Abstract
There is evidence for enlargement of association cortex in humans compared to other primate species. Expansion of temporal association cortex appears to have displaced extrastriate cortex posteriorly and inferiorly in humans compared to macaques. However, the details of the organization of these recently expanded areas are still being uncovered. Here, we used diffusion tractography to examine the organization of extrastriate and temporal association cortex in chimpanzees, humans, and macaques. Our goal was to characterize the organization of visual and auditory association areas with respect to their corresponding primary areas (primary visual cortex and auditory core) in humans and chimpanzees. We report three results: (1) Humans, chimpanzees, and macaques show expected retinotopic organization of primary visual cortex (V1) connectivity to V2 and to areas immediately anterior to V2; (2) In contrast to macaques, chimpanzee and human V1 shows apparent connectivity with lateral, inferior, and anterior temporal regions, beyond the retinotopically organized extrastriate areas; (3) Also in contrast to macaques, chimpanzee and human auditory core shows apparent connectivity with temporal association areas, with some important differences between humans and chimpanzees. Diffusion tractography reconstructs diffusion patterns that reflect white matter organization, but does not definitively represent direct anatomical connectivity. Therefore, it is important to recognize that our findings are suggestive of species differences in long-distance white matter organization rather than demonstrations of direct connections. Our data support the conclusion that expansion of temporal association cortex, and the resulting posterior displacement of extrastriate cortex, occurred in the human lineage after its separation from the chimpanzee lineage. It is possible, however, that some expansion of the temporal lobe occurred prior to the separation of humans and chimpanzees, reflected in the reorganization of long white matter tracts in the temporal lobe that connect occipital areas to the fusiform gyrus, middle temporal gyrus, and anterior temporal lobe.
Keywords: Visual Cortex, Evolution, Auditory Cortex, Diffusion, Tractography
Introduction
Human brain expansion has not been uniform across neocortical regions: association areas have expanded relatively more than non-association areas (reviewed in Schoenemann 2006; Preuss 2017a; Rilling 2006; Schoenemann 2006; Mars et al. 2017; Hrvoj-Mihic et al. 2013). However, the organizational changes that have accompanied these expansions are still being characterized. Detailed information about chimpanzee cortical neuroanatomy is critical for resolving this and many other issues about human brain evolution because chimpanzees, along with bonobos, are the closest relatives of humans, and by definition, specializations of the human brain are features that evolved in our lineage after the separation of the human and chimpanzee lineages (fig. 1). At present, however, we know very little about chimpanzee cortical organization: almost all studies of great ape neuroanatomy that yielded maps of cortical regions predate the modern era of neuroscience (Campbell 1905; Mauss 1911; Brodmann 1912; Walker 1938; Bailey et al. 1950) and lack the degree of detail needed to rigorously compare chimpanzees to humans and to other nonhuman primates. The paucity of research on chimpanzees and other great apes in subsequent years reflects in part the lack of availability of captive populations for research compared to more widely used model animals, and the fact that great apes have been effectively off-limits for research using the invasive tract-tracing and microelectrode mapping studies that proved so useful for delineating cortical areas and systems of connectivity in macaques and other Old World and New World monkeys (Preuss 2010).
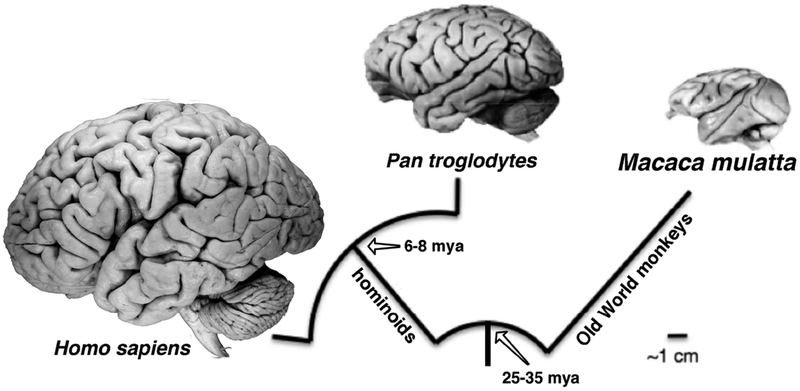
Figure 1.
Evolutionary relationships and divergence times among the three primate species examined here. Chimpanzees (Pan troglodytes), along with bonobos, are the closest living relatives of humans (Homo sapiens); both belong to the hominoid group. Rhesus macaques (Macaca mulatta) belong to the Old World monkey group, which is the sister group of the hominoids. Divergence dates are from (Steiper and Seiffert 2012), (Finstermeier et al. 2013), (Perelman et al. 2011), and (Wilkinson et al. 2011). Brain photographs are from the Comparative Mammalian Brain Collections website (www.brainmuseum.org).
Recent developments in neuroimaging, including structural magnetic resonance imaging (MRI) and diffusion-weighted imaging (DWI), provide new tools for comparing cortical organization between humans, chimpanzees, macaques, and other nonhuman primate species because they are non-invasive (Rilling 2008; Preuss 2017a) and easy to measure (Behrens, 2003). To date, published studies include comparisons of cortical morphometry (e.g., Hopkins et al. 1998; Rilling and Insel 1999; Bruner et al. 2017), connectivity (Rilling et al. 2008b; Hecht et al. 2015; Li et al. 2013), and myeloarchitecture (Glasser et al. 2014). Currently, however, it is impractical to carry out functional MRI studies in chimpanzees (Hecht et al. 2013). Here, we apply neuroimaging methods to address the question of association cortex expansion in the human lineage.
Recent macaque-human comparisons with DWI have been carried out in the parietal lobe (Catani et al. 2017) and frontal lobe (Neubert et al. 2015). Here, to investigate the pattern of association cortex expansion in humans, we focused on the relationship between extrastriate cortex and temporal association cortex, as there is a rich body of data on visual cortical organization in macaques and the rapidly expanding dataset for humans. Comparison of temporal lobe morphology suggests that chimpanzees are more like humans than macaques (fig. 2). For one, macaques possess only a single, well-developed temporal sulcus, whereas humans and chimpanzees possess both an inferior and a superior temporal sulcus. Macaques also lack a discrete fusiform gyrus, which is found on the ventral surface of the temporal lobe in chimpanzees and humans.
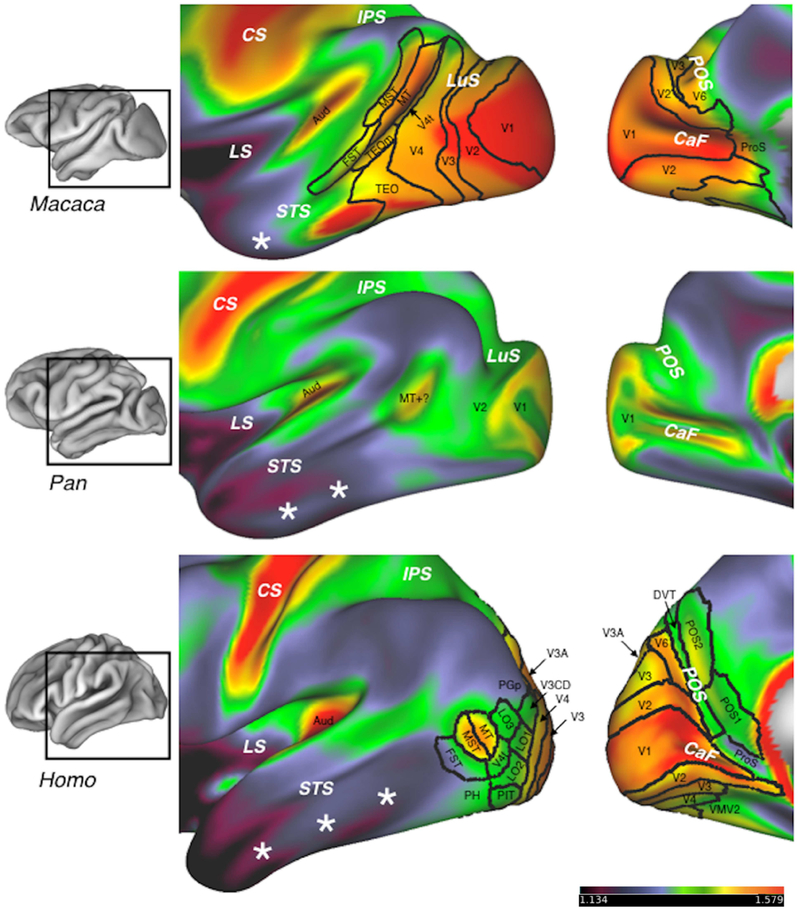
Figure 2.
Myeloarchitecture and areal organization of occipital and temporal cortex in macaques (Macaca), chimpanzees (Pan), and humans (Homo). Color maps show the myeloarchitecture of the lateral (middle column) and medial (right column) surfaces of the left hemisphere in the HCP Workbench inflated view, with the sulci partly opened. The figures in the left column show the approximate locations the enlarged regions without inflation (Workbench’s pial view). Hotter colors represent regions of dense myelination, while cool colors represent lightly myelinated regions. Territories demarcated with black lines are areas recognized in recent studies of macaques (Markov et al. 2014) and humans (Matthew F. Glasser et al. 2016). There are no modern studies of chimpanzee visual areas, although the primary visual area, V1, can be identified with reasonable confidence in myeloarchitecture, and V1 and the second visual area, V2, were described in classical cytoarchitectonic studies (Bailey et al. 1950). Chimpanzees also possess a small territory of relatively dense myelination in the posterior STS that may correspond to the MT complex (MT+) of humans and macaques (Glasser et al. 2014). Note that the relative proportions of lightly myelinated cortex in temporal cortex, denoted with white stars, increase dramatically from macaques to chimpanzees to humans. In humans, this increase is accompanied by a posterior and inferior displacement of the extrastriate cortex, so that the MT complex (areas MT, MST, and FST) is located posterior to the STS, and very little of area V1 extends onto the lateral surface of the occipital lobe. In addition, humans lack a deep lunate sulcus (LuS), unlike chimpanzees and macaques. Abbreviations: Aud - auditory core; CaF - calcarine fissure; CS - central sulcus; DVT - dorsal visual transitional cortex; FST - area of the fundus of the STS; LO1, LO2, LO3 - lateral occipital areas 1, 2, and 3; LS - lateral sulcus; MT - middle temporal visual area; MST - medial superior temporal area; PGp - posterior area of the PG complex; PH - area PH; PIT - posterior inferotemporal cortex; POS - parieto-occipital sulcus; POS1, POS2 - parieto-occipital sulcus areas 1 and 2; ProS - prostriate area; TEOm - area TEOm; VMV2 - ventromedial visual area 2. Additional visual areas: V3, V3A, V3CD, V4, V4t.
On the other hand, MR (magnetic resonance) morphometric and myeloarchitectonic studies suggest a large increase in temporal association cortex in humans compared to chimpanzees and macaques (see Glasser et al. 2014; Passingham and Smaers 2014; Rilling and Seligman 2002; Donahue et al. 2018 and reviews by Orban 2017; Rilling 2006; Preuss 2017b; Preuss 2011). One indication of this is that in humans, the V1/V2 border lies close to the occipital pole and is not marked by a deep sulcus (e.g., (Benson et al. 2012; Horton and Hoyt 1991; Allen et al. 2006), whereas in chimpanzees, as in macaques and other gyrencephalic monkeys, the primary visual area, V1, extends for a considerable distance onto the lateral surface of the occipital lobe, where it borders area V2 along the posterior rim of the deep lunate sulcus (Mauss 1911; Bailey et al. 1950). In humans, furthermore, the extrastriate visual areas are situated more posteriorly and inferiorly than their counterparts in macaques and other Old World and New World monkeys (Orban 2017). Area MT, for example, which is located in the posterior part of the superior temporal sulcus (STS) in macaques, is located inferior and posterior to the STS in humans, and is not associated with a deep sulcus (Tootell and Taylor 1995; Watson et al. 1993). In chimpanzees, MR myeloarchitecture indicates the presence of a discrete patch of heavily myelinated cortex in the posterior part of STS that could be area MT+ (Glasser et al. 2014). If that is the case, the layout of extrastriate visual areas in chimpanzees probably resembles that of macaques more than humans, and most of the expansion of temporal cortex in humans compared to macaques evolved in the human lineage after it separated from the chimpanzee lineage.
The present study used diffusion tractography to characterize the organization of chimpanzee extrastriate and temporal association cortex, and to compare it to that of humans and macaques. The main aims of this study were, first, to localize extrastriate visual areas in humans and chimpanzees, and second, to characterize the apparent connectivity of primary sensory areas (V1 and auditory core) with temporal association areas. More detailed information on the organization of extrastriate and temporal areas in chimpanzees, in combination with the wealth of data available for humans and macaques, will provide insight into how chimpanzee and human cortical organization changed, and more specifically about the expansion of human temporal association cortex, since our evolutionary divergence from the chimpanzee lineage approximately 6–8 million years ago (fig. 1).
Materials and Methods
Dataset
We examined in vivo T1-weighted and diffusion-weighted MR scans from humans (Homo sapiens; n=10, age range 22–35 yrs), chimpanzees (Pan troglodytes; n=15, 23 ± 12 yrs), and rhesus macaques (Macaca mulatta; n=10, 10 ± 7 yrs). All the individuals were female. Pre-processed human scans were obtained from the Human Connectome Project (HCP) 500 Subjects Release (Jenkinson et al. 2002; Andersson et al. 2003a; Glasser and Van Essen 2011; JAndersson et al. 2012; Fischl 2012; Jenkinson et al. 2012a; Van Essen et al. 2012; Glasser et al. 2013a). Chimpanzee and macaque scans were selected from larger scan sets of both species collected as part of a comparative study on brain aging in females; these scan sets have been used in previous studies (e.g., Chen et al. 2013; Autrey et al. 2014). A subset of cases was selected for tractography analysis from larger sets by K. Bryant based on criteria for high quality data, including strong grey matter/white matter contrast and lack of white matter lesions. All chimpanzees and macaques were housed at the Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia. All procedures were carried out in accordance with protocols approved by the YNPRC and the Emory University Institutional Animal Care and Use Committee (IACUC, approval # YER-2001206).
Nonhuman Primate Brain Imaging
Prior to MR scanning, chimpanzee and macaque subjects were immobilized with ketamine injections (2–6 mg/kg, i.m.), and then anesthetized with an intravenous propofol drip (10 mg/kg/h), following standard YNPRC veterinary procedures. Subjects remained sedated for the duration of the scans as well as the time required for transport between their home cage and the scanner location. Upon scan completion, primates were housed in a single cage for 6–12 h to recover from the effects of anesthesia before being returned to their home cage and cage mates. The well-being (activity and food intake) of the chimpanzees and macaques was evaluated twice daily after the scan for possible post-anesthesia distress by the veterinary and research staff.
