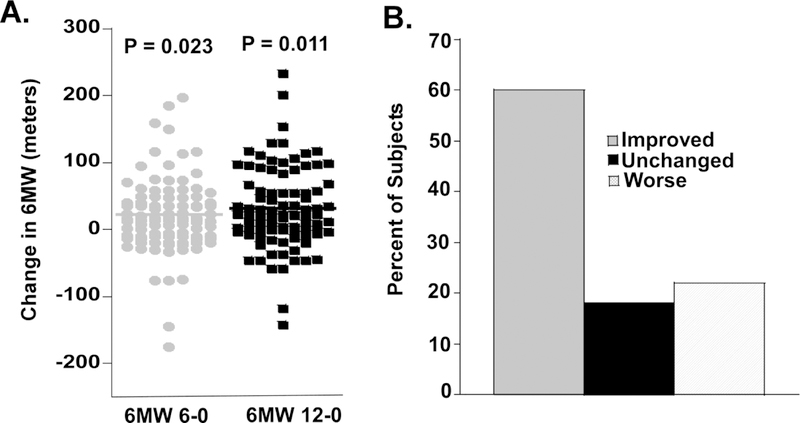
As previously mentioned, intensive glucose control has not been convincingly shown to reduce the incidence or progression of neuropathy in type 2 diabetes mellitus. However, a small prospective cohort study of patients with type 2 diabetes mellitus who underwent gastric bypass surgery found that the patients with neuropathy had an improvement in their Neuropathy Symptom Score and Neuropathy Deficit Score 6 months post-operatively. Interestingly, this improvement was independent of improved glucose control [48]. There have also been a number of other small studies that have found that exercise interventions have a positive effect on quality of life, balance, walking speed, and reaction time [21, 32, 33, 46]. Importantly, even with current “standard of care recommendations” that are widely used in clinical practice, there is a significant improvement in the 6 minute walk test, which is an excellent measure of change in mobility [47, 68] (Figure 2). “Standard of care recommendations” were provided prospectively to participants over a one-year period and the 6-minute walk test was performed by physiologist masked to the patient intervention. These general recommendations are that the patient loses 7 percent of body weight, sustain this loss, and undertake aerobic exercise for 150 minutes/week, up to 30 minutes per session. Participants were encouraged to follow the intervention but had to lose weight and exercise by themselves without the assistance of an interventionist. There was a significant improvement in the mean 6 minute walk test for the intervention group at 6 months compared to baseline, with 61 percent improving (≥5% improvement in the 6 minute walk test), 19 percent remaining unchanged (<5 percent improvement in the 6 minute walk test, and 19 percent with a worse 6 minute walk test. These improvements were sustained for 1 year (Figure 2). Furthermore, over 78 percent of participants improved or did not show a decline in the 6-minute walk test over 12 months.
Figure 2. Improvement in the 6 minute walk in subjects with diabetic neuropathy receiving “standard of care” dietary and exercise counselling over a 12 month period. Participants had mild, recently diagnosed type 2 diabetes mellitus or impaired glucose tolerance.
(A) Change in the 6MW between baseline and 6 months (n = 74) and baseline and 12 months (n = 64). The 6MW was performed prospectively, was masked, and completed in accordance with a standardized protocol. “Standard of care” advice included recommendations for 150 minutes per week of aerobic exercise, up to 30 minutes per session, and a goal of reducing baseline weight by 7 percent. The change in the 6MW was statistically significant at 6 months (P = 0.023) and 12 months (P = 0.011). (B) Percent subjects showing an improvement in the 6MW at 12 months (≥5% improvement in the 6MW), no change (<5% improvement), or worse 6MW with “standard of care” dietary and exercise counselling.
Overall, dietary and exercise interventions have been shown to slow progression to diabetes and to be of benefit in diabetic neuropathy. These were uncontrolled studies and there is a need for controlled, randomized trials. The data shows improvement in the IENFD, as well as symptom scores and mobility. While there is some improvement in pain, evidence of impact may be limited by the size of the studies.
