Abstract
Our aim was to estimate how long-term mortality following breast cancer diagnosis depends on age at diagnosis, tumor estrogen receptor (ER) status, and the time already survived. We used the population-based Australian Breast Cancer Family Study which followed-up 1,196 women enrolled during 1992–99 when aged <60 years at diagnosis with a first primary invasive breast cancer, over-sampled for younger ages at diagnosis, for whom tumor pathology features and ER status were measured. There were 375 deaths (median follow-up=15.7; range=0.8–21.4, years). We estimated the mortality hazard as a function of time since diagnosis using a flexible parametric survival analysis with ER status a time-dependent covariate. For women with ER-negative tumors compared with those with ER-positive tumors, 5-year mortality was initially higher (P<.001), similar if they survived to 5 years (P=0.4), and lower if they survived to 10 years (P=0.02). The estimated mortality hazard for ER-negative disease peaked at ~3 years post-diagnosis, thereafter declined with time, and at 7 years post-diagnosis became lower than that for ER-positive disease. This pattern was more pronounced for women diagnosed at younger ages. Mortality was also associated with lymph node count (hazard ratio (HR) per 10 nodes=2.52 [95% CI:2.11–3.01]) and tumor grade (HR per grade=1.62 [95% CI:1.34–1.96]). The risk of death following a breast cancer diagnosis differs substantially and qualitatively with diagnosis age, ER status and time survived. For women who survive >7 years, those with ER-negative disease will on average live longer, and more so if younger at diagnosis.
Keywords: Breast cancer, cohort study, estrogen receptor, mortality, survival, time-dependent effects
Introduction
Breast cancer is a heterogeneous disease, not only in terms of its clinicopathological characteristics, gene expression patterns and oncogenic signaling pathways, but also in terms of outcome.1 The estrogen receptor (ER) status of a tumor is a predictor of endocrine responsiveness.2 Studies have reported higher mortality in the first 5 years post-diagnosis for women diagnosed with ER-negative tumors compared with those with ER-positive disease; this was no longer evident or reversed for women who survived past that time.3, 4 Although lower long-term survival and shorter disease-free interval have also been reported for women with ER-positive tumors,5, 6 how the risk of death differs by ER status and time since diagnosis over a long time period is less well established.
Differences in risk of death by ER status have also been observed for women diagnosed at a young age3 and consequently more likely to have ER-negative tumors.1 Other factors potentially associated with mortality following breast cancer include axillary node involvement, tumor grade and size.2 Whether ER status and younger age at diagnosis are independent predictors of mortality is not well understood.1, 7–9 Studying this has been complicated by the fact that a differential mortality hazard by ER status over time violates the proportional hazards assumption of the Cox regression model.
The aim of this study was to examine how mortality following a breast cancer diagnosis depends on age at diagnosis, tumor estrogen receptor (ER) status and the time already survived. We used an Australian population-based breast cancer family study that over-sampled women diagnosed at younger ages. Specifically, we estimated the mortality hazard following a breast cancer diagnosis as a flexible function of time since diagnosis to determine the extent to which long-term mortality depends on how long a woman has already survived, and to see if and how this depends on the ER status of her tumor and her age at diagnosis. Once we established an appropriate way to model mortality hazard as a function of ER status by age at diagnosis, we tried to identify other predictors of mortality.
Material and methods
Subjects
Subjects were women recruited to the Australian Breast Cancer Family Study (ABCFS), a population-based case-control-family study of breast cancer,10–13 who were aged <60 years and living in metropolitan Melbourne or Sydney when they were diagnosed between 1992 and 1999 with an incident, histologically confirmed, first primary invasive breast cancer reported to the population-complete Victorian and New South Wales cancer registries to which notification is compulsory under state legislation. Selection for recruitment was at random, irrespective of family history, but stratified by age at diagnosis as described in detail previously.13 Briefly, all women aged <40 years at diagnosis during January 1992-September 1999 in Melbourne and during January 1993-December 1998 in Sydney were selected, and random samples of women aged 40–59 years at diagnosis in 5 year categories were selected from January 1996 onward.
Institutional ethics committees of The University of Melbourne, Cancer Council Victoria and New South Wales Cancer Council approved the study. Written informed consent was obtained from all study participants.
We excluded women with missing ER status (n=129), with a known BRCA1 or BRCA2 gene mutation (n=71), or for whom there was no follow-up (n=132), leaving 1,196 women with breast cancer for this analysis. They were followed up for a total of 15,762 person-years (median = 15.7 years; range = 0.8 to 21.4 years) during which time 375 deaths were identified.
Table 1 shows that the median age at breast cancer diagnosis was 42 years (range = 23 to 59 years). Nearly 45% of all women were aged <40 years at baseline, reflecting that sampling of this age at diagnosis group was carried out over a longer time period, and by design the remainder were approximately equally made up of those aged 40–49 and 50–59 years. Women aged <35 years at diagnosis accounted for 22% of deaths while those aged 35–39, 40–49 and 50–59 years accounted for 34%, 24% and 20% of deaths, respectively. The corresponding percentages of the total sample at baseline were 17%, 28%, 28% and 27%, respectively. Overall, 32% of tumors were ER-negative, 40% were grade 3, the median tumor diameter was 15 mm and 40% of women had positive lymph nodes. Of tumors diagnosed during 1992–95, 42% were ER-negative, compared with 29% of tumors diagnosed thereafter.
Table 1.
