Abstract
Purpose
The aim of the present study was to evaluate the effectiveness of the ultramicronized-palmitoylethanolamide (um-PEA) and co-micronised palmitoylethanolamide/polydatin m(PEA/PLD) in the management of chronic pelvic pain related to endometriosis in patients desiring pregnancy.
Patients and methods
Thirty symptomatic women with laparoscopic diagnosis of endometriosis and pregnancy desire were enrolled. Patients were treated with um-PEA twice daily for 10 days followed by m(PEA/PLD) twice daily for 80 days. Intensity of chronic pelvic pain, dyspareunia, dysmenorrhea, dyschezia, and dysuria were evaluated at baseline, after 10, 30, 60, 90 days and after 30 days from the end of treatment, by VAS. Quality of life and women’s psychological well-being were evaluated at baseline and at the end of the treatment after 90 days with 36-Item Short Form Health Survey questionnaire and Symptom Check list-90 questionnaire, respectively. All collected data were analyzed with the non-parametric Wilcoxon test.
Results
At the end of the treatment, all patients showed a significant improvement in chronic pelvic pain, deep dyspareunia, dysmenorrhea, dyschezia, as well as in quality of life and psychological well-being.
Conclusion
In spite of the study’s limited sample size and the open-label design, this research suggests the efficacy of um-PEA and m(PEA/PLD) in reducing painful symptomatology and improving quality of life as well as psychological well-being in patients suffering from endometriosis. Additionally, this treatment did not show any serious side effect, proving particularly suitable for women with pregnancy desire and without other infertility factors.
Keywords: endometriosis, chronic pelvic pain, psychological well-being, quality of life, ultramicronized-palmitoylethanolamide, co-micronized palmitoylethanolamide and polydatin
Introduction
Endometriosis is an estrogen-dependent chronic benign inflammatory disease characterized by the presence and growth of endometrial tissue outside uterine cavity, most commonly in pelvic organs and peritoneum and in some rare cases in extra-abdominal locations.1,2 The presence of ectopic endometrium associated with cellular activity in the lesions and progression leads to formation of adhesions and/or interference with the normal reproductive processes.3 The etiology of the disease is not clearly defined and is probably multifactorial.4 Endometriosis affects approximately 10–20% of women in the reproductive age, causing pain and infertility.5 Moreover, this chronic disease importantly affects the quality of life as well as sexual and psychological health.6,7 Severe and chronic pain causes functional disability and requires medical/surgical treatment.8,9 For many years, surgical treatment was considered the best approach due to its effectiveness on symptoms and lesion eradications.10 Unfortunately, recent data indicate a high recurrence rate after surgery and possible complications related to extensive surgery especially on the ovarian reserve.11 Current treatments are aimed primarily at resolving symptoms in a non-invasive and palliative way. Hormonal therapies are considered the primary choice. These drugs achieve an acyclic, hypo-estrogenic environment avoiding ovarian estrogen production (GnRH agonists and antagonists), inducing pseudo-pregnancy, or locally inhibiting ectopic endometrium estrogenic stimulation (progestins).12,13
In recent years, considerable experimental and clinical evidence has emerged to support the involvement of mast cells in pain associated with endometriosis.14,15 Increased numbers of activated mast cells have been found in endometriotic lesions and in particular in deep infiltrating lesions (typically associated with more severe pelvic pain), proximal to nerves, suggesting that mast cells may contribute to endometriotic pain by a direct effect on nerve structures.16,17 In fact, mast cells dysregulation may cause an excessive release of pro-inflammatory products responsible of peripheral neuronal sensitization processes which are amplified by microglial activation at the central level, with the development of chronic pelvic pain.18,19 Moreover, cytokines and growth factors secreted appear to promote invasion and growth of ectopic endometrium by inducing proliferation and angiogenesis.20 Therefore, mast cells may be an innovative target for therapeutic strategies designed to reduce both inflammation and the consequent hyperalgesia and allodynia in endometriosis patients.21
Among the molecules acting on immune inflammatory cells, palmitoylethanolamide (PEA) is of particular interest. PEA is an endogenous fatty acid amide belonging to the family of fatty acid ethanolamides, which plays a role in the resolution of inflammatory processes by down-modulating mast cell activation and by controlling microglia behavior.22,23 Another important element in the pathophysiology of endometriosis is oxidative stress, probably involved in the growth and adhesion of endometrial cells in the peritoneal cavity and consequent onset of the disease.24 Polydatin (PLD), a glycoside of resveratrol, is a molecule with well-known antioxidant and anti-chemotactic activities, often combined with PEA in a co-micronized form for the treatment of pain associated with endometriosis.25,26 Micronization and ultra-micronization of PEA produce a crystalline structure with higher energy content and lower particle size, which contributes to better distribution and diffusion than the naïve form.27,28
Pre-clinical studies have shown that ultra-micronized PEA (um-PEA) by itself and PEA co-micronized with polydatin m(PEA/PLD) significantly reduce behavioral indices of both uterine and ureteral pain and decrease cyst formation.29,30 Importantly, m(PEA/PLD) markedly reduced chronic pelvic pain associated with endometriosis in woman either by itself or in combination with hormonal or anti-inflammatory drugs.26,31–34
The aim of our study was to investigate whether um-PEA followed by m(PEA/PLD) treatment in symptomatic patients with laparoscopic diagnosis of endometriosis improves symptoms and quality of life in these patients.
