Abstract
BACKGROUND:
Cue-induced relapse to drug use is a primary symptom of cocaine addiction. Cue-induced transient excitatory synaptic potentiation (t-SP) induced in nucleus accumbens mediates cued cocaine seeking in rat models of relapse. Cue-induced t-SP depends on extracellular signaling by matrix metalloproteases (MMPs), but it is unknown how this catalytic activity communicates with accumbens neurons to induce t-SP and cocaine seeking.
METHODS:
Male Sprague-Dawley rats (n=125) were trained to self-administer cocaine, extinguished and reinstated by cocaine-conditioned cues. We used a morpholino antisense strategy to knock-down the β1- or β3-integrin subunit, or inhibitors to prevent phosphorylation of integrin signaling kinases focal adhesion kinase (FAK) or integrin-linked kinase (ILK). We quantified protein changes with immunoblotting, and t-SP by measuring dendritic spine morphology and AMPA/NMDA glutamate currents. Integrin signaling was stimulated by microinjecting an MMP activator or integrin peptide ligand into the accumbens.
RESULTS:
Knock-down of β3-integrin or FAK inhibitor, but not β1-integrin or ILK inhibitor, prevented cue-induced cocaine seeking, but not sucrose seeking. β3-integrin knock-down prevented t-SP as measured by preventing the cue-induced increases in both AMPA/NMDA ratio and spine head diameter. Activating MMP gelatinases with tissue plasminogen activator potentiated cue-induced reinstatement, which was prevented by β3-integrin knock-down and FAK inhibition. Stimulating integrin receptors with the RGD ligand liberated by MMP gelatinase activity also potentiated cued cocaine seeking.
CONCLUSIONS:
Activation of MMP gelatinase in the extracellular space is necessary for and potentiates cued cocaine seeking. This extracellular catalysis stimulates β3-integrins and activates FAK to induce t-SP and promote cue-induced cocaine seeking.
Keywords: cocaine, integrin, focal adhesion kinase, synaptic plasticity, nucleus accumbens, relapse, drug abuse
INTRODUCTION
The negative social and personal consequences of drug addiction have inspired researchers to identify drug-induced pathogenic neuroplasticity in glutamatergic brain synapses, particularly in the nucleus accumbens(1). Human imaging and rodent studies of cue-induced drug seeking reveal enduring adaptations in accumbens glutamatergic synapses(2–4). Rodent experiments that model cue-reactivity studies in humans reveal that drug-conditioned cues elicit drug-seeking and transient synaptic potentiation (t-SP) across all drug classes tested to date (cocaine, opioids and nicotine)(4). Transient-SP is quantified as postsynaptic enlargement in dendritic spine heads (dh) and increases in the ratio of AMPA to NMDA (A/N) receptor currents(5). Importantly, t-SP elicited by drug cues is not recapitulated by cue-induced sucrose seeking(4, 6); thereby identifying t-SP as a potential pathological addiction process that is not recruited by conditioned cues motivating biological reward seeking.
In addition to adaptations in the canonical pre- and postsynapse, t-SP and drug-seeking induced by conditioned cues requires transient remodeling of the extracellular matrix (ECM). The ECM is an extracellular signaling domain composed of proteins that are catalytically activated by matrix metalloprotease (MMP) activity(7). Cues associated with cocaine, heroin or nicotine self-administration transiently increase MMP-9 activity(6), and MMP-9 activation is required for cue-induced induction of t-SP and drug seeking(8). However, it is unknown how MMP-9 activity in the ECM signals into accumbens medium spiny neurons (MSNs) to increase A/N and dh, and thereby promote cue-induced cocaine seeking.
MMP-9 is a gelatinase whose activation is necessary for inducing long-term potentiation (LTP) in the hippocampus(9, 10), and for developing fear conditioning and spatial learning(11, 12). Cleavage of gelatin sequences by MMP-9 reveals an Arg-Gly-Asp (RGD) binding motif that signals into neurons by binding to integrins(13). Integrins are heterodimeric (α and β-subunits), transmembrane cell adhesion receptors that support the interface between cell membranes and the ECM(14, 15). The β1 and β3 integrin subunits are most abundant in brain, and acute or repeated cocaine administration changes the amount of β1-and β3-containing integrins in the nucleus accumbens(16, 17). Many studies show that signaling through either subunit contributes to synaptic plasticity(18–20). Specifically, integrin subunits regulate spine morphology and synaptic strength by linking to actin cytoskeleton and regulating glutamate receptor surface expression through focal adhesion (FAK) and integrin linked kinases (ILK)(14, 15, 21, 22). Thus, β1- and β3-integrin subunits and associated kinases may constitute the MMP-9 signaling pathway necessary for cue-induced t-SP and reinstatement.
We tested the hypothesis that MMP-9 acts through the integrin signaling cascade in MSNs of the core subcompartment of the accumbens (NAcore) to initiate cue-induced t-SP and cocaine seeking. We used an integrin subunit knock-down strategy and FAK or ILK inhibitors to disrupt cued cocaine seeking and t-SP. We also activated NAcore MMP-9 with tissue plasminogen activator (tPA)(23, 24), and directly modulated integrins with the RGD peptide liberated by catalytic MMP-9 activity (13). From these studies, we conclude that MMP-9 activation by cocaine-associated cues signals through β3 Integrin to activate FAK, produce t-SP and reinstate cocaine seeking.
METHODS
(see Supplemental Methods for great detail)
Animal housing and surgery
Male Sprague Dawley rats (250 g; Charles River Laboratories) were individually housed using a 12:12 hr dark/light cycle with ad libitum food and water. All experimentation occurred in the dark cycle. For most experiments, rats were stereotaxically implanted immediately after catheterization with bilateral guide cannulae aimed at the NAcore. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animal Care.
Drugs and reagents used
Cocaine hydrochloride was supplied by the National Institute of Drug Abuse. Morpholinos were purchased from Gene Tools, LLC (Summerton Way, Philomath, OR). The antisense sequence used for β3 integrin morpholino (5’-TCTCTGCCTCAGAACTCGCCCCGCT-3’); β1 integrin morpholino (5’-TGCAAATTCATCTTTTCGCAGCGTC-3’) and standard scrambled control (5’-CCTCTTACCTCAgTTACAATTTATA-3’). MMP-9 inhibitor was purchased from EMD Bioscience (#444278; Ki=5nM). The FAK inhibitor prevents autophosphorylation at Y397, and was microinjected at 1, 3 or 10 nmol/0.5 μl/side, (FAK inhibitor 14; Tocris, #3414, Ki=1μM). The ILK inhibitor prevents phosphorylation at Ser473, and was microinjected at 1 or 10 nmol/0.5 μl/side (Cpd 22; Milipore #407331; Ki=0.6μM). All the reagents were dissolved in sterile saline, except for MMP-9 inhibitor that was dissolved in 1% DMSO.