Characteristics of participants by age at diagnosis: Australian Breast Cancer Family Study
| Characteristic | Age <35 years (n=201, 16.8%) |
Age 35–39 years (n=336, 28.1%) |
Age 40–49 years (n= 337, 28.2%) |
Age 50–59 years (n=322, 26.9%) |
All (n=1,196, 100%) |
|---|---|---|---|---|---|
| Age at diagnosis (median, IQR), years | 32 (30–33) | 37 (36–38) | 45 (43–48) | 54 (52–57) | 42 (36–50) |
| Year of diagnosis, (n, %) | |||||
| 1992–95 | 88 (43.8) | 150 (44.6) | 46 (13.7) | 35 (10.9) | 319 (26.7) |
| 1996–99 | 113 (56.2) | 186 (55.4) | 291 (86.3) | 287 (89.1) | 877 (73.3) |
| Age at menarche (median, IQR), years | 13 (12–14) | 13 (12–14) | 13 (12–14) | 13 (12–14) | 13 (12–14) |
| Age at first birth (median, IQR), yearsa | 26 (23–28) | 27 (24–30) | 25 (22–29) | 24 (21–27) | 25 (22–29) |
| No. with children (n, %) | 128 (63.7) | 255 (75.9) | 278 (82.5) | 278 (86.3) | 939 (78.5) |
| No. of pregnancies (median, IQR) | 2 (0–3) | 2 (1–3) | 2 (2–3) | 3 (2–4) | 2 (1–3) |
| Use of contraceptive pill (n, %) | |||||
| Ever | 184 (91.5) | 303 (90.2) | 292 (86.7) | 252 (78.3) | 1,031 (86.2) |
| Never | 17 (8.5) | 33 (9.8) | 45 (13.3) | 70 (21.7) | 165 (13.8) |
| Menopausal status (n, %) | |||||
| Premenopausal | 197 (98.0) | 328 (97.6) | 303 (89.9) | 126 (39.1) | 954 (79.8) |
| Postmenopausal | 4 (2.0) | 8 (2.4) | 34 (10.1) | 196 (60.9) | 242 (20.2) |
| Hormone replacement therapy (n, %) | |||||
| No | 199 (99.0) | 323 (96.1) | 299 (88.7) | 140 (43.5) | 961 (80.4) |
| Yes | 2 (1.0) | 11 (3.3) | 38 (11.3) | 181 (56.2) | 232 (19.4) |
| Missing/unknown | 0 (0.0) | 2 (0.6) | 0 (0.0) | 1 (0.3) | 3 (0.2) |
| No. with first- or second-degree blood relatives with invasive breast cancer (n, %) |
|||||
| First degree | 20 (10.0) | 30 (8.9) | 42 (12.5) | 44 (13.7) | 136 (11.4) |
| Second degree | 44 (21.9) | 82 (24.4) | 74 (22.0) | 68 (21.1) | 268 (22.4) |
IQR = interquartile range.
For those with at least one live birth.
Table 2 shows that tumors were ER-negative for 42% of the women aged <35 years at diagnosis compared with 24% for those aged 50–59 years at diagnosis. The histological grade distribution differed substantially between ER-negative and ER-positive tumors.
Table 2.
Tumor characteristics of participants by age at diagnosis and estrogen receptor (ER) status: Australian Breast Cancer Family Study
| Characteristic | Age <35 years | Age 35–39 years | Age 40–49 years | Age 50–59 years | ||||
|---|---|---|---|---|---|---|---|---|
| ER-negative (n=84, 41.8%) |
ER-positive (n=117, 58.2%) |
ER-negative (n=124, 36.9%) |
ER-positive (n=212, 63.1%) |
ER-negative (n=102, 30.3%) |
ER-positive (n=235, 69.7%) |
ER-negative (n=77, 23.9%) |
ER-positive (n=245, 76.1%) |
|
| Histological type (%) | ||||||||
| Mixed ductal & lobular | 2 (2.4) | 6 (5.1) | 4 (3.2) | 24 (11.3) | 4 (3.9) | 13 (5.5) | 5 (6.5) | 12 (4.9) |
| Ductal | 74 (88.1) | 96 (82.1) | 107 (86.3) | 162 (76.4) | 82 (80.4) | 189 (80.4) | 59 (76.6) | 185 (75.5) |
| Lobular | 0 (0.0) | 10 (8.6) | 3 (2.4) | 19 (9.0) | 7 (6.7) | 19 (8.1) | 5 (6.5) | 31 (12.7) |
| Other | 8 (9.5) | 5 (4.2) | 10 (8.1) | 7 (3.3) | 9 (9.0) | 14 (6.0) | 8 (10.4) | 17 (6.9) |
| Histological grade (%) | ||||||||
| 1 | 2 (2.4) | 15 (12.8) | 2 (1.6) | 33 (15.6) | 6 (5.9) | 49 (20.8) | 5 (6.5) | 72 (29.4) |
| 2 | 16 (19.1) | 47 (40.2) | 32 (25.8) | 101 (47.6) | 25 (24.5) | 99 (42.1) | 23 (29.9) | 108 (44.1) |
| 3 | 60 (71.4) | 47 (40.2) | 79 (63.7) | 62 (29.3) | 63 (61.8) | 77 (32.8) | 41 (53.2) | 50 (20.4) |
| Missing/unknown | 6 (7.1) | 8 (6.8) | 11 (8.9) | 16 (7.5) | 8 (7.8) | 10 (4.3) | 8 (10.4) | 15 (6.1) |
| PR status (%) | ||||||||
| PR-negative | 56 (66.7) | 10 (8.5) | 82 (66.1) | 21 (9.9) | 62 (60.8) | 22 (9.4) | 57 (74.0) | 45 (18.4) |
| PR-positive | 28 (33.3) | 106 (90.6) | 42 (33.9) | 191 (90.1) | 40 (39.2) | 213 (90.6) | 20 (26.0) | 199 (81.2) |
| Missing/unknown | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Tumor size (median, IQR), mm | 19 (13–25) | 15 (10–20) | 15 (12–20)a | 15 (10–25) | 19 (13–25) | 15 (11–24) | 16.5 (10–20) | 13 (9–20) |
| Missing/unknown (%) | 36 (42.9) | 52 (44.4) | 56 (45.2) | 86 (40.6) | 46 (45.1) | 114 (48.5) | 43 (55.8) | 142 (58.0) |
| No. with ≥1 axillary lymph node positive (%) | 33 (39.3) | 52 (44.4) | 43 (34.7) | 95 (44.8) | 44 (43.1) | 100 (42.6) | 30 (39.0) | 85 (34.7) |
| Missing/unknown | 7 (8.3) | 11 (9.4) | 15 (12.1) | 17 (8.0) | 3 (2.9) | 8 (3.4) | 5 (6.5) | 13 (5.3) |
| No. of positive lymph nodes (mean, SD) | 3 (6) | 2 (3) | 2 (4) | 2 (3) | 2 (3) | 2 (5) | 2 (3) | 2 (4) |
| Missing/unknown (%) | 7 (8.3) | 11 (9.4) | 15 (12.1) | 17 (8.0) | 3 (2.9) | 8 (3.4) | 5 (6.5) | 13 (5.3) |
ER = estrogen-receptor; PR = progesterone-receptor; IQR = interquartile range; SD = standard deviation.