For both species, anatomical MRI and DWI scans were acquired in a Siemens 3T Trio scanner (Siemens Medical System, Malvern, PA, USA). Diffusion-weighted MRI data were collected with a single-shot, spin-echo echo-planar imaging (EPI) sequence. A dual spin-echo technique combined with bipolar gradients was used to minimize eddy-current effects. High-resolution T1-weighted MRI images were acquired with a 3D magnetization-prepared rapid gradient-echo (MP-RAGE) sequence for all subjects. For chimpanzee subjects, a standard circularly polarized birdcage coil was used to accommodate the chimpanzee jaw, which is too large to fit into the standard phase-array coil designed for human subjects. In macaques, a standard 8-channel human knee coil was used. In both species, head motion was minimized with foam cushions and elastic straps. For macaques, a specially designed plastic holding device was used to secure subjects’ ear canals.
In chimpanzees, 2 diffusion-weighted images were acquired for each of 60 diffusion directions, each with 1 of the possible left–right phase-encoding directions and 8 averages, allowing for correction of susceptibility-related distortion (Andersson et al. 2003b). For each average of diffusion-weighted images, 6 images without diffusion weighting (b = 0 s/mm2) were also acquired with matching imaging parameters. In macaques, the procedure was similar: diffusion-weighted images were acquired with phase-encoding directions of opposite polarity (left–right), each with 4 averages, and for each average of diffusion-weighted images, 5 images without diffusion weighting (b = 0 s/mm2) were acquired. Detailed imaging parameters of the T1-weighted and diffusion MRI for chimpanzees and macaques are listed in Table 1.
Table 1:
Imaging parameters of the T1-weighted and diffusion MRI for chimpanzees and macaques.
| MPRAGE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | iPAT | Flip angle | Slice thickness (mm) | Voxel size (mm3) | TR/TI/TE (ms) | NEX | Matrix size | FOV | Scan time (min) |
| chimpanzee | 1 | 8 | 0.8 | 0.8×0.8×0.8 | 2600/900/3.06 | 2 | 256 × 256 × 192 | 205 × 205 × 154 | 16 |
| macaque | 2 | 8 | 0.5 | 0.5×0.5×0.5 | 2500/900/3.37 | 3 | 320 × 320 × 176 | 160 × 160 × 88 | 25 |
| Double spin echo diffusion EPI | |||||||||
| Species | iPAT | Partial Fourier | Slices | Voxel size (mm3) | TR/TE (ms) | DWI/total b0s | Matrix size | FOV | Scan time (min) |
| chimpanzee | 1 | 5/8 | 41 | 1.8×1.8×1.8 | 5900/86 | 8/40 | 72×128 | 130×230 | 60 |
| macaque | 3 | N/A | 43 | 1.1×1.1 × 1.1 | 7000/108 | 10/50 | 128×120 | 141×132 | 86 |
Notes: iPAT: GRAPPA parallel imaging factors; TR/TI/TE: repetition time/inversion time/echo time; NEX: number of excitations; FOV: field of view.
Pre-processing
Anatomical and diffusion MR data were analyzed using tools from the FSL software library of the Oxford Center for Functional Magnetic Resonance Imaging of the Brain (www.fmrib.ox.ac.uk/fsl/; (Smith et al. 2004). T1-weighted images were skull-stripped using BET, with some manual correction (Smith 2002) for chimpanzee data, especially in the posterior occipital lobe. FAST (Zhang et al. 2001) and SUSAN (Smith and Brady 1997) were used to correct for intensity bias and to reduce noise, respectively. Diffusion-weighted MR data were corrected for eddy current and susceptibility distortion using FSL’s eddy_correct (Graham et al. 2016) and topup (Andersson et al. 2003b) implemented in Matlab (Matlab7, Mathworks, Needham, MA).
Template Generation and HCP Pipeline Processing
Human, chimpanzee, and macaque scans were processed using the HCP pipeline (Van Essen et al. 2013) or a version modified for non-human primates. The HCP pipeline reconstructs the pial and white/gray matter interface surface by combining T1 and T2-weighted scans (Glasser et al. 2013b). For humans, each individual’s pial and WM/GM interface surfaces were subsequently registered to those of the HCP 440 human template using the landmark-based surface registration technique implemented in FreeSurfer (Reuter et al. 2012). For macaques and chimpanzees, sample-specific surface templates were first iteratively derived based on 19 macaque data sets and 29 chimpanzee data sets. The templates were then used as targets for registering the macaque and chimpanzee data sets employing a similar surface-based method. Chimpanzee cortical surfaces and template are available by request through the National Chimpanzee Brain Resource (chimpanzeebrain.org).
In all three species, cortical myelin density maps were computed by dividing T1-weighted scan data with T2-weighted data using the method first described for humans in Glasser and Van Essen (Glasser and Van Essen 2011). The method was subsequently used to create myeloarchitectonic maps for chimpanzees and macaques (Glasser et al. 2014). Recent advancements in this methodology have improved the accuracy of the reconstruction of the white matter and pial surfaces (Robinson et al. 2014).
Surface-based ROIs were manually drawn on the surface template for each species using the HCP Workbench View application (Marcus et al. 2011). From there, ROIs were registered onto individual surface space using the transformations derived earlier, where surface-based tractography was performed in FSL (see next section). After the tractography analysis, connectivity maps representing the probability of two ROIs being connected were projected on each individual’s surface space, where they were subsequently averaged in the surface templates for visualization in HCP Workbench View.
Diffusion Tractography
We used probabilistic diffusion tractography implemented in FSL’s diffusion toolbox (FDT) to track anatomical connections between ROIs in each hemisphere. We used a partial-volume model with automatic relevance detection (ARD; Behrens et al. 2007a) for delineating subsidiary fibers in each voxel, as a recent study shows that 63 — 90% of white matter voxels contain crossing fibers (Jeurissen et al. 2013). Moreover, probabilistic fiber tracking, rather than deterministic fiber tracking was employed for quantified reproducibility of the tracking results (Behrens et al. 2003, 2007). The details used in our fiber-tracking process were as follows: Region-of-interest (ROI) masks were manually drawn on the white matter/gray matter interface in an inflated projection view in HCP Workbench View. For each tract between any two ROIs (R1, R2), one ROI was first used as a seed mask and the other was used as a waypoint mask. The process was repeated with the seed mask and the waypoint mask reversed.
For each individual tracking process, the FDT program function probtrackx (Behrens et al. 2003, 2007) was used to compute a raw histogram for the spatial distribution of streamlines from the seed mask (R1) that pass through any given voxel x and the waypoint mask (R2; i.e., fdt_paths(R1→x→R2).
Probtrackx also calculates the total number of samples that are not rejected by the tracking conditions (waypoint masks, exclusion masks, etc.), called the waytotal. Each individual subject’s histogram was normalized by its waytotal in order to directly compare data across subjects and hemisphere. Thus, for probability p([R1→x→R2] or [R2→x→R1]), the probability that the path of least hindrance to diffusion from seed mask R1 (or R2) passes through x and waypoint mask R2 (or R1) is:
For a given voxel, a value of 1 represents the path of least hindrance to diffusion from either mask R1 to R2 or from mask R2 to R1 for a streamline under a given threshold in all subjects. Conversely, a value of 0 indicates that no streamline traveling between the two ROIs successfully passed through the voxel, in any subject under the given threshold.
For this study, the tractography parameters were set as follows: Each vertex was sampled 100,000 times per symmetrical tracking process. A curvature threshold of 0.2 and distance correction were used. Maximally, three crossing fibers were modeled. The pial gray matter surface without the medial wall was used as the stop mask to prevent streamlines from jumping across gyri via CSF (Li et al. 2013). All other parameters were set as defaults. After the connection probability maps for each tract between an ROI pair in each hemisphere were derived, they were constrained by a series of increasingly strict threshold values (0.5, 1.0, 1.5, 2.0 per voxel per 1000 streamline hits), which serve as an index of robustness against noise. The thresholded tractogram was then binarized and averaged across subjects to form a probability map for the whole sample population. This probability map was then projected onto the species-appropriate template. In the resulting population map, each voxel on the pial surface contains a value between 0 and 10 (for humans and macaques) or 1 and 15 (for chimpanzees). These values represent the number of individual subjects that had streamlines successfully pass through the voxel in question after normalization and thresholding. As an index of reproducibility across subjects, the final mean tractogram result for each species was thresholded at the population level so as to include only voxels from the majority subset of individuals (70–90%).
Seed masks in V1, auditory core, and extrastriate-plus-temporal cortex
The connections of V1 with extrastriate cortex were examined in humans, chimpanzees, and macaques, using a set of seed masks placed in different retinotopic divisions of area V1 (fig. 3A–3B’) and with a large mask that covered the entire extent of temporal cortex except for the auditory cortex, but including the parahippocampal gyrus (fig. 3D). Cortical surface masks were drawn on each species template. In V1, a mask covering the central representation (V1c) was created to reflect the portion of V1 most thoroughly described in macaque tract-tracing studies, and included the foveal plus parafoveal regions (fig. 3A). Additional masks covering different retinotopic portions of V1 were created for each species (foveal, lower and upper parafoveal, and lower and upper peripheral V1; fig. 3B, 3B’). Drawing of the human and macaque V1 masks was guided by the considerable information about the retinotopic organization of V1 available for these species (for humans, e.g., Horton and Hoyt 1991; Benson et al. 2012; Sereno et al. 1995a; Engel et al. 1997); for macaques (e.g., Tootell et al. 1988).
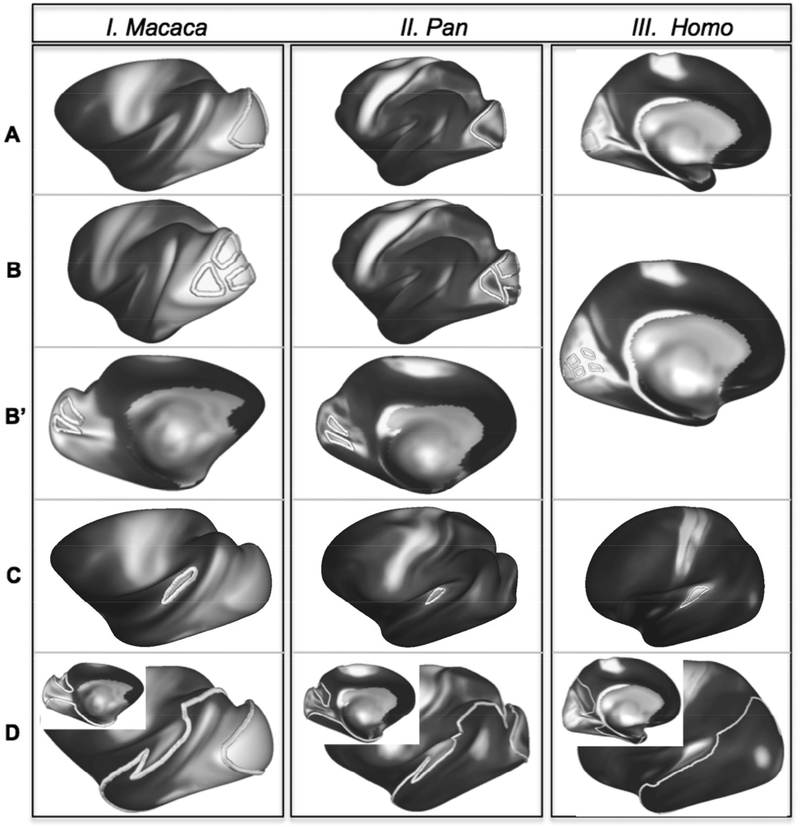
Figure 3:
Location of experimental ROIs in macaques, chimpanzees, and humans. (A) Central V1 ROIs (V1c); (B) foveal and parafoveal V1 ROIs; (B’) peripheral V1 ROIs; (C) large occipito-temporal cortex ROIs.
To explore the connections between V1 and MT+, masks covering MT+ and the intraparietal sulcal (IPS) cortex were drawn. These served to explore in more detail the connections between V1 and MT+, between IPS and MT+, and between IPS and extrastriate cortex more generally. In humans and macaques, large extrastriate masks encompassing MT+ were drawn using the MR myeloarchitectonic maps as guides, and based on published areal maps for these species, to include the heavily myelinated portions of MT, MST, and FST; the chimpanzee masks covered the myeloarchitectonically similar region located in the posterior STS (supplementary figs. S1A, S2A, & S3A). IPS masks covered both banks of the sulcus and included the well-myelinated cortex within (supplementary figs. S1B, S2B, & S3B), including the areas of intraparietal cortex reported to have strong connections with MT+ in macaques, specifically, the lateral and ventral intraparietal areas (LIP, VIP; e.g., (Maunsell and Van Essen 1983a; Boussaoud et al. 1990; Ungerleider and Desimone 1986a; Weller and Kaas 1983; Andersen et al. 1990; Lewis and Van Essen 2000; Morel and Bullier 1990; Rosa et al. 1993), as well as homologous divisions of IPS cortex in humans (Orban 2017). The connections of auditory core with temporal association and extrastriate cortex were examined using the aforementioned temporal cortex mask (fig. 3D) and a seed mask placed on the auditory core (fig 3C), the borders of which were informed by previous identification of this cortical area in humans, chimpanzees, and macaques (Hackett et al., 2001) and cortical myelin density maps (Glasser and Van Essen, 2011b).
In both species, the horizontal meridian is represented within the calcarine fissure, extending to V1-V2 border, located near the occipital pole (in humans) or on the posterior bank of the lunate sulcus (in macaques). In humans and macaques, the lower visual field is represented superior to the horizontal meridian and the upper field inferior to it, while foveal vision is represented at the intersection of the horizontal meridian with the V1-V2 border. Despite the absence of any modern studies of retinotopy in chimpanzees, all primates examined experimentally exhibit a similar pattern (reviewed by Rosa and Tweedale 2005; D. C. Lyon 2007; Krubitzer and Kaas 1990), so it is highly likely that chimpanzees share that organization. Two older studies of geniculostriate projections in chimpanzees confirm that, as in macaques, the central representation is located on the lateral surface of the occipital lobe while the peripheral representation is located medially (Poljak and Hayashi 1936; Walker 1938). Also, because chimpanzees, like macaques, have a well-developed lunate sulcus, and the border between V1 and V2 on the lateral surface of the occipital lobe lies along the posterior lip of the lunate sulcus (Bailey et al. 1950); see also (Tigges and Tigges 1979), we have drawn retinotopic ROIs in chimpanzee V1 that closely resemble our macaque ROIs (fig. 3B, 3B’).
