Abstract
Purpose:
BN-CV301 is a poxviral-based vaccine comprised of recombinant (rec.) Modified vaccinia Ankara (MVA-BN-CV301; prime) and rec. fowlpox (FPV-CV301; boost). Like its predecessor PANVAC, BN-CV301 contains transgenes encoding tumor-associated antigens, MUC1 and CEA, as well as costimulatory molecules (B7.1, ICAM-1, LFA-3). PANVAC was re-engineered to make it safer and more antigenic.
Experimental Design:
This open-label, 3+3 design, dose-escalation trial evaluated three dose levels (DL) of MVA-BN-CV301: one, two or four subcutaneous injections of 4×108 infectious units (Inf.U)/0.5 mL on weeks 0 and 4. All patients received FPV-CV301 subcutaneously at 1×109Inf.U/0.5 mL every 2 weeks for 4 doses, then every 4 weeks. Clinical and immune responses were evaluated.
Results:
There were no dose limiting toxicities. Twelve patients enrolled on trial (DL1=3, DL2=3, DL3=6). Most side effects were seen with the prime doses and lessened with subsequent boosters. All treatment-related adverse events were temporary, self-limiting, grade ½, and included injection site reactions and flu-like symptoms. Antigen-specific T-cells to MUC1 and CEA, as well as to a cascade antigen, brachyury, were generated in most patients. Single agent BN-CV301 produced a confirmed partial response (PR) in one patient and prolonged stable disease (SD) in multiple patients, most notably in KRAS mutant gastrointestinal tumors. Furthermore, two patients with KRAS mutant colorectal cancer had prolonged SD when treated with an anti-PD-L1 antibody following BN-CV301.
Conclusions:
The BN-CV301 vaccine can be safely administered to patients with advanced cancer. Further studies of the vaccine in combination with other agents are planned.
Keywords: cancer vaccines, MUC1-C, CEA, TRICOM, immunotherapy, BN-CV301
Introduction
BN-CV301 is a poxviral-based vaccine comprised of recombinant Modified vaccinia Ankara (MVA-BN-CV301, prime) and recombinant fowlpox (FPV-CV301, boost). BN-CV301 contains transgenes encoding two tumor-associated antigens (TAA), mucin 1 (MUC1) and carcinoembryonic antigen (CEA), as well as three costimulatory molecules (B7.1, ICAM-1 and LFA-3, designated TRICOM). CEA and MUC1 have been identified on numerous adenocarcinomas including colorectal cancer (CRC), breast cancer, non-small cell lung cancer (NSCLC), bladder cancer and pancreatic cancer.
The transmembrane MUC1 C-terminal (MUC1-C) has been shown to induce an epithelial-mesenchymal transition (EMT) leading to stemness, drug resistance and immune evasion (1–3). MUC1-C also acts as an oncogene and overexpression is associated with a poor prognosis (4–7). Several studies have demonstrated that MUC1-C interacts with various receptor tyrosine kinases (i.e., EGFR and HER2) and promotes activation and downstream pathways (1,2,8). Furthermore, MUC1-C overexpression has been associated with immune evasion from anti-PD-L1 agents in triple negative breast cancer and NSCLC (3,9).
An earlier version of BN-CV301, PANVAC, has been evaluated in the preclinical and clinical settings. Preclinical studies have demonstrated that PANVAC activated antigen-specific human T-cells in vitro (10), cellular immune responses in vivo, and antitumor efficacy against tumors expressing either human MUC1 or CEA in mice. Phase 1 and 2 clinical trials involving PANVAC as a single agent, or in combination with granulocyte macrophage colony-stimulating factor (GM-CSF) +/− chemotherapy, demonstrated an acceptable safety profile with some hints of clinical benefit (11–14). Treatment with PANVAC was well tolerated with only low grade injection site reactions and transient flu-like symptoms (15). The pilot trial of PANVAC had four prolonged responders (one patient with CRC, one patient with appendiceal and two patients with ovarian cancer) but multiple patients had prolonged survival after coming off trial and several patients had unexpected clinical responses to subsequent therapies (15). Based on this pilot trial there was an expansion cohort for patients with breast or ovarian cancer, again resulting in a few prolonged responders. One breast cancer patient continues to have a complete response on-going for 9.5 years and received a total of 55 PANVAC vaccines prior to being taken off trial due to lack of available vaccine (13,16). Encouraging responses in breast cancer patients led to a small randomized, controlled phase 2 trial showing that docetaxel + PANVAC resulted in longer progression-free survival than docetaxel alone (7.9 months vs 3.9 months, respectively) (12). Furthermore, in a phase 2 trial, patients with complete resection of oligometastatic CRC who received PANVAC had improved survival compared with unvaccinated historical and contemporary controls (14). Collectively, these trials demonstrated preliminary evidence of clinical efficacy of PANVAC as an adjunct to other therapies.
To improve safety and immune response, PANVAC was modified in several ways, resulting in the new version of the vaccine, called BN-CV301. Like its predecessor PANVAC, BN-CV301 utilizes a prime-boost dosing regimen. Due to the replication-competency of the vaccinia vector employed in the priming dose of PANVAC, its administration offered particular risks to specific segments of the general population, including immunocompromised individuals and persons diagnosed with atopic dermatitis (17–19). In addition, traditional replication-competent smallpox vaccines are known to carry the risk of rare but serious cardiac complications, including myo/pericarditis. Considering these potential safety issues, a second-generation cancer immunotherapy strategy using an attenuated vaccinia virus, MVA, was developed. MVA-BN can infect mammalian cells and express transgenes, but it cannot produce infective viral particles, alleviating most of the safety concerns of PANVAC. More than 13,000 patients have been vaccinated with various MVA-BN-based infectious disease vaccines and no inflammatory cardiac adverse reactions have been reported. The expected improved safety profile has allowed the dosing regimen to be changed for BN-CV301 compared with PANVAC. Specifically, priming doses of MVA-BN-CV301 are given in four separate injection sites, resulting in a higher total virus dose and potential for exposure to a greater number of dendritic cells (DCs) in four draining lymph node regions. Additionally, two priming doses of MVA-BN-CV301 are given prior to switching to heterologous boosting with FPV-CV301, which is then given for a longer duration than in some previous trials with PANVAC.
The transgenes in the original PANVAC vectors were modified to contain one CD8+-- enhancer epitope for CEA (10,20) and one CD8+-- enhancer for MUC1 in the VNTR region of the molecule (21). The BN-CV301 vectors contain additional MUC1 enhancer epitopes, especially in the MUC1-C region. These enhancer epitopes have been described in detail previously (22) and span HLA-A2, HLA-A3 and HLA-A24 MHC Class I alleles, which encompass the majority of the population. All of the CEA and MUC1 agonist epitopes in BN-CV301 were also shown to enhance T-cell responses and subsequent tumor lysis versus the use of the corresponding native epitopes (20–22). Consequently, BN-CV301 is expected to promote a stronger antigen-specific targeted immune response in comparison to PANVAC.
The primary objective of this phase 1 trial was to assess the safety of BN-CV301 and to identify the recommended dose of BN-CV301 for use in future clinical trials.
Materials and Methods
Preclinical evaluation of antigenicity
Culture and infection of dendritic cells.
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized blood of HLA-A2 healthy donors obtained from the NIH Blood Bank by centrifugation on a Ficoll density gradient (Lymphocyte Separation Medium, LSM, MP Biomedicals, Santa Ana, CA). DCs were prepared using a modification of a previously published procedure (23); briefly, PBMCs (1.5×108) were resuspended in 50 ml AIM-V medium (Thermo Fisher Scientific, Waltham, MA) and allowed to adhere to T-150 flasks (Corning Costar Corp., Cambridge, MA). After 2 hours at 37°C, the non-adherent cells were removed with a gentle rinse. Adherent cells were cultured for 6 to 7 days in AIM-V medium containing 100ng/mL of recombinant human– (rh) GM-CSF and 20ng/mL of recombinant human interleukin 4 (rhIL-4). The culture medium was replenished every 3 days. For infection, DCs (1×106) were incubated in 1mL of Opti-MEM medium (Thermo Fisher Scientific) at 37°C with MVA-BN-CV301, FPV-CV301, or the control empty vectors, MVA-wildtype (WT) and FPV-WT. DCs were infected for 2 hours with FPV-based constructs at a multiplicity of infection (MOI) of 20 or 40, and for 1 hour with MVA-based constructs at an MOI of 5 or 10, followed by the addition of 10mL of fresh, warmed RPMI-1640 complete medium containing 100ng/mL of rhGM-CSF and 20ng/mL of rhIL-4. After 24 hours, DCs were analyzed by flow cytometry or used as antigen presenting cells (APCs) for in vitro stimulation of human antigen-specific T-cells.