n=3 marked as <1mm not included.
Questionnaire
Women with breast cancer completed an interviewer-administered baseline questionnaire that asked about epidemiological risk factors (demographic background, personal characteristics, medical and reproductive histories, anthropometric measures, and environmental and lifestyle factors) up until one year prior to diagnosis.11–13 Information on tumor- and adjuvant treatment-related factors was obtained by abstracting from medical records and administering a self-reported questionnaire.14
Breast cancer pathology review
A central review of tumor histologic type, grade and axillary nodal status was performed.15, 16 ER and progesterone receptor (PR) status of primary tumors was obtained from centralized immunohistochemical testing of tumor tissue17 or abstracted from diagnostic histopathology reports held by the cancer registry or in medical records.18
Follow-up and ascertainment of deaths
Systematic re-contact was sought with all women approximately 10 years and 15 years after their baseline interview. Questionnaires updating baseline information and personal and family cancer histories were either interviewer-administered by telephone or self-administered, with a telephone interview used to obtain additional details if required. Vital status and cancer-related outcomes were also updated through linkage to cancer registries and the national death index.
Statistical analysis
Observation time started at date of diagnosis of breast cancer and ended at either date of death or the last follow-up date, whichever occurred earliest. Overall survival, defined as the time from date of invasive breast cancer diagnosis to death from any cause, was assessed using Kaplan-Meier curves. We estimated the mortality hazard as a function of time since diagnosis. Mortality by tumor ER status and length of survival (to baseline, and to 5 and 10 years, respectively) was estimated using the cumulative hazard function.
To assess differences in mortality hazard by tumor ER status, we fitted a flexible parametric survival model using restricted cubic splines within the stpm2 command in Stata, where ER status was treated as a covariate with time-dependent effects to overcome issues related to time-dependency of differential survival by ER status.19, 20 The model did not adjust for other factors. Varying degrees of freedom for the baseline mortality hazard and time-dependent effect were explored, with the best model fit assessed using the Akaike Information Criterion21 found to come from setting the number of degrees of freedom to 3 and 1 for the baseline mortality hazard and the time-dependent effect, respectively.
We plotted the estimated time-dependent mortality hazards and hazard ratio (HR), and their 95% confidence intervals (CIs), over time since diagnosis by tumor ER status. We did this for women overall, and separately for women diagnosed at age 35, 45 and 55 years.
To examine additional predictors of survival, we devised a multiple imputation model to account for missing values of tumor size (n=575; 48.1%), tumor grade (n=82; 6.9%) and the number of positive lymph nodes (n=79; 6.6%), assuming these data were missing at random, conditional on the variables in the imputation model. The outcome variable (mortality) and predictor variables (ER status, age at breast cancer diagnosis, year of diagnosis) were also included in the imputation model. Tumor size and the number of positive lymph nodes were imputed using linear regression, and tumor grade using ordinal logistic regression. Missing values were sampled and replaced with a set of plausible values randomly drawn from their predicted distribution based on the other observed variables, thus creating 10 complete data sets.
Unadjusted and Adjusted HRs and 95% CIs were calculated using flexible parametric regression. Regression models were run separately for each imputed data set and estimates of the predictor variables were combined using the programs written by Carlin et al.22 Multivariable models were adjusted for age at diagnosis (continuous, per 10-year increment), year of diagnosis (1992–95/1996–99), tumor size (continuous, per 10 mm), tumor grade (continuous) and number of positive lymph nodes (continuous, per 10 nodes), and fitting ER status as a covariate with time-dependent effects, setting the number of degrees of freedom to 2 for the time-dependent effect. Models were also run separately by ER status. We fitted interaction terms for age with each of grade, tumor size and number of nodes to test for differences in associations by age at diagnosis. We repeated the analysis using the complete case series.