Tractography procedure and interpretation of results
For each species, 8 symmetric tractography runs were performed in the left hemisphere, one for each striate mask to the large temporal lobe mask, and one from the auditory core mask to the large temporal mask. Two symmetric tractography runs were performed in the right hemisphere between (1) a central V1 mask and a large temporal lobe mask, and (2) an auditory core mask and a large temporal lobe mask; both corresponded to the left hemisphere masks. Individual tracking results were processed and averaged to produce population connectivity maps for each species, as described in the diffusion tractography section. Surface results (figs. 3–8; S1–S3) were visualized using Workbench View, part of the HCP Connectome Workbench software package (Marcus et al. 2011); volume results (fig. 9) were visualized with FSLeyes, part of the FSL software package (Jenkinson et al. 2012b). In all three species, tracking results were overlaid on myelin-density maps. Because humans and macaques have been so intensively investigated, we were able to assess both the areal localization and broad retinotopy of streamlines terminations in those species, overlaying tractography results directly on areal parcellations viewable in the HCP Workbench applications. In humans and macaques, tract termini were also assigned to specific areas, using the parcellation of Glasser and co-workers (2016) for humans and the M132 parcellation of Markov and colleagues (2014) for macaques. A one-way analysis of variance (ANOVA) was conducted on the summed tractogram volumes from symmetric tractography corrected for average species brain volumes. The volumes analyzed were from tractography between V1c and large temporal lobe masks (left and right hemispheres) and between auditory core and the large temporal lobe mask (left hemisphere).
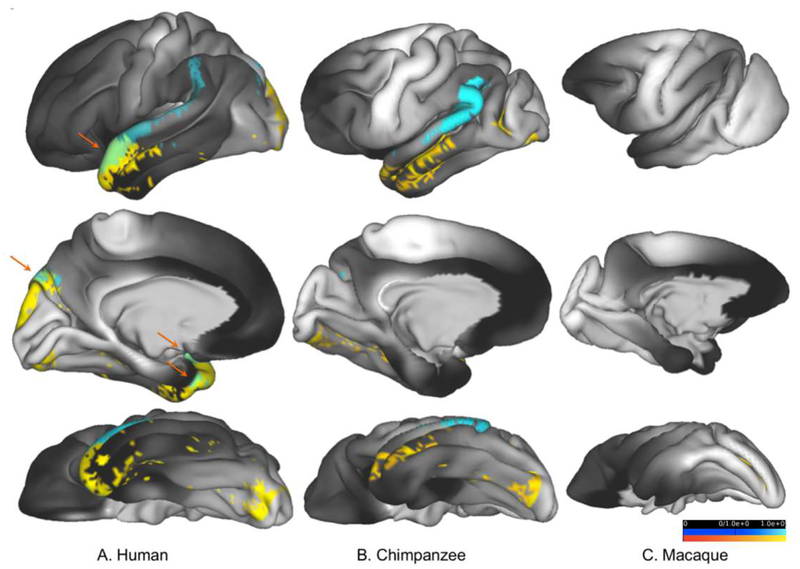
Figure 8:
Results shown in Figure 4 & 5 (tracking between V1c and occipito-temporal cortex) and 7 (tracking between auditory core and occipito-temporal cortex) are shown here on uninflated cortical representations of the left hemisphere in humans (A), chimpanzees (B), and macaques (C). V1c-occipitotemporal cortex results are shown in yellow, auditory core-temporal cortex results are shown in blue. Areas of streamline termini overlap in humans are indicated by orange arrows.
Figure 9:
Individual tractogram volumes for a subsample of macaques (A), chimpanzees (B), and humans (C). Tractography between V1c and occipito-temporal cortex is shown in red; between auditory core and occipitotemporal cortex is shown in blue. Threshold for all subjects=0.5 hits per vertex per 1000 streamlines.
In humans and macaques, as well as other nonhuman primates that have been examined experimentally, there is a set of retinotopically organized extrastriate areas located anterior to area V1 (Lyon 2007; Rosa and Tweedale 2005; Gattass et al. 2005a). The homologies of these areas have not been completely resolved, but macaques and humans evidently share V2, V3, V3A, V4, V6, and the areas of the MT complex, or MT+, consisting of the middle temporal area (MT), medial superior temporal area (MST), and fundal superior temporal area (FST; (Orban 2017). Moreover, in the species that have been examined experimentally, there is a pattern of retinotopy that spans the V1—MT territories, with lower-field representation dominating superiorly and upper-field representation dominating inferiorly, and foveal vision being represented in a band that extends across the lateral part of the occipital lobe from V1, through V2, V3, V4, and into the region containing MT+ and posterior inferotemporal cortex (e.g., (Sereno et al. 1995a; Abdollahi et al. 2014; Kolster et al. 2014; Krubitzer and Kaas 1990; Zeki 1969).
There currently exists almost no published data about extrastriate cortex in chimpanzees, or other great apes, and it is the location and retinotopy of these areas that are at issue in the present study. The most comprehensive studies from the classical era of architectonics, the cytoarchitectonic study of chimpanzees by Bailey and colleagues (1950) and the myeloarchitectonic study of orangutans by Mauss (1911), suggest an organization broadly similar to macaques, with the border between area 17 (V1) and area 18 (V2), located along the posterior rim of the lunate sulcus, results consistent with MR myeloarchitecture (Glasser et al. 2014). However, the cortex anterior to V2, which in species studied experimentally contains multiple extrastriate areas, was simply denoted as area 19 (or its equivalent in other nomenclatures). No information is available about the retinotopic organization of extrastriate areas in these animals.
Results
Central V1 (V1c)
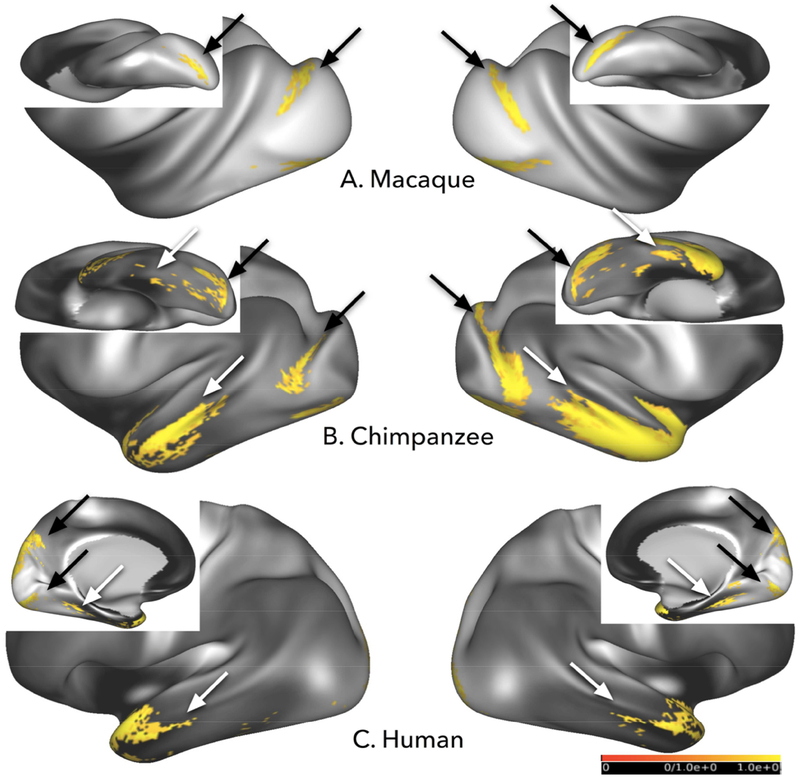
In all three species examined, V1c streamline connectivity extended into the extrastriate region immediately anterior to V1c, extending to, or close to, the MT complex, consistent with the known location of central visual field representation in humans and macaques. In macaques, streamlines connected V1c with dorsal and ventral V2, and dorsal V3 after thresholding (fig. 4A, 5A, black arrows); human V1c streamlines reached the same visual areas, as well as ventral V4 and V6 (fig. 4C, 5C, dark arrows). In chimpanzees, the similarities of apparent connectivity in the lateral occipital cortex suggest that these locations include homologs of dorsal and ventral V2, and possibly V3 and V4 (fig. 4B, 5B, dark arrows). Notably, no significant connectivity with MT+ was observed in any of the three species.
Figure 4:
Averaged results for tracking between V1c and the occipito-temporal cortex masks in macaques (A), chimpanzees (B), and humans (C) in both left hemisphere (left panel) and right hemisphere (right panel). Black arrows point to streamline results in unimodal extrastriate territories. White arrows indicate streamlines reaching higher order extrastriate and multimodal temporal cortex. Threshold for all subjects=0.5 hits per vertex per 1000 streamlines; population threshold=80% of subjects.
Figure 5:
The same results shown in Figure 4 are shown here on uninflated cortical representations of the left hemisphere and right hemispheres. Abbreviations: STG: Superior Temporal Gyrus; STS: Superior Temporal Sulcus; MTG: Middle Temporal Gyrus; ITS: Inferior Temporal Sulcus; ITG: Inferior Temporal Gyrus; LS: Lunate Sulcus; FG: Fusiform Gyrus; LG: Lingual Gyrus; PHG: Parahippocampal Gyrus; EC: Entorhinal Cortex.
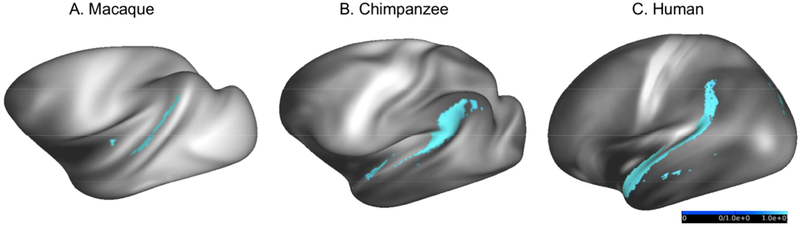
In addition to these similarities, some V1c results differed between species. Macaques do not show any significant streamline connectivity with V1c beyond V2 and V3 (fig. 4A, 5A). The most prominent difference, however, is that chimpanzees and humans, but not macaques, showed apparent major connectivity between V1c and anterior, lateral, and inferior temporal association regions (fig. 4B, 4C; 5B, 5C). One-way ANOVA revealed a significant effect of species on proportional tract volumes. Proportionally, human V1c to temporal lobe tract volumes were significantly larger than macaques (right hemisphere: F(1,18)=85.862, p<0.00001; left hemisphere: F(1,18)=49.784, p<0.00001), and significantly smaller than chimpanzees (right hemisphere: F(1,23)=36.905, p<0.00001; left hemisphere: F(1,23)=53.564, p<0.00001). Chimpanzee tract volumes were also significantly larger than macaques right hemisphere: F(1,23)=178.268, p<0.00001; left hemisphere: F(1,23)=128.929, p<0.00001). Patterns of streamline connectivity in these temporal regions will be discussed in more detail below.
Retinotopic organization of streamline terminations in extrastriate cortex
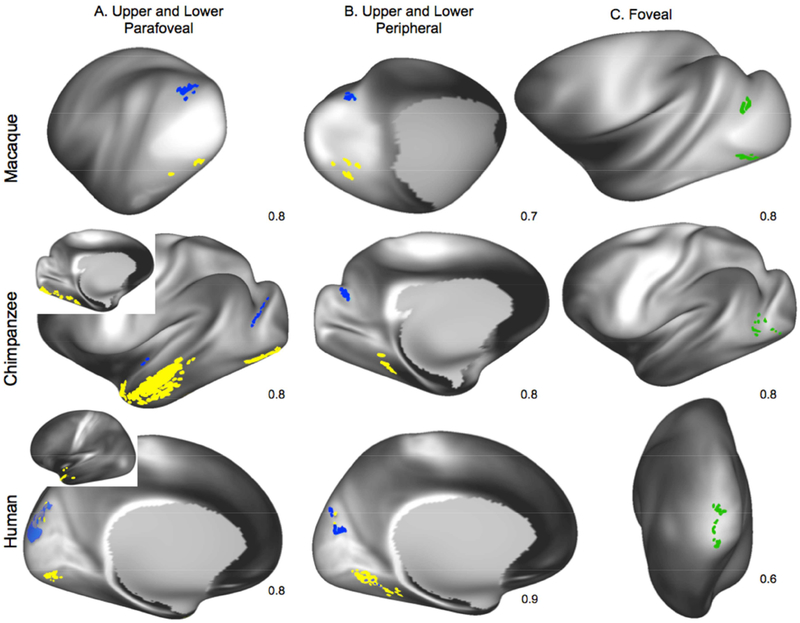
We investigated whether the retinotopic organization of extrastriate cortex could be revealed using smaller V1 ROIs. Tractography results between foveal seeds and the large temporal seeds were similar across species, with the strongest streamline connectivity concentrated in lateral V2 in humans and macaques, and to the cortex immediately anterior to area V1 in chimpanzees (fig. 6). Streamlines from upper parafoveal seeds, placed in the ventral parts of V1, preferentially reached ventral V2 in macaques and humans, and the corresponding region of chimpanzees (fig. 6A). Similarly, lower parafoveal seeds, placed dorsally in V1, showed the highest probability of connectivity with dorsal V2 in macaques and humans, and the corresponding region of chimpanzees, after thresholding results to display 80% of the individuals sampled (fig. 6A; 8/10 macaques and humans; 12/15 chimpanzees).
Figure 6:
Averaged retinotopic tracking results in humans, chimpanzees, and macaques. Blue areas temporal seed voxels with apparent major connectivity with lower parafoveal (A) and peripheral (B) V1 ROIs; yellow areas represent apparent major connectivity to upper parafoveal (A) and peripheral (B) V1 ROIs. Green areas in C represent results with foveal ROIs. Numbers in lower right corner of each map indicate the percentage of individual results included to produce the final averaged result. Threshold for all subjects=0.5 hits per vertex per 1000 streamlines except for human peripheral and parafoveal results, which are at 0.25 hits per 1000 streamlines; population threshold=80% of subjects.
In the humans, the majority of dorsal V2 connectivity involved lower parafoveal field streamlines, although some upper parafoveal streamlines also reached dorsal V2. Macaque and chimpanzee parafoveal connectivity was concentrated in the lateral part of V2, including ventromedial and dorsomedial V2 for upper peripheral and lower peripheral masks, respectively. In these averaged projections, macaque upper and lower parafoveal connectivity was limited to V2, while in the humans, in addition to V2 connections, we found hotspots of high probability connectivity to V3 with some extension into V6 (fig. 6A).
Streamlines connected with ROIs located in the peripheral visual field representation of area V1 were concentrated in medial extrastriate territories in all 3 species, and as well as further anterior to the parafoveal connections in humans (fig. 6B). In macaques, ROIs in the peripheral representation of area V1 yielded fewer streamlines when compared to parafoveal and foveal ROIs, so the number of individuals included in the averaged results was reduced to 7/10 individuals. With this modification, streamlines from upper peripheral and lower peripheral seeds reached ventral and dorsal extrastriate cortices, respectively. These extrastriate territories included ventral parts of V2, V3, and V4 for upper peripheral seed streamlines and dorsal parts of V2, V3, and V4 for lower peripheral streamlines. In humans, streamlines between peripheral V1 and extrastriate regions were slightly more numerous than those related to parafoveal ROIs; so averaged results were thresholded higher, using averaged results from 9/10, rather than 8/10 individuals. Human upper peripheral connectivity included ventral parts of V2, V3, and V6; lower peripheral seeds tracked to dorsal parts of V2, V3, and V6. As with parafoveal data in humans, some “cross-contamination” of connectivity patterns was visible, with some upper peripheral streamlines connecting with dorsal extrastriate cortex and some lower peripheral with ventral extrastriate cortex (lower panel in fig 6A, B). Chimpanzee peripheral visual connectivity patterns were most similar to macaque results, although some streamlines from upper peripheral ROIs appeared in the lingual and fusiform gyri, which might include ventral V4.