FACS analysis of infected DCs.
Flow cytometry was performed on infected DCs using phycoerythrin (PE)-labeled antibodies against human B7–1 (CD80), ICAM-1 (CD54) and LFA-3 (CD58) or a control isotype IgG (BD Biosciences, San Jose, CA). The anti-CEA monoclonal antibody COL-1 (24) and anti–MUC1 antibodies DF3 and DF3-P (25,26) were also used.
Activation of human CEA- and MUC1-specific T-cells.
For T-cell stimulation, DCs (2×104) were co-incubated with HLA-A2‒restricted T-cell lines specific for CEA or two different epitopes of MUC1, at a ratio of T-cell-to-DCs of 1:10. Culture supernatants were collected at 24 hours and evaluated for secretion of IFNγ by ELISA (BioSource International, San Diego, CA).
Clinical
Patients.
Patients were eligible for trial if they had evaluable (not necessarily measurable) metastatic or unresectable locally advanced solid tumors with no known curative therapy. When available, KRAS status was determined using historical genomic profile reports (i.e., FoundationOne, Caris). Patients with surgically resected or ablated metastatic disease at high risk of relapse were also eligible. Patients must have completed at least one prior line of standard therapy at least 4 weeks prior to enrollment on trial, with resolution of any grade ≥2 adverse events (AEs). Patients could continue maintenance therapy where appropriate (i.e., endocrine therapy for ER+/PR+ breast cancer; HER2-targeted therapy for HER2+ breast cancer, capecitabine +/− bevacizumab for CRC; erlotinib for EGFR-mutated NSCLC; androgen deprivation therapy for prostate cancer) as long as the patient had been receiving this treatment ≥ 2 months prior to the start of trial treatment. Patients were required to be ≥18 years old with a good performance status (ECOG 0–1) and normal organ function. Exclusion criteria included chronic infection, including hepatitis B or C and HIV, active brain metastases, leptomeningeal disease, autoimmune disorders of clinical significance, concurrent systemic corticosteroids (physiological doses defined as ≤ prednisone 5mg per day or equivalent allowed), history of allergic reaction to components of vaccines, serious uncontrolled medical issues and pregnancy.
Trial design and oversight.
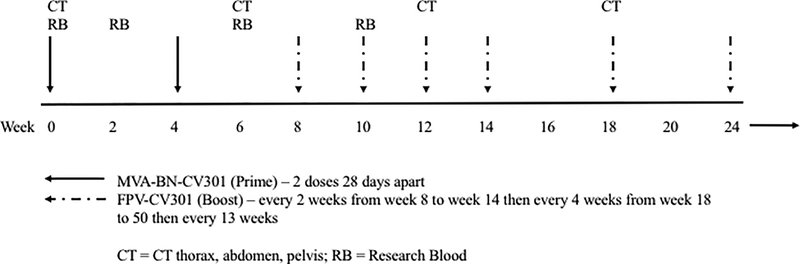
This phase 1 dose-escalation trial followed a standard 3+3 design to demonstrate the safety and immunogenicity of the BN-CV301 vaccine in patients with advanced solid malignancies (). Three vaccine dose levels (DLs) of the priming dose of MVA-BN-CV301were evaluated with patients receiving one (DL1), two (DL2) or four (DL3) injections. Each of these injections contained 4×108 infectious units per 0.5ml (Inf.U/0.5ml) and was injected in a different arm or leg. Following two priming doses at week 0 and week 4, a booster dose of the FPV-CV301 (1×109 Inf.U/0.5ml) was given subcutaneously at one site every 2 weeks from week 8 to week 14, every 4 weeks from week 18 to week 50 and every 13 weeks in all three dose levels (Fig. 1A).
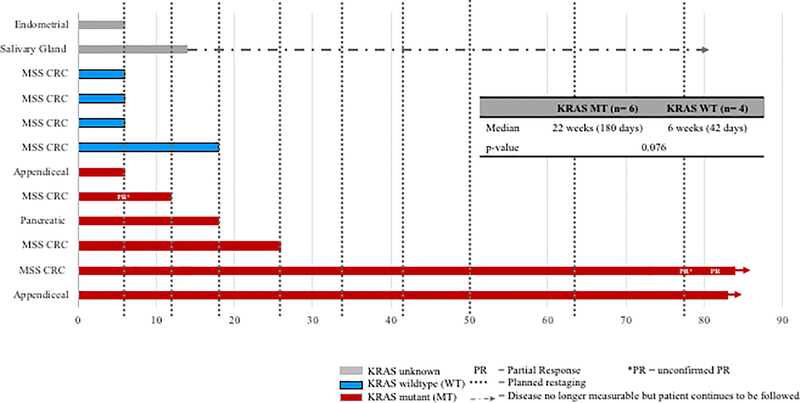
Figure 1. BN-Phase I schema and clinical outcomes.
(A) BN-CV301 Phase I study schema. (B) Progression-free survival on BN-CV301 for individual patients by KRAS status. Post-hoc KRAS analysis performed with an exact two-tailed log-rank test suggests there is a trend towards a difference by KRAS status but is limited by sample size.
The phase 1b component of this trial is on-going and is evaluating the safety and tolerability of BN-CV301 plus anti-PD-1 therapy (nivolumab or pembrolizumab) in patients with NSCLC who relapsed after or who are refractory to first-line chemotherapy. This phase 1 trial was conducted in accordance with the Declaration of Helsinki after approval by the Scientific Review Committee and Institutional Review Board (IRB) of the Intramural National Cancer Institute (NCI) and the Center for Cancer Research, NCI. All patients provided informed written consent. This trial was sponsored by Bavarian Nordic and the NCI. Ongoing safety oversight was conducted by the IRB and an appointed Safety Monitoring Team. Any serious AEs were reported to the U.S. Food and Drug Administration for review, per guidelines. Informed consent was obtained from each participant, including consent for treatment, primary and secondary endpoints, and correlative studies.
Safety assessment.
All patients were monitored for dose limiting toxicities (DLTs) during the 7 days after the first dose of BN-CV301. On day 7, patients were called by trained research staff to elicit symptoms of interest. A 12-lead EKG was performed at baseline, as well as during the week 2 clinic visit. Safety was assessed based on reported AEs through time on trial. Reported AEs were graded according to the NCI Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Treatment-emergent adverse events occurring from the first dose of BN-CV301 through 30 days after the last dose of BN-CV301 were recorded. All BN-CV301‒related serious AEs or AEs of special interest were collected until 100 days following administration of the last dose of BN-CV301.
Clinical assessment.
All patients were assessed in the clinic every 2 weeks +/− 3 days for the first 14 weeks on trial, every 4 weeks +/− 7 days through week 50, then every 13 weeks +/−7 days for the duration of treatment. Routine laboratory tests and targeted physical exams were performed at each time point. Tumor markers were also monitored as appropriate. Restaging with CT of the chest, abdomen and pelvis was performed at baseline, week 6, week 12, week 18, every 8 weeks until week 50 and then every 13 weeks using RECIST 1.1 criteria to assess the response.
Exploratory analyses.