All statistical tests were two-sided, and P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using Stata 14.2 (StataCorp, College Station, TX).
Results
Mortality overall
Figure 1 shows the Kaplan-Meier survival curves from which the 5- and 10-year overall survivals were estimated to be 86.3% (95% CI: 84.2–88.2%) and 76.3% (95% CI: 73.7–78.6%), respectively. Overall, women diagnosed with ER-negative breast cancers had lower survival than those with ER-positive disease (P <.001), and the 10-year survivals were 68.9 (95% CI: 64.0–73.3) and 79.8 (95% CI: 76.8–82.4), respectively (Figure 1A). Higher survival with later age at diagnosis was more obvious for women with ER-positive tumors (Figure 1B).
Figure 1.

Kaplan-Meier overall survival estimates from a population-based sample of women with breast cancer in the Australian Breast Cancer Family Study as a function of: estrogen receptor (ER) status of their tumors (ER-positive, blue; ER-negative, red; 95% confidence intervals, shaded area) (A); and age at diagnosis (<35, blue; 35–39, red; 40–49, green; 50–59, yellow) for ER-positive (B) and ER-negative (C) disease.
Five-year mortality by length of survival
Figure 2 shows that, post-diagnosis, women diagnosed with ER-negative tumors had higher 5-year mortality than those diagnosed with ER-positive tumors (P<.001); see Figure 2A. For those women who survived to 5 years, the subsequent 5-year mortality did not differ appreciably by ER status (P=0.4); see Figure 2B. However, for women who survived to 10 years, those with ER-positive tumors had a higher 5-year mortality thereafter than those with ER-negative tumors (P = 0.02); see Figure 2C.
Figure 2.
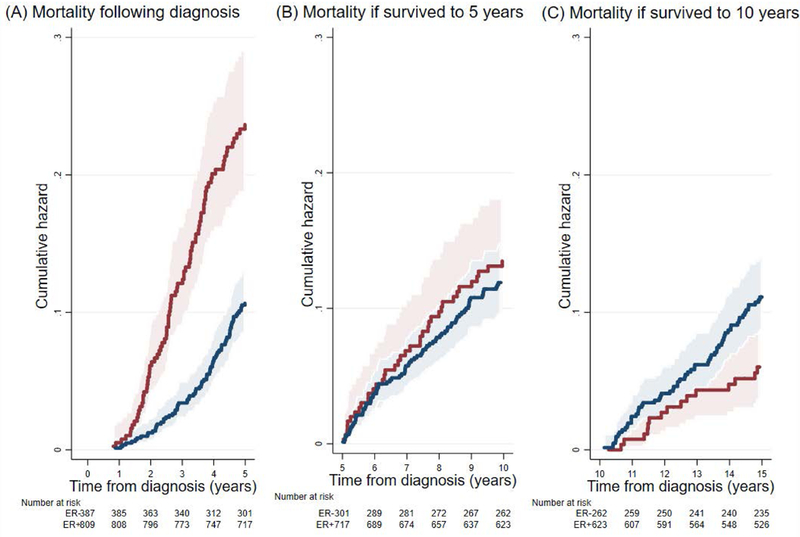
Five-year mortality estimated from a population-based sample of women with breast cancer in the Australian Breast Cancer Family Study in terms of time since: they were diagnosed with breast cancer (A); if they survived to 5 years (B); and if they survived to 10 years (C). Estrogen receptor (ER)-positive, blue; ER-negative, red; 95% confidence intervals, shaded area.
Mortality hazard as a function of time since diagnosis by ER status
Figure 3 shows graphically that the mortality hazard predicted by the model fitting differed by ER-status and, especially for women with ER-negative tumors, by age at diagnosis. Figures 3A, 3B and 3C show that the mortality hazard for women with ER-negative and ER-positive tumors both peaked at about 3 years post diagnosis. The mortality hazards were higher, and the differences in risk between ER-negative and ER-positive tumors were greater, for women diagnosed at younger ages. For example, for women diagnosed with breast cancer at age 35 years, the mortality hazard was initially higher for women with ER-negative tumors and peaked at about 93/1,000 person-years compared with 43/1,000 person-years for women with ER-positive tumors (Figure 3A). The mortality hazards declined with time since diagnosis, more rapidly for women with ER-negative tumors, and crossed-over at about 7 years post-diagnosis, irrespective of the age at diagnosis. The mortality hazards and HR over time by ER status, estimated from combining data on all women with breast cancer, are shown in Supplementary Figure 1. After about 10 years post-diagnosis, the mortality hazard for women with ER-positive tumors was higher than for those with ER-negative tumors.
Figure 3.

Predicted time-varying mortality hazard from a population-based sample of women with breast cancer in the Australian Breast Cancer Family Study at: age 35 years (A); age 45 years (B); and age 55 years (C) for estrogen receptor (ER)-positive (thick blue line) and ER-negative (thick red line) tumors. 95% confidence limits, thin blue and red lines.