Materials and methods
This interventional study was conducted based on a single-arm, non-randomized, open-label design. Symptomatic women with laparoscopic diagnosis of endometriosis were enrolled at the Department of the Obstetrics and Gynecology, University of Cagliari, Italy, between October 2013 and August 2015. Inclusion criteria were: age between 18 and 48 years, diagnosis of endometriosis on the base of laparoscopic and histological examinations in last 3 years, chronic pain score ≥4 and ≤8 measured with the VAS, non-assumption of any drug in the month before enrollment (in particular estro-progestins, progestins, GnRH agonists, and antagonists), persistence of symptoms for at least 1 month. The exclusion criteria were: presence of other associated diseases, assumption of drugs, menopause, pregnancy, adverse reaction, or hyper sensitivity to active substance or excipients, unavailability to sign informed consent. This study was performed in accordance with the Good Clinical Practice guidelines and the principles of the Declaration of Helsinki of 1964 and its subsequent revisions; it was approved by our institutional ethics committee and registered in the clinicaltrial.gov website at the Number NCT02372903.
After written informed consents were provided, um-PEA 600 mg (Normast 600; Epitech Group, Italy) micro granules were administered twice daily for 10 days followed by m(PEA/PLD) (400 mg+40 mg) (Pelvilen Forte, Epitech Group, Italy) micro granules, twice daily for 80 days. The drugs were taken orally 12 hrs apart, not necessarily related to meals.
In all patients, a trans-vaginal ultrasound exam and cancer antigen 125 (CA 125) were performed to rule out other possible causes of gynecological pain, unrelated to endometriosis.
The main outcomes were the effect of the treatment on:
1) Intensity of pain, dyspareunia, dysmenorrhea, dyschezia, and dysuria evaluated with VAS. VAS is represented by a line 10 cm long with one extremity (0) indicating no pain and the other (10) the worst pain imaginable;35
2) Quality of life evaluated with the 36-Item Short Form Health Survey questionnaire (SF-36). The SF-36 consist of 36 items which tap eight different health concepts such as physical functioning, body pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. Score calculation consists of two steps. First, the pre-coded numerical values assigned to each question are recoded in a score from 0 to 100 according to a pre-established scoring key. In step 2, Items belonging to the same scale are averaged together to create the 8 scale scores. A high scale score indicates a more favorable health state;36,37
3) Psychological well-being was evaluated with Symptom Check list-90 (SCL-90). SCL-90 is a self-report measurement scale of 90 questions covering a wide range of psycho-pathological symptoms such as somatization, obsessive-compulsive syndrome, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism, and sleep disorders. Each item of the questionnaire is scored from 0 (not at all) to 4 (extremely) (Likert scale). All items with symptoms (score≥1) are counted indicating the “Positive Symptoms Total”. A high scale score defines a more psycho-pathologic state.38
VAS was administered at baseline, after 10, 30, 60 and 90 days and 30 days after the end of treatment (120 days), while SF-36 and SCL-90 were administered at baseline and at the end of treatment after 90 days.