Drug self-administration and reinstatement
Seven days after surgery, 125 rats began daily 2-hour self-administration sessions for cocaine and 11 rats for heroin self-administration. During self-administration, drug was delivered using an FR1 schedule with a 20 s timeout following each infusion. Active lever presses resulted in cocaine infusions (0.25 mg/infusion) or heroin (100 μg/infusion for day 1–2, 50 μg/infusion for day 3–4, 25 μg/infusion for day 5–12) and simultaneous presentation of a compound light (above the active lever) and tone (2900 Hz) conditioning stimulus. An inactive lever was also provided to control for nonspecific responding. Following at least 10 self-administration sessions at ≥10 infusions/day, rats began extinction training, during which programmed consequences were removed from lever pressing. Extinction training lasted at least 10 days, or until two consecutive days revealed ≤25 active lever presses.
Five days after the last morpholino microinjection the light/tone-conditioned cue was restored to active lever pressing and reinstated lever pressing quantified. For integrin knock-down experiments rats were only reinstated once and the session lasted 15 min when tissue was obtained for spine analysis and electrophysiology, or 120 min for full behavioral analysis. A total of 125 rats began cocaine self-administration, 11 rats were heroin-trained, 18 rats were yoked-saline, 16 were eliminated from the study due to catheter failure or microinjection cannula placements outside the NAcore.
Sucrose self-administration
Sixteen rats were trained in daily 2h-sessions on FR1 reinforcement schedule during which active lever presses resulted in delivery of a sucrose pellet (45 mg, Bio-Serv, Flemington, NJ) paired with light/tone cues. Following a minimum of 10 days of ten pellets or more, rats entered extinction training for 11–14 days, followed by cue-induced reinstatement test. Morpholino microinjections were made as described above.
Microinjection procedures and histology
Rats were stereotaxically implanted immediately after catheterization with bilateral guide cannulae aimed above NAcore (AP +1.5, ML ±1.7, DV −5.5)Palombi, Shin (26). Bilateral microinjections of 0.5 μl were made into NAcore over 2 min of β3, β1 integrin subunit or scrambled control (25 pmol × 5 days), small molecule inhibitors for MMP-9 (0.1 nmol), FAKi (1.0, 3.0, 10.0 nmol) and ILK (1.0, 3.0, 10.0 nmol), or peptides tPA (1 ng), RAD and RGD peptide (1 nmol). At the end of experimentation, rats were perfused and coronal slices (100 μm thick) of NAcore were mounted and stained with cresyl violet to verify guide cannulae placement.
Membrane Fractionation and Western Blotting
Rats were rapidly decapitated and the NAcore was dissected and homogenized. 15 μg protein was added to each lane of 10% Bis-Tris gels (Bio-Rad), and transferred to PVDF membranes via the Invitrogen iBlot transfer system. Primary antibodies used were for β1 integrin subunit (1:500, Abcam ab1952), β3 integrin subunit (1:250, Millipore 05769), ILK (1:1000, Abcam ab76468), Calnexin (Chemicon International), GADPH (Cell Signaling 5174).
Immunohistochemistry on dendrites and dendritic spines analysis
NAcore tissue slices and DiI-labeling dendrite spine morphology was performed as described previously(27). Confocal Z-series data sets of spine morphology were acquired using a Leica SP5 laser-scanning confocal microscope and images were acquired at 63x oil (1024×256 frame; 0.21 μm/Z step). Following deconvolution, individual dendrites were isolated and all image acquisition, cropping and isolating dendrites was performed by an investigator unaware of the treatment groups (Figure S1).
Slice preparation and whole cell recordings
Rats were anesthetized with ketamine and coronal slices (250 μm) collected into oxygenated artificial cerebrospinal fluid. Inhibitory synaptic transmission was blocked with picrotoxin (50 μM) and whole cell patch clamp recordings in voltage clamp mode were performed in NAcore using glass microelectrodes (1–2 MΩ) filled with a cesium-based internal solution. Postsynaptic currents were evoked at 0.05 Hz via a bipolar stimulating electrode placed ~300 μm dorsomedial of the recorded cell. The stimulation intensity chosen evoked a ~50% of maximal AMPA current. Recordings began >10 min after the cell membrane was ruptured, to allow diffusion of the internal solution into the cell. AMPA currents were first measured at −80 mV, and then the membrane potential was gradually increased to +40 mV. Recordings were resumed 5 min later. AP5 (50 μM) was bath-applied to block NMDA currents and currents recorded at +40 mV. NMDA currents were obtained by subtracting the AMPA currents from the total current. All recordings were made by an individual blinded to the treatment groups.
In Vivo Zymography
MMP activity was measured using an in vivo zymography assay as described previously(8). Briefly, rats were microinjected with intramolecularly dye-quenched fluorescein conjugated gelatin (Life Technologies) in the NAcore (1.5 μl/hemisphere at a rate of 0.5 μl/min) with a 20 min incubation time. Proteolytic cleavage by the gelatinases (MMP-2,9) results in an activity-dependent increase in green fluorescence. Coronal brain sections (100 μm) were imaged using a Leica SP5 laser-scanning confocal microscope using through a 10x objective. ImageJ was used to quantify images.
Statistics
All statistics were done using GraphPad Prism Version 6 or a two-level nested ANOVA in Excel. Behavioral and AMPA/NMDA ratio data were analyzed by two-way ANOVA followed by Bonferroni post-hoc tests for multiple comparisons. Protein was compared using paired Student’s t-test. All spine density, dh and immunohistochemistry data were statistically analyzed with a nested ANOVA, and post-hoc comparisons conducted using a Bonferroni post hoc. All data except behavior was obtained and quantified by individuals unaware of the treatment groups. See supplemental Table S1 for complete statistical information.
RESULTS
Knockdown of β3, but not β1 integrin, prevented cued cocaine seeking.