Diabetic autonomic neuropathy
Cardiac autonomic neuropathy is a serious complication of diabetes that is strongly associated with an increased risk of morbidity and mortality. It is associated with a high risk of cardiac arrhythmias and sudden death, possibly due silent myocardial ischemia and infarction [6]. Although currently under recognized, cardiac autonomic neuropathy is a common complication of type 1 diabetes mellitus and type 2 diabetes mellitus. However, the reported prevalence varies widely (<5% - 90%) depending on the criteria used to define cardiac autonomic neuropathy and the population studied. The cardiac autonomic neuropathy Subcommittee of the Toronto Consensus Panel on diabetic neuropathy established criteria for cardiac autonomic neuropathy diagnosis and staging as: (1) one abnormal cardiovagal test result identifies possible or early cardiac autonomic neuropathy; (2) at least two abnormal cardiovagal test results are required for definite or confirmed cardiac autonomic neuropathy; and (3) the presence of orthostatic hypotension in addition to abnormal heart rate test results identifies severe or advanced cardiac autonomic neuropathy [65]. Using the above definition of confirmed cardiac autonomic neuropathy the prevalence in diabetes mellitus is estimated to be approximately 20%, but the prevalence increases with age and duration of diabetes mellitus.
Symptoms of cardiac autonomic neuropathy include sinus tachycardia, exercise intolerance, orthostatic hypotension with dizziness, presyncope or syncope, abnormal blood pressure regulation, asymptomatic or “silent” myocardial ischemia and infarction, and sudden death. V may be present at the time of diagnosis of diabetes mellitus and it is important to diagnose early because early treatment may affect long term outcomes. Although cardiovascular autonomic reflex tests are not widely available, the presence of autonomic symptoms in early diabetic neuropathy can be assessed by the Survey of Autonomic Symptoms, which is a validated and easily administered tool to measure autonomic symptoms that is sensitive enough to detect mild autonomic neuropathy [78]. The Survey of Autonomic Symptoms consists of two components, the reported autonomic symptom score and the Total Impact Score that determines the patient reported severity of each symptom and the overall impact of symptoms of dysautonomia [78].
Type 2 diabetes mellitus and obesity are associated with an impairment of parasympathetic function. This results in an imbalance of sympathetic vs. parasympathetic tone and sympathetic overactivity. The underlying mechanism of this change is still unknown. Part of this reason may be that animal models of diabetic autonomic neuropathy have significant differences in terms of the changes in cardiovascular parameters that likely reflect different underlying mechanisms. In humans increases in heart rate and blood pressure are commonly seen in early autonomic neuropathy, but in the mouse model of type 1 diabetes mellitus decreased blood pressure and heart rate are reported [30]. In humans, the earliest sign of cardiac autonomic neuropathy is frequently a reduction in heart rate variability, which is associated with an increased risk of death. Exercise is a potential treatment for diabetic cardiac autonomic neuropathy because aerobic training is known to result in increased parasympathetic predominance and good aerobic fitness is associated with increased vagal modulation [73]. In overweight patients without diabetes, exercise has been shown to improve heart rate variability [25, 26, 37].
Lifestyle interventions in diabetic autonomic neuropathy
The Diabetes Control and Complications Trial showed that intensive glucose control reduced the prevalence of cardiac autonomic neuropathy in patients with type 1 diabetes mellitus by 53% compared to conventional therapy [1]. The long-term outcomes of the participants were followed and the former participants in the intensive glucose control cohort continued to have a significantly lower prevalence and risk of cardiac autonomic neuropathy [54]. These results indicate that intensive treatment of type 1 diabetes mellitus should be started as early as possible to prevent or delay the development of cardiac autonomic neuropathy. Similar to diabetic somatic peripheral neuropathy, strict glucose control has been shown to reduce the risk of developing cardiac autonomic neuropathy in type 1 diabetes mellitus. However, the benefits are not as clear for patients with type 2 diabetes mellitus and the beneficial effect of intensive glycemic control on cardiac autonomic neuropathy in type 2 diabetes mellitus has not been proven [2, 35].
In patients with type 2 diabetes mellitus, the Steno 2 study utilized an intensive multifactorial treatment with lifestyle modification and pharmacologic therapy to treat hyperglycemia, hypertension, dyslipidemia, and microalbuminuria as well as aspirin. This study found that this multifactorial intervention reduced the risk of cardiac autonomic neuropathy by 60% in type 2 diabetes mellitus compared to conventional therapy [27, 53]. Looking at the effects of an exercise intervention, the previously discussed Italian supervised treadmill study of patients with type 2 diabetes mellitus but without neuropathy found that the 4-year intense aerobic exercise program resulted in an improved resting heart rate compared to control subjects [8]. Resting tachycardia is a frequent finding in cardiac autonomic neuropathy with vagal impairment and this finding suggests an improvement in autonomic function. Even though these results are intriguing and suggest that lifestyle interventions may improve autonomic function the results need to be interpreted with caution. In general, most studies support the use of exercise interventions in the treatment of cardiac autonomic neuropathy in type 2 diabetes mellitus. However, this area requires further research since studies are small with a variety of interventions and there is a lack of conformity of outcome measures. High quality studies with tests of cardiac autonomic function as a primary outcome measure as well as long -term follow up to determine the impact on morbidity and mortality are needed.