Analgesic use was strictly monitored. Ketoprofen lysine salt sachet 80 mg was the FANS drug allowed for a maximum of twice a day. Patients were invited to collect data in a diary provided to them about every analgesic assumption and even about any side effect of the treatment.
Statistical analysis
Data were analyzed using the statistical software package IBM SPSS Statistics Desktop 22.0. The Kolmogorov–Smirnov test was used to evaluate whether values had a Gaussian distribution, in order to choose between parametric and non-parametric statistical tests. They were summarized as the mean and SD for continuous data and as the frequency for categorical data. Within-group variations between baseline and follow-up-values were evaluated using Wilcoxon matched pairs test. Significance level was accepted at p<0.05.
Results
Thirty symptomatic women with laparoscopic diagnosis of endometriosis were enrolled. Only two patients withdrew from the study, one due to pelvic inflammatory disease and, the other for non-compliance with therapy.
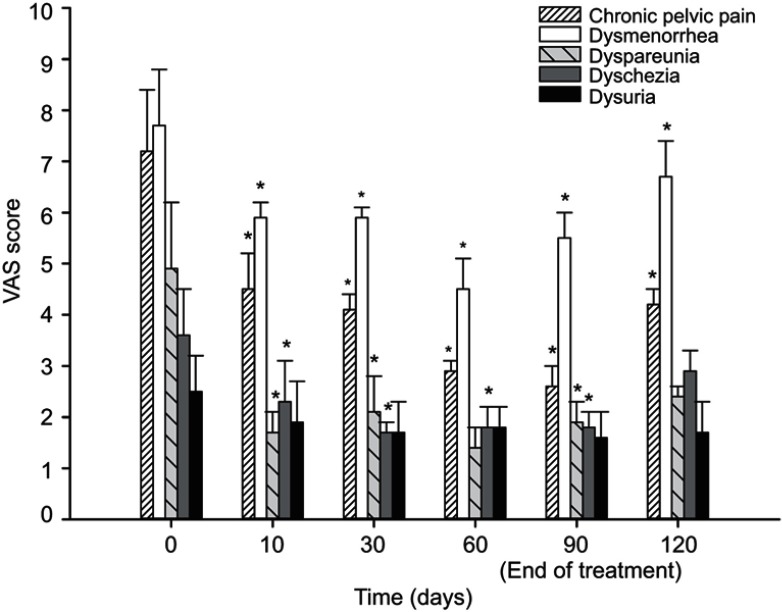
Statistical analysis showed that all pain symptoms decreased between baseline and 90 days (end of treatment). In particular, the VAS mean score of chronic pelvic pain significantly decreased (p<0.0001) from 7.2±1.2 (CI 6.0–8.4) to 2.6±0.4 (CI 2.2–3) (p<0.05); further, dysmenorrhea, dyspareunia, and dyschezia mean scores significantly decreased from 7.7±1.1 (CI 6.6–8.8) to 5.5±0.5 (CI 5–6) (p<0.05), from 4.9±1.3 (CI 3.6–6.2) to 1.9±0.4 (CI1.5–2.3) (p<0.05), and from 3.6±0.9 (CI 2.7–4.5) to 1.8±0.3 (CI 1.5–2.1) (p<0.05), respectively. Dysuria mean score showed a reduction trend from 2.5±0.7 (CI 1.8–3.2) to 1.6±0.5 (CI 1.1–2.1) (n.s). Thirty days after the end of treatment, VAS mean scores of all evaluated variables slightly increased; however, chronic pelvic pain and dysmenorrhea mean scores maintained a statistically significant difference compared to baseline, moving to 4.2±0.3 (CI 3.9–3.9) (p<0.05) and to 6.7±0.7 (CI 6–7.4) (p<0.05), respectively. All the VAS mean scores at each time point are presented in Figure 1. Obviously, the 10-day follow-up for menstrual and sexual pain was analyzed only in the patients who had periods or intercourse.
Figure 1.