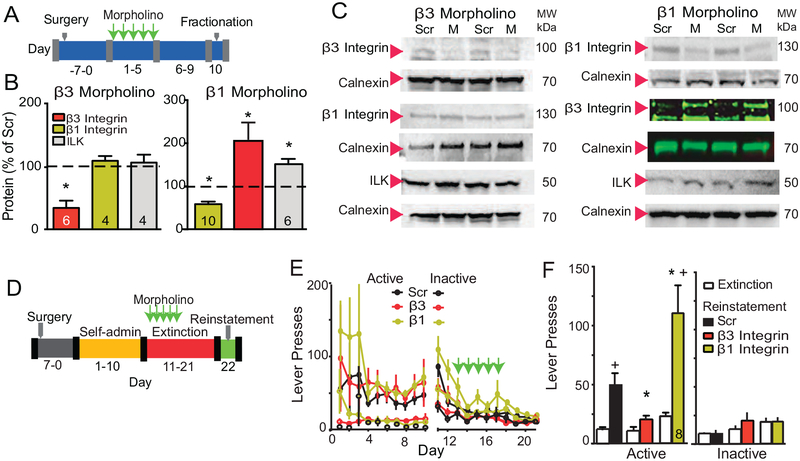
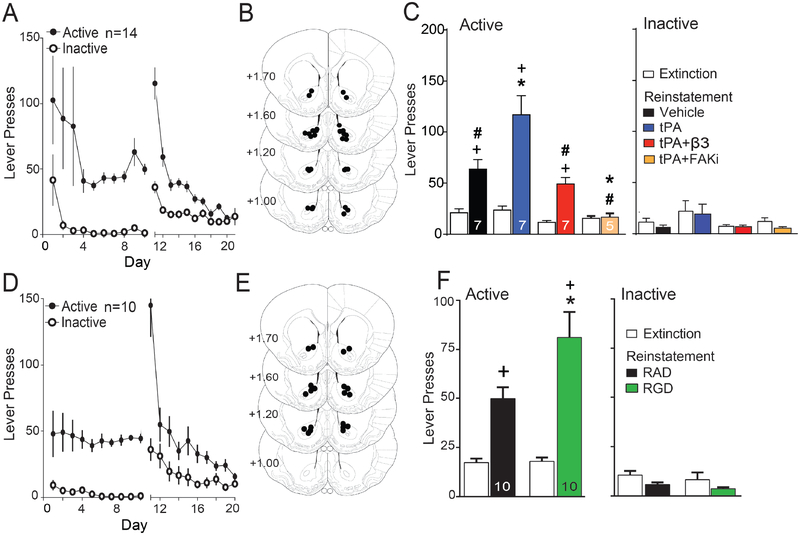
We used a morpholino knockdown strategy to determine if integrins containing β3 or β1 subunits were mediating cue-induced t-SP and cocaine seeking. Morpholinos have been used to modulate drug seeking(28, 29), and are synthetic antisense oligonucleotides with a backbone consisting of a morpholine ring and phosphorodiamidate group that increases in vivo stability and cell penetration(30). Five daily microinjections of morpholino antisense or control scrambled-sequence morpholino (Scr) were made into the NAcore in opposite hemispheres of drug naïve rats to validate the efficacy and specificity of the morpholino sequences at reducing β3 or β1 integrin subunit expression. Five days after the last microinjection, β3 or β1 were reduced compared with Scr by ~70% or ~60%, respectively (Figure 1B/C and S2). The β3 morpholino selectively reduced the β3 subunit without altering the β1 subunit or ILK. In contrast, the morpholino targeting the β1 subunit caused a compensatory elevation in β3 integrin and ILK.
Figure 1: Knockdown of β3 Integrin, but not β1 Integrin, blocked cue-induced cocaine seeking.
(A) Treatment protocol for morpholino experiments to assess the efficacy of morpholino-knockdown of β3 or β1 Integrin. (B) Scr or β integrin morpholino were microinjected into opposite hemispheres permitting within animal comparison. After β3 morpholino treatment, β3 subunit expression was reduced (paired Student’s t-test t(5)=8.91, p<0.001) without changing the β1 subunit or ILK. After β1 morpholino treatment, the β1 subunit expression was reduced (t-test t(5)=5.75, p<0.01), but β3 subunit (t(5)=3.98, p=0.050) and ILK (t(5)=4.24, p=0.008) expression were elevated. (C) Representative Western blots of each protein and the respective calnexin or GADPH loading control are shown below each bar. Note that because of the relatively weak β3 integrin antibody, a fluorescent secondary was used to augment detection after β1 morpholino treatment. *p<0.05. (D) Diagram of the experimental time line for drug self-administration. (E) Time course of active and inactive lever pressing for self-administration and extinction in cocaine-treated rats used in Figure 1 was equivalent between treatment groups. (F) Cue-induced reinstatement of cocaine seeking was reduced after knockdown of β3 subunit compared to Scr morpholino and potentiated after β1 Integrin knockdown (2-way repeated measures ANOVA, EXT/RST F(1,22)=20.05, p<0.001; Scr/β3/β1 F(2,22)=8.63, p=0.002, interaction F(2,22)=4.78, p=0.019). All data are shown as mean ± SEM, and N is shown within the bars.
*p< 0.05, compared to Scr or Scr/RST, using a Bonferroni’s post hoc test
+p< 0.05, compared to EXT responding within each treatment group
To determine if knockdown of either β subunit altered cue-induced cocaine seeking and t-SP, rats were trained to self-administer cocaine, and lever pressing extinguished (Figure 1D). A light/tone compound stimulus was paired with cocaine infusions during self-administration and lever pressing during extinction training yielded neither cocaine nor light/tone stimulus. Rats received 5 daily bilateral infusions into NAcore of β1, β3 or Scr morpholino during extinction training. Five days after the last morpholino microinjection the light/tone-conditioned cue was restored to active lever pressing and reinstated lever pressing quantified over 120 min (Figure 1E). Cocaine infusions, and active and inactive lever pressing during self-administration and extinction training were statistically equivalent between animals assigned to Scr, β3 or β1 morpholino groups (Figures 1E, S3A), and all rats had bilateral microinjection cannula tips in the NAcore (Figure S3B).
Exposing extinguished rats to cocaine-associated cues reinstated active lever pressing in the Scr-treated animals compared to active lever pressing averaged over the last 2 days of extinction training (Figure 1F). Morpholino knockdown of β3 integrin abolished reinstated lever pressing, while β1 integrin knockdown potentiated pressing (Figure 1F). These data support a necessary role by β3-containing integrins in signaling cue-induced cocaine seeking. The reduction in cocaine seeking after β3 integrin knockdown was without effect on inactive lever pressing (Figure 1F) or open field motor activity (Figure S3C). The paradoxical increase in reinstated seeking after β1 knockdown may arise from the compensatory increase in β3 integrin and ILK (Figure 1B).
β3 integrin knockdown did not alter cued sucrose seeking.
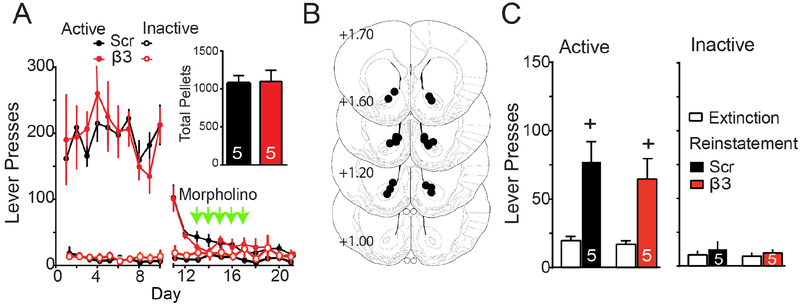
We next determined if β3 integrin signaling in NAcore was selective for regulating drug seeking or was also necessary for cued reinstatement of seeking a biological reward. Rats were trained to self-administer sucrose pellets, and active lever pressing extinguished in combination with β3 or Scr morpholino microinjections (Figure 1D). The β3 and Scr groups demonstrated equivalent amounts of active lever pressing during self-administration and extinction training, and consumed an equal number of pellets over the training period (Figure 2A). All microinjection cannula were bilaterally targeted into NAcore (Figure 2B). Sucrose-trained rats showed robust cue-induced reinstatement of active lever pressing, and there was no difference between rats pretreated with β3 or Scr morpholino (Figure 2C). These data reveal that in NAcore, β3 integrin subunits are recruited to mediate cue-induced cocaine, but not sucrose seeking.
