Abstract
The morbidity and mortality rates of human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfection are higher than that of either infection alone. Outcomes and the virological response to antiretrovirals (combination antiretroviral therapy, cART) were explored in HIV/HBV subjects in a cohort of Italian patients treated with cART. A single-center retrospective analysis of patients enrolled from January 2007 to June 2018 was conducted by grouping patients by HBV status and recording baseline viro-immunological features, the history of virological failure, the efficacy of cART in achieving HIV viral undetectability, viral blip detection and viral rebound on follow up. Among 231 enrolled patients, 10 (4.3%) were HBV surface (s) antigen (HBsAg)-positive, 85 (36.8%) were positive for antibodies to HBV c antigen (HBcAb) and with or without antibodies to HBV s antigen (HBsAb), and 136 were (58.9%) HBV-negative. At baseline, HBcAb/HBsAb+/−-positive patients had lower CD4+ cell counts and CD4+ nadirs (188 cell/mmc, IQR 78–334, p = 0.02 and 176 cell/mmc, IQR 52–284, p = 0,001, respectively). There were significantly higher numbers of AIDS and non-AIDS events in the HBcAb+/HBsAb+/−-positive subjects than in the HBV-negative patients (41.1% vs 19.1%, p = 0.002 and 56.5% vs 28.7%, respectively, p ≤ 0.0001); additionally, HIV viremia undetectability was achieved a significantly longer time after cART was begun in the former than in the latter population (6 vs 4 months, p = 0.0001). Cox multivariable analysis confirmed that after starting cART, an HBcAb+/HBsAb+/−-positive status is a risk factor for a lower odds of achieving virological success and a higher risk of experiencing virological rebound (AHR 0.63, CI 95% 0.46–0.87, p = 0.004 and AHR 2.52, CI 95% 1.09–5.80, p = 0.030). HBcAb-positive status resulted in a delay in achieving HIV < 50 copies/mL and the appearance of viral rebound in course of cART, hence it is related to a poor control of HIV infection in a population of coinfected patients.
Subject terms: Outcomes research, Infectious diseases
Introduction
Due to the introduction of combined antiretroviral therapy (cART), and in particular the introduction of drugs with activity against hepatitis B virus (HBV), such as Lamivudine (LAM), Tenofovir (TDF) and other nucleoside/nucleotide analogues (NA), the ability to control HBV infection has strongly increased in HIV-coinfected patients. However, compared to HIV mono-infected patients, HIV/HBV coinfected patients reach immune-virologic control with more difficulty and have a higher rate of acquired immunodeficiency syndrome (AIDS) events and end-stage liver disease (ESLD) despite the introduction of cART1,2. HBsAg-positive cART-treated patients demonstrate impaired CD4 recovery and accelerated evolution towards AIDS3 in addition to increased HBV replication, liver disease progression, hepatocellular carcinoma (HCC) prevalence and liver-related mortality4,5.
Based on these data, the European Clinical Practice Guidelines for the management of HBV infection6 recommended the use of anti-HBV active drugs (NAs) containing cART in HIV-positive patients and strongly suggested avoiding the interruption of this type of treatment. Conversely, no suggestions were provided regarding the management of HIV-positive patients with HBV-resolved infections (i.e., the presence of anti-HBc and/or anti-HBs in the absence of HBsAg). Resolved HBV infection is known to be associated with HBV reactivation in immunocompromised patients subsequent to transplantation or chemotherapy for solid or hematological neoplasia7. As a component of resolved infection, occult hepatitis B Infection (OBI), which is defined as the absence of the HBs antigen (HBsAg) in the presence of intrahepatic or plasma HBV replication, is also known to be a risk factor for the evolution of HBV infection in cirrhosis, ESLD and HCC in immunocompromised patients8. Very few data are available regarding the influence of resolved HBV infection and OBI on HIV infection control9,10, especially in people with low-prevalence HBV infection.
The goal of this study was to explore whether having a resolved HBV infection affects overall health outcomes by comparing a group of cART-treated, HBV/HIV-coinfected Italian subjects to a group of HIV mono-infected patients. Clinical, virological and immunological data of patients seen from January 2007 until July 2018 at the Infectious Diseases Unit of the Policlinico Tor Vergata were retrospectively evaluated.