Pain symptom scores obtained for the VAS at baseline, after 10, 30, 60, 90 days and after 30 days from the end of the treatment with um-PEA followed by m(PEA/PLD) in patients affected from endometriosis. Intensity of chronic pelvic pain, dysmenorrhea, dyspareunia, and dyschezia showed a significant reduction at each time point compared to baseline. Thirty days after the end of the treatment, only chronic pelvic pain and dysmenorrhea mean score maintained a statistically significant difference compared to baseline. Data values are expressed as means±SD *A p-value of <0.05 was considered significant.
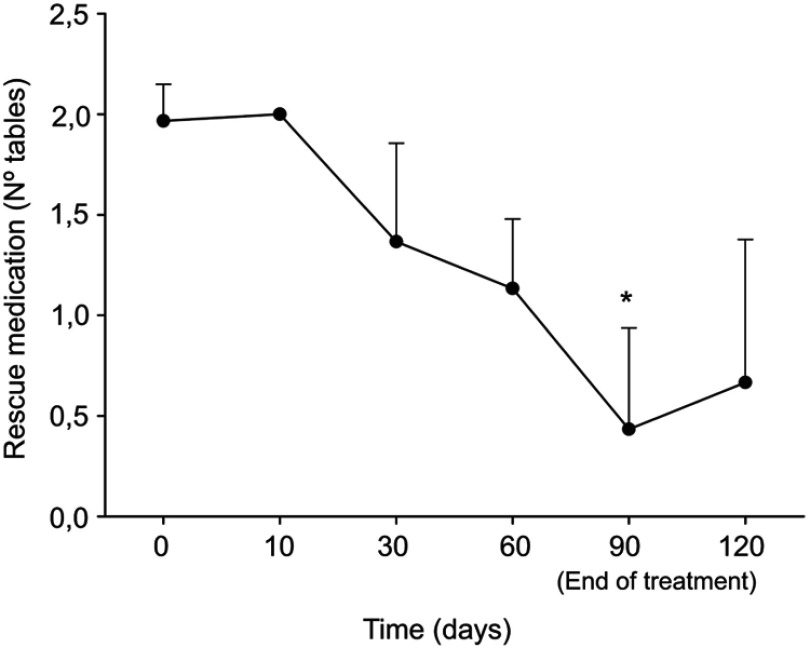
At the end of treatment, average values from self-administered questionnaires were indicative of a positive change in the quality of life and psychological well-being: the score of all SF-36 questionnaire subscale as well as the Positive Symptoms Total of the SCL-90 significantly improved (p<0.0005) (Tables 1, 2). The general improvement on the variables considered was accompanied by a significant reduction in the use of the analgesic ketoprofen at the end of the treatment (Figure 2). Treatment was well tolerated and adverse events were not observed.
Table 1.
SF-36 subscale score at baseline and after 90 days of treatment with um-PEA followed by m(PEA/PLD) in patients suffering from chronic pelvic pain due to endometriosis
| SF-36 | Baseline | 90 days of treatment | p-Value |
|---|---|---|---|
| Physical functioning | 67±26 | 88±14 | <0.0005 |
| Role limitations as a result of physical problems | 36±26 | 76±35 | <0.0005 |
| Bodily pain | 54±21 | 72±20 | <0.0005 |
| General health perception | 59±20 | 73±20 | <0.0005 |
| Vitality | 46±18 | 65±15 | <0.0005 |
| Social functioning | 61±26 | 83±20 | <0.0005 |
| Role limitations resulting from emotional problems | 45±36 | 75±32 | <0.0005 |
| General mental health | 53±17 | 60±15 | <0.0005 |
Note: Data values are expressed as means±SD.
Abbreviations: SF-36, 36-Item Short Form Health Survey questionnaire; um-PEA, ultramicronized-palmitoylethanolamide; m(PEA/PLD), co-micronized palmitoylethanolamide/polydatin;.
Table 2.
SCL-90 score (Positive Symptoms Total) at baseline and after 90 days of treatment with um-PEA followed by m(PEA/PLD) in patients suffering from chronic pelvic pain due to endometriosis
| SCL-90 | Baseline | 90 days of treatment | p-Value |
|---|---|---|---|
| Positive Symptoms Total | 75±8 | 37±6 | p<0.001 |
Notes: Data values are expressed as means±SD A p-value of <0.05 was considered significant.