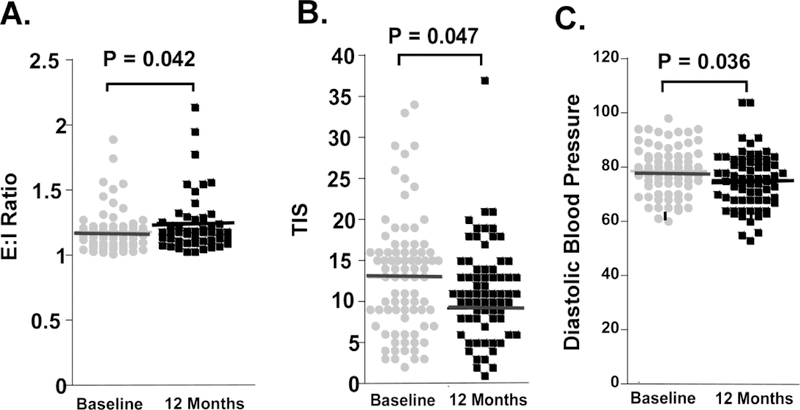
In patients with type 2 diabetes mellitus who do not have established cardiac autonomic neuropathy, exercise has been shown to improve autonomic function as measured by heart rate variability and the expiration:inspiration ratio [10, 29, 71, 80]. In subjects with recently diagnosed type 2 diabetes mellitus or with impaired glucose tolerance based on American Diabetes Association criteria [4], even a mild “standard of care lifestyle intervention” showed improvement over 1 year in some measures of autonomic function (Figure 3). The protocol was performed prospectively, was masked, and standardized. “Standard of care” advice included recommendations for 150 minutes per week of aerobic exercise, up to 30 minutes per session, and a goal of reducing baseline weight by 7 percent. This “standard of care lifestyle intervention” is provided to patients with impaired glucose tolerance and type 2 diabetes mellitus in clinical practice. Using a similar protocol, there was improvement in several measures of autonomic function at 1 year compared to baseline values, namely: (1) the expiration:inspiration ratio, a sensitive test of cardiac autonomic function, (2) the Total Impact Score, a validated measure of symptoms of dysautonomia, and (3) the change in the diastolic blood pressure (Figure 3). The technician performing the outcome measures was masked to the patient intervention. Participants were encouraged to follow the intervention but had to lose weight and exercise by themselves without the assistance of an interventionist. It is possible that “training bradycardia” could affect the heart rate variability as determined by the expiration:inspiration ratio. However, this is unlikely as the exercise intervention was minimal. Although there are outliers for the expiration:inspiration ratio at both baseline and 1 year (Figure 3), the confidence intervals for both are narrow with minimal overlap and would have a 0.95 probability of containing the population mean from subsequent population samples. Furthermore, even with transformation of the sample distribution, the means were statistically different between the baseline and 1 year samples.
Figure 3. Improvement in measures of autonomic function in subjects with neuropathy and impaired glucose tolerance or type 2 diabetes mellitus receiving “standard of care” dietary and exercise counselling over a 12 month period.
The protocol was performed prospectively, was masked, and standardized. “Standard of care” advice included recommendations to undertake aerobic exercise for 150 minutes/week, up to 30 minutes per session, and a goal of reducing baseline weight by 7 percent. However, none of the recommendations were enforced or monitored. The technician performing the outcome measures was masked to the patient intervention.
(A) Increased expiration:inspiration (E:I ratio, n = 50) after 1 year (P=0.042). Base 95% CI:1.14, 1.22. 1 year 95% CI: 1.20, 1.44.
(B) Decreased Total Impact Score (TIS) on the Survey of Autonomic Symptoms (SAS, n = 71),
(C) Decreased resting diastolic blood pressure (n = 72).