Materials and Methods
Study design
An observational retrospective study of 671 HIV-positive patients collected from January 2007 to July 2018 at the Infectious Diseases Unit of the Policlinico Tor Vergata in Rome, Italy was conducted. Specifically, for this study, a database was built that included all patients’ data at baseline, at the start of cART treatment, and at follow-up visits. In particular, the following data were collected for all of the patients: hematochemical data and HBV and HCV serology at baseline, HIV-RNA viral load, CD4+ cell count at baseline and pre-cART, time since the HIV infection diagnosis and the start time of cART treatment. Data on flares in transaminases (defined as an increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 50 IU/L), HIV viral blips (VB, defined as a single detection of HIV-RNA > 50 cp/mL), virological rebound (VR, defined as two consecutive HIV-RNA values ≥ 50 cp/ml or one > 1000 cp/mL with consequent changes in cART) and time required to achieve viral undetectability (virological success [VS], defined as two consecutive HIV-RNA < 50 cp/mL) were collected during follow-up examinations. Moreover, data regarding the antiviral treatment for chronic hepatitis B (CHB) (LAM, TDF, Tenofovir Alafenamide [TAF] or Entecavir [ETV]) were also gathered. When available, HBV-DNA viral load data were collected.
No specific ethics committee’s consent was required due to the retrospective nature of the study, which is based on information available from existing clinical documentation [Determination of the Italian Drug Agency (AIFA) of 20 March 2008]. With regard for privacy, all personal information was treated in a confidential manner, and all clinical data were anonymously analyzed.
Inclusion and exclusion criteria
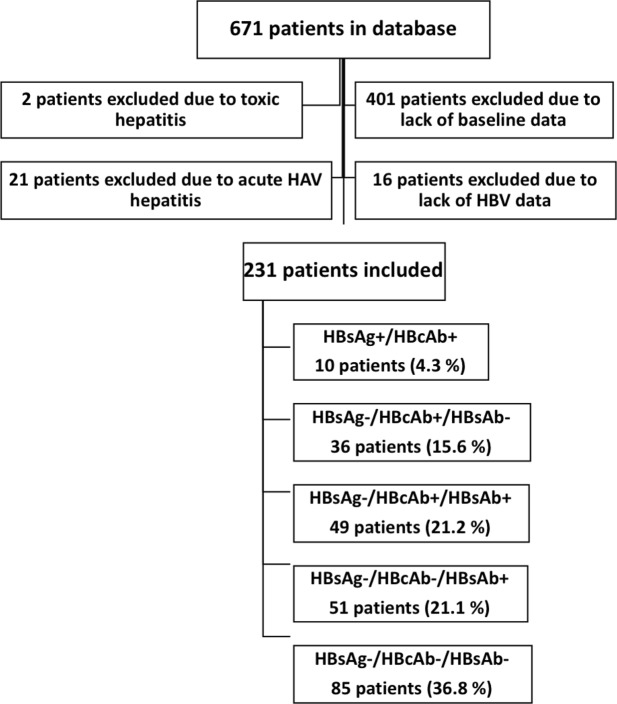
As shown in Fig. 1, of the 671 patients followed in our center, 401 were excluded due to a lack of virological or immunological data at the time of diagnosis (including patients who were non-adherent to follow-up visits, who transferred from other clinical centers and who voluntarily stopped first-line cART treatment), and 16 patients were excluded because of the loss of HBV serology data before the start of ART. Twenty-three patients were excluded due to the presence of transaminase flares dependent on hepatic drug toxicity (2 patients) or acute hepatitis A virus (HAV) infection (21 patients), which would invalidate the evaluation of transaminase flares. Two hundred thirty-one patients were ultimately studied.
Figure 1.
Algorithm of the study population inclusion and exclusion criteria.
Endpoints
The primary endpoint of this study was to investigate the influence of HBcAb+/HBsAb+/− status on time required to achieve an HIV viral load < 50 copies/mL after the initiation of cART and the onset of VB or VR after HIV VS. Subsequently, the effects of HBV antigen/antibody profiles (i.e., HBsAg, HBcAb and/or HBcAb/HBsAb positivity) on overall mortality and the appearance of AIDS-related events were also evaluated.
Furthermore, non-AIDS events, including hypertension, diabetes and lipidic disorders, bone disorders (osteopenia, osteoporosis), renal impairment and non-AIDS-related neoplastic pathologies, were investigated.
Laboratory testing for the diagnosis of HBV and HIV infection
HBV serological markers were measured using immune-enzymatic assays (Roche/Cobas Diagnostics, Rotkreuz, Switzerland). Plasma HBV-DNA was identified using real-time polymerase chain reaction (lower limit of quantification: 20 IU/ml) (Roche/Cobas Ampliprep/Cobas Taqman, Rotkreuz, Switzerland). Plasma HIV-RNA levels were measured using a commercial test, with 20 copies/ml of HIV-RNA defined as the lower limit of quantification (COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0).