Predictors of mortality hazard
Table 3 shows that, when analyzing all women combined, age at diagnosis, year of diagnosis, ER status, tumor grade, tumor size and the number of positive lymph nodes were all univariately associated with mortality hazard (Unadjusted model). From the Adjusted models (Models 1–8), all of which included age at and year of diagnosis and fitted ER status as a covariate with time-dependent effects, older age at diagnosis was consistently associated with lower mortality hazard (e.g. HR=0.79 [95% CI: 0.70–0.90] per 10-year increment in Model 8). Part of this was explained statistically by year of diagnosis and tumor grade (Unadjusted model versus Model 2). Year of diagnosis was not associated with mortality after adjusting for age at diagnosis (HR=0.84 [95% CI: 0.67–1.06] for 1996–99 compared with 1992–95 in Model 1). Tumor size was not associated with mortality hazard after adjusting for number of positive lymph nodes (HR=1.07 [95% CI: 0.97–1.19] per 10 mm in Model 7 and HR=1.04 [95% CI: 0.93–1.16] per 10 mm in Model 8). The associations of tumor grade (HR=1.62 [95% CI: 1.34–1.96] per grade in Model 8) and especially the number of positive lymph nodes (HR=2.52 [95% CI: 2.11–3.01] per 10 nodes in Model 8) with mortality were little altered after adjusting for other covariates. The best fitting model was Model 6.
Table 3.
Mortality hazard ratio (HR) following a diagnosis of breast cancer for potential predictors: Australian Breast Cancer Family Study
| Models | Age at diagnosis (per 10-year increment) HR (95% CI) |
Year of diagnosis (1996–99 cf. 1992–95) HR (95% CI) |
Tumor grade (per grade) HR (95% CI) |
Tumor size (per 10 mm) HR (95% CI) |
No. of positive lymph nodes (per 10 nodes) HR (95% CI) |
|---|---|---|---|---|---|
| Unadjusted | 0.71 (0.63–0.80) | 0.68 (0.55–0.84) | 1.89 (1.60–2.23) | 1.23 (1.13–1.34) | 2.68 (2.32–3.09) |
| Adjusteda | |||||
| Model 1 | 0.75 (0.66–0.85) | 0.84 (0.67–1.06) | - | - | - |
| Model 2 | 0.79 (0.69–0.90) | 0.85 (0.68–1.07) | 1.76 (1.48–2.11) | - | - |
| Model 3 | 0.76 (0.66–0.86) | 0.82 (0.65–1.03) | - | 1.24 (1.13–1.36) | - |
| Model 4 | 0.76 (0.67–0.86) | 0.79 (0.63–1.00) | - | - | 2.75 (2.38–3.18) |
| Model 5 | 0.79 (0.69–0.90) | 0.84 (0.67–1.05) | 1.67 (1.38–2.01) | 1.20 (1.08–1.32) | - |
| Model 6 | 0.79 (0.69–0.90) | 0.80 (0.64–1.01) | 1.64 (1.36–1.97) | - | 2.61 (2.24–3.03) |
| Model 7 | 0.76 (0.67–0.87) | 0.78 (0.62–0.99) | - | 1.07 (0.97–1.19) | 2.58 (2.17–3.07) |
| Model 8 | 0.79 (0.70–0.90) | 0.80 (0.63–1.01) | 1.62 (1.34–1.96) | 1.04 (0.93–1.16) | 2.52 (2.11–3.01) |
CI = confidence interval.
Adjusted for variables shown under each model and estrogen receptor status fitted as a covariate with time-dependent effects.
Results were similar when using the complete case series (Supplementary Table 1). The best fitting model was Model 6 (AIC = 909; Supplementary Table 1). There was no evidence of differences in these associations by age at diagnosis (Pinteraction>0.05).
Supplementary Table 2 shows that, when considering only women with ER-positive tumors, a similar pattern of associations was found as in Table 3. Supplementary Table 3 shows that, when considering only women with ER-negative tumors, associations with age at diagnosis and tumor grade were less evident and there was no association with year of diagnosis.
Discussion
From following women with a breast cancer diagnosis, more than 50% for longer than 15 years post-diagnosis, we have found that the risk of death differs substantially and qualitatively between ER-negative and ER-positive disease in terms of both length of survival and age at diagnosis. Our modelling predicts that for women who survive >7 years, those with ER-negative disease will on average live longer, the more so the younger the age at diagnosis.
The number of positive lymph nodes was an independent predictor of mortality hazard and of the same magnitude for both ER-negative and ER-positive breast cancer, whereas associations with age at diagnosis and tumor grade were less evident for ER-negative breast cancer. An association between a more recent diagnosis and lower mortality hazard was limited to ER-positive tumors. Adjusting for number of positive lymph nodes resulted in attenuation of the HR for tumor size.
Our study is one of only a few population-based studies that have examined variation in the rate at which women die following a diagnosis of either ER-negative or ER-positive breast cancer over a long period of time. From our analysis which took into account time-dependency of differential mortality by ER status, we found that the mortality hazard was initially higher for women with ER-negative tumors compared with those with ER-positive tumors, but this declined with time to eventually become reversed after about 7 years post-diagnosis. These differences in mortality hazard over time according to tumor ER status were more pronounced for women diagnosed at younger ages.