PBMCs collected from patients before and during treatment were separated by Ficoll-Hypaque density gradient centrifugation and cryopreserved in human AB serum and 10% DMSO (1×107 cells/mL) and stored in liquid nitrogen until assayed. Antigen-specific responses were assessed by intracellular cytokine staining following a period of in vitro stimulation (IVS) with overlapping 15-mer peptide pools encoding MUC1 and CEA, as well as the cascade antigen brachyury, as previously described (27). The TAA peptide pools contain agonist epitopes that had previously been identified (20,22). Antigen-specific responses to KRAS were assessed in patients where the specific mutation was known and compared to wildtype KRAS. Peptide pools encoding for HLA and CEFT (a mixture of peptides of cytomegalovirus, Epstein-Barr virus, influenza and tetanus toxin) served as negative and positive controls, respectively. Peptide mixes were purchased from JPT (Berlin, Germany) and GenScript (Piscataway, NJ), reconstituted in DMSO, and used immediately.
Cryopreserved PBMCs from patients before therapy and on weeks 6 (2 weeks after 2nd MVA-BN-CV301 prime), 10 (2 weeks after 1st FPV-CV301 boost) and 18 or 22 (4 weeks after 4th or 5th FPV-CV301 boost, where available) were assayed as previously described (27). Using a BD Fortessa flow cytometer equipped with a UV, violet, blue, red, and yellow/green laser, 3×105 events in the live gate were acquired. FCS files were analyzed with FlowJo V9.7 for Macintosh (TreeStar, Ashland, OR). Non-viable cells were excluded, and fluorescence minus one control were used for gating. The absolute number of CD4+ or CD8+ T lymphocytes producing cytokine or positive for the degranulation marker CD107a was calculated per 1×106 cells plated at the start of the IVS. The background signal (obtained with the HLA peptide pool) and any value obtained prior to vaccination were subtracted from those obtained after vaccination ([post-TAA – post-HLA] – [pre-TAA – pre HLA]). A response to each TAA was scored as positive if a patient had more than 250 CD4+ or CD8+ T-cells that produced IFN-γ, TNF, IL2, or were positive for CD107a at the end of the stimulation assay per 1×106 cells that were plated at the start of the assay.
Statistical analysis.
This was a dose escalation phase 1 clinical trial. Patients with presumed CEA/MUC1-expressing tumors were enrolled using a 3+3 design. Descriptive statistics are reported throughout the manuscript. Due to differing clinical outcomes noted among patients with KRAS mutant and wildtype cancers, an exploratory, post-hoc analysis of the impact of KRAS mutation status on clinical outcome was evaluated using an exact two-tailed log-rank test with a priori p-value of significance <0.05.
Results
Preclinical antigenicity
MVA-BN-CV301– and FPV-CV301–infected DCs stimulate CEA- and MUC1-specific CD8+ T-cells.
The ability of the recombinant BN-CV301 vectors to infect human DCs in vitro was first evaluated. As shown in Supplementary Table S1A, expression of the encoded costimulatory molecules B7–1, ICAM-1, and LFA-3 was markedly enhanced in MVA-BN-CV301 and FPV-CV301 verse the corresponding MVA-WT– and FPV-WT–infected cells, respectively. Expression of the encoded CEA and MUC1 tumor antigens was observed with both MVA-BN-CV301 and FPV-C301 vectors above the low levels observed in control-infected or uninfected DCs.
MVA-BN-CV301– and FPV-CV301–infected human DCs were subsequently used in vitro as APCs to stimulate HLA-A2–restricted human CD8+ T-cells specific for an epitope of CEA (T-CEA) (28) or two distinct T-cell lines directed against an epitope of MUC1 (T-MUC1) or an epitope located on the C-terminal region of MUC1 (T-MUC1-C) (22). As shown in Supplementary Table S1B, MVA-BN-CV301 and FPV-CV301 were equally efficient at stimulating T-cell lines directed against CEA or MUC1 epitopes, as denoted by the secretion of high levels of IFN-γ, compared to the levels observed with control MVA-WT– or FPV-WT–infected DCs.
Altogether these results demonstrated that MVA-BN-CV301 and FPV-CV301 are able to efficiently infect and direct the expression of the encoded transgenes CEA, MUC1, B7–1, ICAM-1 and LFA-3 in human DCs. Moreover, the antigens CEA and MUC1 encoded by the vectors are being processed and presented in the context of MHC-class I molecules, leading to the effective stimulation of antigen-specific CD8+ T-cells.
Patient demographics.
In total, 12 patients were enrolled on trial between December 2016 to May 2017 (DL1, n = 3; DL2, n =3; DL3, n = 6). The data cutoff date for analysis was December 11, 2018. At this time, two patients remain on trial and receive FPV-CV301 every 13 weeks.
Half of patients enrolled were female, and median age was 56 years (Table 1). Due to referral patterns, most patients had a gastrointestinal tumor, including seven patients with microsatellite stable (MSS) CRC, three of whom had KRAS mutations and four were KRAS wildtype. Two patients had appendiceal cancer (>50% mucinous; both with KRAS mutations) and one patient had pancreatic cancer (presumed KRAS mutation since > 90% of pancreatic cancer have KRAS mutations) (29). Other patients enrolled had endometrial cancer (n = 1) and salivary gland cancer (n = 1); KRAS status was unknown. Half of patients were initially diagnosed with cancer in the metastatic setting (de novo). Patients had a mean of 4.2 prior regimens in the advanced cancer setting. Fifty percent of patients had at least one prior therapy qualifying as immunotherapy (i.e., prior therapeutic cancer vaccine, cytokine or anti-PD-1/L1 agent). One appendiceal cancer patient remained on maintenance standard therapy with capecitabine + bevacizumab while receiving BN-CV301. All patients enrolled were evaluable for clinical, safety and immune responses.
Table 1.
Patient demographics (n = 12)
| n (%) | |
|---|---|
| Female | 6 (50.0%) |
| Age in years, mean (range) | 56.0 (39 to 77) |
| Race | |
| White | 6 (50.0%) |
| Black | 3 (25.0%) |
| Other | 3 (25.0%) |
| Ethnicity: Hispanic | 1 (8.3%) |
| Tumor Type | |
| MSS Colorectal Cancer | 7 (58.3%) |
| Appendiceal Cancer | 2 (16.7%) |
| Pancreatic Cancer | 1 (8.3%) |
| Endometrial Cancer | 1 (8.3%) |
| Salivary Gland Cancer | 1 (8.3%) |
| De novo metastatic at diagnosis | 6 (50.0%) |
| Cancer Treatment | |
| Prior number of regimens, mean (range) | 4.2 (1 to 8) |
| Patients with Prior Immunotherapy | 6 (50.0%) |
| Patients on maintenance chemotherapy | 1 (8.3%) |
n (%) unless otherwise stated; MSS, microsatellite stable
Toxicity.
BN-CV301 was well tolerated with no DLTs (Supplementary Table S2). The maximum tolerated dose was not reached. The recommended phase 2 dose (R2PD) is DL3 of MVA-BN-CV301 (four injections of 4×108 Inf.U/0.5mL, each one administered in a different arm and leg) followed by the FPV-CV301 dose of 1×109 Inf.U/0.5mL. No deaths occurred on trial. There were no grade ≥3 AEs reported that were attributed to the BN-CV301 vaccine. The majority of AEs were reported during the priming doses of the vaccine (MVA-BN-CV301) and lessened over time. All reported AEs that were attributed to BN-CV301 were temporary, self-limiting and grade 1 or 2 in severity. Grade 1 or 2 injection site reactions were reported in all 12 patients (more common and severe with MVA- than FPV-BN-CV301 priming doses). These reactions generally occurred within 24 hours of administration, resolved within 7 days and were managed with supportive care. The majority of patients also reported systemic symptoms including fatigue (n=11; 91.7%), myalgias (n=9; 75.0%), chills (n=7; 58.3%) and headache (n=6; 50.0%). Non-neutropenic fevers were reported in slightly more than half of patients (n=7; 58.3%). Arthralgia, nausea, vomiting, and headache were also reported.
Clinical activity and outcomes.