Statistical methods
All statistical analyzes were conducted using STATA 14.2 (College Station, TX).
The study population is described using proportions and percentages for categorical values and median measurements and interquartile ranges (IQRs) for continuous values. The comparison between HBcAb+/HBsAb+/−-positive and HBV-negative patients was performed with the Kruskal-Wallis test for continuous variables and with the Chi-squared test or Fisher’s exact test, when appropriate, for categorical variables.
Kaplan-Meier curves were used to estimate the time required to achieve and the probability of achieving VS after the start of cART and the probabilities of experiencing VB and VR in the HBcAb+/HBsAb+/−-positive and HBV-negative groups of patients.
Cox regression analysis was performed to evaluate the association between HBcAb and the risk of achieving VS or experiencing VR after controlling for other potential confounding factors under the assumption of proportionality of the hazards. The following variables were considered potential confounders: age, AST levels, hepatitis C virus (HCV) coinfection, pre-cART plasma HIV-RNA, pre-cART CD4 count, anti-HBV treatment, risk factor for HIV acquisition, calendar year, and type of cART.
Results
Description of the study population
In Table 1, the characteristics of the study population are reported: 231 patients were included in the study, the median age was 42 years old (IQR 32–52), 75% were males, and 200 patients (86.6%) had acquired HIV through a sexual relationship. The median baseline HIV viral load was 85.605 cp/mL (IQR 31.199–285.442), and the median baseline CD4+ cell count was 236.5 (IQR 100–410). The patients were followed for a median duration of 59.4 months (4.9 years) (IQR 35.5–88.4). Regarding the serological status of HBV infection, ten (4.3%) of patients were HBsAg-positive, and 85 (36.8%) were HBcAb-positive/HBsAg-negative; among these, 36 (15.6%) were HBsAb-negative, and 49 (21.2%) were HBsAb-positive. One hundred thirty-six patients (58.9%) were HBV-negative, and 51 (22.1%) of these patients had received prior vaccinations.
Table 1.
Study population characteristics.
| Total study population, patients, n. | 231 |
| Age, years, median (IQR) | 42 (32–52) |
| Sex ratio, M/F (M%) | 175 (75.5%) |
| Origin, n (%) | |
| -Italian | 183 (79.2%) |
| -European | 13 (56%) |
| -African | 31 (13.4%) |
| -Other | 4 (1.7%) |
| Risk factors | |
| -Sexual, n (%) | 200 (86.6%) |
| -IDUs, n. (%) | 31 (13.4%) |
| Months from cART initiation, median (IQR) | 1,5 (0.4–4) |
| Duration of cART, months, median (IQR) | 50,2 (32.7–82.8) |
| Follow-up, months, median (IQR) | 59,4 (35.5–88.4) |
| AST, UI/L, median (IQR) | 22 (1.34) |
| ALT, UI/L, median (IQR) | 29 (23–43) |
| Platelet count, 10^6 cells/µL, median (IQR) | 196 (137–248) |
| Transaminases flare, median (IQR) | 115 (49.8%) |
| FIB-4 at baseline, median (IQR) | 0,87 (0.57–1.50) |
| CD4+ at baseline, cells/mmc, median (IQR) | 236,5 (100–410) |
| HIV-RNA VL at baseline, cp/mL median (IQR) | 85.605 (31.199–285.442) |
| Undetectability after 6 months of cART, Yes/No, n. (%) | 171 (74.3%) |
| Undetectability, months, median (IQR) | 5 (3-.,5) |
| AIDS-related event, Yes/No, n. (%) | 64 (27.7%) |
| Non-AIDS related event, Yes/No, n. (%) | 90 (38.9%) |
| HIV mono-infected people, patients, n. | 136 |
| Vaccinated for HBV (antiHBc-negative/anti-HBs-positive), n. | 51 |
| Not vaccinated (antiHBc-negative/anti-HBs-negative), n. | 85 |
| Coinfected HIV/HBV, patients, n. | 95 |
| -HBsAg+/AntiHBc+, n. (%) | 10 (4.3%) |
| -AntiHBc+/AntiHBs−, n. (%) | 36 (15.6%) |
| -AntiHBc+/AntiHBs+, n. (%) | 49 (21.2%) |
| Anti-HCV+, n. (%) | 13 (5.6%) |
| -HCV-RNA positive, n. (%) | 3 (1.2%) |
| cART strategy, n. (%): | |
| -Lamivudine | 38 (16.4%) |
| -Tenofovir/TAF | 176 (76.2%) |
| -No anti-HBV therapy | 17 (7.4%) |
| >1 year without anti-HBV agent, Yes/No | 23 (10%) |
| HIV VR, n. (%) | 34 (14.8%) |
| HIV VB, n. (%) | 101 (44.1%) |
| Deaths, n. (%) | 10 (4.3%) |
cART: combined antiretroviral therapy; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Undetectability: HIV-RNA < 50 copies/mL VR: virological rebound; VB: HIV viral blips.