Findings similar to ours had previously been observed for women aged 18–40 years at diagnosis who were enrolled in the UK-based Prospective Study of Outcomes in Sporadic and Hereditary Breast Cancer (POSH),3 which also found an increasing mortality hazard for women diagnosed with ER-positive tumors beyond 5 years post-diagnosis which a study using US-based Surveillance Epidemiology and End Results data for non-age-selected patients did not find.23 A recent publication of data from a hospital-based cohort in North America also found that mortality according to ER status differed by age at diagnosis.24 Compared with women with ER-negative disease, women with ER-positive breast cancer had higher mortality if diagnosed before age 40 years, while those aged 40–75 years at diagnosis had lower mortality to 15 years post-diagnosis.24 That study did not fit ER status as a covariate with time-dependent effects. The population-based Danish Breast Cancer Cooperative Group also found higher mortality for women with ER-negative, compared with ER-positive, primary breast cancer up to 5 years from diagnosis (HR=2.08 [95% CI: 1.95–2.22]) that changed to a small overall survival advantage >5 years post-diagnosis (HR=0.89 [95% CI: 0.79–1.00]).4 Their finding was unchanged after excluding patients receiving adjuvant systemic treatment, suggesting that adjuvant treatment was not solely responsible for the time varying survival by tumor ER status seen in their study.4 We were unable to assess this issue, as our self-administered treatment questionnaire did not capture details on adjuvant systemic treatment. Many ER-positive breast cancers recur during or following adjuvant endocrine therapy.25 They eventually become resistant to ER-targeted therapies through several mechanisms.26–29 A novel mechanism via FGFR1 amplification, which confers resistance to antiestrogens in these breast cancers,30 at least partly explains the limited effects of estrogen deprivation on ER+/FGFR1–amplified breast cancers.25
Endocrine responsiveness has been a key factor when determining treatment choices for breast cancer for more than a decade; i.e. endocrine therapies for women with clearly endocrine responsive disease (steroid hormone receptor positive), chemotherapy alone for endocrine non-responsive disease, and combined chemotherapy and endocrine therapy for uncertain endocrine responsiveness disease (low expression of steroid hormone receptors).2 Based on findings primarily from the Adjuvant Tamoxifen: Longer Against Shorter randomized trial,31 tamoxifen alone has been advocated as the default adjuvant endocrine therapy for premenopausal women diagnosed with breast cancer, with the recommended treatment duration extended to 10 years (instead of the standard 5 years) for at least some women.32 In recent times, a continuation of endocrine therapy for up to 10 years for both pre- and postmenopausal women with node-positive disease who are disease-free following 5 years of tamoxifen has been suggested.33 The underlying biological mechanisms for the efficacy of extending the duration of adjuvant endocrine therapy for ER-positive breast cancer might relate to the timing of recurrences; in spite of highly effective modern treatments, half of all recurrences in ER-positive breast cancer occur more than 5 years from the initial diagnosis34 and there is a 25% risk of recurrence after 10 years.35
Intricacies in adjuvant endocrine therapy for premenopausal women that are still considered contentious include whether to add ovarian function suppression and whether to combine that with an aromatase inhibitor.33, 34 Additionally, women who are premenopausal at diagnosis but postmenopausal after five years of tamoxifen have been found to benefit more from extended hormone therapy with letrozole.36 For postmenopausal women in general, tamoxifen is considered adequate but inclusion of an aromatase inhibitor might be indicated,33, 34 with an aromatase inhibitor started upfront for those at higher risk or with lobular cancer.34
Overall, we have found that younger age at diagnosis is associated with a higher mortality, which is consistent with existing evidence.9, 37–43 We also found that the higher mortality initially for women with ER-negative tumors, and later on for women with ER-positive tumors, is more pronounced for women diagnosed at younger ages. Several aggressive clinical and tumor-related characteristics have been found to be more common for women with breast cancer diagnosed at younger ages, although they might not fully explain the underlying worse prognosis. These include higher tumor grade,1, 7, 9, 38, 41, 43–47 greater lymph node involvement1, 9, 38, 44, 46 and vascular invasion,7, 44 and human epidermal growth factor receptor 2 (HER2) overexpression1, 5, 47 in addition to an absence of hormone receptors.1, 7, 9, 38, 39, 41, 44, 46, 47 Greater axillary node involvement (not nodal status) remains by far the major attribute defining risk status (high risk: involvement of ≥4 axillary nodes or 1–3 nodes with HER2 overexpression/amplification) while tumor grade and size are also considered useful for predicting survival.2 In addition, prognostic gene expression signatures have been developed to predict survival more efficiently for women with breast cancer.48, 49
Strengths of our study include the population-based sampling and the over-sampling of women diagnosed at younger ages for whom the years of life lost to disease is greater. The 5-year overall survival estimated from the ABCFS cohort of 86.3% (standard error = 1.0%) compares with the 2008–12 5-year relative survival estimate for Australia of 89.8%,50 given that this cohort is over-sampled for younger ages at diagnosis. Another major strength was our analysis using flexible parametric modeling to estimate the mortality hazard as a function of time since diagnosis. The ability to fit complex time-dependent effects and investigate absolute as well as relative effects are some of the advantages of this approach over the Cox model.19 The extended length of follow-up is also a strength of our study.
Limitations of our study include the unavailability of information on HER2 status (HER2 testing was not conducted routinely at the time of diagnosis) in light of advances in managing women diagnosed with HER2 positive breast cancer. The POSH study did not find a statistically significant difference in overall survival by HER2 status (either at 5 or 8 years post-diagnosis) although the point estimate was lower for women with HER2 positive compared with HER2 negative breast cancer.3 Further, POSH observed only weak evidence of lower 8-year overall survival for women with ER-negative and HER2 positive (58.4%) compared with ER-negative and HER2-negative breast cancer (68.3%; P =.05), and concluded that lower long term survival for young women with ER-positive tumors was independent of their HER2 status.3 Considering that the ABCFS participants were diagnosed during 1992–99, before adjuvant trastuzumab was used routinely in the treatment of HER2 positive breast cancer, cautious interpretation of our findings is recommended when comparing with breast cancers diagnosed in current oncology settings.