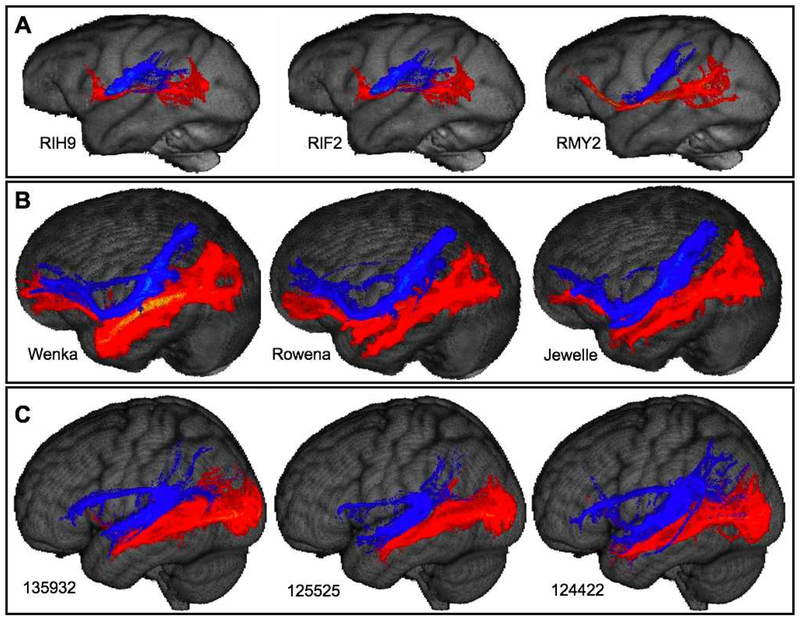
In all 3 species, tractography between area V1 and a large occipito-temporal cortex mask revealed few terminations within the MT+ complex (supplementary figs S1–3). This is surprising, given that V1 is known to possess strong connections with MT+ in macaques and other nonhuman primates that have been studied. We explored this result in more detail in chimpanzees and macaques by drawing more circumscribed MT+ masks and then tracking connections to these seeds from V1c (supplementary figs S1A & S2A). Further, to determine whether the apparent weak connectivity between V1 and MT+ reflected some property of V1 or of MT+, we also carried out tractography with masks drawn on IPS cortex, another region known to have strong connections with MT+ in macaques, comparing results with V1c masks (supplementary figs. S1B, S2B & S3B). In these follow-up experiments, certain individuals did have streamlines reaching MT+, for example RHZ8, RMY2, and RIH9 in macaques, Suwanee and Bo in chimpanzees, and 100307 in humans (supplementary figs. S1C & S2C). In all cases and in all species, however, streamlines had higher probabilities of terminating in the more lightly myelinated territories immediately surrounding MT+. Tractography between IPS masks and temporal areas yielded numerous streamlines dispersed in the inferior parietal lobule of all three species, but, after thresholding, in no species did streamlines terminate in MT+ (supplementary figs. S1D, S2D & S3D). Thus, neither large nor small masks of V1 or large masks of IPS yielded substantial numbers of streamlines to MT+ in any species.
Apparent anterior and ventral temporal lobe connectivity with V1c
As noted above, seeding V1 in chimpanzees and humans, but not macaques, resulted in large numbers of streamlines in anterior and ventral parts of the temporal lobe. In human left hemispheres (figs. 4C, 5C), streamlines from the large V1c mask reached ventral temporal areas: the ventral visual areas (VMV areas) and parahippocampal gyrus (PHG). Numerous streamlines reached anterior temporal areas, including temporopolar and parainsular areas. Lateral temporal areas that received streamlines include anterior STG and STS. Parafoveal and peripheral V1 seeds also reached temporopolar areas. After thresholding, we did not see streamline connectivity reaching the right fusiform gyrus in any of the averaged results. Results for the human right hemisphere (figs. 4C, 5C) were similar to the left. Although we do not have chimpanzee cortex parcellations that are comparable to human parcellations, the distribution of streamlines to anterior and ventral temporal cortex was similar to that in humans, except that they extended more posteriorly along the superior temporal gyrus (STG), superior temporal sulcus (STS), and middle temporal gyrus (MTG; figs. 4B, 5B, 6).
Apparent anterior and posterior temporal lobe connectivity with auditory core
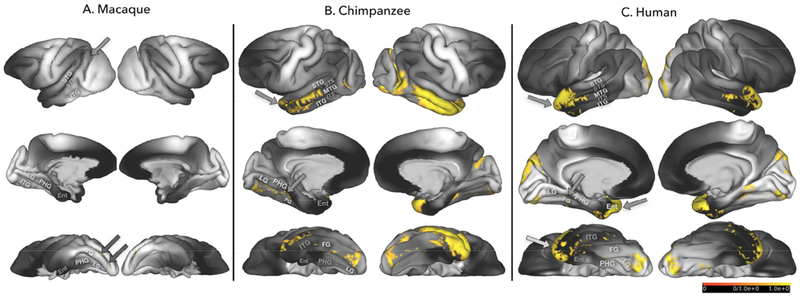
Seeding the auditory core produced apparent streamline connectivity with territories immediately adjacent to the auditory belt in macaques, chimpanzees, and humans (fig. 7). In macaques, this includes medial, caudal, and possibly the posterior-most portion of the anterior parabelt (fig. 7A), based on the auditory cortex mappings of Hackett and colleagues (1998). Humans also show apparent connectivity with parabelt and A4 (Moerel et al. 2014) In both humans and chimpanzees, streamlines reached posterior STG and supramarginal gyrus (SMG; fig 7B & 7C). Additionally, humans showed streamline connectivity to dorsal extrastriate area V3 in anterior STG and superior and medial ATL (fig. 8, arrows). Posterior territories in humans included posterior STG and STS. Areas of overlap of streamline connectivity between V1c and auditory core protocols was seen in humans only (fig. 8A, arrows) and were localized in dorsal V3, medial and superior ATL. One-way ANOVA revealed a significant effect of species on tract volumes between auditory core and temporal cortex. When corrected for total brain size, human auditory core to temporal lobe tract volumes were significantly larger than macaques (F(1,18)=49.784, p<0.00001), and significantly smaller than chimpanzees (F(1,23)=92.510, p<0.00001). Chimpanzee tract volumes were also significantly larger than macaques (F(1,23)=365.670, p<0.00001).
Figure 7:
Averaged results for tracking between auditory core and the occipito-temporal cortex masks in macaques (A), chimpanzees (B), and humans (C) in the left hemisphere. Threshold for all subjects=0.5 hits per vertex per 1000 streamlines; population threshold=80% of subjects.
Fascicular contributions to temporal cortex organization
In order to gain insight into the fascicular contributions to the differences in the distribution of streamline termini across species, we examined the tracts that were produced from the V1c and auditory core to temporal cortex tractography in volume space (fig. 9). Streamlines from the occipitotemporal mask to the V1c mask bear similarities with the inferior longitudinal fasciculus (ILF) as previously described by for macaques (Schmahmann et al., 2007), and the ILF and inferior fronto-occipital fasciculus (IFOF), as previously described in chimpanzees (Bryant et al. 2018), and humans (Catani and Thiebaut de Schotten 2008). In humans and chimpanzees, these tracts also extended further anteriorly than our macaque sample, and after correcting for average brain volumes, were significantly larger than in macaques. The location of streamlines from the occipitotemporal mask to auditory core are consistent with the organization of the middle longitudinal fasciculus (MdLF) in all three species (macaques: Schmahmann and Pandya 2006; chimpanzees: Bryant et al., 2018; humans: Menjot de Champfleur et al. 2013), and in humans and chimpanzees, the arcuate fasciculus (Rilling et al. 2008a). Notably, streamlines in chimpanzees for both auditory core and V1c conditions reached prefrontal areas to a greater degree than humans (fig. 9B & 9C).
Discussion
In this study, we used probabilistic diffusion tractography to investigate the organization of extrastriate cortex and adjacent temporal cortex in humans, chimpanzees, and macaques. In humans and macaques, streamlines connected area V1 with retinotopically matched parts of extrastriate cortex, and chimpanzees showed a pattern of streamline distribution consistent with this. However, in the consensus results for all three species, few streamlines were observed to pass between V1 and the MT+ complex, or between MT+ and the IPS. Although the three species were similar in these respects, in humans and chimpanzees only, streamlines were observed to connect area V1 with ventral and anterior regions of the temporal lobe, including the multimodal anterior temporal lobe (ATL) region of humans and similarly located cortex in chimpanzees. Humans and chimpanzees also showed differences in streamline connectivity between auditory core and occipitotemporal cortex: while macaque streamlines were restricted to parabelt areas, streamlines in chimpanzees and humans reached posterior STG and SMG; further, streamlines in humans exclusively reached anterior STG and ATL via the ILF/IFOF. Here, we discuss how these results bear on the organization of occipital and temporal cortex in chimpanzees, as well as methodological issues arising from some of the unexpected results, including the apparent absence of substantial connectivity between V1 and MT+, and the large number of streamlines extending between primary sensory cortices (V1 and auditory core) and temporal association areas in humans and chimpanzees.
Methodological considerations
Cortico-cortical tractography with diffusion-weighted tractography presents specific challenges. Previously, diffusion tensor imaging (DTI) has been the standard for local modeling of diffusion MRI signals in diffusion tractography, but the more recently developed HARDI technique encodes 50 or more directions (in our case, 60) enabling higher angular resolution of diffusion MRI data when compared with DTI, which only requires a minimal diffusion direction of 6 to estimate tensors. Diffusion MRI data collected using the HARDI scheme and constructed using models supporting multiple peaks of diffusion has been shown to outperform data constructed using diffusion tensor models for difficult-to-reconstruct tracts like the acoustic radiation (Berman et al. 2013) that are intercepted by other tracts. It is important to note that while multifiber algorithms increase sensitivity and capture portions of known tracts that were unresolvable with traditional DTI (Behrens et al. 2007b), with this increased sensitivity comes increased risk of false positives (Maier-Hein et al. 2017). The problem of gyral bias (Van Essen et al. 2014), in which tracking is biased away from sulcal fundi and slightly toward gyral crowns, has also been documented extensively (Donahue et al. 2016a; Reveley et al. 2015a). Despite these challenges, several studies comparing macaque tract-tracing with diffusion tractography have found a favorable comparison between the two techniques (Jbabdi et al. 2013; Azadbakht et al. 2015; van den Heuvel et al. 2015; Donahue et al. 2016a), although a few failed to effectively replicate tract-tracing patterns when examining smaller cortical seeds (Thomas et al. 2014; Reveley et al. 2015a). It is important to keep in mind that the use of probabilistic diffusion tractography does not imply an attempt to replicate individual cortical connections but rather estimations of the probability of terminations and courses of larger-scale white matter tracts. The risk of false positives is a concern for cortical probabilistic tractography, but as argued by Zalesky and colleagues (2016), diffusion MRI researchers should first ask themselves what is more detrimental to their study - false positives or false negatives - and then adjust their methods accordingly. For the purposes of this study, we feel it is reasonable to trade some risk of false positives for the reward of greater sensitivity to putative differences in white matter organization between species.
Connections between area V1 and retinotopically organized extrastriate areas
As expected, selectively seeding different parts of the visual field representation of V1 yielded patterns of connectivity consistent with the known retinotopic organization of macaques and humans (Brewer et al. 2002; Sereno et al. 1995b; Tootell et al. 2003). In all individual macaques and humans, streamlines connected the central representation of area V1 with the portion of area V2 on the lateral surface, immediately adjacent to V1, which is also consistent with known foveal representation in primates, with additional streamlines extending anteriorly, in the territory between V2 and the heavily myelinated MT+ region, which includes V3 and V4 (Wandell et al. 2007). The pattern of streamline distribution was very similar after seeding the presumed central representation of chimpanzee V1. Seeding of the presumed upper-field parafoveal and peripheral representations of V1 in humans, chimpanzees, and macaques showed connectivity with ventral extrastriate areas, which represent the upper visual field; seeds in lower parafoveal and peripheral visual fields reached dorsal extrastriate territories, representing the lower visual field.
Although the distribution of streamlines was broadly in keeping with the known retinotopy of extrastriate cortex, in humans, we did observe some “cross-contamination” in the distribution of streamlines between V1 and V2, with some streamlines connecting seeds in the upper and lower visual field representations of V1 with the lower and upper field representations of V2, respectively, although the largest number of streamlines were in the expected locations. Although these mismatches could represent false positives, even in experimental tracer studies of nonhuman primates, connections are sometimes observed between upper and lower visual field representations (Lyon and Kaas 2002). In macaques and other non-human primates, numerous studies using traditional tract-tracing methods have demonstrated that the MT complex is a major target of V1 connections (Ungerleider and Desimone 1986b; Maunsell and van Essen 1983b; Shipp and Zeki 1989; Boyd and Casagrande 1999). Surprisingly, we observed few streamlines between V1 and MT+ in any of the species we examined, including macaques, a result consistent with a recent diffusion imaging study by Donahue and colleagues (2016b). Tracking between masks covering the MT+ region and IPS cortex—which, like V1, is strongly connected with MT+ in experimental tracer studies (Rosa et al. 1993; Lewis and Van Essen 2000; Maunsell and Van Essen 1983b; Morel and Bullier 1990) — also yielded few streamline termini in MT+ compared to surrounding areas. One possible explanation for this null result is the geometry of fibers in superficial white matter, which has been shown to impact the ability to track connections (Reveley et al. 2015b). Another possibility is gyral bias, a phenomenon reported previously (Jbabdi and Johansen-Berg 2011; Reveley et al. 2015a; Van Essen et al. 2014), including a study which also found weak apparent connectivity between V1 and MT+ in high resolution post-mortem macaque scans (Donahue et al. 2016b). Thus, it is possible that the organization of fibers in the white matter immediately deep to MT+, or the gyral topography surrounding the area, or some combination, derail tractography.
Apparent connectivity between V1 and anterior and ventral temporal cortex
Apparent connectivity between primary visual cortex ROIs and temporal cortex was more extensive in humans and chimpanzees than in macaques. In humans, streamlines from V1 reached higher-order visual areas and association regions of anterior and inferior temporal cortex. In particular, streamlines reached the region commonly referred to as the ATL, which includes the temporal pole as well as anterior portions of the STG, STS, MTG, lateral inferotemporal (IT) cortex, and portions of the parahippocampal gyrus (PHG). There is no suitable map for identifying the chimpanzee areas that receive streamlines, although the broad pattern was similar to that of humans except that in chimpanzees the streamline terminations are concentrated further posteriorly along the middle temporal gyrus. Human apparent connectivity was sparser and localized more anteriorly than in chimpanzees, concentrated in territories at the extreme anterior end of the temporal pole. It is important to note that although macaques do not possess a morphological MTG, we did not see connectivity in any portion of the temporal lobe anterior to unimodal extrastriate areas that might encompass putative homologs to hominoid MTG (fig. 5A & 6). Although some tract-tracing studies have indicated the existence of thinly dispersed connections from V1 to IT cortex in macaques (Doty 1983; Rockland and Van Hoesen 1994; Rockland, Saleem, and Tanaka 1994), comprehensive inter-areal connectivity analyses demonstrate these connections are relatively weak compared to connections to other extrastriate areas (Felleman and Van Essen 1991; Markov et al. 2011, 2014), and the majority of studies indicate that connections from occipital areas to inferior and anterior temporal territories in macaques come mainly from extrastriate areas and not V1 (reviewed in Gattass et al. 2005b).