In diabetic patients with established cardiac autonomic neuropathy it also appears that moderately intense aerobic exercise can improve cardiac autonomic function. However, a longer and/or more intense intervention seems to be required to see a benefit with more severe cardiac autonomic neuropathy. In a study of patients with type 1 diabetes mellitus and early cardiac autonomic neuropathy, 3 months of endurance training improved heart rate variability. However, in patients with definite-severe cardiac autonomic neuropathy, there was no effect of exercise training on heart rate variability [36]. In patients with type 2 diabetes mellitus and definite cardiac autonomic neuropathy, 6 months of high intensity aerobic exercise at 70–85% heart rate reserve improved cardiac autonomic activity as well as glycemic control, lipid profile and aerobic capacity [50]. These findings highlight the fact that it is important to diagnose cardiac autonomic neuropathy early and support the idea that late cardiac autonomic neuropathy may be less amenable to change. A recent systemic review of research on the effects of exercise programs on heart rate variability in type 2 diabetes mellitus reviewed 15 human studies and concluded that more than 3 days per week of aerobic training, along with strength training, for at least 3 months seems to improve heart rate variability in type 2 diabetes. The exercise regimens in many of the studies were different, but weekly frequency may be the most important variable [72].
It is difficult to draw conclusions from these multiple small studies because, in general, the evidence is of moderate to low level and the study populations, interventions, and endpoint measures are not consistent. However, overall there is evidence of benefit of lifestyle interventions in cardiac autonomic neuropathy in both type 1 and 2 diabetes mellitus.
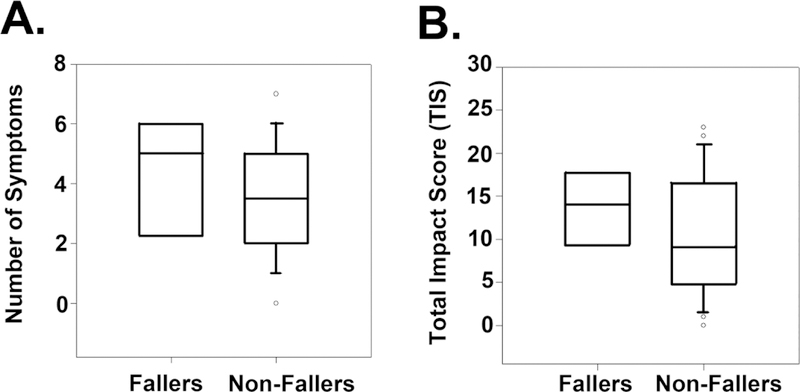
One of the major concerns with impaired autonomic function is the concern with increasing falls. Risk of falls is associated with orthostatic hypotension and with an increasingly abnormal Survey of Autonomic Symptoms (Figure 4). In a prospective study of impaired glucose tolerance and type 2 diabetes mellitus, subjects who reported frequent falls had a median Survey of Autonomic Symptoms symptom score of 5 compared to a median score of 3.5 in those who did not report a history of falls (Figure 4). The median Survey of Autonomic Symptoms Total Impact Score in fallers compared to non-fallers was 14 and 9 respectively, indicating that an increase in symptoms of dysautonomia parallels an increase in falls.
Figure 4. An increase in the symptom score and the Total Impact Score (TIS) on the Survey of Autonomic Symptoms (SAS) is associated with worse symptoms of dysautonomia and an increase in falls.
(A) Number of symptoms. (B) TIS.
Safety of exercise in diabetic neuropathy
In general, regular physical exercise is recommended for patients with diabetes. However, patients with proliferative nephropathy should avoid exercise that can cause hypertension [11] and weight bearing exercises should be performed with caution in patients with more advanced neuropathy and insensate feet due to risk of developing a diabetic foot ulcer. In general, supervised exercise programs in patients with diabetes are well tolerated. An effective lifestyle intervention that has a multisystemic effect would be preferable to a pharmaceutical therapy not only due to cost, but also because of relative lack of potential side effects. In addition to the side effects themselves, many frequent medication side effects, for example fatigue, imbalance, or gastrointestinal upset, may result in patients being less able to participate in physical activity. This is of particular concern in those with chronic disease and lower levels of physical activity because the lack of physical activity negatively impacts health related quality of life in diabetes [70].
It is important to be aware of treatment induced diabetic neuropathy. This is an acute onset, reversible, painful autonomic neuropathy that may present acutely shortly after starting intensive glucose control in individuals with previously poorly controlled diabetes. It can occur in either type 1 diabetes mellitus or type 2 diabetes mellitus and while often associated with intense control of blood glucose with medication, may be observed due to excessively rapid dietary control. Severe restriction in calories alone may result in treatment induced neuropathy [28].