All patients completed the first two priming doses of MVA-BN-CV301 (Fig. 1A and B). The median progression-free time was 15 weeks (range: 6 to on-going at 82 weeks). Due to disease progression (PD) at the first restaging point at 6 weeks, five patients (42%) received only the two priming doses. Seven patients received at least one dose of FPV-CV301 booster (range: 2 to on-going at 15 FPV-CV301 doses). Of these seven patients, one patient with MSS CRC had a partial response (PR) at 78 weeks and later confirmed at 82 weeks on trial after a period of prolonged stable disease (SD). Another patient with MSS CRC had an unconfirmed PR at 6 weeks but progressed with a new lesion at the next restaging visit (Fig. 1B; Table 2). The remaining five patients had SD as the best clinical response. Nine of the 12 patients eventually had PD, including the patient with the unconfirmed PR at 6 weeks. One patient with salivary gland cancer underwent resection of the tumor and continued to receive the vaccine for 6 months in the adjuvant setting due to high risk of recurrence. After 6 months, the patient was taken off trial but continues to be monitored for recurrence.
Table 2:
Individual patient responses
| Patient information | DL | Time to progression (weeks) |
Best clinical response (Δ target lesions from baseline) |
|---|---|---|---|
| KRAS mutation | |||
| MSS colorectal cancer | 1 | 12 | PR* at 6 wk (−38%) |
| MSS colorectal cancer | 1 | 26 | SD at 12 wk (−4%) |
| Appendiceal cancer | 3 | 6 | PD at 6 wk (+6% + new) |
| Pancreatic cancer | 3 | 18 | SD at 6 wk (−20%) |
| Appendiceal cancer on capecitabine + bevacizumab | 3 | 80+ | SD at 12 wk (−8%) |
| MSS colorectal cancer | 3 | 82+ | PR at 82 wk (−30.6%) |
| KRAS wildtype | |||
| MSS colorectal cancer | 2 | 6 | PD at 6 wk (+23%) |
| MSS colorectal cancer | 2 | 6 | PD at 6 wk (+7% + new) |
| MSS colorectal cancer | 2 | 18 | SD at 6 wk (+11%) |
| MSS colorectal cancer | 3 | 6 | PD at 6 wk (+27%) |
| KRAS unknown or not tested | |||
| Endometrial cancer | 1 | 6 | PD at 6 wk (+13%) |
| Salivary gland cancer | 3 | 12+ | SD at 12 wk (−2%)** |
PR*, unconfirmed partial response; + ongoing treatment on trial; DL, dose level (DL1 = 1 MVA-BN-CV301 injection, DL2 = 2 MVA-BN-CV301 injections, DL3 = 4 MVA-BN-CV301 injections); MSS, microsatellite stable; SD, stable disease; PD, progressive disease; PR, partial response; wk, week; new, new lesion; **tumor resected at 13 weeks on trial. Patient remained on trial with no measurable disease but at high risk of recurrence.
Two patients remain on trial at this time. One patient with KRAS mutant, MSS CRC on vaccine along with maintenance capecitabine + bevacizumab maintains SD at 81+ weeks. Another patient with KRAS mutant, MSS CRC on single agent vaccine achieved a PR. In this patient, the target lesion in the liver decreased by 30.6% from baseline at week 78 and was confirmed 4 weeks later (Table 2, Fig. 1B).
Preliminary signal in KRAS mutated cancers.
While this is a small sample of patients with known KRAS status (n=10), it is notable that patients with KRAS mutations (three MSS CRC, two appendiceal and one pancreatic cancer) remained stable longer than patients with KRAS wildtype cancers. The six KRAS mutation carriers remained on study for 6 weeks, 12 weeks, 18 weeks, and 26 weeks and two patients remain on study at 80+ weeks while the four KRAS wildtypes remained on study for 5 weeks, 6 weeks, 6 weeks and 18 weeks, respectively (KRAS mutation median progression-free time of 22 weeks vs KRAS wildtype 6 weeks; p=0.076). Furthermore, most patients with the KRAS mutation had SD with a decrease in the size of index lesions (although not more than a 30% reduction as required for a PR) for their best clinical response (Table 2, Fig. 1B). T-cell responses to KRAS mutations were compared to KRAS wildtype in three patients where the specific mutation was known, and peptides could be designed (two patients with KRAS G12D mutation, one patient with KRAS G12C mutation). There was a slightly greater response to the specific KRAS mutations than the wildtype peptides in two of the three patients (Supplementary Fig. S1), with patient 10 achieving a PR at week 78.
The two patients remain on trial at the time of the data cutoff point and continue to receive FPV-CV301 every 13 weeks as long as they tolerate treatment and there is no evidence of disease progression.