Abbreviations: SCL-90, Symptom Check list-90 questionnaire; um-PEA, ultramicronized-palmitoylethanolamide; m(PEA/PLD), co-micronized palmitoylethanolamide/polydatin;.
Figure 2.
Consumption of rescue drugs at baseline, after 10, 30, 60, 90 days and after 30 days from the end of the treatment with um-PEA followed by m(PEA/PLD) in patients affected from endometriosis. Consumption of rescue drugs (tablets) showed a significant reduction at the end of treatment compared to baseline. Data values are expressed as means±SD *A p-value of <0.05 was considered significant.
Discussion
Endometriosis is increasingly being recognized as an inflammatory condition, where the increased presence of activated and degranulating mast cells, in particular in infiltrating lesions, proximal to nerves, play a role in the development of chronic pelvic pain.
The present study shows that treatment with um-PEA followed by m(PEA/PLD) in patients affected from symptomatic endometriosis leads to a significant reduction in the intensity of painful symptoms and improvement in quality of life and psychological well-being.
These results confirm the efficacy of um-PEA and m(PEA/PLD) for the management of chronic pelvic pain in endometriosis. Past clinical studies have shown that both um-PEA and m-PEA reduce chronic pain in patients suffering from various pathological conditions including pelvic pain in the bladder pain syndrome, menstrual pain in adolescents, and in particular in endometriosis.31,32,33,39,40 Cobellis et al in a double-blind study with three arms showed that the beneficial effects of m(PEA/PLD) are comparable to those obtained with anti-inflammatory drugs and more effective than placebos.32 Di Francesco and Pizzigallo in an open-label controlled study obtained significant reduction in pelvic pain with m(PEA/PLD) as well as with hormonal drugs (leuprorelin acetate or ethinylestradiol+drospirenone), irrespective of the treatment administered.33
PEA is an important anti-inflammatory drug with pain-relieving properties, mainly involved in the downregulation of mast cell activity.22 Further evidence support the hypothesis that PEA can act directly, stimulating either an as-yet uncharacterized cannabinoid CB2-like receptor, or the nuclear receptor peroxisome proliferator-activated receptor-alpha; moreover, it has been proposed that PEA acts indirectly through an “entourage effect”, ie, enhancing the anti-inflammatory and anti-nociceptive effects exerted by anandamide.41
Apart from the characteristic symptomatic picture, women with endometriosis present mood disorders (viz., depression and anxiety) and overall poor quality of life.8,9
In the present study, we were able to show that um-PEA and m(PEA/PLD) treatment led to a significant improvement in patients’ global quality of life evaluated by the SF-36 questionnaire and in particular of all its scale scores related to physical functioning, body pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. Similar results were obtained by Di Francesco and Pizzigallo, where m(PEA/PLD) improved quality of life on the basis of the SF-12 assessment (a 12-item subset of the SF-36) for the physical (PCS-12) and mental (MCS-12) health summary scores, while hormonal treatment improved only the PCS-12 summary score.33 Recently, the pilot study of Dell’Anna and De Marzi investigated the efficacy of a product based on the combination of um-PEA+m(PEA/PLD) on the quality of life in patients suffering from endometriosis with the Endometriosis Health Profile (EHP-30) questionnaire. The results of this study showed a significant improvement of the “Emotional well-being” component over time,34 parallel to an improvement of endometriosis pain symptoms, comparable to ours.
In our study, we also evaluated the effect of the treatment on psychological well-being, using the SCL-90, a scale that examines a wide range of psycho-pathological symptoms such as somatization, obsessive-compulsive syndrome, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism, and sleep disorders. The Positive Symptom Total of the SCL-90 significantly improved after 3 months of treatment and this effect persisted after the end of treatment.
The finding that um-PEA and m(PEA/PLD) improve women’s quality of life and psychological well-being might be linked to the pain relief properties of the treatment. However, recent medical research have demonstrated the link between inflammatory diseases and mood disorders.42,43 In particular, as regards endometriosis patients, it was detected an immunological shift resulting in the imbalance of pro-(IL-1β, IL-2, IFN-γ) and anti-inflammatory (IL-4) cytokine production, correlated with mood disorders such as depression and anxiety.44 Some indications have shown that mast cells might act peripherally and centrally coordinate the inflammatory processes in neuropsychiatric diseases supporting the hypothesis of common pathways/mechanisms in the etiopathogenesis of both chronic pelvic pain and depression.21,34,45,46 Therefore, targeting mast cells may represent a therapeutic strategy to contrast the symptomatology of both diseases.