Figure 2: Morpholino knockdown of the β3 integrin subunit did not alter cued sucrose seeking.
(A) Time course of active lever pressing for self-administration and extinction in sucrose-trained rats was equivalent between treatment groups. Inset shows equivalent pellets taken by each treatment group. (B) Location of microinjection cannula tips in the NAcore corresponding to the data shown in Figure 2. (C) Sucrose-trained rats show cue-induced reinstatement and no difference between treatment groups (EXT/RST F(1,8)=22.95, p<0.001; Scr/β3 F(1,8)=0.51, p=0.494; interaction F(1,8)=0.20, p=0.667). Data shown as mean ± SEM, and N is shown within the bars.
+p<0.05 compared to EXT lever presses using a Bonferroni’s post hoc test.
β3 integrin knockdown blocked cue-induced t-SP: AMPA/NMDA ratio.
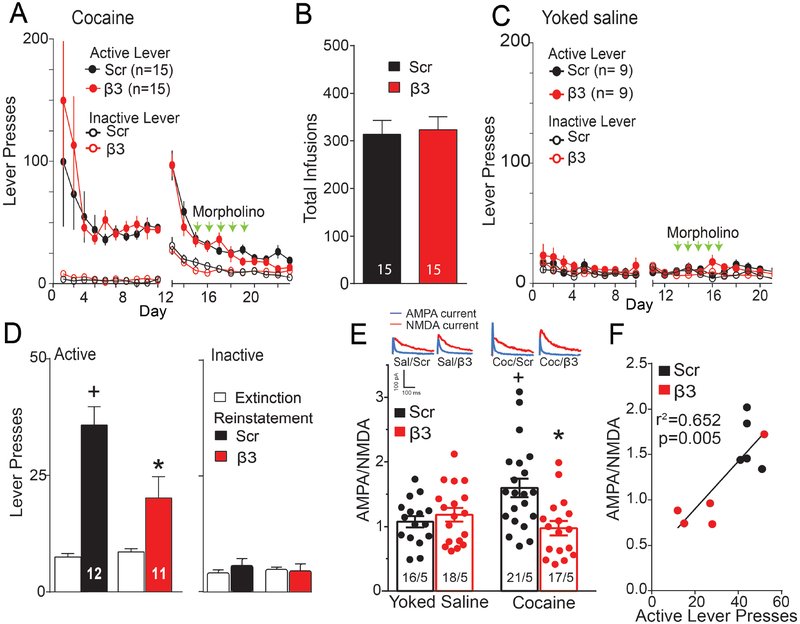
Fifteen min after initiating cue-induced drug seeking for a variety of addictive drugs, the AMPA/NMDA (A/N) ratio in NAcore MSNs is elevated and returns to baseline levels within 120 min(4). The increase in A/N is positively correlated with the number of cue-induced active lever presses and depends on activation of MMP-9(5, 8). To determine if cued increases in A/N result from stimulating β3 integrin, rats were trained to self-administer cocaine, extinguished, and morpholino injections made into NAcore during extinction training (Figure 1D). Rats assigned to Scr or β3 integrin groups showed equal active lever pressing and drug infusions during cocaine self-administration, yoked-saline and extinction training (Figure 3A–C). NAcore slices were obtained 24 hr after the last extinction session for yoked-saline and cocaine extinction groups, or 15-min after beginning a cued reinstatement trial. Cue-induced active lever pressing over the 15 min prior to making tissue slices was reduced in the β3 morpholino compared to the Scr treatment group without altering inactive lever presses (Figure 3D). Figure 3A–D shows behavior for rats that were then randomly assigned to quantify A/N (Figure 3) or spine morphology (Figure 4). The reinstated lever pressing in Figure 3D was measured for 15 min compared to Figure 1F where behavior was recorded for 120 min. Whole cell patch clamp recordings were made from MSNs and revealed increased A/N during 15 min of cue-induced reinstatement in Scr treated rats compared with yoked-saline controls. The cued increase in A/N was abolished in reinstated rats pretreated with β3 morpholino (Figure 3E). Supporting a relationship between β3 integrin knockdown and A/N in regulating cued reinstatement, increased A/N was correlated with the number of reinstated lever presses (Figure 3F).
Figure 3: β3 Integrin knockdown prevented synaptic potentiation initiated by cued cocaine seeking.
(A) Time course of active and inactive lever pressing for self-administration and extinction in cocaine-treated rats used for A/N (Figure 3) and spine morphology measurement (Figure 4) was equivalent between treatment groups. (B) An equivalent number of infusions taken by each treatment group. (C) Time course of active and inactive lever pressing for yoked saline and extinction in rats used for A/N and spine morphology measurement (D) Cue-induced reinstatement of cocaine seeking increased lever presses over 15 min prior to euthanizing rats for morphological measurements and A/N measurements, and this was reduced by β3 integrin morpholino (EXT/RST F(1,21)=48.81, p<0.001; Scr/β3 F(1,21)=4.78, p=0.040, interaction F(1,21)=8.66, p=0.008). (E) Two-way ANOVA revealed elevated A/N during 15 min cue-induced reinstatement in Scr morpholino treated rats compared to β3 morpholino and yoked saline animals (Scr/β3 F(1,68)=4.647, p<0.05; Sal/Coc F(1,68)=1.743, p=0.191; interaction F(1,68)=9.297, p=0.010). N in bars represents number of cells recorded over number of animals in each condition. Representative traces are shown for each treatment. Data are shown as mean ± SEM. (F) The A/N measured after 15 min of cued reinstatement was correlated with the number of active lever presses.
*p< 0.05 comparing to Scr/RST using a Bonferroni post hoc.
+p< 0.05, comparing to EXT or Scr/yoked saline.