It is important to note that both of these prolonged responders received BN-CV301 at the highest DL tested (DL3). More KRAS mutants were enrolled in the highest DL compared to the lower DLs (DL3 had four KRAS mutants; DL2 had zero KRAS mutants; DL1 had two KRAS mutants). However, the two KRAS mutants in DL1 had prolonged SD on single agent vaccine followed by prolonged SD when transitioned to an anti-PD-L1 agent as will be discussed next. Only one of the KRAS wildtype patients (DL2) experienced SD beyond the first restaging at 6 weeks. While it is possible the prolonged responses are due to dose-effect, it is also plausible that the clinical responses are due to immune responses to KRAS mutations or other cascade TAAs.
Preliminary signal of BN-CV301 followed by anti-PD-L1.
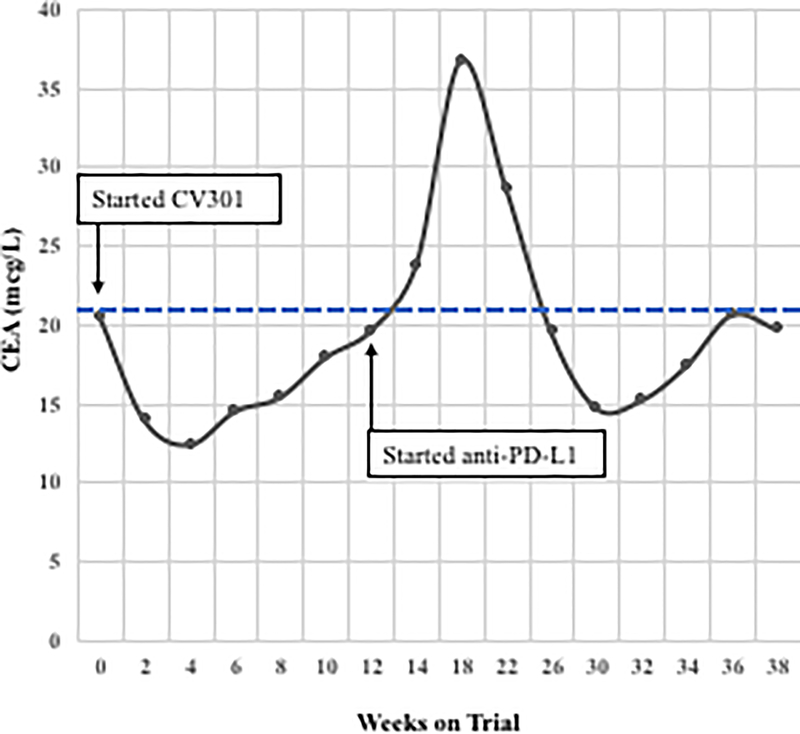
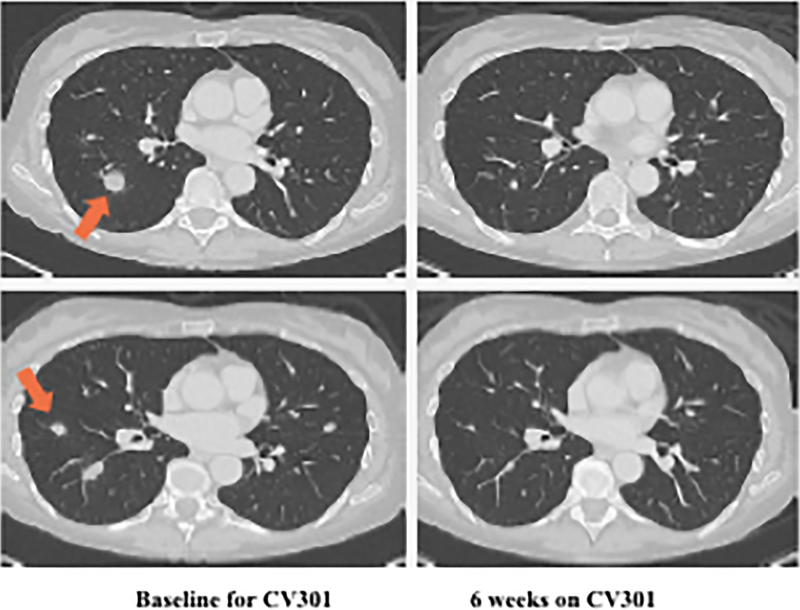
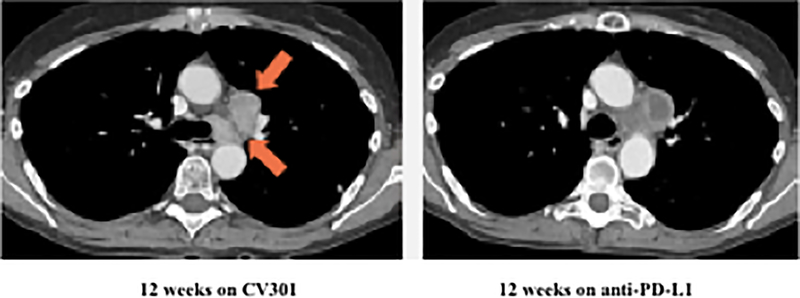
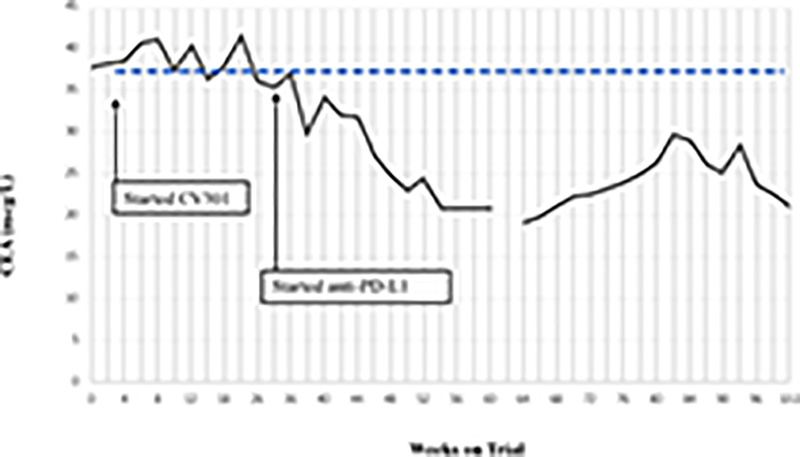
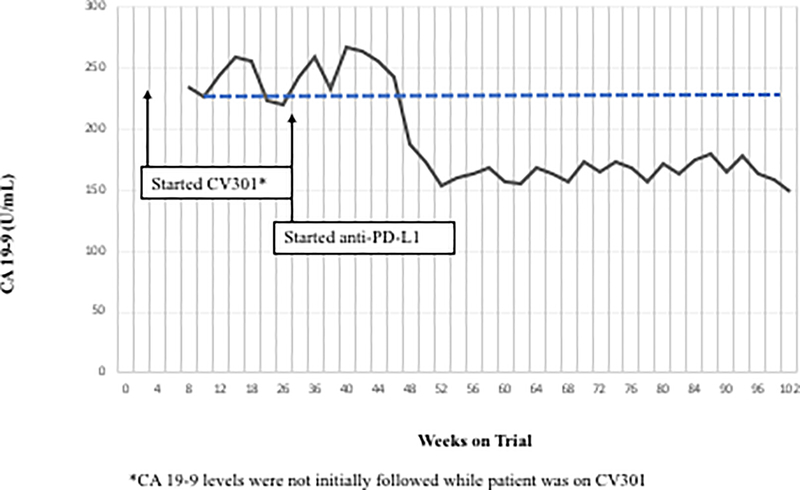
Following BN-CV301 treatment, three patients were transitioned to another trial evaluating an anti-PD-L1 agent. While mostly driven by patient interest in clinical trial participation, the clinical decision to enroll these patients on the anti-PD-L1 trial was supported by preclinical data demonstrating synergy with the concurrent (or sequential) use of therapeutic cancer vaccines and anti-PD-1/L1 agents. One patient with pancreatic cancer quickly progressed. The patient with a KRAS mutant, MSS CRC with an unconfirmed PR at 6 weeks on trial was transitioned to an anti-PD-L1 trial when the 12-week scan showed a new mediastinal lymph node. Shortly after starting on the anti-PD-L1 trial, tumor markers started to decline. At the 12-week restaging visit on the anti-PD-L1 trial, this patient had central necrosis of the affected mediastinal lymph node (Fig. 2A–C). Another patient with KRAS mutation, MSS CRC has prolonged SD per RECIST after switching to anti-PD-L1 therapy (SD on-going at 80 weeks). Both patients had a notable decrease (≥35%) in tumor markers (CEA, CA19–9) shortly after starting on anti-PD-L1 (Fig. 2D–E) and a prolonged period of SD, both of which are uncommon in patients with KRAS mutant, MSS CRC on checkpoint inhibitors.
Figure 2.
Two patients with KRAS mutation, MSS mCRC had a ≥ 35% decrease in tumor markers associated with prolonged stable disease after treatment with BN-CV301 followed by an anti-PD-L1 antibody.
Patient #1: A 62-year-old female with KRAS MT, MSS CRC with progressive disease despite 6 prior regimens (DL=1). She had an initial decrease in CEA (A) and an unconfirmed partial response at the first restaging (6 weeks; B) followed by growth of a non-target lesion (new mediastinal adenopathy) at the 12-week restaging. The patient then enrolled on an anti-PD-L1 trial and experienced a subsequent decrease in CEA as well as a radiographic response (C) to treatment with necrosis of mediastinal adenopathy and decreasing tumor markers at week 12 of treatment with the anti-PD-L1 antibody. This patient had stable disease for 43 weeks while on an anti-PD-L1 antibody. Patient #2: A 54-year-old female with KRAS MT, MSS CRC with progressive disease despite 8 prior regimens (DL=1). While on BN-CV301 trial, CEA and tumor burden were stable but the patient eventually developed progressive disease at 26 weeks. Patient was then enrolled on an anti-PD-L1 trial and experienced a subsequent decrease in tumor markers (D and E). Radiographically the patient has continued stable disease on the anti-PD-L1 antibody ongoing at 71 weeks. Prior trials have found a median progression-free survival of 10 weeks (2.2 months) in patients with MSS, mCRC who receive an anti-PD-L1 antibody. Blue dotted lines represent baseline tumor markers.
Identification of MUC1, CEA, and brachyury-specific T-cells.
Sufficient PBMCs were available to analyze MUC1-, CEA-, and brachyury-specific T-cells prior to vaccination (n=12) and at weeks 6 (n=12), 10 (n=7), and 18 or 22 (n=4) of the study. The FACS-based assay for T-cells expressing the type I cytokines IFNγ, IL2, TNF, and/or the degranulation marker CD107a following stimulation with overlapping peptide pools is described in detail in the Materials and Methods section. All assays for a given patient’s samples before and after vaccine were performed in the same controlled experiment.