Thus, humans and chimpanzees, but not macaques, showed apparent major connectivity between V1 (including V1c) and the ATL, MTG, and PHG (fig. 4 & 5). How can we account for these differences in apparent connectivity between the hominoids and macaques? The lack of evidence from tract-tracing studies for strong connections in macaques between V1 and the middle, anterior, and ventral temporal lobe suggests that the apparent connections seen in humans are simply false positives. Alternatively, it is possible that these regions evolved increased connectivity with the great expansion of temporal association cortex that occurred in hominoid and/or human evolution (Hill et al. 2010). In either case, the present results should be considered as a possible source of information on hominid specializations. Tractography results, when viewed in volume space, indicate that the streamlines between V1c and occipital temporal areas differ across species, and are consistent with known differences in fasciculi between species. In both macaques and humans, the inferior longitudinal fasciculus spans the occipital lobe, parietal lobe, and inferior temporal gyrus (ILF; Catani et al., 2003; Forkel et al., 2014; Schmahmann & Pandya, 2009). The reconstruction of human ILF by Catani and colleagues (2003) suggests that its occipital lobe termini originate in the posterior lingual and fusiform gyri, and the cuneus, but may not reach the calcarine fissure, which may explain this result.
By contrast, the inferior fronto-occipital fasciculus (IFOF), which also connects visual areas with temporal cortex and prefrontal areas, extends to occipital polar areas close to V1, namely Brodmann area 18 (Thiebaut de Schotten et al. 2012; Forkel et al. 2014). The IFOF runs parallel and medial to the ILF along its occipito-temporal course (Ashtari 2012; Catani and Thiebaut de Schotten 2008). Its equivalent in macaques, sometimes referred to as the fronto-occipital fasciculus (Thiebaut de Schotten et al. 2012) or as part of the extreme capsule system (Schmahmann et al. 2007) does not reach the occipital lobe but instead terminates along the STS. One might expect that this difference may underlie the differences in V1c-temporal streamline connectivity we observed between apes and macaques, and indeed, the tractograms are consistent with the temporal portion of IFOF in humans. Although formal tractography identifying IFOF in chimpanzees has not been carried out, preliminary results suggest IFOF is similar in size and organization to humans (Bryant et al., 2018), and the streamlines between chimpanzee V1 and occipitotemporal cortex look similar to human IFOF (fig. 9B).
Apparent connectivity between auditory core and anterior and posterior temporal cortex
Like V1, apparent connectivity between auditory core ROIs and temporal cortex was more extensive in humans and chimpanzees than in macaques. Macaque streamline connectivity was restricted to the immediate territories surrounding auditory core, including the anterior and posterior portions of auditory belt. In chimpanzees and humans, streamlines from auditory core reached higher-order auditory areas and association regions of posterior temporal cortex, including SMG and posterior STG (fig. 7 & 8). Only humans showed streamline connectivity reaching ATL, specifically in the superior and medial portions of ATL (fig. 5, arrows). Human apparent connectivity also reached dorsal extrastriate areas.
Thus, humans and chimpanzees, but not macaques, showed apparent major connectivity between auditory core and posterior STG and SMG. Humans alone show apparent major connectivity with anterior STG, extending into superior and medial ATL. Combined with our results from V1 tracking, it seems possible that these differences reflect evolutionary modifications to connectivity between primary sensory areas and the expanded temporal association cortex that appeared in hominoid and/or human lineage (Hill et al. 2010).
Streamline connectivity from auditory core reached an area roughly corresponding to parabelt in all three species (fig. 7). Although to our knowledge, no diffusion tractography studies have examined the cortico-cortical connectivity of auditory core in macaques, tract-tracing work in this species has found the major connections from A1 to be the cortex immediately medial and lateral (Morel et al. 1993), similar to our findings (fig. 7A). Diffusion tractography studies in humans have identified connectivity between human auditory core and anterior and posterior STG (Upadhyay et al. 2008). In both humans and chimpanzees, streamlines reached posterior STG and SMG, suggestive of these two putative processing streams. Only in humans did streamlines between the occipito-temporal mask and auditory core reach dorsal extrastriate area V3 and superior and medial ATL - further, only in humans did these streamline connections overlap with those from the V1c-occipito-temporal cortex tractography. For humans and chimpanzees, streamline organization most closely resembles the MdLF and the arcuate fasciculus. Previous work has established important modifications and expansions to the arcuate fasciculus in the hominoid and human lineages (Rilling et al. 2008a, 2011), which may explain the more expansive pattern of streamline termini in the temporal cortex of chimpanzees and particularly humans. A comparable comparative analysis of the MdLF across species has not been carried out, however, in humans the MdLF is reported to extend along the STG, reaching the inferior parietal lobule and the temporal pole (Makris et al. 2013; Menjot de Champfleur et al. 2013). This is similar to the organization in macaques, investigated using tract-tracing (Schmahmann et al. 2007; Seltzer and Pandya 1984). One possible explanation for why streamlines that were picked up in putative MdLF in macaques did not reach similar territories in STG and the inferior parietal lobule, as they did in humans and macaques, is that the MdLF, like the arcuate, has become larger and more robust in the hominoid lineage; however, comparative diffusion tractography would be needed to ascertain this.
Modifications to Temporal Association Cortex
What is the evidence for the evolutionary modification of temporal lobe association cortex in hominoid evolution? In addition to the expansion of poorly myelinated cortex shown in fig. 2, there is evidence that humans possess expansions or even new subdivisions that are lacking in macaques. For example, it is currently unclear whether macaques possess a homologue of the human parahippocampal place area (PPA). The PPA was first identified in humans as responding preferentially to scenes rather than objects, with a critical factor being the presence of a spatial layout (Epstein and Kanwisher 1998); further, the PPA specializes in responding to layout but not navigation or movement within scenes (Epstein et al. 1999). A macaque homologue to human PPA was first identified on the ventral IT cortex using stimuli with high spatial frequencies, but not scenes (Rajimehr et al. 2011). A later study with identical scene stimuli for humans and macaques showed a small area of ventral IT activation in macaques (Nasr et al. 2011). In both macaques (Saleem et al. 2007) and humans (Baldassano, Beck, and Fei-Fei 2013), an antero-posterior gradient within the PPA is seen based on both differences in connectivity and functional specializations, with anterior portions activating preferentially for specific objects and posterior portions for abstract objects. In a recent review, Orban has argued that macaques do possess a PPA (Orban 2017). If that is the case, however, the PPA would appear to be much larger, relatively and absolutely, in humans than in macaques (see Figure 10 in Orban 2017), and, unlike macaques, appears to be composed of no fewer than three distinct areas (Glasser et al. 2016).
Whereas in macaques, the cortex inferior to the superior temporal sulcus contains higher-order visual cortex (inferior temporal cortex), in humans, the cortex spanning the MTG and extending into the ATL is known to possess multiple territories of semantic representation. The posterior MTG is well-documented in humans as an important language-related semantic center (Bates et al. 2003; Dronkers et al. 2004; Hickok and Poeppel 2004, 2007; Turken and Dronkers 2011; Glasser and Rilling 2008). The anterior MTG (aMTG) is less well-studied, but available literature suggests that in humans, this territory is also part of a multimodal association area (Binder et al., 2009) involved in a semantic processing network (Copland et al. 2003; Schwartz et al. 2009; Butler et al. 2014). Human imaging studies have implicated aMTG in lexical decision-making, for example, in exception word reading tasks (Wilson et al. 2012), visual word recognition (Pammer et al. 2004), and spoken word recognition (Roxbury et al. 2014). Anterior MTG appears to be recruited for recognition of famous faces (Leveroni et al. 2000) and proper names of famous individuals (Gorno-Tempini et al. 1998), tasks that may be considered as tapping into semantic “meaningfulness” (Binder et al. 2009). The latter two findings are similar to functions that have been localized in the ATL broadly, perhaps reflecting conflicting interpretations regarding the posterior limits of the ATL (reviewed in Bonner and Price 2013).
The ATL, a large swathe of cortex encompassing the temporal pole, is a multimodal association center that plays an important role in both semantic memory and affective cognition in humans. This encompasses language functions, including production and comprehension of spoken and written words and pictures (Coccia et al. 2004; Pobric, Jefferies, and Ralph 2007), taste recognition (Small et al. 1997), olfactory memory (Rausch et al. 1977; Eskenazi et al. 1986), stimulus-invariant perception of emotional facial expressions (Schmolck and Squire 2001; Cancelliere and Kertesz 1990), generation of emotions in response to visual cues (Reiman et al. 1997), storage of unique, socially relevant entities, such as familiar people and landmarks (Damasio et al. 2004; Frith 2007; Kriegeskorte et al. 2007); emotional memory retrieval (Dolan et al. 2000), comprehension of social concepts (Zahn et al. 2007, 2009; Ross and Olson 2010; Mars et al. 2013a), and coherent conceptual categorization of objects (Rogers et al. 2004; Lambon Ralph et al. 2010). The presence of such a diverse array of functions suggests that ATL is comprised of multiple divisions in humans, as the recent cortical map of Glasser et al. (2016) suggests. In macaques, the temporopolar cortex corresponds in location approximately to the human ATL; however, there appear to be some differences in the functions of this region in the two species. For example, there is evidence for at least one area in the region involved social cognition that is present in humans but not macaques (Mars et al. 2013b).
Conclusions
Our results highlight both the potential value as well as the shortcomings of comparative studies of cerebral connectivity using diffusion imaging. The shortcomings reflect the fact that diffusion tractography yields both false positives and false negatives (Thomas et al. 2014; de Reus and van den Heuvel 2013). These can complicate the interpretation of connectivity differences between species, because there are both extrinsic and intrinsic factors that can affect tractography results, such as image quality and white matter organization, and these are bound to differ even between relatively closely related species. Nevertheless, there were cross-species similarities in our tractography results that support several conclusions about the organization of chimpanzee cortex compared to humans and macaques. Specifically, they indicate that the retinotopic organization of extrastriate areas in the lateral occipital lobe of chimpanzees closely resembles that of humans and other nonhuman primates, with a band of central field visual representation extending anteriorly from the central representation of V1. Following from this, we can interpret the lack of streamlines between V1 and the heavily myelinated MT+ region of macaques and humans, and the heavily myelinated region of the posterior STS in chimpanzees, as evidence that chimpanzee MT+ is located within the STS, similar to macaques. That is, while the lack of streamlines between V1 and MT+ in all three species is almost certainly a false negative, the pattern of streamline distribution is similar enough between all three species to permit interpretation of chimpanzee extrastriate organization, a conclusion reinforced by the comparative myeloarchitectonic evidence.
By contrast, the apparent high-probability connectivity between V1 and anterior and ventral temporal cortex, and auditory core and anterior and posterior temporal cortex in humans and chimpanzees is more difficult to interpret, given the absence of streamlines between these regions in macaques. These results likely reflect differences in fascicular organization along the temporal lobe in humans, chimpanzees, and macaques, more so than absolute differences in connectivity between unimodal and multimodal areas. Finally, patterns of overlap between auditory and visual cortex streamline connectivity with anterior temporal cortex in humans support the possibility that organizational changes to cortico-cortical connectivity have accompanied cortical expansion of the human temporal pole. Thus, while the organization of chimpanzee extrastriate cortex appears to be more similar to that of macaques than humans, the organization of temporal association cortices in chimpanzees may share features with humans that are lacking in macaques, and some features of ATL organization may be unique to humans.
Supplementary Material
Supplemental Figure S1: Macaque MT+ convergence results: A. Central V1 and Large MT+ masks; B. Intraparietal sulcus and Extrastriate masks; C. Representative subject results of central V1 connectivity to large MT+ mask; D: Representative subject results of IPS connectivity with extrastriate mask. Surface projection results are outlined in black to enhance visibility.
Supplemental Figure S2: Chimpanzee MT+ convergence results: A. Central V1 and Large MT+ masks; B. Intraparietal sulcus and Extrastriate masks; C. Representative results of central V1 connectivity to large MT+ mask; D: Representative results of IPS connectivity with extrastriate mask. Results are thresholded at 99% for all subjects. IPS-Extrastriate results (D) further exclude the upper 15 percent. Surface projection results are outlined in black to enhance visibility. E: Detail of Suwannee V1-MT+ results. F: Detail of Bo V1-MT+ results. G: Detail of Suwannee IPS-Extrastriate results. H: Detail of Agatha IPS-Extrastriate results. Asterisk indicates center of densely-myelinated area MT.
Supplemental Figure S3: Human MT+ convergence results: A. Central V1 and Large MT+ masks; B. Intraparietal sulcus and Extrastriate masks; C. Representative results of central V1 connectivity with large MT+ mask; D: Representative results of IPS connectivity with extrastriate mask. Surface projection results are outlined in black to enhance visibility. E-H: Close-up of MT+ lacunae. E. V1c, subject 103414; F. V1c, subject 103414; G. IPS, subject 117122; H. IPS, subject 151223. Asterisk indicates center of densely-myelinated area MT.