Treatment induced neuropathy improves with sustained glucose control, although there is greater improvement seen in patients with type 1 diabetes mellitus [28]. There are other acute severe neuropathic pain syndromes that are rarely seen in diabetic patients: diabetic neuropathic cachexia, diabetic anorexia, and idiopathic acute painful neuropathy. Diabetic neuropathic cachexia presents in association with severe, unintentional weight loss with or without a change in glucose control. Pain in association with intentional weight loss unrelated to changes in glucose control is referred to as diabetic anorexia.
In particular, patients with autonomic neuropathy can have an increased risk of exerciseinduced injury due to decreased cardiac responsiveness to exercise, impaired thermoregulation, orthostatic hypotension and greater susceptibility to hypoglycemia. In addition, because cardiac autonomic neuropathy is an independent risk factor for the development of silent myocardial infarction and cardiovascular death, patients with cardiac autonomic neuropathy should be screened and possibly undergo an exercise stress test before starting an exercise program. Once cleared to exercise, exercise intensity is best prescribed using the heart rate reserve method with direct measurement of maximal heart rate [15, 17]. Heart rate reserve is the percentage of the difference between the resting heart rate and maximum heart rate during exercise, added to the resting value. It can be used to accurately predict Vo2 reserve, which is a percentage of the difference between resting and maximal Vo2 at which a person is exercising, in diabetics with and without clinical diabetic autonomic neuropathy [16] Moderate intensity aerobic exercise is 40–60% heart rate reserve. Another method to ensure that patients are exercising at the correct intensity level is to rely on perceived exertion and not heart rate since heart rate variability is likely to be reduced. Patients should also be educated that hypotension can occur after vigorous activities. Patients with autonomic dysfunction should also avoid exercising in hot or cold environments because of the risk of dehydration due to difficulty with thermoregulation. Finally, in patients with symptomatic orthostatic hypotension, recumbent exercises or water aerobics may be safer activities.
Conclusion
There is increasing evidence that dietary and physical activity interventions are effective in reducing the severity of both somatic and autonomic neuropathy. However, the results from these studies should be interpreted with caution because most studies are small, not randomized, and not all of the outcome measures are reliable or clinically meaningful. Specifically, further studies are needed to determine if changes in laboratory measures of autonomic neuropathy translate to differences in long term morbidity and mortality in diabetic individuals. Lifestyle interventions also improve mobility and may improve patient reported outcome measures. Of note, there is a paucity of studies that examine neuropathic pain and other quality of life factors as endpoint measures. Lifestyle interventions have profound effects on muscle energy balance and this may translate into lasting benefits to both muscle and nerve function. However, randomized, masked, controlled trials are needed to confirm the benefit of diet and physical activity lifestyle interventions and more data is needed on challenges and solutions to implementation, long term benefits of the intervention, and analysis of medical costs.
Acknowledgements
Supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health 1R01DK107007–01A1, Office of Research Development, Department of Veterans Affairs (Biomedical and Laboratory Research Service and Rehabilitation Research and Development, 101RX001030), Diabetes Action Research and Education Foundation, and the Baltimore GRECC (JWR), 1K2RX001651 (LAZ).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.DCCT (1998) The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 41:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UKPDS (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853 [PubMed] [Google Scholar]
- 3.Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B (2007) Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care 30:2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes A (2018) 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 41:S13–S27 [DOI] [PubMed] [Google Scholar]
- 5.Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, Low PA, Nehrdich D, Novosadova M, O’Brien PC, Reljanovic M, Samigullin R, Schuette K, Strokov I, Tritschler HJ, Wessel K, Yakhno N, Ziegler D (2003) The sensory symptoms of diabetic polyneuropathy are improved with a-lipoic acid: The Sydney trial. Diabetes Care 26:770–776 [DOI] [PubMed] [Google Scholar]
- 6.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH (2006) Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 29:334–339 [DOI] [PubMed] [Google Scholar]