The main limitations of this open-label study include lack of blindness and small patient sizes, which increases the difficulty in estimating the size effect of the treatment. Further, a treatment period >3 months would allow one to better appreciate the effectiveness and tolerability of the compound. Despite these limitations, this open-label study provides encouraging findings on the potential benefits of um-PEA and m(PEA/PLD) in patients affected by endometriosis.
Conclusion
Our research indicates the efficacy and safety of um-PEA and m(PEA/PLD) in the treatment of endometriosis symptomatology. Their administration to patients who were not taking any standard therapy provided a significant improvement in pain symptoms, in the quality of life and in their psychological well-being. Given its effectiveness, and absence of adverse events, um-PEA and m(PEA/PLD) may be promising therapeutic strategies for the treatment of endometriosis symptomatology, in particular for patients unable to receive hormonal therapy or who desire pregnancy. Future placebo-controlled, double-blind clinical studies need to be conducted to evaluate the benefits of these treatments in larger populations.
Availability of data and materials
The data supporting our findings are available upon request from the corresponding author.
Abbreviations
m(PEA/PLD), co-micronized palmitoylethanolamide/polydatin; OS, oxidative stress;
PEA, palmitoylethanolamide; PLD, polydatin; um-PEA, ultramicronized-palmitoylethanolamide;
SCL-90, Symptom Check list-90; SF-36, 36-Item Short Form Health Survey questionnaire.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Angioni S. New insights on endometriosis. Minerva Ginecol. 2017;69(5):438–439. [DOI] [PubMed] [Google Scholar]
- 2.Pontis A, Arena I, Angioni S. Umbilical endometriosis primary site without pelvic endometriosis and previous surgery: a case report. G Ital Ostet E Ginecol. 2014;36(2):336–338. [Google Scholar]
- 3.Angioni S, Cela V, Sedda F, et al. Focusing on surgery results in infertile patients with deep endometriosis. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2015;31(8):595–598. doi: 10.3109/09513590.2015.1062868 [DOI] [PubMed] [Google Scholar]
- 4.Locci R, Nisolle M, Angioni S, Foidart J-M, Munaut C. Expression of the gamma 2 chain of laminin-332 in eutopic and ectopic endometrium of patients with endometriosis. Reprod Biol Endocrinol. 2013;11:94. doi: 10.1186/1477-7827-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourdel N, Alves J, Pickering G, Ramilo I, Roman H, Canis M. Systematic review of endometriosis pain assessment: how to choose a scale? Hum Reprod Update. 2015;21(1):136–152. doi: 10.1093/humupd/dmu046 [DOI] [PubMed] [Google Scholar]
- 6.Melis I, Agus M, Pluchino N, et al. Alexithymia in women with deep endometriosis? A pilot study. J Endometr Pelvic Pain Disord. 2014;6(1):26–33. [Google Scholar]
- 7.Melis I, Litta P, Nappi L, Agus M, Melis GB, Angioni S. Sexual function in women with deep endometriosis: correlation with quality of life, intensity of pain, depression, anxiety, and body image. Int J Sex Health. 2015;27(2). doi: 10.1080/19317611.2014.952394 [DOI] [Google Scholar]
- 8.Angioni S, Cofelice V, Sedda F, et al. Progestins for symptomatic endometriosis: results of clinical studies. Curr Drug Ther. 2015;10:2. doi: 10.2174/157488551002151222160051 [DOI] [Google Scholar]
- 9.Mereu L, Florio P, Carri G, Pontis A, Petraglia F, Mencaglia L. Clinical outcomes associated with surgical treatment of endometrioma coupled with resection of the posterior broad ligament. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2012;116(1):57–60. doi: 10.1016/j.ijgo.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 10.Angioni S, Peiretti M, Zirone M, et al. Laparoscopic excision of posterior vaginal fornix in the treatment of patients with deep endometriosis without rectum involvement: surgical treatment and long-term follow-up. Hum Reprod Oxf Engl. 2006;21(6):1629–1634. doi: 10.1093/humrep/del006 [DOI] [PubMed] [Google Scholar]