Acknowledgements
This study was supported by NIH/NIDCD (RO1 DC04318), the National Chimpanzee Brain Resource (R24 NS092988), the James S. McDonnell Foundation (JSMF 21002093), and the John Templeton Foundation (Award 40463), the NIH Office of Research Infrastructure Programs (OD P510D1132), and the Yerkes National Primate Research Center base grant (2P51 RR000165–51) and NIMH (P50, MH100029). The authors thank Mary Ann Cree for advice and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdollahi Rouhollah O., Kolster Hauke, Glasser Matthew F., Robinson Emma C., Coalson Timothy S., Dierker Donna, Jenkinson Mark, Van Essen David C., and Orban Guy A.. 2014. “Correspondences between Retinotopic Areas and Myelin Maps in Human Visual Cortex.” NeuroImage 99 (October): 509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen John S., Bruss Joel, and Damasio Hanna. 2006. “Looking for the Lunate Sulcus: A Magnetic Resonance Imaging Study in Modern Humans.” The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology 288 (8): 867–76. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, and Siegel RM. 1990. “Corticocortical Connections of Anatomically and Physiologically Defined Subdivisions within the Inferior Parietal Lobule.” The Journal of Comparative Neurology 296 (1): 65–113. [DOI] [PubMed] [Google Scholar]
- Andersson Jesper L. R., Skare Stefan, and Ashburner John. 2003a. “How to Correct Susceptibility Distortions in Spin-Echo Echo-Planar Images: Application to Diffusion Tensor Imaging.” NeuroImage 20 (2): 870–88. [DOI] [PubMed] [Google Scholar]
- Andersson Jesper L. R., Skare Stefan, and Ashburner John. 2003b. “How to Correct Susceptibility Distortions in Spin-Echo Echo-Planar Images: Application to Diffusion Tensor Imaging.” NeuroImage 20 (2): 870–88. [DOI] [PubMed] [Google Scholar]
- Andersson Jlr, Xu J, Yacoub E, Auerbach E, Moeller S, and Ugurbil K. 2012. “A Comprehensive Gaussian Process Framework for Correcting Distortions and Movements in Diffusion Images.” In Proceedings of the 20th Annual Meeting of ISMRM, 2426. [Google Scholar]
- Ashtari Manzar. 2012. “Anatomy and Functional Role of the Inferior Longitudinal Fasciculus: A Search That Has Just Begun.” Developmental Medicine and Child Neurology 54 (1): 6–7. [DOI] [PubMed] [Google Scholar]
- Autrey Michelle M., Reamer Lisa A., Mary Catherine Mareno Chet C. Sherwood, Herndon James G., Preuss Todd, Schapiro Steve J., and Hopkins William D.. 2014. “Age-Related Effects in the Neocortical Organization of Chimpanzees: Gray and White Matter Volume, Cortical Thickness, and Gyrification.” NeuroImage 101 (November): 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadbakht Hojjatollah, Parkes Laura M., Haroon Hamied A., Augath Mark, Logothetis Nikos K., de Crespigny Alex, D’Arceuil Helen E., and Parker Geoffrey J. M.. 2015. “Validation of High-Resolution Tractography Against In Vivo Tracing in the Macaque Visual Cortex.” Cerebral Cortex 25 (11): 4299–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey Percival, Von Bonin Gerhardt, and McCulloch Warren S.. 1950. The Isocortex of the Chimpanzee. Univ. Illinois Press. [Google Scholar]
- Baldassano Christopher, Beck Diane M., and Fei-Fei Li. 2013. “Differential Connectivity within the Parahippocampal Place Area.” NeuroImage 75 (July): 228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates Elizabeth, Wilson Stephen M., Ayse Pinar Saygin Frederic Dick, Sereno Martin I., Knight Robert T., and Dronkers Nina F.. 2003. “Voxel-Based Lesion–symptom Mapping.” Nature Neuroscience 6 (5): 448–50. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen Berg H, Jbabdi S, Rushworth MFS, and Woolrich MW. 2007a. “Probabilistic Diffusion Tractography with Multiple Fibre Orientations: What Can We Gain?” NeuroImage 34 (1): 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen Berg H, Jbabdi S, Rushworth MFS, and Woolrich MW. 2007b. “Probabilistic Diffusion Tractography with Multiple Fibre Orientations: What Can We Gain?” NeuroImage 34 (1): 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, and Smith SM. 2003. “Characterization and Propagation of Uncertainty in Diffusion-Weighted MR Imaging.” Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 50 (5): 1077–88. [DOI] [PubMed] [Google Scholar]
- Benson Noah C., Butt Omar H., Datta Ritobrato, Radoeva Petya D., Brainard David H., and Aguirre Geoffrey K.. 2012. “The Retinotopic Organization of Striate Cortex Is Well Predicted by Surface Topology.” Current Biology: CB 22 (21): 2081–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Lanza MR, Blaskey L, Edgar JC, and Roberts TPL. 2013. “High Angular Resolution Diffusion Imaging Probabilistic Tractography of the Auditory Radiation.” AJNR. American Journal of Neuroradiology 34 (8): 1573–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder Jeffrey R., Desai Rutvik H., Graves William W., and Conant Lisa L.. 2009. “Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies.” Cerebral Cortex 19 (12): 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner Michael F., and Price Amy R.. 2013. “Where Is the Anterior Temporal Lobe and What Does It Do?” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 33 (10): 4213–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, and Desimone R. 1990. “Pathways for Motion Analysis: Cortical Connections of the Medial Superior Temporal and Fundus of the Superior Temporal Visual Areas in the Macaque.” The Journal of Comparative Neurology 296 (3): 462–95. [DOI] [PubMed] [Google Scholar]
- Boyd Jamie D., and Casagrande Vivien A.. 1999. “Relationships between Cytochrome Oxidase (CO) Blobs in Primate Primary Visual Cortex (V1) and the Distribution of Neurons Projecting to the Middle Temporal Area (MT).” The Journal of Comparative Neurology 409 (4): 573–91. [DOI] [PubMed] [Google Scholar]
- Brewer Alyssa A., Press William A., Logothetis Nikos K., and Wandell Brian A.. 2002. “Visual Areas in Macaque Cortex Measured Using Functional Magnetic Resonance Imaging.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 22 (23): 10416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann Korbinian. 1912. “Neue Ergebnisse über Die Vergleichende Histologische Lokalisation Der Grosshirnrinde Mit Besonderer Berücksichtigung Des Stirnhirns.” Anatomischer Anzeiger 41 (Suppl): 157–216. [Google Scholar]
- Bruner Emiliano, Preuss Todd M., Chen Xu, and Rilling James K.. 2017. “Evidence for Expansion of the Precuneus in Human Evolution.” Brain Structure & Function 222 (2): 1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant Katherine L., Li Longchuan, and Mars Rogier B.. 2018. “White Matter Projection Maps in Chimpanzees in Comparison with Humans and Macaques” Cortical Evolution Conference. Las Palmas, Spain. [Google Scholar]
- Butler Rebecca A., Lambon Ralph Matthew A., and Woollams Anna M.. 2014. “Capturing Multidimensionality in Stroke Aphasia: Mapping Principal Behavioural Components to Neural Structures.” Brain: A Journal of Neurology 137 (Pt 12): 3248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell Alfred Walter. 1905. Histological Studies on the Localisation of Cerebral Function. University Press. [Google Scholar]
- Cancelliere AE, and Kertesz A. 1990. “Lesion Localization in Acquired Deficits of Emotional Expression and Comprehension.” Brain and Cognition 13 (2): 133–47. [DOI] [PubMed] [Google Scholar]
- Catani Marco, Robertsson Naianna, Beyh Ahmad, Huynh Vincent, de Santiago Requejo Francisco, Howells Henrietta, Barrett Rachel L. C., et al. 2017. “Short Parietal Lobe Connections of the Human and Monkey Brain.” Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 97 (December): 339–57. [DOI] [PubMed] [Google Scholar]
- Catani Marco, and Thiebaut de Schotten Michel. 2008. “A Diffusion Tensor Imaging Tractography Atlas for Virtual in Vivo Dissections.” Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 44 (8): 1105–32. [DOI] [PubMed] [Google Scholar]
- Chen Xu, Errangi Bhargav, Li Longchuan, Glasser Matthew F., Westlye Lars T., Fjell Anders M., Walhovd Kristine B., et al. 2013. “Brain Aging in Humans, Chimpanzees (Pan Troglodytes), and Rhesus Macaques (Macaca Mulatta): Magnetic Resonance Imaging Studies of Macro- and Microstructural Changes.” Neurobiology of Aging 34 (10): 2248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia Michela, Bartolini Marco, Luzzi Simona, Provinciali Leandro, and Lambon Ralph Matthew A.. 2004. “Semantic Memory Is an Amodal, Dynamic System: Evidence from the Interaction of Naming and Object Use in Semantic Dementia.” Cognitive Neuropsychology 21 (5): 513–27. [DOI] [PubMed] [Google Scholar]
- Copland David A., de Zubicaray Greig I., McMahon Katie, Wilson Stephen J., Eastburn Matt, and Chenery Helen J.. 2003. “Brain Activity during Automatic Semantic Priming Revealed by Event-Related Functional Magnetic Resonance Imaging.” NeuroImage 20 (1): 302–10. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, and Damasio A. 2004. “Neural Systems behind Word and Concept Retrieval.” Cognition 92 (1–2): 179–229. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, and Fletcher P. 2000. “Dissociable Temporal Lobe Activations during Emotional Episodic Memory Retrieval.” NeuroImage 11 (3): 203–9. [DOI] [PubMed] [Google Scholar]
- Donahue Chad J., Glasser Matthew F., Preuss Todd M., Rilling James K., and Van Essen David C.. 2018. “Quantitative Assessment of Prefrontal Cortex in Humans Relative to Nonhuman Primates.” Proceedings of the National Academy of Sciences of the United States of America 115 (22): E5183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue Chad J., Sotiropoulos Stamatios N., Jbabdi Saad, Moises Hernandez-Fernandez Timothy E. Behrens, Dyrby Tim B., Coalson Timothy, et al. 2016a. “Using Diffusion Tractography to Predict Cortical Connection Strength and Distance: A Quantitative Comparison with Tracers in the Monkey.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 36 (25): 6758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue Chad J., Sotiropoulos Stamatios N., Jbabdi Saad, Moises Hernandez-Fernandez Timothy E. Behrens, Dyrby Tim B., Coalson Timothy, et al. 2016b. “Using Diffusion Tractography to Predict Cortical Connection Strength and Distance: A Quantitative Comparison with Tracers in the Monkey.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 36 (25): 6758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RW 1983. “Nongeniculate Afferents to Striate Cortex in Macaques.” The Journal of Comparative Neurology 218 (2): 159–73. [DOI] [PubMed] [Google Scholar]
- Dronkers Nina F., Wilkins David P., Van Valin Robert D. Jr, Redfern Brenda B., and Jaeger Jeri J.. 2004. “Lesion Analysis of the Brain Areas Involved in Language Comprehension.” Cognition 92 (1–2): 145–77. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, and Wandell BA. 1997. “Retinotopic Organization in Human Visual Cortex and the Spatial Precision of Functional MRI.” Cerebral Cortex 7 (2): 181–92. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, and Kanwisher N. 1999. “The Parahippocampal Place Area: Recognition, Navigation, or Encoding?” Neuron 23 (1): 115–25. [DOI] [PubMed] [Google Scholar]
- Epstein R, and Kanwisher N. 1998. “A Cortical Representation of the Local Visual Environment.” Nature 392 (6676): 598–601. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Cain WS, Novelly RA, and Mattson R. 1986. “Odor Perception in Temporal Lobe Epilepsy Patients with and without Temporal Lobectomy.” Neuropsychologia 24 (4): 553–62. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, and Van Essen DC. 1991. “Distributed Hierarchical Processing in the Primate Cerebral Cortex.” Cerebral Cortex 1 (1): 1–47. [DOI] [PubMed] [Google Scholar]
- Finstermeier Knut, Zinner Dietmar, Brameier Markus, Meyer Matthias, Kreuz Eva, Hofreiter Michael, and Roos Christian. 2013. “A Mitogenomic Phylogeny of Living Primates.” PloS One 8 (7): e69504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl Bruce. 2012. “FreeSurfer.” NeuroImage 62 (2): 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel Stephanie J., Thiebaut de Schotten Michel, Kawadler Jamie M., Dell’Acqua Flavio, Danek Adrian, and Catani Marco. 2014. “The Anatomy of Fronto-Occipital Connections from Early Blunt Dissections to Contemporary Tractography.” Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 56 (July): 73–84. [DOI] [PubMed] [Google Scholar]
- Frith Chris D. 2007. “The Social Brain?” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 362 (1480): 671–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass Ricardo, Sheila Nascimento-Silva Juliana G. M. Soares, Lima Bruss, Ana Karla Jansen Antonia Cinira M. Diogo, Farias Mariana F., et al. 2005a. “Cortical Visual Areas in Monkeys: Location, Topography, Connections, Columns, Plasticity and Cortical Dynamics.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 360 (1456): 709–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass Ricardo, Sheila Nascimento-Silva Juliana G. M. Soares, Lima Bruss, Ana Karla Jansen Antonia Cinira M. Diogo, Farias Mariana F., et al. 2005b. “Cortical Visual Areas in Monkeys: Location, Topography, Connections, Columns, Plasticity and Cortical Dynamics.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 360 (1456): 709–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser Matthew F., Coalson Timothy S., Robinson Emma C., Hacker Carl D., Harwell John, Yacoub Essa, Ugurbil Kamil, et al. 2016. “A Multi-Modal Parcellation of Human Cerebral Cortex.” Nature 536 (7615): 171–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser Matthew F., Goyal Manu S., Preuss Todd M., Raichle Marcus E., and Van Essen David C.. 2014. “Trends and Properties of Human Cerebral Cortex: Correlations with Cortical Myelin Content.” NeuroImage 93 Pt 2 (June): 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser Matthew F., Sotiropoulos Stamatios N., Wilson J. Anthony, Coalson Timothy S., Fischl Bruce, Andersson Jesper L., Xu Junqian, et al. 2013a. “The Minimal Preprocessing Pipelines for the Human Connectome Project.” NeuroImage 80 (October): 105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser Matthew F., Sotiropoulos Stamatios N., Wilson J. Anthony, Coalson Timothy S., Fischl Bruce, Andersson Jesper L., Xu Junqian, et al. 2013b. “The Minimal Preprocessing Pipelines for the Human Connectome Project.” NeuroImage 80 (October): 105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser Matthew F., and Van Essen David C.. 2011. “Mapping Human Cortical Areas in Vivo Based on Myelin Content as Revealed by T1- and T2-Weighted MRI.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 31 (32): 11597–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, and Van Essen DC. 2011. “Mapping Human Cortical Areas In Vivo Based on Myelin Content as Revealed by T1- and T2-Weighted MRI.” Journal of Neuroscience 31 (32): 11597–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS, and Tempini ML. 1998. “The Neural Systems Sustaining Face and Proper-Name Processing.” Brain: A Journal of Neurology 121 (Pt 11) (November): 2103–18. [DOI] [PubMed] [Google Scholar]