- 7.Avinash Rao S, Priyanka S, Chen C, Chandrasekaran K, Russell JW (2014) Administration of either nicotinamide mononucleotide (NMN) or over expression of SIRT1 prevents and treats peripheral neuropathy in type 1 and type 2 diabetic mouse models (abstract). In: 3rd International conference and exhibition on Neurology and Therapeutics. Journal of Neurology and Neurophysiology, Philadelphia, Pennsylvania, p 98 [Google Scholar]
- 8.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F (2006) Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 20:216–223 [DOI] [PubMed] [Google Scholar]
- 9.Bergström B, Lilja B, Osterlin S, Sundkvist G (1990) Autonomic neuropathy in non-insulin dependent (type II) diabetes mellitus. Possible influence of obesity. J Intern Med 227:57–63 [DOI] [PubMed] [Google Scholar]
- 10.Bhagyalakshmi S, Nagaraja H, Anupama B, Ramesh B, Prabha A, Niranjan M, Shreedhara A (2007) Effect of supervised integrated exercise on heart rate variability in type 2 diabetes mellitus. Kardiol Pol 65:363–368; discussion 369 [PubMed] [Google Scholar]
- 11.Burr JF, Shephard RJ, Riddell MC (2012) Physical activity in type 1 diabetes mellitus: assessing risks for physical activity clearance and prescription. Can Fam Physician 58:533–535 [PMC free article] [PubMed] [Google Scholar]
- 12.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J (2012) The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15:838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekaran K, C C, Sagi AR, Russell JW (2016) A Nicotinamide Adenine Nucleotide (NAD+) Precursor is a Potential Therapy for Diabetic Neuropathy (abstract). In: International Conference of Neuromuscular Diseases. J Neuromuscular Dis, Toronto, Canada, p S86 [Google Scholar]
- 14.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B (2018) IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281 [DOI] [PubMed] [Google Scholar]
- 15.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B, Medicine ACoS, Association AD (2010) Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 33:2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colberg SR, Swain DP, Vinik AI (2003) Use of heart rate reserve and rating of perceived exertion to prescribe exercise intensity in diabetic autonomic neuropathy. Diabetes Care 26:986–990 [DOI] [PubMed] [Google Scholar]
- 17.Colberg SR, Vinik AI (2014) Exercising with peripheral or autonomic neuropathy: what health care providers and diabetic patients need to know. Physiology of Sports Medicine 42:15–23 [DOI] [PubMed] [Google Scholar]
- 18.Cooper MA, Menta BW, Perez-Sanchez C, Jack MM, Khan ZW, Ryals JM, Winter M, Wright DE (2018) A ketogenic diet reduces metabolic syndrome-induced allodynia and promotes peripheral nerve growth in mice. Exp Neurol 306:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppey L, Davidson E, Shevalye H, Torres ME, Yorek MA (2018) Effect of dietary oils on peripheral neuropathy-related endpoints in dietary obese rats. Diabetes Metab Syndr Obes 11:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa LA, Canani LH, Lisbôa HR, Tres GS, Gross JL (2004) Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med 21:252–255 [DOI] [PubMed] [Google Scholar]
- 21.Dixit S, Maiya A, Shastry B (2014) Effect of aerobic exercise on quality of life in population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, randomized controlled trial. Qual Life Res 23:1629–1640 [DOI] [PubMed] [Google Scholar]
- 22.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O’Brien PC, Melton LJ III, Service FJ (1993) The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 43:817–824 [DOI] [PubMed] [Google Scholar]
- 23.Dyck PJB, Dyck PJ (1999) Diabetic polyneuropathy. In: Dyck PJ, Thomas PK (eds) Diabetic Neuropathy W.B. Saunders Company, Philadelphia, p 255–278 [Google Scholar]
- 24.Emdin M, Gastaldelli A, Muscelli E, Macerata A, Natali A, Camastra S, Ferrannini E (2001) Hyperinsulinemia and autonomic nervous system dysfunction in obesity: effects of weight loss. Circulation 103:513–519 [DOI] [PubMed] [Google Scholar]
- 25.Facchini M, Malfatto G, Sala L, Silvestri G, Fontana P, Lafortuna C, Sartorio A (2003) Changes of autonomic cardiac profile after a 3-week integrated body weight reduction program in severely obese patients. J Endocrinol Invest 26:138–142 [DOI] [PubMed] [Google Scholar]
- 26.Figueroa A, Baynard T, Fernhall B, Carhart R, Kanaley JA (2007) Endurance training improves post-exercise cardiac autonomic modulation in obese women with and without type 2 diabetes. Eur J Appl Physiol 100:437–444 [DOI] [PubMed] [Google Scholar]
- 27.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348:383–393 [DOI] [PubMed] [Google Scholar]