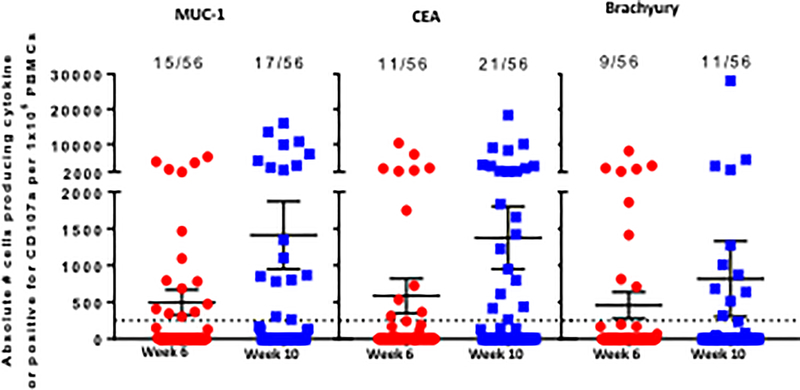
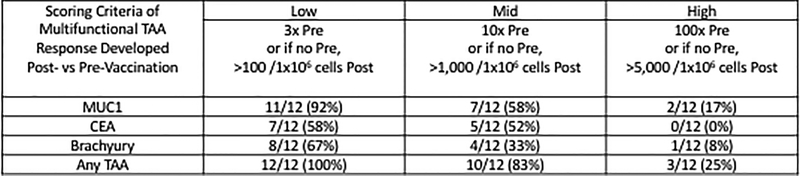
Including all DLs and time points examined, 11/12 patients (92%) developed CD4+ and/or CD8+ T-cell responses after vaccination to the antigens MUC1 and CEA that were encoded in the vaccine, as well as to brachyury, a “cascade” antigen not encoded in the vaccine. The induction of antigen-specific T-cells was rapid, with most patients having responses to MUC1 (75%), CEA (67%), or brachyury (58%) within 2 weeks after the second MVA-BN-CV301 priming vaccination (week 6). CEA-, MUC1-, and brachyury-specific T-cells were observed at all DLs at this early timepoint; MUC1-specific T-cells were generated in 1/3 patients at DL1, 2/3 at DL2, and 6/6 at DL3; CEA-specific T-cells were induced in 1/3 patients at DL1, 3/3 at DL2, and 4/6 at DL3; and brachyury-specific T-cells were developed in 1/3 patients at DL1, 2/3 at DL2, and 4/6 at DL3. TAA responses were evaluated in seven patients with sufficient PBMCs after the first FPV-CV301 boost; in these patients, although not statistically significant, the magnitude of the MUC1-, CEA-, and brachyury-specific T-cells producing cytokine or positive for CD107a was increased on average 2.8-, 2.3-, and 1.8-fold, respectively, after the booster vaccination compared to the results after the second priming vaccination (Fig. 3A). In light of the heterogenous nature of the patients on this study in terms of cancer type, and number and type of prior therapies, it is difficult to define which type of immune cell responses are most prominent post-vaccination. In some patients, immune responses to either CEA or MUC1 declined post-vaccination, which is not unexpected due to progression of disease (Supplementary Table S3). In general, however, IFNγ+ CD4+ and CD8+ CEA-specific T-cells and IFNγ+ MUC1-specific CD4+ T-cells increased in the majority of patients post-vaccination (Supplementary Table S3). CEA-specific CD107a CD8+ T-cells also increased post-vaccination in 5/7 patients (Supplementary Table S3). Polyfunctional TAA responses, defined as CD4 or CD8 T cells that express two or more of the following markers: IFNγ, TNF, IL-2, or CD107a, were measured in all patients before and after vaccination. Using the criteria of a >10 fold increase post versus pre-vaccination, or the presence of >1,000 polyfunctional cells at post per 1×106 PBMCs (if negative at pre), polyfunctional T-cells specific for MUC1, CEA, or brachyury were generated after BN-CV301 in 58%, 52%, and 33% of patients, respectively (Fig. 3B).
Figure 3.
Magnitude and breadth of combined antigen-specific CD4+ and CD8+ T-cell responses post- (vs pre) BN-CV301 (MVA-BN-CV301/FPV-CV301) vaccine.
(A) Seven patients were tested and TAA responses compared both at 6 weeks (2 weeks after the 2nd MVA-BN-CV301 prime, red) and 10 weeks (2 weeks after the 1st FPV-CV301 boost, blue). The absolute number of CD4+ and/or CD8+ T-cells producing IFNγ, TNF, or IL-2, or positive for the degranulation marker CD107a per 1 × 106 PBMC plated at the start of the stimulation assay was calculated. Any background signal (obtained with the HLA peptide pool) and any signal obtained prior to vaccination was subtracted ([post-TAA – post-HLA] – [pre-TAA – pre-HLA]). Each point indicates the magnitude of a cytokine/CD107a measure, with 8 measures assessed per patient (CD8+IFNγ+, CD8+TNF+, CD8+IL-2+, CD8+CD107a+, CD4+IFNγ+, CD4+TNF+, CD4+IL-2+, CD4+CD107a+). Frequency of positive measures (>250 CD4+ and CD8+ T-cells producing cytokine and/or positive for CD107a) is indicated. (B) Polyfunctional TAA responses (CD4+ and CD8+ T cells expressing 2 or more of the following: IFNγ, TNF, IL-2, or CD107a) were measured before and after any time point post-vaccination in all 12 patients. The frequency of patients developing a low, mid, or high magnitude of multifunctional TAA-specific T-cells after vaccination at any time point post- vs pre- is indicated. (C) TAA responses in 4 patients with known wild type KRAS (Black) and six patients with known/presumed KRAS mutations (Purple) were compared at 6 weeks (2 weeks after the 2nd MVA Prime). The absolute number of CD4+ or CD8+ T-cells producing IFNg, TNF, or IL-2, or positive for the degranulation marker CD107a per 1×106 PBMC plated at the start of the stimulation assay was calculated. Each point indicates the magnitude of a cytokine/CD107a measure, with 8 measures assessed per patient. Frequency of positive measures (>250 CD4+ and CD8+ T-cells producing cytokine and/or positive for CD107a) is indicated.
TAA responses were also compared between four patients with KRAS wildtype and six patients with known/presumed KRAS mutations. At week 6, a similar frequency of patients with KRAS wildtype (3/4) and KRAS mutations (4/6) developed MUC1-specific T-cells after vaccination; however, those with mutated KRAS displayed a trend of a greater magnitude (on average 4.9-fold) of MUC1-specific T-cells producing cytokine or positive for CD107a compared to those with KRAS wildtype tumors (Fig. 3C). CEA-specific T-cells, on the other hand, were generated in a slightly higher frequency of patients with KRAS wildtype (4/4) than KRAS mutations (3/6); however, the magnitude of CEA responses generated was similar between these groups. Brachyury T-cells were generated in 3/4 patients with KRAS wildtype and 3/6 patients with KRAS mutations, and the two groups of patients displayed a similar magnitude of brachyury-specific T-cells.
Discussion
The prime-boost regimen of BN-CV301 was well tolerated by all patients and demonstrated prolonged SD for some patients with advanced solid tumors as well as one confirmed PR. Side effects were minimal and were more common with MVA-BN-CV301 prime doses (limited to two doses) than with the FPV-CV301 booster doses. All side effects were self-limited and required only supportive care measures to manage symptoms. The safety profile of BN-CV301 was similar to that observed with the first generation PANVAC vaccine; however, the use of the replication defective MVA- and FPV-recombinant vectors eliminates the potential toxicity issues in the use of the replication competent vaccinia as a prime in the original PANVAC regimen.
Immunologic analyses demonstrated that BN-CV301 was able to generate MUC1- and CEA-specific T-cells in most patients (92%) after vaccination, demonstrating the immunogenicity of the BN-CV301 vaccine. In addition, T-cell responses against brachyury, an antigen not encoded in the vaccine, were generated after vaccination, suggesting that BN-CV301 induces immunologically relevant tumor cell destruction. Furthermore, we observed a trend indicating a potential dose-related response, resulting in the highest dose being selected for use in future phase II trials. We additionally observed that the magnitude of MUC1-, CEA-, and brachyury-specific immune responses was increased after the FPV-CV301 booster vaccine, supporting the use of a diversified prime-boost approach to generate a TAA immune response. It has been shown that long-lasting polyfunctional T-cells can be induced by vaccination and associate with improved overall survival (30). It is interesting in this study that the majority of patients generated polyfunctional T-cell responses to both CEA and MUC1 post-vaccination; moreover, the majority of patients also generated polyfunctional T-cell responses to the cascade antigen brachyury. Finally, we observed a trend in the magnitude of MUC1-specific T-cell responses being enhanced following vaccination to a greater extent in patients with KRAS mutations compared to those with KRAS wildtype tumors. The relationship between MUC1-C and KRAS mutations has been described previously (31,32). The immunogenicity demonstrated with the BN-CV301 vaccine described here in advanced and diverse cancer patients in this phase 1 trial supports the use of this vaccine in combination immunotherapy studies in more homogeneous patients and the safety supports its use in less advanced cancer settings.