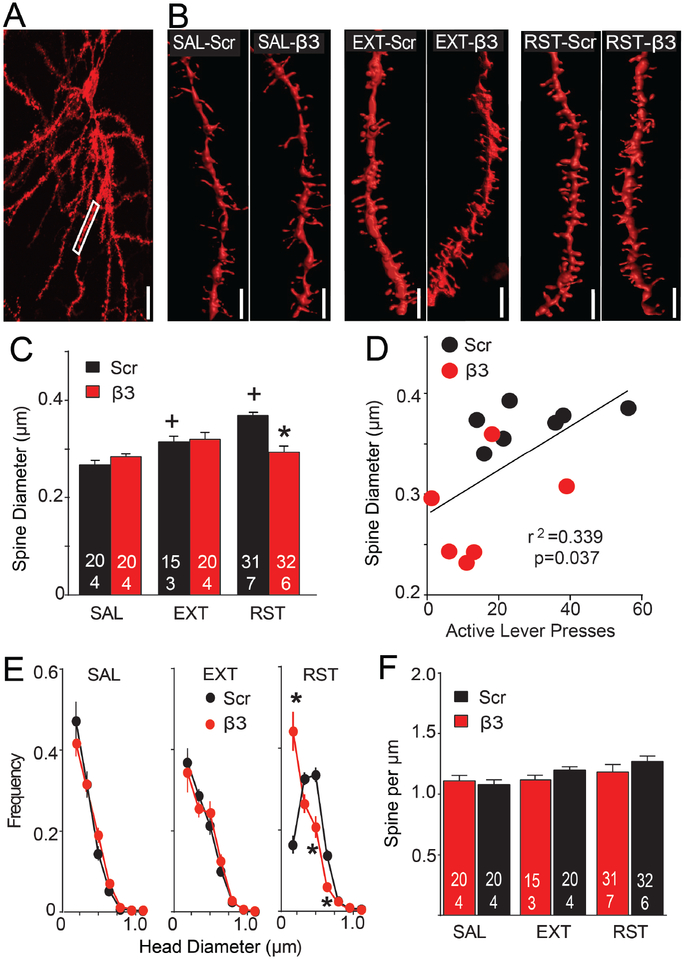
Figure 4: β3 Integrin knockdown blocked increases in dendritic spine head diameter (dh) during cue-induced cocaine seeking.
(A) Representative DiI filled MSN. Box shows area sampled from this neuron for spine analysis. Bar= 20 μm. (B) Representative dendritic segments from each treatment group. Bar= 2 μm. (C) Spine dh was increased during 15 min reinstatement and β3 morpholino treatment inhibited the increase in dh. Nested ANOVA revealed an overall interaction between groups for spine dh (F(5,22)=6.68, p<0.001). (D) Correlation between spine dh and active lever presses during 15 min cue-induced reinstatement. (E) The decreased dh after β3 treatment was associated with a shift in the frequency distribution compared to Scr treatment only in reinstated animals (2-way ANOVA with repeated measures over dh, Scr/β3 F(1,61)=0.26, p=0.612; dh F(6,366)=95.58, p<0.001; interaction F(6,366)=20.61, p<0.001). N in bars represents number of neurons quantified over number of animals in each condition. Three to 7 dendrite segments from separate neurons were analyzed from each animal. (F) No change was measured in spine density between any of the treatment groups. Data are shown as mean ± SEM.
*p<0.05 comparing to Scr/RST using Bonferroni post hoc.
+p<0.05 compared to Scr/SAL in each morpholino group.
β3 integrin knockdown blocked cue-induced t-SP: Spine morphology.
Self-administration, extinction and cued reinstatement data are shown in Figure 3 together with rats used for measuring A/N. To quantify spine density and dh, Z-series confocal images were made of diolistically labeled neurons in the NAcore (Figures 4A/S1). Cued cocaine seeking increased dh in the reinstated (RST) compared to the extinguished (EXT) or yoked saline (SAL) rats that were pretreated with Scr morpholino, and the increase in dh during reinstatement was abolished in rats pretreated with β3 morpholino (Figure 4B,C). Supporting a relationship between β3 integrin knockdown and dh in regulating cued reinstatement, reinstated lever pressing was positively correlated with spine dh (Figure 4D). Analysis of dh frequency distribution revealed no effect of β3 morpholino in yoked-saline or extinguished rats. However, the decrease in thin spines (dh<0.20 μm) and increase in thick spines (0.35>dh<0.65 μm) elicited during cued reinstatement in rats pretreated with Scr control was eliminated after β3 morpholino, resulting in a dh frequency distribution equivalent to yoked-saline and extinguished rats (Figure 4E). No difference in spine density was found between any of the treatment groups (Figure 4F).
Inhibiting FAK phosphorylation prevented cue-induced drug seeking.
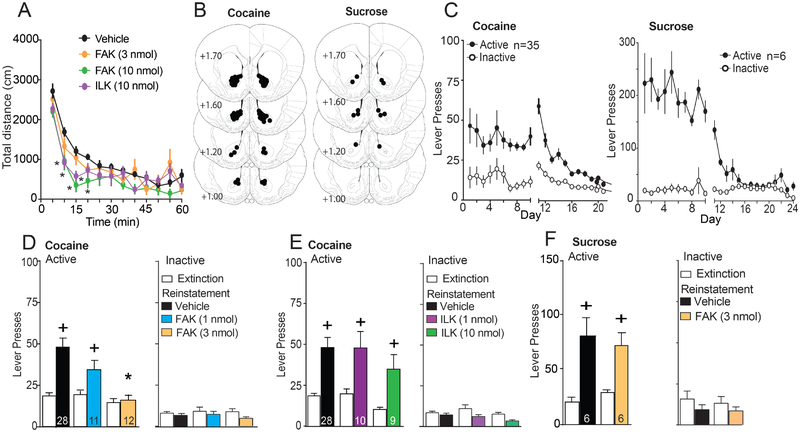
Two kinases mediating β3 integrin signaling are FAK and ILK. The phosphorylation of FAK on Tyr397(31) or ILK at Ser473(32) activates the kinases, and enzyme selective small molecule inhibitors of phosphorylation were used to determine which kinase mediated the capacity of β3 integrin to initiate t-SP and reinstate cocaine seeking. An initial study conducted in drug-naïve rats determined that bilateral microinjection of a FAK (FAKi) or ILK inhibitor (ILKi) into NAcore reduced locomotor activity at the highest dose tested (10 nmol/side) (Figure 5A). Accordingly, lower doses were evaluated for effects on cued drug seeking to avoid motor depression. Rats were implanted with microinjection cannulae over the NAcore (Figure 5B and S4B for heroin) and trained to self-administer cocaine, sucrose or heroin and extinguished (Figure 5C and S4A). Using a randomized cross-over design between vehicle and FAKi treatment, NAcore microinjection of FAKi (1 or 3 nmol/side) 10 min before initiating cued reinstatement dose-dependently inhibited active lever pressing in cocaine-trained rats (Figure 5D), without altering inactive lever responding (Figure 5D) or locomotor activity in an open field (Figure 5A). The specificity of FAK involvement in cued drug seeking was further demonstrated by showing that FAKi (3 nmol/side) microinjection also reduced cued heroin seeking, but not cue-induced reinstatement of sucrose seeking (Figure 5G/S2C).
Figure 5: Inhibiting FAK, but not ILK, prevented cue-induced cocaine and heroin, but not sucrose seeking.