- 11.Angioni S, Pontis A, Cela V, Sedda F, Genazzani AD, Nappi L. Surgical technique of endometrioma excision impacts on the ovarian reserve. Single-port access laparoscopy versus multiport access laparoscopy: a case control study. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2015;31(6):454–457. doi: 10.3109/09513590.2015.1017812 [DOI] [PubMed] [Google Scholar]
- 12.Angioni S, Nappi L, Pontis A, et al. Dienogest. A possible conservative approach in bladder endometriosis. Results of a pilot study. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2015;31(5):406–408. doi: 10.3109/09513590.2015.1006617 [DOI] [PubMed] [Google Scholar]
- 13.Alessandro P, Luigi N, Felice S, Maria PA, Benedetto MG, Stefano A. Research development of a new GnRH antagonist (Elagolix) for the treatment of endometriosis: a review of the literature. Arch Gynecol Obstet. 2017;295(4):827–832. doi: 10.1007/s00404-017-4328-6 [DOI] [PubMed] [Google Scholar]
- 14.Uchiide I, Ihara T, Sugamata M. Pathological evaluation of the rat endometriosis model. Fertil Steril. 2002;78(4):782–786. doi: 10.1016/S0015-0282(02)03327-7 [DOI] [PubMed] [Google Scholar]
- 15.Kirchhoff D, Kaulfuss S, Fuhrmann U, Maurer M, Zollner TM. Mast cells in endometriosis: guilty or innocent bystanders? Expert Opin Ther Targets. 2012;16(3):237–241. doi: 10.1517/14728222.2012.661415 [DOI] [PubMed] [Google Scholar]
- 16.Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol N Y N 1989. 2004;52(4):267–275. [DOI] [PubMed] [Google Scholar]
- 17.Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, Noël J-C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril. 2006;86(5):1336–1343. doi: 10.1016/j.fertnstert.2006.03.057 [DOI] [PubMed] [Google Scholar]
- 18.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. Microglial regulation of neuropathic pain. J Pharmacol Sci. 2013;121(2):89–94. doi: 10.1254/jphs.12R14CP [DOI] [PubMed] [Google Scholar]
- 19.Skaper SD, Facci L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3312–3325. doi: 10.1098/rstb.2011.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oral E, Olive DL, Arici A. The peritoneal environment in endometriosis. Hum Reprod Update. 1996;2(5):385–398. doi: 10.1093/humupd/2.5.385 [DOI] [PubMed] [Google Scholar]
- 21.Graziottin A, Skaper SD, Fusco M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2014;30(7):472–477. doi: 10.3109/09513590.2014.911280 [DOI] [PubMed] [Google Scholar]
- 22.Cerrato S, Brazis P, Della Valle MF, Miolo A, Puigdemont A. Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFalpha release from canine skin mast cells. Vet Immunol Immunopathol. 2010;133(1):9–15. doi: 10.1016/j.vetimm.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 23.Bettoni I, Comelli F, Colombo A, Bonfanti P, Costa B. Non-neuronal cell modulation relieves neuropathic pain: efficacy of the endogenous lipid palmitoylethanolamide. CNS Neurol Disord Drug Targets. 2013;12(1):34–44. doi: 10.2174/1871527311312010008 [DOI] [PubMed] [Google Scholar]
- 24.Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod Oxf Engl. 2005;20(7):2014–2020. doi: 10.1093/humrep/dei001 [DOI] [PubMed] [Google Scholar]
- 25.Bertelli AA, Ferrara F, Diana G, et al. Resveratrol, a natural stilbene in grapes and wine, enhances intraphagocytosis in human promonocytes: a co-factor in antiinflammatory and anticancer chemopreventive activity. Int J Tissue React. 1999;21(4):93–104. [PubMed] [Google Scholar]
- 26.Indraccolo U, Barbieri F. Effect of palmitoylethanolamide-polydatin combination on chronic pelvic pain associated with endometriosis: preliminary observations. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):76–79. doi: 10.1016/j.ejogrb.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 27.Impellizzeri D, Bruschetta G, Cordaro M, et al. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J Neuroinflammation. 2014;11(1):136. doi: 10.1186/s12974-014-0139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrosino S, Cordaro M, Verde R, et al. Oral ultramicronized palmitoylethanolamide: plasma and tissue levels and spinal anti-hyperalgesic effect. Front Pharmacol. 2018;9:249. doi: 10.3389/fphar.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iuvone T, Affaitati G, De Filippis D, et al. Ultramicronized palmitoylethanolamide reduces viscerovisceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis: role of mast cells. Pain. 2016;157(1):80–91. doi: 10.1097/j.pain.0000000000000473 [DOI] [PubMed] [Google Scholar]