With the limitations of the small number of patients treated, a correlation between the dose of vaccine administered and percentage of patients developing an immune response is noted in this trial, consistent with a previous phase 1 trial studying a similar vaccine construct encoding a different TAA, brachyury (33). In both trials the three dose levels explored used one, two and four injection sites with the aim of minimizing the severity of the injection site reactions, resulting in the activation of an increasing number of lymph node regions. Experimental evidence in mice has shown a significant correlation between the number of draining lymph node areas and the number of induced T-cells (34). This intriguing observation raises the possibility of some confounding between a pure dose-effect and the number of lymph nodes involved as determinants of the T-cell response magnitude.
Mutations in the KRAS proto-oncogene are found in many cancers including CRCs (30–40%), pancreatic cancers (90%) and NSCLC (25%) (35,36). Due to our referral pattern, this trial was unintentionally enriched for gastrointestinal tumors. Generally, KRAS mutations are associated with worse clinical outcomes and resistance to traditional therapies (35,37–40). Moreover, despite their prevalence, to the best of our knowledge no therapeutic agent to date has shown clinical benefit specifically for KRAS mutant cancers.
While this trial was small (n = 12 total with six KRAS mutant, four KRAS wildtype and two tumors with undetermined KRAS status), there appears to be a trend to longer time to progression for patients with KRAS mutant cancers as compared to patients with KRAS wildtype cancers (median time on trial 22 weeks in KRAS mutated vs 6 weeks in KRAS wildtype; p=0.076; Fig. 1B). This is worth noting since anti-PD-L1 therapies to date have shown no efficacy in MSS CRC with objective response rates of 0% and a median progression-free survival of 10 weeks (41). This early evidence suggests that a MUC1/CEA vaccine has the potential to produce durable clinical benefit (prolonged SD or PR) in KRAS mutant cancers. To our knowledge, no one to date has described this finding of clinical benefit with a vaccine targeting MUC1 or CEA in this population. Previously, Takahashi et al. demonstrated that KRAS mutated tumors have higher MUC1 expression than KRAS wildtype tumors (42). Downregulation of MUC1 expression is associated with reversal of EMT, reversal of an immunosuppressive tumor microenvironment and decreased tumor cell growth in KRAS mutant, NSCLC (2,3,43). We hypothesize that higher MUC1 expression enabled a more robust immune response to the TAAs contained in the vaccine. Vaccine-induced MUC1 immune responses also trended higher in tumors with KRAS mutations compared to those with KRAS wildtype.
Due to the heterogeneity in patient populations and the relatively small number of patients in each trial, it is difficult to draw strong conclusions in comparing the original PANVAC vaccine to BN-CV301. An ELISPOT assay was used to measure immune responses to CEA and MUC1 in the PANVAC trials. This assay was capable of measuring only CD8+ responses to HLA-A2 Class I patients using 9-mer peptides. Subsequent to those studies, we have developed a flow cytometry–based assay employing 15-mer peptides; this assay is capable of measuring both CD4+ and CD8+ T-cell responses, regardless of Class I or Class II type (27,33). Nonetheless, in the pilot study of PANVAC (15), 3/8 (38%) of patients analyzed developed T-cell responses to CEA, and 4/14 (29%) of patients developed T-cell responses to MUC1. In two additional studies of PANVAC (13,14), 5/33 (15%) of patients developed CEA-specific T-cell responses and 1/3 developed MUC1-specific T-cell responses. In this BN-CV301 trial, 11/12 (92%) of patients developed T-cell responses to CEA, and 11/12 (92%) of patients also developed T-cell responses to MUC1.
As previously mentioned, the phase 1b component of this trial combines BN-CV301 with pembrolizumab or nivolumab. Following progressive disease on BN-CV301, two patients with KRAS mutant, MSS CRC were transitioned to another trial evaluating an anti-PD-L1 agent and subsequently had prolonged SD. BN-CV301 is hypothesized to induce TAA-specific T-cells to migrate to the tumor. Combining BN-CV301 with anti-PD-1/L1 blockade therapy may augment these T-cell‒mediated clinical responses. These early clinical data further support the potential clinical benefit of combining BN-CV301 with anti-PD-L1 therapies. Both patients remained on this anti-PD-L1 therapy ≥ 40 weeks, exceeding expectations based upon published data.
In conclusion, the BN-CV301 vaccine can be safely administered to patients with advanced cancer in a prime-boost regimen. Side effects were mild to moderate and self-limited. Immune analysis demonstrated a high level of immunogenicity induced by the BN-CV301 vaccine. The BN-CV301 vaccine may have clinical benefit as monotherapy or in combination with anti-PD-1/L1 agents. A recent study by Massarelli et al (44) has shown the clinical benefit in the use of a therapeutic cancer vaccine with checkpoint inhibition in patients with HPV+ cancers. Further trials of the vaccine in combination with other agents are planned based on the results of preclinical data.
Supplementary Material
Translational Relevance:
BN-CV301 is a poxviral-based vaccine containing transgenes encoding for MUC1 and CEA. The predecessor of BN-CV301, PANVAC, was modified to improve safety and immune responses. The amino acid sequences in the CEA and MUC1 tumor associated antigens, including those in the MUC1-C region, were modified to produce a stronger immune response. The C-terminal transmembrane unit of MUC1 (MUC1-C) is important for cell growth, cell survival and functions as an oncoprotein. In this trial, BN-CV301 activated CD8+ and CD4+ T-cells against MUC1 and CEA and to a cascade antigen brachyury. Furthermore, we saw preliminary evidence of clinical efficacy in colorectal cancer, a traditionally “cold” tumor, both as a single agent and followed by an anti-PD-L1 antibody. This phase 1 trial demonstrated that the BN-CV301 vaccine can be safely administered to patients with advanced cancer. Further trials of the vaccine in combination with other agents are planned.
Acknowledgements:
We would like to thank Angie Schwab for her technical assistance with immune assays, Dr. Seth Steinberg for his statistical input, and Debra Weingarten for her editorial assistance.
Funding: Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health, and a Cooperative Research and Development Agreement (CRADA) between the NCI and Bavarian Nordic.
Footnotes
Disclosure of Potential Conflicts of Interest:
M. Gatti-Mays, J. Strauss , R. Donahue, C. Palena, J. Del Rivero, J. Redman, R. Madan, J. Marté, L. Cordes, E. Lamping, J. Schlom and J. Gulley declare they have no potential conflicts to disclose. A. Orpia and A. Burmeister are employees of Leidos Biomedical Research. E. Wagner is an employee of Bavarian Nordic GmbH. C. Pico Navarro and C. Heery are employees of Bavarian Nordic.