- 28.Gibbons CH, Freeman R (2010) Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann. Neurol 67:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goit RK, Pant BN, Shrewastwa MK (2018) Moderate intensity exercise improves heart rate variability in obese adults with type 2 diabetes. Indian Heart J 70:486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisé KN, Olver TD, McDonald MW, Dey A, Jiang M, Lacefield JC, Shoemaker JK, Noble EG, Melling CW (2016) High Intensity Aerobic Exercise Training Improves Deficits of Cardiovascular Autonomic Function in a Rat Model of Type 1 Diabetes Mellitus with Moderate Hyperglycemia. J Diabetes Res 2016:8164518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE (2013) Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain 154:2658–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handsaker JC, Brown SJ, Bowling FL, Cooper G, Maganaris CN, Boulton AJ, Reeves ND (2014) Contributory factors to unsteadiness during walking up and down stairs in patients with diabetic peripheral neuropathy. Diabetes Care 37:3047–3053 [DOI] [PubMed] [Google Scholar]
- 33.Handsaker JC, Brown SJ, Bowling FL, Maganaris CN, Boulton AJ, Reeves ND (2016) Resistance exercise training increases lower limb speed of strength generation during stair ascent and descent in people with diabetic peripheral neuropathy. Diabet Med 33:97–104 [DOI] [PubMed] [Google Scholar]
- 34.Hinder LM, O’Brien PD, Hayes JM, Backus C, Solway AP, Sims-Robinson C, Feldman EL (2017) Dietary reversal of neuropathy in a murine model of prediabetes and metabolic syndrome. Dis Model Mech 10:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR (2008) Long-term follow-up after tight control of blood pressure in type 2 diabetes. N. Engl. J. Med 359:1565–1576 [DOI] [PubMed] [Google Scholar]
- 36.Howorka K, Pumprla J, Haber P, Koller-Strametz J, Mondrzyk J, Schabmann A (1997) Effects of physical training on heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Cardiovasc Res 34:206–214 [DOI] [PubMed] [Google Scholar]
- 37.Ito H, Ohshima A, Tsuzuki M, Ohto N, Yanagawa M, Maruyama T, Kaji Y, Kanaya S, Nishioka K (2001) Effects of increased physical activity and mild calorie restriction on heart rate variability in obese women. Jpn Heart J 42:459–469 [DOI] [PubMed] [Google Scholar]
- 38.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma NK, Wright DE (2012) The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications 26:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H (2014) AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem 21:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Sole J, European Federation of Neurological S, Peripheral Nerve S (2010) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 17:903–912, e944–909 [DOI] [PubMed] [Google Scholar]
- 42.Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TM, Bril V (2017) Using in vivo corneal confocal microscopy to identify diabetic sensorimotor polyneuropathy risk profiles in patients with type 1 diabetes. BMJ Open Diabetes Res Care 5:e000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis EJH, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V (2017) Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology 88:2294–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP (2009) Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular. Med 11:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min SW, Sohn PD, Cho SH, Swanson RA, Gan L (2013) Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front Aging Neurosci 5:53 eCollection;%2013.:53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison S, Colberg SR, Parson HK, Vinik AI (2014) Exercise improves gait, reaction time and postural stability in older adults with type 2 diabetes and neuropathy. J Diabetes Complications 28:715–722 [DOI] [PubMed] [Google Scholar]
- 47.Mueller MJ, Tuttle LJ, Lemaster JW, Strube MJ, McGill JB, Hastings MK, Sinacore DR (2013) Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil 94:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller-Stich BP, Fischer L, Kenngott HG, Gondan M, Senft J, Clemens G, Nickel F, Fleming T, Nawroth PP, Büchler MW (2013) Gastric bypass leads to improvement of diabetic neuropathy independent of glucose normalization--results of a prospective cohort study (DiaSurg 1 study). Ann Surg 258:760–765; discussion 765–766 [DOI] [PubMed] [Google Scholar]
- 49.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S, Group DPPR (2005) The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagkalos M, Koutlianos N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A (2008) Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med 42:47–54 [DOI] [PubMed] [Google Scholar]
- 51.Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA (1988) Body fat and the activity of the autonomic nervous system. N Engl J Med 318:1077–1083 [DOI] [PubMed] [Google Scholar]
- 52.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC (2004) The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 127:1606–1615 [DOI] [PubMed] [Google Scholar]
- 53.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D (2017) Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH (2009) Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N (2015) Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care 38:671–675 [DOI] [PubMed] [Google Scholar]