- 30.Di Paola R, Fusco R, Gugliandolo E, et al. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front Pharmacol. 2016;7:382. doi: 10.3389/fphar.2016.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giugliano E, Cagnazzo E, Soave I, Lo Monte G, Wenger JM, Marci R. The adjuvant use of N-palmitoylethanolamine and transpolydatin in the treatment of endometriotic pain. Eur J Obstet Gynecol Reprod Biol. 2013;168(2):209–213. doi: 10.1016/j.ejogrb.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 32.Cobellis L, Castaldi MA, Giordano V, et al. Effectiveness of the association micronized N-Palmitoylethanolamine (PEA)-transpolydatin in the treatment of chronic pelvic pain related to endometriosis after laparoscopic assessment: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):82–86. doi: 10.1016/j.ejogrb.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 33.Di Francesco A, Pizzagallo D. Use of micronized palmitoylethanolamide and trans-polydatin in chronic pelvic pain associated with endometriosis. An open-label study. G Ital Ostet E Ginecol. 2014;XXXVI(2):353–358. [Google Scholar]
- 34.Dell’Anna A, De Marzi CA. Mast cells and microglia: new therapeutic targets for the treatment of endometriosis-associated symptomatology. Giorn It Ost Gin. 2017;2017;39(4): 193-201. [Google Scholar]
- 35.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4 [DOI] [PubMed] [Google Scholar]
- 36.Apolone G, Mosconi P. The Italian SF-36 health survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–1036. doi: 10.1016/S0895-4356(98)00094-8 [DOI] [PubMed] [Google Scholar]
- 37.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/(ISSN)1099-1050 [DOI] [PubMed] [Google Scholar]
- 38.Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the scl‐90: A study in construct validation. J Clin Psychol. 1977;33(4):981–989. doi: 10.1002/(ISSN)1097-4679 [DOI] [Google Scholar]
- 39.Skaper SD, Facci L, Fusco M, et al. Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology. 2014;22(2):79–94. doi: 10.1007/s10787-013-0191-7 [DOI] [PubMed] [Google Scholar]
- 40.Sommariva M, Schievano C, Saleh O. Micronized palmitoylethanolamide reduces bladder chronic pelvic pain due to different etiologies and improves bladder functions. Pelviperineology. 2017;36:92–96. [Google Scholar]
- 41.Petrosino S, Iuvone T, Di Marzo V. N-palmitoyl-ethanolamine: biochemistry and new therapeutic opportunities. Biochimie. 2010;92(6):724–727. doi: 10.1016/j.biochi.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 42.Steiner J, Walter M, Gos T, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 10 agosto, 2011;8:94. doi: 10.1186/1742-2094-8-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70(2):176–184. doi: 10.1001/2013.jamapsychiatry.102 [DOI] [PubMed] [Google Scholar]
- 44.Nasyrova RF, Sotnikova LS, Baystrukova NV, et al. Psychoimmune interactions in women of reproductive age with endometriosis. Bull Exp Biol Med. 2011;152(1):93–97. doi: 10.1007/s10517-011-1463-0 [DOI] [PubMed] [Google Scholar]
- 45.Nelissen S, Lemmens E, Geurts N, et al. The role of mast cells in neuroinflammation. Acta Neuropathol (Berl). 2013;125(5):637–650. doi: 10.1007/s00401-013-1092-y [DOI] [PubMed] [Google Scholar]
- 46.Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA. Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev. 2017;79:119–133. doi: 10.1016/j.neubiorev.2017.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting our findings are available upon request from the corresponding author.