- Graham Mark S., Drobnjak Ivana, and Zhang Hui. 2016. “Realistic Simulation of Artefacts in Diffusion MRI for Validating Post-Processing Correction Techniques.” NeuroImage 125 (January): 1079–94. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, and Kaas JH. 1998. “Subdivisions of Auditory Cortex and Ipsilateral Cortical Connections of the Parabelt Auditory Cortex in Macaque Monkeys.” The Journal of Comparative Neurology 394 (4): 475–95. [DOI] [PubMed] [Google Scholar]
- Hecht Erin E., Gutman David A., Bradley Bruce A., Preuss Todd M., and Stout Dietrich. 2015. “Virtual Dissection and Comparative Connectivity of the Superior Longitudinal Fasciculus in Chimpanzees and Humans.” NeuroImage 108 (March): 124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht Erin E., Murphy Lauren E., Gutman David A., Votaw John R., Schuster David M., Preuss Todd M., Orban Guy A., Stout Dietrich, and Parr Lisa A.. 2013. “Differences in Neural Activation for Object-Directed Grasping in Chimpanzees and Humans.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 33 (35): 14117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel Martijn P., de Reus Marcel A., Feldman Barrett Lisa, Scholtens Lianne H., Coopmans Fraukje M. T., Schmidt Ruben, Preuss Todd M., Rilling James K., and Li Longchuan. 2015. “Comparison of Diffusion Tractography and Tract-Tracing Measures of Connectivity Strength in Rhesus Macaque Connectome.” Human Brain Mapping 36 (8): 3064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok Gregory, and Poeppel David. 2004. “Dorsal and Ventral Streams: A Framework for Understanding Aspects of the Functional Anatomy of Language.” Cognition 92 (1–2): 67–99. [DOI] [PubMed] [Google Scholar]
- Hickok Gregory, and Poeppel David. 2007. “The Cortical Organization of Speech Processing.” Nature Reviews. Neuroscience 8 (5): 393–402. [DOI] [PubMed] [Google Scholar]
- Hill Jason, Inder Terrie, Neil Jeffrey, Dierker Donna, Harwell John, and Van Essen David. 2010. “Similar Patterns of Cortical Expansion during Human Development and Evolution.” Proceedings of the National Academy of Sciences of the United States of America 107 (29): 13135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, and MacGregor LA. 1998. “Planum Temporale Asymmetries in Great Apes as Revealed by Magnetic Resonance Imaging (MRI).” Neuroreport 9 (12): 2913–18. [DOI] [PubMed] [Google Scholar]
- Horton JC, and Hoyt WF. 1991. “The Representation of the Visual Field in Human Striate Cortex. A Revision of the Classic Holmes Map.” Archives of Ophthalmology 109 (6): 816–24. [DOI] [PubMed] [Google Scholar]
- Hrvoj-Mihic Branka, Bienvenu Thibault, Stefanacci Lisa, Muotri Alysson R., and Semendeferi Katerina. 2013. “Evolution, Development, and Plasticity of the Human Brain: From Molecules to Bones.” Frontiers in Human Neuroscience 7 (October): 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi Saad, and Johansen-Berg Heidi. 2011. “Tractography: Where Do We Go from Here?” Brain Connectivity 1 (3): 169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi Saad, Lehman Julia F., Haber Suzanne N., and Behrens Timothy E.. 2013. “Human and Monkey Ventral Prefrontal Fibers Use the Same Organizational Principles to Reach Their Targets: Tracing versus Tractography.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 33 (7): 3190–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson Mark, Bannister Peter, Brady Michael, and Smith Stephen. 2002. “Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images.” NeuroImage 17 (2): 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson Mark, Beckmann Christian F., Behrens Timothy E. J., Woolrich Mark W., and Smith Stephen M.. 2012a. “FSL.” NeuroImage 62 (2): 782–90. [DOI] [PubMed] [Google Scholar]
- Jenkinson Mark, Beckmann Christian F., Behrens Timothy E. J., Woolrich Mark W., and Smith Stephen M.. 2012b. “FSL.” NeuroImage 62 (2): 782–90. [DOI] [PubMed] [Google Scholar]
- Jeurissen Ben, Leemans Alexander, Tournier Jacques-Donald, Jones Derek K., and Sijbers Jan. 2013. “Investigating the Prevalence of Complex Fiber Configurations in White Matter Tissue with Diffusion Magnetic Resonance Imaging.” Human Brain Mapping 34 (11): 2747–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster Hauke, Janssens Thomas, Orban Guy A., and Vanduffel Wim. 2014. “The Retinotopic Organization of Macaque Occipitotemporal Cortex Anterior to V4 and Caudoventral to the Middle Temporal (MT) Cluster.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 34 (31): 10168–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte Nikolaus, Formisano Elia, Sorger Bettina, and Goebel Rainer. 2007. “Individual Faces Elicit Distinct Response Patterns in Human Anterior Temporal Cortex.” Proceedings of the National Academy of Sciences of the United States of America 104 (51): 20600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, and Kaas JH. 1990. “Cortical Connections of MT in Four Species of Primates: Areal, Modular, and Retinotopic Patterns.” Visual Neuroscience 5 (2): 165–204. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph Matthew A., Sage Karen, Jones Roy W., and Mayberry Emily J.. 2010. “Coherent Concepts Are Computed in the Anterior Temporal Lobes.” Proceedings of the National Academy of Sciences of the United States of America 107 (6): 2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, and Rao SM. 2000. “Neural Systems Underlying the Recognition of Familiar and Newly Learned Faces.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 20 (2): 878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis James W., and Van Essen David C.. 2000. “Mapping of Architectonic Subdivisions in the Macaque Monkey, with Emphasis on Parieto-Occipital Cortex.” The Journal of Comparative Neurology 428 (1): 79–111. [DOI] [PubMed] [Google Scholar]
- Lewis JW, and Van Essen DC. 2000. “Corticocortical Connections of Visual, Sensorimotor, and Multimodal Processing Areas in the Parietal Lobe of the Macaque Monkey.” The Journal of Comparative Neurology 428 (1): 112–37. [DOI] [PubMed] [Google Scholar]
- Li Longchuan, Hu Xiaoping, Preuss Todd M., Glasser Matthew F., Damen Frederick W., Qiu Yuxuan, and Rilling James. 2013. “Mapping Putative Hubs in Human, Chimpanzee and Rhesus Macaque Connectomes via Diffusion Tractography.” NeuroImage 80 (October): 462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon David C., and Kaas Jon H.. 2002. “Evidence from V1 Connections for Both Dorsal and Ventral Subdivisions of V3 in Three Species of New World Monkeys.” The Journal of Comparative Neurology 449 (3): 281–97. [DOI] [PubMed] [Google Scholar]
- Lyon DC 2007. “The Evolution of Visual Cortex and Visual Systems” In Evolution of Nervous Systems, Volume 3: Mammals, edited by Kaas Jon H., 267–306. Oxford: Academic Press. [Google Scholar]
- Maier-Hein Klaus H., Neher Peter F., Houde Jean-Christophe, Marc-Alexandre Côté Eleftherios Garyfallidis, Zhong Jidan, Chamberland Maxime, et al. 2017. “The Challenge of Mapping the Human Connectome Based on Diffusion Tractography.” Nature Communications 8 (1): 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Preti MG, Asami T, Pelavin P, Campbell B, Papadimitriou GM, Kaiser J, et al. 2013. “Human Middle Longitudinal Fascicle: Variations in Patterns of Anatomical Connections.” Brain Structure & Function 218 (4): 951–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Daniel S., Harwell John, Olsen Timothy, Hodge Michael, Glasser Matthew F., Prior Fred, Jenkinson Mark, Laumann Timothy, Curtiss Sandra W., and Van Essen David C.. 2011. “Informatics and Data Mining Tools and Strategies for the Human Connectome Project.” Frontiers in Neuroinformatics 5 (June): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz MM, Ribeiro Gomes AR, Lamy C, Magrou L, Vezoli J, Misery P, et al. 2014. “A Weighted and Directed Interareal Connectivity Matrix for Macaque Cerebral Cortex.” Cerebral Cortex 24 (1): 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Misery P, Falchier A, Lamy C, Vezoli J, Quilodran R, Gariel MA, et al. 2011. “Weight Consistency Specifies Regularities of Macaque Cortical Networks.” Cerebral Cortex 21 (6): 1254–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Passingham RE, Neubert F-X, Verhagen L, and Sallet J. 2017. “Evolutionary Specializations of Human Association Cortex” In Evolution of Nervous Systems (Second Edition), Volume 4: The Evolution of the Human Brain: Apes and Other Ancestors, edited by Preuss TM Kaas J, 185–205. Oxford: Academic Press. [Google Scholar]
- Mars Rogier B., Sallet Jérôme, Neubert Franz-Xaver, and Rushworth Matthew F. S.. 2013a. “Connectivity Profiles Reveal the Relationship between Brain Areas for Social Cognition in Human and Monkey Temporoparietal Cortex.” Proceedings of the National Academy of Sciences of the United States of America 110 (26): 10806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars Rogier B., Sallet Jérôme, Neubert Franz-Xaver, and Rushworth Matthew F. S.. 2013b. “Connectivity Profiles Reveal the Relationship between Brain Areas for Social Cognition in Human and Monkey Temporoparietal Cortex.” Proceedings of the National Academy of Sciences of the United States of America 110 (26): 10806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, and van Essen DC. 1983a. “The Connections of the Middle Temporal Visual Area (MT) and Their Relationship to a Cortical Hierarchy in the Macaque Monkey.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 3 (12): 2563–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, and van Essen DC.. 1983b. “The Connections of the Middle Temporal Visual Area (MT) and Their Relationship to a Cortical Hierarchy in the Macaque Monkey.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 3 (12): 2563–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss Th. 1911. “Die Faserarchitektonische Gliederung Des Cortex Cerebri Der Anthropomorphen Affen.” J Psychol Neurol 18: 410–67. [Google Scholar]
- Menjot de Champfleur Nicolas, Igor Lima Maldonado Sylvie Moritz-Gasser, Machi Paolo, Emmanuelle Le Bars Alain Bonafé, and Duffau Hugues. 2013. “Middle Longitudinal Fasciculus Delineation within Language Pathways: A Diffusion Tensor Imaging Study in Human.” European Journal of Radiology 82 (1): 151–57. [DOI] [PubMed] [Google Scholar]
- Moerel Michelle, De Martino Federico, and Formisano Elia. 2014. “An Anatomical and Functional Topography of Human Auditory Cortical Areas.” Frontiers in Neuroscience 8 (July): 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, and Bullier J. 1990. “Anatomical Segregation of Two Cortical Visual Pathways in the Macaque Monkey.” Visual Neuroscience 4 (6): 555–78. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, and Kaas JH. 1993. “Tonotopic Organization, Architectonic Fields, and Connections of Auditory Cortex in Macaque Monkeys.” The Journal of Comparative Neurology 335 (3): 437–59. [DOI] [PubMed] [Google Scholar]
- Nasr Shahin, Liu Ning, Devaney Kathryn J., Yue Xiaomin, Rajimehr Reza, Ungerleider Leslie G., and Tootell Roger B. H.. 2011. “Scene-Selective Cortical Regions in Human and Nonhuman Primates.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 31 (39): 13771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert Franz-Xaver, Mars Rogier B., Sallet Jérôme, and Rushworth Matthew F. S.. 2015. “Connectivity Reveals Relationship of Brain Areas for Reward-Guided Learning and Decision Making in Human and Monkey Frontal Cortex.” Proceedings of the National Academy of Sciences of the United States of America 112 (20): E2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA 2017. “Evolution of Human Visual System Specializations” In Evolution of Nervous Systems (Second Edition), Vol. 4: The Evolution of the Human Brain. Apes and Other Ancestors, edited by Preuss TM Kaas J, 227–47. Oxford: Academic Press. [Google Scholar]
- Pammer Kristen, Hansen Peter C., Kringelbach Morten L., Holliday Ian, Barnes Gareth, Hillebrand Arjan, Singh Krish D., and Cornelissen Piers L.. 2004. “Visual Word Recognition: The First Half Second.” NeuroImage 22 (4): 1819–25. [DOI] [PubMed] [Google Scholar]
- Passingham Richard E., and Smaers Jeroen B.. 2014. “Is the Prefrontal Cortex Especially Enlarged in the Human Brain Allometric Relations and Remapping Factors.” Brain, Behavior and Evolution 84 (2): 156–66. [DOI] [PubMed] [Google Scholar]
- Perelman Polina, Johnson Warren E., Roos Christian, Seuánez Hector N., Horvath Julie E., Moreira Miguel A. M. Kessing Bailey, et al. 2011. “A Molecular Phylogeny of Living Primates.” PLoS Genetics 7 (3): e1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides Michael, and Pandya Deepak N.. 2007. “Efferent Association Pathways from the Rostral Prefrontal Cortex in the Macaque Monkey.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 27 (43): 11573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric Gorana, Jefferies Elizabeth, and Lambon Ralph Matthew A.. 2007. “Anterior Temporal Lobes Mediate Semantic Representation: Mimicking Semantic Dementia by Using rTMS in Normal Participants.” Proceedings of the National Academy of Sciences of the United States of America 104 (50): 20137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak S, and Hayashi Red. 1936. “The Cerebral Representation of the Retina in the Chimpanzee.” Brain: A Journal of Neurology 59 (1): 51–60. [Google Scholar]
- Preuss TM 2017a. “An Introduction to Human Brain Evolutionary Studies” In Evolution of Nervous Systems (Second Edition), Volume 4:, edited by Preuss Todd M Kaas Jon, 1–18. Oxford: Academic Press. [Google Scholar]
- Preuss TM 2017b. “The Human Brain: Evolution and Distinctive Features” In On Human Nature: Biology, Psychology, Ethics, Politics, and Religion, edited by Tibaynenc M and Ayala FJ, 129–49. Academic Preuss. [Google Scholar]
- Preuss Todd M. 2010. “Reinventing Primate Neuroscience for the Twenty-First Century” In Primate Neuroethology, edited by Platt ML Ghazanfar A, 422–54. Oxford University Press. [Google Scholar]
- Preuss Todd M. 2011. “The Human Brain: Rewired and Running Hot.” Annals of the New York Academy of Sciences 1225 Suppl 1 (May): E182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajimehr Reza, Devaney Kathryn J., Bilenko Natalia Y., Young Jeremy C., and Tootell Roger B. H.. 2011. “The ‘Parahippocampal Place Area’ Responds Preferentially to High Spatial Frequencies in Humans and Monkeys.” PLoS Biology 9 (4): e1000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch R, Serafetinides EA, and Crandall PH. 1977. “Olfactory Memory in Patients with Anterior Temporal Lobectomy.” Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 13 (4): 445–52. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, and Chen K. 1997. “Neuroanatomical Correlates of Externally and Internally Generated Human Emotion.” The American Journal of Psychiatry 154 (7): 918–25. [DOI] [PubMed] [Google Scholar]
- de Reus Marcel A., and van den Heuvel Martijn P.. 2013. “Estimating False Positives and Negatives in Brain Networks.” NeuroImage 70 (April): 402–9. [DOI] [PubMed] [Google Scholar]