Another limitation relates to the information we had on hormone receptor status. While the definition of ER status may have changed over time, it is also possible that missing ER records in some instances could have been misinterpreted as ER-negative during 1992–95; in our study this information was abstracted from diagnostic histopathology reports for some women. We tried to account for this by adjusting for the year of diagnosis (1992–95 compared with 1996–99) in multivariable-adjusted models. Also, missing data (7% for grade; 7% for number of positive lymph nodes; 48% for tumor size) could potentially bias survival estimates. To try to minimize any bias, we have used multiple imputation to replace the missing data.
In summary, age at diagnosis, ER status and time since diagnosis all influence breast cancer mortality in a dynamic manner so that no one factor dominates. The mortality hazard as a function of time since diagnosis differs substantially between women diagnosed with ER-negative and ER-positive breast cancers, particularly for women diagnosed at younger ages. Mortality is higher for women diagnosed with ER-positive breast tumors if they survive to 7 years post-diagnosis. While the number of positive lymph nodes, tumor grade and age at diagnosis are associated with mortality hazard, the association with tumor size was explained statistically by the associations with tumor grade and lymph node involvement, consistent with tumor size being determined by those two factors. Our findings suggest extended treatment with adjuvant endocrine therapy beyond 5 years might be beneficial for women diagnosed with ER-positive breast cancer. Whether to use an aromatase inhibitor and/or tamoxifen, and the sequence and duration of each treatment component could depend on age at diagnosis, tumor biology, number of positive lymph nodes and genetic profile. Evidence from clinical trials and large observational studies will be needed to establish clinical consensus on these matters.
Supplementary Material
Novelty and Impact.
To better understand long-term mortality following breast cancer diagnosis, we conducted a novel study that was population-based, large and over-sampled for young age at diagnosis, with a median follow-up of 15 years, and analyzed using a flexible model. We found that the risk of dying differed substantially between estrogen receptor (ER)-negative and ER-positive disease, and more so the younger the age at diagnosis. For women who survive >7 years, those with ER-negative disease are predicted to live longer, and more so if younger at diagnosis.
Acknowledgements
The Australian Breast Cancer Family Study (ABCFS) was supported in Australia by the National Health and Medical Research Council, the New South Wales Cancer Council, the Victorian Health Promotion Foundation, the Victorian Breast Cancer Research Consortium, Cancer Australia, and the National Breast Cancer Foundation. The ABCFS was also supported by the National Cancer Institute, US National Institutes of Health, under Request for Application CA-06–503 and through cooperative agreements with members of the Breast Cancer Family Registry: the University of Melbourne (Melbourne, Victoria, Australia) (grant U01 CA69638); the Fox Chase Cancer Center (Philadelphia, Pennsylvania) (grant U01 CA69631); the Huntsman Cancer Institute (Salt Lake City, Utah) (grant U01 CA69446); Columbia University (New York, New York) (grant U01 CA69398); the Cancer Prevention Institute of California (Fremont, California) (grant U01 CA69417); and Cancer Care Ontario (Toronto, Ontario, Canada) (grant U01 CA69467). JLH is a National Health and Medical Research Council Senior Principal Research Fellow. MCS is a National Health and Medical Research Council Senior Research Fellow. AKW is a National Health and Medical Research Council Early Career Fellow. We thank Prof. Margaret McCredie for her key role in the establishment and leadership of the ABCFS in Sydney, Australia.
The content of this manuscript does not necessarily reflect the views or policies of the US National Cancer Institute or any of the collaborating centers of the Breast Cancer Family Registry. The mention of trade names, commercial products, or organizations does not imply endorsement by the US government or the Breast Cancer Family Registry.