- 56.Russell JW, Chandrasekaran K, Choi J, Chen H (2013) Nicotinamide adenine nucleotide (NAD+) regulation of sirtuin 1 (SIRT1) in the treatment of diabetic neuropathy (abstract). In: American Neurological Association; Annals of Neurology, New Orleans, Louisiana, p 2406 [Google Scholar]
- 57.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR (1998) Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci 18:8947–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singleton JR, Marcus RL, Jackson JE, Lessard K, Graham TE, Smith AG (2014) Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann. Clin. Transl. Neurol 1:844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG (2015) Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann. Neurol 77:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singleton JR, Smith AG, Russell JW, Feldman EL (2003) Microvascular complications of impaired glucose tolerance. Diabetes 52:2867–2876 [DOI] [PubMed] [Google Scholar]
- 61.Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, McArthur J (2005) The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J. Neurol. Sci 228:65–69 [DOI] [PubMed] [Google Scholar]
- 62.Smith AG, Rose K, Singleton JR (2008) Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci 273:25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AG, Russell JW, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR (2006) Lifestyle intervention for prediabetic neuropathy. Diabetes Care 29:1294–1299 [DOI] [PubMed] [Google Scholar]
- 64.Smith AG, Singleton JR (2013) Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications 27:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P (2011) Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev:10 [DOI] [PubMed] [Google Scholar]
- 66.Stevens MJ, Li F, Drel VR, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG (2007) Nicotinamide reverses neurological and neurovascular deficits in streptozotocin diabetic rats. J Pharmacol. Exp. Ther 320:458–464 [DOI] [PubMed] [Google Scholar]
- 67.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M (2003) The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 60:108–111 [DOI] [PubMed] [Google Scholar]
- 68.Taveggia G, Villafane JH, Vavassori F, Lecchi C, Borboni A, Negrini S (2014) Multimodal treatment of distal sensorimotor polyneuropathy in diabetic patients: a randomized clinical trial. J Manipulative Physiol Ther 37:242–252 [DOI] [PubMed] [Google Scholar]
- 69.Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C (2016) Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci. Rep 6:26933. doi: 10.1038/srep26933.:26933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vadstrup ES, Frølich A, Perrild H, Borg E, Røder M (2011) Health-related quality of life and self-related health in patients with type 2 diabetes: effects of group-based rehabilitation versus individual counselling. Health Qual Life Outcomes 9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanninen E, Uusitupa M, Länsimies E, Siitonen O, Laitinen J (1993) Effect of metabolic control on autonomic function in obese patients with newly diagnosed type 2 diabetes. Diabet Med 10:66–73 [DOI] [PubMed] [Google Scholar]
- 72.Villafaina S, Collado-Mateo D, Fuentes JP, Merellano-Navarro E, Gusi N (2017) Physical Exercise Improves Heart Rate Variability in Patients with Type 2 Diabetes: A Systematic Review. Curr Diab Rep 17:110. [DOI] [PubMed] [Google Scholar]
- 73.Voulgari C, Pagoni S, Vinik A, Poirier P (2013) Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 62:609–621 [DOI] [PubMed] [Google Scholar]
- 74.Yoshino J, Mills KF, Yoon MJ, Imai S (2011) Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14:528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R (2006) Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care 29:2365–2370 [DOI] [PubMed] [Google Scholar]
- 76.Ziegler D, Low PA, Freeman R, Tritschler H, Vinik AI (2016) Predictors of improvement and progression of diabetic polyneuropathy following treatment with alpha-lipoic acid for 4 years in the NATHAN 1 trial. J Diabetes Complications 30:350–356 [DOI] [PubMed] [Google Scholar]
- 77.Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schutte K, Dyck PJ (2011) Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 34:2054–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zilliox L, Peltier AC, Wren PA, Anderson A, Smith AG, Singleton JR, Feldman EL, Alexander NB, Russell JW (2011) Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology 76:1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW (2016) Diabetes and Cognitive Impairment. Curr. Diab. Rep 16:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zoppini G, Cacciatori V, Gemma ML, Moghetti P, Targher G, Zamboni C, Thomaseth K, Bellavere F, Muggeo M (2007) Effect of moderate aerobic exercise on sympatho-vagal balance in Type 2 diabetic patients. Diabet Med 24:370–376 [DOI] [PubMed] [Google Scholar]