(A) Novel open field locomotor activity revealed decreased activity after the highest doses FAKi or ILKi (10 nmol) microinjected 10 min before the test, compared to vehicle (two-way ANOVA repeated measures over time, inhibitor F(3,21)=4.27, p=0.017; time F(11,231)=44.43, p<0.001; interaction F(33,231)=4.61, p=0.017). Intra-NAcore FAKi (3 nmol) microinjection did not alter locomotor activity. (B) Histological verification of NAcore guide cannula for cocaine and sucrose-seeking experiments. Circles drawn on the modified stereotaxic atlas indicate the location of the tip of the microinjector (2 mm below the end of the guide cannula). Numbers refer to mm from bregma. (C) Time course of active and inactive lever pressing for self-administration and extinction in cocaine and sucrose-treated rats. (D) FAKi bilateral microinjection into NAcore prevented cue-induced cocaine seeking (2-way ANOVA, repeated measures over extinction/reinstatement, dose F(2,48)=8.60, p<0.001; EXT/RST F(1,48)=16.57, p<0.001; interaction F(2,48)=5.54, p=0.007). No statistical difference was observed in inactive lever presses during cued-induced cocaine-seeking after VEH or FAKi microinjections in NAcore (left panel). (E) ILKi microinjection in NAcore did not prevent cocaine seeking (Dose F(2,44)=1.93, p=0.16; EXT/RST F(1,44)=30.50, p<0.001; interaction F(2,44)=0.08, p=0.92). No statistical difference was observed in inactive lever presses during cued-induced cocaine-seeking after VEH or ILKi microinjections in NAcore (left panel). (F) FAKi (3 nmol) microinjection in NAcore did not prevent the cue-induced reinstatement in sucrose-trained rats (VEH/FAK F(1,10)=0.01, p=0.99; EXT/RST F(1,10)=34.96, p<0.001; interaction F(1,10)=1.049, p=0.33). No statistical difference was observed in inactive lever presses during cued-induced cocaine-seeking after VEH or FAKi microinjections in NAcore (left panel). Rats were reinstated 2–3 times each in random order with VEH and one or two doses of inhibitor. N in bars represents the number of animals in each condition. Data are shown as mean ± SEM.
+p<0.05 compared to EXT using a Bonferroni’s post hoc test
*p<0.05 compared to Vehicle using a Bonferroni’s post hoc test.
Bilateral microinjection of ILKi into NAcore 10 min before cued-induced cocaine reinstatement did not alter reinstated cocaine seeking compared to vehicle injection (Figure 5E), even at a dose (10 nmol) that decreased locomotor activity (Figure 5A). Inactive lever pressing was not altered by any dose of ILKi (Figure 5E). Taken together, these data are consistent with FAK, not ILK signaling being downstream from MMP-9 and β3 integrin in mediating cue-induced reinstatement of cocaine and heroin, but not sucrose seeking.
MMP activity potentiated cue-induced reinstatement by signaling through β3 integrin.
Drug cues increase MMP-9 activity in NAcore and MMP-9 inhibition prevents both t-SP and cued reinstatement(8). MMP-9 is a gelatinase that catalytically liberates arginine-glycine-aspartate (RGD) peptide sequences from brain ECM proteins, and RGD is a high affinity integrin binding motif(13). To further link MMP-9 activity with β3 integrin signaling, we used tissue plasminogen activator (tPA) to activate MMP-9(23, 24). Gelatinase (MMP-2,9) activity was quantified using in vivo zymography after FITC-quenched gelatin microinjection into NAcore in drug-naïve animals. tPA produced dose-dependent increases in fluorescence that were abolished by intra-NAcore pretreatment with an MMP-9 inhibitor (Figure S5). Rats were trained to self-administer cocaine and extinguished (Figure 6A). The lowest dose of tPA (1 ng) that increased gelatinase activity was microinjected bilaterally into NAcore (Figure 6B). tPA microinjection potentiated cue-induced cocaine seeking compared to vehicle and the potentiated response was inhibited when rats were pretreated with β3 integrin morpholino or FAKi (Figure 6C). The partial inhibition by β3 morpholino may arise from tPA producing effects in addition to activating the MMP9-b3-FAK signaling cascade (23, 24) or because morpholino treatment did not abolish protein expression (Figure 1B/C). There was no effect of tPA, β3 morpholino or FAKi on inactive lever responding (Figure 6C).
Figure 6: Stimulating MMPs with tissue plasminogen activator (tPA) or integrins with RGD peptide potentiated cue-induced cocaine seeking.
(A) Time course of active and inactive lever pressing for self-administration and extinction in cocaine-treated rats for tPA experiment. (B) Location of microinjection cannula tips in the NAcore for all rats used in figure 6C. Numbers refer to mm to bregma. (C) tPA bilateral microinjection into NAcore potentiate cue-induced cocaine seeking (2-way ANOVA, repeated measures over extinction/reinstatement, Veh/tPA/β3/FAKi F(3,22)=10.05, p<0.001; EXT/RST F(1,22)=40.66, p<0.001; interaction F(3,22)=6.99, p=0.002) and β3 Integrin or FAKi microinjections in NAcore prevented tPA induced potentiation in cued-cocaine seeking. No statistical difference was observed in inactive lever presses during cued-induced cocaine-seeking after Veh, tPA, β3 or FAKi microinjections in NAcore. (D) Time course of active and inactive lever pressing for self-administration and extinction in cocaine-treated rats for RAD/RGD experiment. (E) Location of microinjection cannula tips in the NAcore for rats used in figure 6F. (F) RGD (10 nmol) microinjection in NAcore potentiate cue-induced reinstatement in cocaine trained rats (RAD/RGD F(1,18)=4.52, p=0.047; EXT/RST F(1,18)=47.31, p<0.001; interaction F(1,18)=4.81, p=0.042). No statistical difference was observed in inactive lever presses during cued-induced cocaine-seeking after RAD or RGD microinjections in NAcore.
+p<0.05 compared to EXT using a Bonferroni’s post hoc test
*p<0.05 compared to VEH or RAD using a Bonferroni’s post hoc test
#p<0.05 compared to tPA treatment in panel C using a Bonferroni’s post hoc test
We also modulated β3 integrin directly using the RGD peptide integrin ligand that is liberated by MMP-2,9-mediate catalysis, and produces LTP-like changes in EPSCs and spine morphology(33–36). After cocaine self-administration and extinction (Figure 6D) rats were randomly assigned to a cross-over design for cue-induced reinstatement testing. Animals were microinjected 10 min before the reinstatement session with either RAD peptide control or RGD into the NAcore (Figure 6E). RGD (1 nmol) potentiated cue-induced cocaine seeking compared to RAD microinjections without altering inactive lever responding (Figure 6F). This dose of RGD does not affect locomotor activity(17).