Abbreviations
- ABCFS
Australian Breast Cancer Family Study
- CI
confidence interval
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
Footnotes
Conflicts of interest: none
References
- 1.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008; 26(20): 3324–3330. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005; 16(10): 1569–1583. [DOI] [PubMed] [Google Scholar]
- 3.Copson E, Eccles B, Maishman T, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18–40 years at diagnosis: the POSH study. J Natl Cancer Inst 2013; 105(13): 978–988. [DOI] [PubMed] [Google Scholar]
- 4.Bentzon N, During M, Rasmussen BB, et al. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer 2008; 122(5): 1089–1094. [DOI] [PubMed] [Google Scholar]
- 5.Liukkonen S, Leidenius M, Saarto T, et al. Breast cancer in very young women. Eu J Surg Oncol 2011; 37(12): 1030–1037. [DOI] [PubMed] [Google Scholar]
- 6.Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 2000; 355(9218): 1869–1874. [DOI] [PubMed] [Google Scholar]
- 7.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol 1994; 12(5): 888–894. [DOI] [PubMed] [Google Scholar]
- 8.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet 1993; 341(8852): 1039–1043. [DOI] [PubMed] [Google Scholar]
- 9.Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: poor survival despite intensive treatment. PLoS One 2009; 4(11): e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopper JL, Giles GG, McCredie MRE, et al. Background, rationale and protocol for a case-control-family study of breast cancer. The Breast 1994; 3(2): 79–86. [Google Scholar]
- 11.McCredie MRE, Dite GS, Giles GG, et al. Breast cancer in Australian women under the age of 40. Cancer Causes Control 1998; 9(2): 189–198. [DOI] [PubMed] [Google Scholar]
- 12.Hopper JL, Chenevix-Trench G, Jolley DJ, et al. Design and analysis issues in a population-based, case-control-family study of the genetic epidemiology of breast cancer and the Co-operative Family Registry for Breast Cancer Studies (CFRBCS). J Natl Cancer Inst Monogr 1999; 26: 95–100. [DOI] [PubMed] [Google Scholar]
- 13.Dite GS, Jenkins MA, Southey MC, et al. Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst 2003; 95(6): 448–457. [DOI] [PubMed] [Google Scholar]
- 14.Phillips KA, Milne RL, Buys S, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol 2005; 23(21): 4679–4686. [DOI] [PubMed] [Google Scholar]
- 15.Armes JE, Egan AJM, Southey MC, et al. The histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer 1998; 83(11): 2335–2345. [PubMed] [Google Scholar]
- 16.Dite GS, Makalic E, Schmidt DF, et al. Tumour morphology of early-onset breast cancers predicts breast cancer risk for first-degree relatives: the Australian Breast Cancer Family Registry. Breast Cancer Res 2012; 14(4): R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armes JE, Trute L, White D, et al. Distinct molecular pathogeneses of early-onset breast cancers in BRCA1 and BRCA2 mutation carriers: A population-based study. Cancer Res 1999; 59(8): 2011–2017. [PubMed] [Google Scholar]
- 18.McCredie MRE, Dite GS, Southey MC, et al. Risk factors for breast cancer in young women by oestrogen receptor and progesterone receptor status. Br J Cancer 2003; 89(9): 1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J 2009; 9(2): 265–290. [Google Scholar]
- 20.Azzato EM, Greenberg D, Shah M, et al. Prevalent cases in observational studies of cancer survival: do they bias hazard ratio estimates? Br J Cancer 2009; 100(11): 1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akaike H Information theory and an extension of the maximum likelihood principle. 2nd International Symposium on Information Theory 1973: 267–281. [Google Scholar]
- 22.Carlin JB, Li N, Greenwood P, et al. Tools for analyzing multiple imputed datasets. Stata J 2003; 3(3): 226–244. [Google Scholar]
- 23.Anderson WF, Chen BE, Jatoi I, et al. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res Treat 2006; 100(1): 121–126. [DOI] [PubMed] [Google Scholar]
- 24.Sopik V, Sun P, Narod SA. The prognostic effect of estrogen receptor status differs for younger versus older breast cancer patients. Breast Cancer Res Treat 2017; 165(2): 391–402. [DOI] [PubMed] [Google Scholar]
- 25.Giltnane JM, Hutchinson KE, Stricker TP, et al. Genomic profiling of ER+ breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci Transl Med 2017; 9(402): pii:eaai7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Na Rev Cancer 2015; 15: 261–275. [DOI] [PubMed] [Google Scholar]
- 27.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer Annu Rev Med 2011; 62: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, et al. D538G mutation in estrogen receptor-α: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 2013; 73(23): 6856–6864. [DOI] [PubMed] [Google Scholar]
- 29.Robinson DR, Wu Y-M, Vats P, Su F, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013; 45: 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formisano L, Stauffer KM, Young CD, et al. Association of FGFR1 with ERα maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER+ breast cancer. Clin Cancer Res 2017; 23: 6138–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381(9869): 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24(9): 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: A brief summary of the consensus discussion. Breast Care 2015; 10(2): 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gnant M, Harbeck N, Thomssen C. St. Gallen/Vienna 2017: A brief summary of the consensus discussion about escalation and de-escalation of primary breast cancer treatment. Breast Care 2017; 12(2): 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378(9793): 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goss PE, Ingle JN, Martino S, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol 2013; 24(2): 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009; 36(3): 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr 1994; 16: 35–42. [PubMed] [Google Scholar]
- 39.Fowble BL, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys 1994; 30(1): 23–33. [DOI] [PubMed] [Google Scholar]
- 40.Chung M, Chang HR, Bland KI, et al. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer 1996; 77(1): 97–103. [DOI] [PubMed] [Google Scholar]
- 41.Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg 2009; 208(3): 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Q, Valero V, Kau V, et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the M.D. Anderson Cancer Center experience. Cancer 2001; 92(10): 2523–2528. [DOI] [PubMed] [Google Scholar]
- 43.El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer 2006; 6: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (< 35 years) with operable breast cancer: features of disease at presentation. Ann Oncol 2002; 13(2): 273–279. [DOI] [PubMed] [Google Scholar]
- 45.Winchester DP, Osteen RT, Menck HR. The National Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer 1996; 78(8): 1838–1843. [DOI] [PubMed] [Google Scholar]
- 46.Gajdos C, Tartter PI, Bleiweiss IJ, et al. Stage 0 to stage III breast cancer in young women. J Am Coll Surg 2000; 190(5): 523–529. [DOI] [PubMed] [Google Scholar]
- 47.Kheirelseid EAH, Boggs JME, Curran C, et al. Younger age as a prognostic indicator in breast cancer: a cohort study. BMC Cancer 2011; 11: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002; 347(25): 1999–2009. [DOI] [PubMed] [Google Scholar]
- 49.Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 2006; 98(17): 1183–1192. [DOI] [PubMed] [Google Scholar]
- 50.Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) books: breast cancer. Canberra: AIHW; 2016. Available from: http://www.aihw.gov.au/acim-books/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