- Reuter Martin, Schmansky Nicholas J., Diana Rosas H, and Fischl Bruce. 2012. “Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis.” NeuroImage 61 (4): 1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveley Colin, Seth Anil K., Pierpaoli Carlo, Silva Afonso C., Yu David, Saunders Richard C., Leopold David A., and Ye Frank Q.. 2015a. “Superficial White Matter Fiber Systems Impede Detection of Long-Range Cortical Connections in Diffusion MR Tractography.” Proceedings of the National Academy of Sciences of the United States of America 112 (21): E2820–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveley Colin, Seth Anil K., Pierpaoli Carlo, Silva Afonso C., Yu David, Saunders Richard C., Leopold David A., and Ye Frank Q.. 2015b. “Superficial White Matter Fiber Systems Impede Detection of Long-Range Cortical Connections in Diffusion MR Tractography.” Proceedings of the National Academy of Sciences of the United States of America 112 (21): E2820–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling James K. 2006. “Human and Nonhuman Primate Brains: Are They Allometrically Scaled Versions of the Same Design?” Evolutionary Anthropology 15 (2): 65–77. [Google Scholar]
- Rilling James K. 2008. “Neuroscientific Approaches and Applications within Anthropology.” American Journal of Physical Anthropology Suppl 47: 2–32. [DOI] [PubMed] [Google Scholar]
- Rilling James K., Glasser Matthew F., Jbabdi Saad, Andersson Jesper, and Preuss Todd M.. 2011. “Continuity, Divergence, and the Evolution of Brain Language Pathways.” Frontiers in Evolutionary Neuroscience 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling James K., Glasser Matthew F., Preuss Todd M., Ma Xiangyang, Zhao Tiejun, Hu Xiaoping, and Behrens Timothy E. J.. 2008a. “The Evolution of the Arcuate Fasciculus Revealed with Comparative DTI.” Nature Neuroscience 11 (March): 426. [DOI] [PubMed] [Google Scholar]
- Rilling James K., Glasser Matthew F., Preuss Todd M., Ma Xiangyang, Zhao Tiejun, Hu Xiaoping, and Behrens Timothy E. J.. 2008b. “The Evolution of the Arcuate Fasciculus Revealed with Comparative DTI.” Nature Neuroscience 11 (4): 426–28. [DOI] [PubMed] [Google Scholar]
- Rilling James K., and Seligman Rebecca A.. 2002. “A Quantitative Morphometric Comparative Analysis of the Primate Temporal Lobe.” Journal of Human Evolution 42 (5): 505–33. [DOI] [PubMed] [Google Scholar]
- Rilling JK, and Insel TR. 1999. “The Primate Neocortex in Comparative Perspective Using Magnetic Resonance Imaging.” Journal of Human Evolution 37 (2): 191–223. [DOI] [PubMed] [Google Scholar]
- Robinson Emma C., Jbabdi Saad, Glasser Matthew F., Andersson Jesper, Burgess Gregory C., Harms Michael P., Smith Stephen M., Van Essen David C., and Jenkinson Mark. 2014. “MSM: A New Flexible Framework for Multimodal Surface Matching.” NeuroImage 100 (October): 414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Saleem KS, and Tanaka K. 1994. “Divergent Feedback Connections from Areas V4 and TEO in the Macaque.” Visual Neuroscience 11 (3): 579–600. [DOI] [PubMed] [Google Scholar]
- Rockland KS, and Van Hoesen GW. 1994. “Direct Temporal-Occipital Feedback Connections to Striate Cortex (V1) in the Macaque Monkey.” Cerebral Cortex 4 (3): 300–313. [DOI] [PubMed] [Google Scholar]
- Rogers Timothy T., Lambon Ralph Matthew A., Garrard Peter, Bozeat Sasha, McClelland James L., Hodges John R., and Patterson Karalyn. 2004. “Structure and Deterioration of Semantic Memory: A Neuropsychological and Computational Investigation.” Psychological Review 111 (1): 205–35. [DOI] [PubMed] [Google Scholar]
- Rosa Marcello G. P., and Tweedale Rowan. 2005. “Brain Maps, Great and Small: Lessons from Comparative Studies of Primate Visual Cortical Organization.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 360 (1456): 665–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MG, Soares JG, Fiorani M Jr, and Gattass R. 1993. “Cortical Afferents of Visual Area MT in the Cebus Monkey: Possible Homologies between New and Old World Monkeys.” Visual Neuroscience 10 (5): 827–55. [DOI] [PubMed] [Google Scholar]
- Ross Lars A., and Olson Ingrid R.. 2010. “Social Cognition and the Anterior Temporal Lobes.” NeuroImage 49 (4): 3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxbury Tracy, McMahon Katie, and Copland David A.. 2014. “An fMRI Study of Concreteness Effects in Spoken Word Recognition.” Behavioral and Brain Functions: BBF 10 (1): 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem Kadharbatcha S., Price Joseph L., and Hashikawa Tsutomu. 2007. “Cytoarchitectonic and Chemoarchitectonic Subdivisions of the Perirhinal and Parahippocampal Cortices in Macaque Monkeys.” The Journal of Comparative Neurology 500 (6): 973–1006. [DOI] [PubMed] [Google Scholar]
- Schmahmann Jeremy D., Pandya Deepak N., Wang Ruopeng, Dai Guangping, D’Arceuil Helen E., de Crespigny Alex J., and Wedeen Van J.. 2007. “Association Fibre Pathways of the Brain: Parallel Observations from Diffusion Spectrum Imaging and Autoradiography.” Brain: A Journal of Neurology 130 (Pt 3): 630–53. [DOI] [PubMed] [Google Scholar]
- Schmolck H, and Squire LR. 2001. “Impaired Perception of Facial Emotions Following Bilateral Damage to the Anterior Temporal Lobe.” Neuropsychology 15 (1): 30–38. [PubMed] [Google Scholar]
- Schoenemann P. Thomas. 2006. “Evolution of the Size and Functional Areas of the Human Brain.” Annual Review of Anthropology 35 (1): 379–406. [Google Scholar]
- Schwartz Myrna F., Kimberg Daniel Y., Walker Grant M., Faseyitan Olufunsho, Brecher Adelyn, Dell Gary S., and Coslett H. Branch. 2009. “Anterior Temporal Involvement in Semantic Word Retrieval: Voxel-Based Lesion-Symptom Mapping Evidence from Aphasia.” Brain: A Journal of Neurology 132 (Pt 12): 3411–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, and Pandya DN. 1984. “Further Observations on Parieto-Temporal Connections in the Rhesus Monkey.” Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale 55 (2): 301–12. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, and Tootell RB. 1995a. “Borders of Multiple Visual Areas in Humans Revealed by Functional Magnetic Resonance Imaging.” Science 268 (5212): 889–93. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, and Tootell RB. 1995b. “Borders of Multiple Visual Areas in Humans Revealed by Functional Magnetic Resonance Imaging.” Science 268 (5212): 889–93. [DOI] [PubMed] [Google Scholar]
- Shipp S, and Zeki S. 1989. “The Organization of Connections between Areas V5 and V1 in Macaque Monkey Visual Cortex.” The European Journal of Neuroscience 1 (4): 309–32. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, and Evans AC. 1997. “A Role for the Right Anterior Temporal Lobe in Taste Quality Recognition.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 17 (13): 5136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Stephen M. 2002. “Fast Robust Automated Brain Extraction.” Human Brain Mapping 17 (3): 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Stephen M., Jenkinson Mark, Woolrich Mark W., Beckmann Christian F., Behrens Timothy E. J., Johansen-Berg Heidi, Bannister Peter R., et al. 2004. “Advances in Functional and Structural MR Image Analysis and Implementation as FSL.” NeuroImage 23 Suppl 1: S208–19. [DOI] [PubMed] [Google Scholar]
- Smith Stephen M., and Michael Brady J. 1997. “SUSAN—A New Approach to Low Level Image Processing.” International Journal of Computer Vision 23 (1): 45–78. [Google Scholar]
- Steiper Michael E., and Seiffert Erik R.. 2012. “Evidence for a Convergent Slowdown in Primate Molecular Rates and Its Implications for the Timing of Early Primate Evolution.” Proceedings of the National Academy of Sciences of the United States of America 109 (16): 6006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten Michel, Flavio Dell’Acqua Romain Valabregue, and Catani Marco. 2012. “Monkey to Human Comparative Anatomy of the Frontal Lobe Association Tracts.” Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 48 (1): 82–96. [DOI] [PubMed] [Google Scholar]
- Thomas Cibu, Ye Frank Q., Irfanoglu M. Okan, Modi Pooja, Saleem Kadharbatcha S., Leopold David A., and Pierpaoli Carlo. 2014. “Anatomical Accuracy of Brain Connections Derived from Diffusion MRI Tractography Is Inherently Limited.” Proceedings of the National Academy of Sciences of the United States of America 111 (46): 16574–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, and Tigges M. 1979. “Ocular Dominance Columns in the Striate Cortex of Chimpanzee (Pan Troglodytes).” Brain Research 166 (2): 386–90. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, and Hamilton SL. 1988. “Functional Anatomy of Macaque Striate Cortex. II. Retinotopic Organization.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 8 (5): 1531–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, and Taylor JB. 1995. “Anatomical Evidence for MT and Additional Cortical Visual Areas in Humans.” Cerebral Cortex 5 (1): 39–55. [DOI] [PubMed] [Google Scholar]
- Tootell Roger B. H., Tsao Doris, and Vanduffel Wim. 2003. “Neuroimaging Weighs In: Humans Meet Macaques in ‘Primate’ Visual Cortex.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 23 (10): 3981–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken And U., and Dronkers Nina F.. 2011. “The Neural Architecture of the Language Comprehension Network: Converging Evidence from Lesion and Connectivity Analyses.” Frontiers in Systems Neuroscience 5 (February): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, and Desimone R. 1986a. “Cortical Connections of Visual Area MT in the Macaque.” The Journal of Comparative Neurology 248 (2): 190–222. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, and Desimone R. 1986b. “Cortical Connections of Visual Area MT in the Macaque.” The Journal of Comparative Neurology 248 (2): 190–222. [DOI] [PubMed] [Google Scholar]
- Upadhyay Jaymin, Silver Andrew, Knaus Tracey A., Lindgren Kristen A., Ducros Mathieu, Kim Dae-Shik, and Tager-Flusberg Helen. 2008. “Effective and Structural Connectivity in the Human Auditory Cortex.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 28 (13): 3341–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen Van, David C, Glasser Matthew F., Dierker Donna L., Harwell John, and Coalson Timothy. 2012. “Parcellations and Hemispheric Asymmetries of Human Cerebral Cortex Analyzed on Surface-Based Atlases.” Cerebral Cortex 22 (10): 2241–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen Van, David C, Jbabdi Saad, Sotiropoulos Stamatios N., Chen Charles, Dikranian Krikor, Coalson Tim, Harwell John, Behrens Timothy E. J., and Glasser Matthew F.. 2014. “Chapter 16 - Mapping Connections in Humans and Non-Human Primates: Aspirations and Challenges for Diffusion Imaging” In Diffusion MRI (Second Edition), 337–58. San Diego: Academic Press. [Google Scholar]
- Essen Van, David C, Smith Stephen M., Barch Deanna M., Behrens Timothy E. J., Yacoub Essa, Ugurbil Kamil, and WU-Minn HCP Consortium. 2013. “The WU-Minn Human Connectome Project: An Overview.” NeuroImage 80 (October): 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE 1938. “The Thalamus of the Chimpanzee: IV. Thalamic Projections to the Cerebral Cortex.” Journal of Anatomy 73 (Pt 1): 37–93. [PMC free article] [PubMed] [Google Scholar]
- Wandell Brian A., Dumoulin Serge O., and Brewer Alyssa A.. 2007. “Visual Field Maps in Human Cortex.” Neuron 56 (2): 366–83. [DOI] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, and Zeki S. 1993. “Area V5 of the Human Brain: Evidence from a Combined Study Using Positron Emission Tomography and Magnetic Resonance Imaging.” Cerebral Cortex 3 (2): 79–94. [DOI] [PubMed] [Google Scholar]
- Weller RE, and Kaas JH. 1983. “Retinotopic Patterns of Connections of Area 17 with Visual Areas V-II and MT in Macaque Monkeys.” The Journal of Comparative Neurology 220 (3): 253–79. [DOI] [PubMed] [Google Scholar]
- Wilkinson Richard D., Steiper Michael E., Soligo Christophe, Martin Robert D., Yang Ziheng, and Tavaré Simon. 2011. “Dating Primate Divergences through an Integrated Analysis of Palaeontological and Molecular Data.” Systematic Biology 60 (1): 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Maximiliano A., Joubert Sven, Perrine Ferré Sylvie Belleville, Ana Inés Ansaldo Yves Joanette, Rouleau Isabelle, and Brambati Simona Maria. 2012. “The Role of the Left Anterior Temporal Lobe in Exception Word Reading: Reconciling Patient and Neuroimaging Findings.” NeuroImage 60 (4): 2000–2007. [DOI] [PubMed] [Google Scholar]
- Zahn Roland, Moll Jorge, Iyengar Vijeth, Huey Edward D., Tierney Michael, Krueger Frank, and Grafman Jordan. 2009. “Social Conceptual Impairments in Frontotemporal Lobar Degeneration with Right Anterior Temporal Hypometabolism.” Brain: A Journal of Neurology 132 (Pt 3): 604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn Roland, Moll Jorge, Krueger Frank, Huey Edward D., Garrido Griselda, and Grafman Jordan. 2007. “Social Concepts Are Represented in the Superior Anterior Temporal Cortex.” Proceedings of the National Academy of Sciences of the United States of America 104 (15): 6430–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky Andrew, Fornito Alex, Cocchi Luca, Gollo Leonardo L., van den Heuvel Martijn P., and Breakspear Michael. 2016. “Connectome Sensitivity or Specificity: Which Is More Important?” NeuroImage 142 (November): 407–20. [DOI] [PubMed] [Google Scholar]
- Zeki SM 1969. “Representation of Central Visual Fields in Prestriate Cortex of Monkey.” Brain Research 14 (2): 271–91. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, and Smith S. 2001. “Segmentation of Brain MR Images through a Hidden Markov Random Field Model and the Expectation-Maximization Algorithm.” IEEE Transactions on Medical Imaging 20 (1): 45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Macaque MT+ convergence results: A. Central V1 and Large MT+ masks; B. Intraparietal sulcus and Extrastriate masks; C. Representative subject results of central V1 connectivity to large MT+ mask; D: Representative subject results of IPS connectivity with extrastriate mask. Surface projection results are outlined in black to enhance visibility.
Supplemental Figure S2: Chimpanzee MT+ convergence results: A. Central V1 and Large MT+ masks; B. Intraparietal sulcus and Extrastriate masks; C. Representative results of central V1 connectivity to large MT+ mask; D: Representative results of IPS connectivity with extrastriate mask. Results are thresholded at 99% for all subjects. IPS-Extrastriate results (D) further exclude the upper 15 percent. Surface projection results are outlined in black to enhance visibility. E: Detail of Suwannee V1-MT+ results. F: Detail of Bo V1-MT+ results. G: Detail of Suwannee IPS-Extrastriate results. H: Detail of Agatha IPS-Extrastriate results. Asterisk indicates center of densely-myelinated area MT.
Supplemental Figure S3: Human MT+ convergence results: A. Central V1 and Large MT+ masks; B. Intraparietal sulcus and Extrastriate masks; C. Representative results of central V1 connectivity with large MT+ mask; D: Representative results of IPS connectivity with extrastriate mask. Surface projection results are outlined in black to enhance visibility. E-H: Close-up of MT+ lacunae. E. V1c, subject 103414; F. V1c, subject 103414; G. IPS, subject 117122; H. IPS, subject 151223. Asterisk indicates center of densely-myelinated area MT.