Discussion
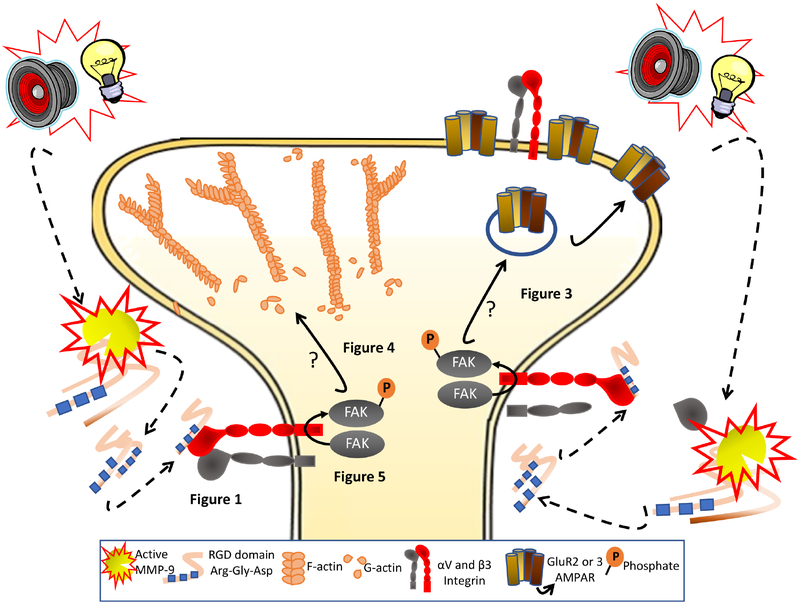
The capacity of cues associated with addictive drug use to induce drug seeking contributes to relapse vulnerability (1). Cue-induced drug seeking in animal models of relapse causes t-SP in NAcore MSNs that is correlated with the intensity of behavioral responding for the cue(5, 8), and is produced only by addictive drug associated cues, not sucrose cues(4). These discoveries point to the importance of understanding how signaling in NAcore initiated by cues is translated into t-SP. Activation of extracellular enzyme MMP-9 is initiated by synaptic glutamate spillover during cued cocaine seeking and is necessary for t-SP(8). Here we show that MMP-9 signaling through β3 integrin to FAK is required for t-SP and cue-induced cocaine and heroin seeking, but not sucrose seeking. The proposed signaling cascade between MMP-9 activation by drug cues and t-SP is illustrated in Figure 7.
Figure 7. Schematic illustration of the MMP-9 to β3 integrin to FAK signaling pathway in NAcore that is proposed to mediate increases in t-SP and is necessary for cue-induced cocaine seeking.
Drug-paired cues (tone and light) increase MMP-9 activity through spillover of synaptic glutamate and increased nitric oxide production (not shown, see(4, 50)). MMP-9 catalytically exposes RGD binding domains during 15 min of cued cocaine seeking. RGD binds to and stimulates β3 integrin subunit to phosphorylate FAK, which initiates spine head expansion (dh) and increases in AMPA:NMDA. How FAK signals to t-SP was not studied (indicate by ?), but FAK promotes LIMK phosphorylation(51) to inactive cofilin via Ser3 phosphorylation and thereby promote elongation of F-actin to increase spine dh. Also β3 integrin-dependent increases in AMPA currents may arise from stabilizing GluA2 subunit containing AMPA receptors through a protein complex between β3 and GluA2 (19).
β1 and β3 Integrins in Synaptic Plasticity and Addiction.
Heterodimeric integrins are mediators of MMP-catalyzed signaling in the ECM, and integrin binding induces both morphological and electrophysiological plasticity at excitatory synapses(37). The two primary β integrin subunits in brain, β1 and β3 integrin, determine intracellular signaling pathways, while α subunits determine extracellular binding partners. The β3 subunit exclusively pairs with the αV subunit, while the β1 subunit pairs promiscuously with different α subunits including αV(38). Activated MMP-9 catalytically creates RGD-containing peptide ligands that are selective for integrins containing the αV subunit(39). Many studies show β1 integrin stimulation regulates spine and dendrite morphology(20, 40) and NMDA or AMPA plasticity(37, 41–43). Moreover, MMP-9 activity can signal through β1 integrin to modulate spine morphology; although the primary effect is a decrease in dh and spine density(44), not the increase observed during cue-induced t-SP. β1 integrins have been linked to the effects of addictive drugs in three preclinical studies. β1 integrin is necessary for the enduring increases in accumbens spine density produced by chronic noncontingent methamphetamine administration(45), knock-down of β1 integrin signaling augments cocaine-induced locomotion(40), and withdrawal from chronic noncontingent cocaine is associated with elevated β1 integrin in the accumbens(16). Given this literature, we were surprised that knocking down β3, not β1 integrin, prevented cue-induced reinstatement of cocaine seeking and the associated t-SP in NAcore MSNs. In fact, consistent with augmenting cocaine locomotion(40), knock-down of β1 integrin in NAcore potentiated cued reinstatement; possibly resulting from compensatory upregulation of the β3 subunit and ILK.
Finding obligatory involvement of β3 integrin in cue-induced t-SP and cocaine seeking is consistent with studies showing that like β1, β3 integrins modulate some forms of synaptic plasticity. For example, modulating β3 integrin levels affects spine maturation and homeostatic synaptic scaling, as well as GluA2 AMPAR expression in the hippocampus(19, 34, 46). The role of β3 over β1 integrins in cue-induced t-SP could be because the literature is largely derived from experimentation in hippocampus, and perhaps β3 integrins are more preferentially involved in NAcore synaptic plasticity. Given the relative abundance of β1 over β3 protein in NAcore(16), this seems unlikely. An intriguing possibility is that β1 integrins signal the induction of more enduring forms of synaptic plasticity, while β3 integrins are more involved in transient forms of plasticity, such as the cue-induced t-SP mediating cocaine seeking(37, 46). For example, rats trained to self-administer cocaine show transient fluctuations in β3, not β1 integrin, during reinstated cocaine seeking(17). Also, β3 integrin is involved in regulating homeostatic synaptic scaling, while β1 is more frequently associated with LTP(46). Finally, β1 integrins are involved in enduring hippocampal plasticity and memory consolidation, while β3 integrins regulate transient acute stress responses(37, 47).
β3 signaling through FAK, but not ILK, in t-SP and reinstated drug seeking
β3 integrin signaling through either ILK or FAK can link integrin signaling to the actin cytoskeleton and modulate synaptic plasticity(21, 32). In contrast to the selective role for FAK in β3 integrin signaling cocaine reinstatement, cocaine behavioral sensitization studies implicate ILK in both augmented locomotion and increases in NAcore spine density after withdrawal from daily noncontingent injections of cocaine(48, 49). The differences in kinase involvement may arise from using distinct models of addiction (sensitization versus cued drug seeking). This is consistent with the hypothesis above that the β3-FAK cascade signals transient plasticity such as t-SP induced by drug cues, while the β1-ILK cascade signals enduring plasticity such as the increased spine density produced by daily cocaine injections. This can also account for the lack of effect by β3 integrin knockdown on spine morphology or A/N in yoked-saline rats, since the knock-down is long-lasting over the course of days, rather than the effect of cue on behavior which typically endures for 60 min.
Conclusions.
We found that t-SP and cue-induced cocaine seeking requires MMP-9 signaling into MSNs via the β3-FAK signaling cascade. Drug associated cues can promote relapse to drug use in human addiction. Identifying β3 integrin to FAK signaling as a necessary component of the cascade that mediates cue-induced cocaine and heroin, but not sucrose seeking, provides new potential pharmacological targets for treating addiction.