One hundred seventy-one patients (74.3%) achieved HIV viremia < 50 copies/mL within a median of 5 months (IQR 3–6.5). The large majority (92.6%) of patients were treated with cART-containing anti-HBV agents: 38 (16.4%) with LAM and 176 (76.2%) with TDF or TAF. Overall, during the observation period, AIDS-related events were reported in 64 (27.7%) patients, and 90 (38.9%) developed non-AIDS-related events. HIV VR occurred in 34 (14.8%) subjects, and 101 (44.1%) showed occasional HIV VB during the follow-up period.
HBsAg-positive patients
Ten (4.3%) out of 231 patients were HBsAg-positive; these patients had a median pre-cART CD4 cell count of 193 cell/mmc (IQR 98-373) and an HIV viral load of 87.100 copies/mL (IQR 31.100–143.000). All had detectable plasma HBV-DNA (median 9.260 UI/L, IQR 2.651–69.300 UI/L) and were taking cART: 2 (20%) as a regimen containing LAM and 8 (80%) as a regimen containing TDF or TAF. Of these 10 patients, 2 (20%) died because of HCC, 1 (10%) had HCC treated with chemoembolization, 2 (20%) developed an AIDS-related event (2 cases of Pneumocystis jirovecii pneumonia [PJP]), and another 3 patients (30%) developed a non-AIDS-related event (2 cases of cardiovascular disease and 1 of diabetes complications) (data not shown).
Comparison between HBcAb+/HBsAb+/−-positive and HBV-negative patients
A comparison between HBV-negative and HBcAb+/HBsAb+/− HIV-positive patients is reported in Table 2. Compared to HBV-negative subjects, HBcAb+/HBsAb+/−-positive patients were older (median, 48 years old [39–55] vs 39 years old [29–48], p 0.0001) and more likely to have a history of intravenous drug use (17 [54.8%] vs 11 [35.5%], p 0.01). Compared to HBV-negative patients, HBcAb+/HBsAb+/−-positive patients had more frequent episodes of transaminase flares (56 [65.9%] vs 49 [36%], p < 0.0001), significantly lower CD4+ cell counts at baseline (188 [IQR 78–334] vs 293 [IQR 127–443], p = 0.02), and lower pre-cART CD4+ cell counts (176 [IQR 52–284] vs 239 [IQR 127–443], p = 0.02).
Table 2.
Comparison between HBcAb/HBsAb+/−-positive vs HBV-negative patient characteristics.
| Study population | HBcAb/HBsAb+/−-positive (n = 85) | HBV-negative (n = 136) | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 48 (39–55) | 39 (29–48) | 0.0001 |
| Risk factors, n. (%) | |||
| Sexual | 68 (34%) | 125 (62.5%) | 0.01 |
| IDUs | 17 (54.8%) | 11 (35.5%) | 0.01 |
| Months from cART initiation, median (IQR) | 1.3 (0.6–4.9) | 1.4 (0.85–3.6) | 0.25 |
| Duration of cART, months, median (IQR) | 49.8 (29.3–84.7) | 49.4 (33.9–79) | 0.89 |
| Calendar years since cART start, median (IQR) | 2013 (2011–2015) | 2013 (2011–2015) | 0.58 |
| Follow-up, months, median (IQR) | 61.9 (34.8–92.3) | 56.3 (36.2–84.3) | 0.56 |
| AST, UI/L, median, (IQR) | 23 (18–34) | 21 (16–31.5) | 0.40 |
| ALT, UI/L, median, (IQR) | 32 (24–43) | 28 (21.5–40) | 0.02 |
| Platelet count, 106/µL median (IQR) | 191 (126–227) | 200 (164–256) | 0.02 |
| Flair of transaminases, median (IQR) | 56 (65,9%) | 49 (36%) | <0.0001 |
| FIB-4 at baseline, median (IQR) | 1.12 (0.68–1.81) | 0.76 (0.48–1.2) | 0.001 |
| Anti-HCV+, n. (%) | 7 (8.2%) | 5 (3.6%) | 0.23 |
| HCV-RNA-positive, n. (%) | 1 (1.1%) | 1 (0.7%) | nc |
| CD4+ at baseline, cell/mmc, median (IQR) | 188 (78–334) | 293 (127–443) | 0.02 |