References:
- 1.Rajabi H, Kufe D. MUC1-C oncoprotein integrates a program of EMT, epigenetic reprogramming and immune evasion in human carcinomas. Biochim Biophys Acta 2017;1868(1):117–22 doi 10.1016/j.bbcan.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharbanda A, Rajabi H, Jin C, Alam M, Wong KK, Kufe D. MUC1-C confers EMT and KRAS independence in mutant KRAS lung cancer cells. Oncotarget 2014;5(19):8893–905 doi 10.18632/oncotarget.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouillez A, Adeegbe D, Jin C, Hu X, Tagde A, Alam M, et al. MUC1-C promotes the suppressive immune microenvironment in non-small cell lung cancer. OncoImmunology 2017;6(9):e1338998 doi 10.1080/2162402X.2017.1338998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Y, Zhang Q, Zhang Y, Lu M, Liu Y, Zheng T, et al. MUC1 predicts colorectal cancer metastasis: a systematic review and meta-analysis of case controlled studies. PLoS One 2015;10(9):e0138049 doi 10.1371/journal.pone.0138049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharbanda A, Rajabi H, Jin C, Raina D, Kufe D. Oncogenic MUC1-C promotes tamoxifen resistance in human breast cancer. Mol Cancer Res 2013;11(7):714–23 doi 10.1158/1541-7786.MCR-12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X, Sun Q, Chen C, Zhang Y, Kang X, Zhang JY, et al. MUC1 overexpression predicts worse survival in patients with non-small cell lung cancer: evidence from an updated meta-analysis. Oncotarget 2017;8(52):90315–26 doi 10.18632/oncotarget.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khodarev NN, Pitroda SP, Beckett MA, MacDermed DM, Huang L, Kufe DW, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res 2009;69(7):2833–7 doi 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, et al. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther 2011;10(5):806–16 doi 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda T, Hiraki M, Jin C, Rajabi H, Tagde A, Alam M, et al. MUC1-C induces PD-L1 and immune evasion in triple-negative breast cancer. Cancer Res 2018;78(1):205–15 doi 10.1158/0008-5472.CAN-17-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang KY, Palena C, Yokokawa J, Arlen PM, Gulley JL, Mazzara GP, et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res 2005;11(4):1597–607 doi 10.1158/1078-0432.CCR-04-1609. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med 2007;5:60 doi 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heery CR, Ibrahim NK, Arlen PM, Mohebtash M, Murray JL, Koenig K, et al. Docetaxel alone or in combination with a therapeutic cancer vaccine (PANVAC) in patients with metastatic breast cancer: a randomized clinical trial. JAMA Oncol 2015;1(8):1087–95 doi 10.1001/jamaoncol.2015.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohebtash M, Tsang KY, Madan RA, Huen NY, Poole DJ, Jochems C, et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res 2011;17(22):7164–73 doi 10.1158/1078-0432.CCR-11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse MA, Niedzwiecki D, Marshall JL, Garrett C, Chang DZ, Aklilu M, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg 2013;258(6):879–86 doi 10.1097/SLA.0b013e318292919e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res 2008;14(10):3060–9 doi 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatti-Mays M Durable Complete Response with PANVAC and Trastuzumab in Metastatic Triple Positive Breast Cancer. 2017; National Harbor, MD. [Google Scholar]
- 17.Greenberg RN, Overton ET, Haas DW, Frank I, Goldman M, von Krempelhuber A, et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis 2013;207(5):749–58 doi 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overton ET, Stapleton J, Frank I, Hassler S, Goepfert PA, Barker D, et al. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect Dis 2015;2(2):ofv040 doi 10.1093/ofid/ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Sonnenburg F, Perona P, Darsow U, Ring J, von Krempelhuber A, Vollmar J, et al. Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atopic dermatitis. Vaccine 2014;32(43):5696–702 doi 10.1016/j.vaccine.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst 1995;87(13):982–90. [DOI] [PubMed] [Google Scholar]
- 21.Tsang KY, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res 2004;10(6):2139–49. [DOI] [PubMed] [Google Scholar]
- 22.Jochems C, Tucker JA, Vergati M, Boyerinas B, Gulley JL, Schlom J, et al. Identification and characterization of agonist epitopes of the MUC1-C oncoprotein. Cancer Immunol Immunother 2014;63(2):161–74 doi 10.1007/s00262-013-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994;179(4):1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muraro R, Wunderlich D, Thor A, Lundy J, Noguchi P, Cunningham R, et al. Definition by monoclonal antibodies of a repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res 1985;45(11 Pt 2):5769–80. [PubMed] [Google Scholar]
- 25.Hayes DF, Sekine H, Ohno T, Abe M, Keefe K, Kufe DW. Use of a murine monoclonal antibody for detection of circulating plasma DF3 antigen levels in breast cancer patients. J Clin Invest 1985;75(5):1671–8 doi 10.1172/JCI111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perey L, Hayes DF, Maimonis P, Abe M, O’Hara C, Kufe DW. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from the DF3 human breast carcinoma-associated antigen. Cancer Res 1992;52(9):2563–8. [PubMed] [Google Scholar]
- 27.Heery CR, Singh BH, Rauckhorst M, Marté JL, Donahue RN, Grenga I, et al. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor brachyury. Cancer Immunol Res 2015;3(11):1248–56 doi 10.1158/2326-6066.CIR-15-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaremba S, Barzaga E, Zhu M, Soares N, Tsang KY, Schlom J. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res 1997;57(20):4570–7. [PubMed] [Google Scholar]
- 29.Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015;6:6744 doi 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wimmers F, Aarntzen EH, Duiveman-deBoer T, Figdor CG, Jacobs JF, Tel J, et al. Long-lasting multifunctional CD8. Oncoimmunology 2016;5(1):e1067745 doi 10.1080/2162402X.2015.1067745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raina D, Agarwal P, Lee J, Bharti A, McKnight CJ, Sharma P, et al. Characterization of the MUC1-C cytoplasmic domain as a cancer target. PLoS One 2015;10(8):e0135156 doi 10.1371/journal.pone.0135156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene 2013;32(9):1073–81 doi 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heery CR, Palena C, McMahon S, Donahue RN, Lepone LM, Grenga I, et al. Phase I study of a poxviral TRICOM-based vaccine directed against the transcription factor brachyury. Clin Cancer Res 2017. doi 10.1158/1078-0432.CCR-17-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mould RC, AuYeung AWK, van Vloten JP, Susta L, Mutsaers AJ, Petrik JJ, et al. Enhancing Immune Responses to Cancer Vaccines Using Multi-Site Injections. Sci Rep 2017;7(1):8322 doi 10.1038/s41598-017-08665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phipps AI, Buchanan DD, Makar KW, Win AK, Baron JA, Lindor NM, et al. KRAS-mutation status in relation to colorectal cancer survival: the joint impact of correlated tumour markers. Br J Cancer 2013;108(8):1757–64 doi 10.1038/bjc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeitouni D, Pylayeva-Gupta Y, Der CJ, Bryant KL. KRAS mutant pancreatic cancer: no lone path to an effective treatment. Cancers (Basel) 2016;8(4) doi 10.3390/cancers8040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Heo JS, Lee J, Lee JY, Lee MY, Lim SH, et al. The impact of KRAS mutations on prognosis in surgically resected colorectal cancer patients with liver and lung metastases: a retrospective analysis. BMC Cancer 2016;16:120 doi 10.1186/s12885-016-2141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghidini M, Personeni N, Bozzarelli S, Baretti M, Basso G, Bianchi P, et al. KRAS mutation in lung metastases from colorectal cancer: prognostic implications. Cancer Med 2016;5(2):256–64 doi 10.1002/cam4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imamura Y, Morikawa T, Liao X, Lochhead P, Kuchiba A, Yamauchi M, et al. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res 2012;18(17):4753–63 doi 10.1158/1078-0432.CCR-11-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zdanov S, Mandapathil M, Abu Eid R, Adamson-Fadeyi S, Wilson W, Qian J, et al. Mutant KRAS conversion of conventional t cells into regulatory T cells. Cancer Immunol Res 2016;4(4):354–65 doi 10.1158/2326-6066.CIR-15-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372(26):2509–20 doi 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R, et al. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene 2015;34(40):5187–97 doi 10.1038/onc.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouillez A, Rajabi H, Pitroda S, Jin C, Alam M, Kharbanda A, et al. Inhibition of MUC1-C suppresses MYC expression and attenuates malignant growth in KRAS mutant lung adenocarcinomas. Cancer Res 2016;76(6):1538–48 doi 10.1158/0008-5472.CAN-15-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5(1):67–73 doi 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.