Supplementary Material
Acknowledgments and contributions
We thank Madhura Athreya for advice and technical assistance. Also, the authors would like to thank Dr. Heather A. Boger for allowing us to perform the rat locomotor measurement using her equipment. This work was supported by the National Institute of Health NIH DA003906, DA12513 and DA015369 (PWK) and DoD UDAK 8B705 (PWK). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Volkow ND, Wang GJ, Fowler JS, Tomasi D (2012): Addiction circuitry in the human brain. Annual review of pharmacology and toxicology. 52:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luscher C, Malenka RC (2011): Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 69:650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein RZ, Volkow ND (2011): Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. (2016): The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev. 68:816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW (2013): Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 77:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulholland PJ, Chandler LJ, Kalivas PW (2016): Signals from the Fourth Dimension Regulate Drug Relapse. Trends Neurosci. 39:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsilibary E, Tzinia A, Radenovic L, Stamenkovic V, Lebitko T, Mucha M, et al. (2014): Neural ECM proteases in learning and synaptic plasticity. Prog Brain Res. 214:135–157. [DOI] [PubMed] [Google Scholar]
- 8.Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, et al. (2014): Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 17:1655–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senkov O, Andjus P, Radenovic L, Soriano E, Dityatev A (2014): Neural ECM molecules in synaptic plasticity, learning, and memory. Prog Brain Res. 214:53–80. [DOI] [PubMed] [Google Scholar]
- 10.Huntley GW (2012): Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 13:743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, et al. (2006): Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 96:1227–1241. [DOI] [PubMed] [Google Scholar]
- 12.Nagy V, Bozdagi O, Huntley GW (2007): The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn Mem. 14:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barczyk M, Carracedo S, Gullberg D (2010): Integrins. Cell and tissue research. 339:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo BH, Carman CV, Springer TA (2007): Structural basis of integrin regulation and signaling. Annu Rev Immunol. 25:619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynes RO (2002): Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. [DOI] [PubMed] [Google Scholar]
- 16.Wiggins AT, Pacchioni AM, Kalivas PW (2009): Integrin expression is altered after acute and chronic cocaine. Neurosci Lett. 450:321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiggins A, Smith RJ, Shen HW, Kalivas PW (2011): Integrins modulate relapse to cocaine-seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 31:16177–16184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babayan AH, Kramar EA, Barrett RM, Jafari M, Haettig J, Chen LY, et al. (2012): Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation. J Neurosci. 32:12854–12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozo K, Cingolani LA, Bassani S, Laurent F, Passafaro M, Goda Y (2012): beta3 integrin interacts directly with GluA2 AMPA receptor subunit and regulates AMPA receptor expression in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramar EA, Lin B, Rex CS, Gall CM, Lynch G (2006): Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci U S A. 103:5579–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouvard D, Pouwels J, De Franceschi N, Ivaska J (2013): Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 14:430–442. [DOI] [PubMed] [Google Scholar]
- 22.Wehrle-Haller B (2012): Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol. 24:569–581. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, et al. (2005): Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 36:1954–1959. [DOI] [PubMed] [Google Scholar]
- 24.Golab P, Kielbus M, Bielewicz J, Kurzepa J (2015): The effect of recombinant tissue plasminogen activator on MMP-2 and MMP-9 activities in vitro. Neurol Res. 37:9–13. [DOI] [PubMed] [Google Scholar]
- 25.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW (2011): Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proceedings of the National Academy of Sciences of the United States of America. 108:19407–19412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palombi O, Shin JW, Watson C, Paxinos G (2006): Neuroanatomical affiliation visualization-interface system. Neuroinformatics. 4:299–317. [DOI] [PubMed] [Google Scholar]
- 27.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW (2009): Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 29:2876–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reissner KJ, Sartor GC, Vazey EM, Dunn TE, Aston-Jones G, Kalivas PW (2012): Use of vivo-morpholinos for control of protein expression in the adult rat brain. Journal of Neuroscience Methods. 203:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW (2015): Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 20:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora V, Devi GR, Iversen PL (2004): Neutrally charged phosphorodiamidate morpholino antisense oligomers: uptake, efficacy and pharmacokinetics. Curr Pharm Biotechnol. 5:431–439. [DOI] [PubMed] [Google Scholar]
- 31.Mitra SK, Hanson DA, Schlaepfer DD (2005): Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 6:56–68. [DOI] [PubMed] [Google Scholar]
- 32.Ghatak S, Morgner J, Wickstrom SA (2013): ILK: a pseudokinase with a unique function in the integrin-actin linkage. Biochem Soc Trans. 41:995–1001. [DOI] [PubMed] [Google Scholar]
- 33.Kramar EA, Bernard JA, Gall CM, Lynch G (2003): Integrins modulate fast excitatory transmission at hippocampal synapses. J Biol Chem. 278:10722–10730. [DOI] [PubMed] [Google Scholar]
- 34.Cingolani LA, Goda Y (2008): Differential involvement of beta3 integrin in pre- and postsynaptic forms of adaptation to chronic activity deprivation. Neuron Glia Biol. 4:179–187. [DOI] [PubMed] [Google Scholar]
- 35.Bourgin C, Murai KK, Richter M, Pasquale EB (2007): The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 178:1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Ethell IM (2006): Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 26:1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YK, Goda Y (2016): Integrins in synapse regulation. Nat Rev Neurosci. 17:745–756. [DOI] [PubMed] [Google Scholar]
- 38.Webb DJ, Zhang H, Majumdar D, Horwitz AF (2007): alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J Biol Chem. 282:6929–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright JW, Harding JW (2009): Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural plasticity. 2009:579382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, et al. (2012): Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J Neurosci. 32:2824–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L (2009): Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 29:6007–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL (2006): Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci. 26:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lonskaya I, Partridge J, Lalchandani RR, Chung A, Lee T, Vicini S, et al. (2013): Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1. PLoS One. 8:e69136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, et al. (2011): Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. Journal of cell science. 124:3369–3380. [DOI] [PubMed] [Google Scholar]
- 45.Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J, Maguire-Zeiss K, et al. (2011): Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. Journal of Neurochemistry. 118:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, et al. (2008): Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 58:749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGeachie AB, Skrzypiec AE, Cingolani LA, Letellier M, Pawlak R, Goda Y (2012): beta3 integrin is dispensable for conditioned fear and hebbian forms of plasticity in the hippocampus. Eur J Neurosci. 36:2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q, Xiong X, Lee TH, Liu Y, Wetsel WC, Zhang X (2008): Neural plasticity and addiction: integrin-linked kinase and cocaine behavioral sensitization. J Neurochem. 107:679–689. [DOI] [PubMed] [Google Scholar]
- 49.Chen Q, Zhu X, Zhang Y, Wetsel WC, Lee TH, Zhang X (2010): Integrin-linked kinase is involved in cocaine sensitization by regulating PSD-95 and synapsin I expression and GluR1 Ser845 phosphorylation. Journal of molecular neuroscience: MN. 40:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, et al. (2017): Accumbens nNOS Interneurons Regulate Cocaine Relapse. J Neurosci. 37:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM (2009): Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 29:8129–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.