| Pre-cART CD4+ cell/mmc, median (IQR) | 176 (52–284) | 239 (97–390) | 0.01 |
| Pre-cART HIV viremia, cp/mL median (IQR) | 109.8 (45.6–330.7) | 63 (22.4–241.1) | 0.14 |
| Undetectability at 6th month, median (IQR) | 50 (59.5%) | 115 (84.6%) | <0.0001 |
| Undetectability, months, median (IQR) | 6 (4–8) | 4 (3–6) | 0.0001 |
| AIDS-related events, Yes/No, n. (%) | 35 (41,1%) | 26 (19,1%) | 0.002 |
| Non-AIDS related events, Yes/No, n. (%) | 48 (56.5%) | 39 (28.7%) | <0.0001 |
| cART anti-HBV active drugs, n. (%): | |||
| -Lamivudine | 14 (16.4%) | 22 (16.1%) | 0.95 |
| -Tenofovir/TAF | 62 (72.9%) | 106 (77.9%) | 0.66 |
| -No anti-HBV agent | 9 (10.6%) | 8 (5.9%) | 0.28 |
| Type of cART | |||
| PI + 2 NRTIs | 54 (39.7) | 42 (49.4) | 0.157 |
| NNRTI + 2 NRTI | 46 (33.8) | 25 (29.4) | 0.494 |
| INI + 2 NRTI | 31 (22.8) | 12 (14.1) | 0.113 |
| Other | 5 (3.7) | 6 (7.1) | 0.342 |
| >1 year without anti-HBV agent, n. (%) | 12 (14.3%) | 11 (8.1%) | 0.18 |
| HIV VR, median (IQR) | 18 (21.7%) | 14 (10.3%) | 0.06 |
| HIV VB, median (IQR) | 62 (74.7%) | 30 (22.1%) | <0.0001 |
| Deaths, n.(%) | 6 (7.1%) | 2 (1.5%) | 0.006 |
IDU: injecting drug users; cART: combined antiretroviral therapy; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Undetectability: HIV-RNA < 50 copies/mL; TAF: Tenofovir alafenamide; VR: virological rebound; VB: HIV viral blips.
Once cART was initiated (89% of HBcAb+/HBsAb+/−-positive patients had an NA in their cART composition), HBV-negative patients achieved HIV-RNA undetectability in a significantly shorter time than was found in HBcAb+/HBsAb+/−-positive subjects (median time, 4 [IQR 3–6] vs 6 months [IQR 4–8], p < 0.0001). A significantly higher number of AIDS-related and non-AIDS-related events were detected in HBcAb+/HBsAb+/−-positive subjects than in HBV-negative patients (35 [41.5%] vs 26 [19.1%] and 48 [56.5%] vs 39 [28.7%], p 0.002 and p < 0.0001, respectively). Among HBcAb+/HBsAb+/− patients, the following AIDS- and non-AIDS-related events were documented: PJP (16 cases), Kaposi Sarcoma (KS) (7 cases), esophageal candidiasis (4 cases), wasting syndrome (4 cases), PJP plus esophageal candidiasis (1 case), non-AIDS tumors (12 cases), lipidic disorders (19 cases), osteoporosis (6 cases), diabetes (5 cases) and more than one non-AIDS disease (7 cases) (data not shown). HBV-negative patients had the following AIDS and non-AIDS pathologies: PJP (14 cases), KS (2 cases), cerebral lymphoma (1 case), esophageal candidiasis (7 cases), more than one of the above-listed AIDS events (2 cases), non-AIDS tumors (7 cases), lipid disorders (13 cases), osteoporosis (2 cases), hypertension (9 cases) or more than one of the previously mentioned non-AIDS comorbidities (8 cases) (data not shown).
At the end of the follow-up period (the duration of which did not differ between HBcAb+/HBsAb+/−-positive and HBV-negative patients [p = 0.56]), 62 (74.7%) of the HBcAb+/HBsAb+/−-positive and 30 (22.1%) of the HBV-negative subjects developed HIV VB (p < 0.0001). Moreover, HBcAb+/HBsAb+/− patients were more prone to VR onset (18 [21.7%] vs 30 [22.1%], p < 0.0001). Virological control of HIV during follow-up was poorer in the HBcAb+/HBsAb+/− group. Overall, at the end of the follow-up period, 8 out of 221 patients had died. Of these, six (7.1%) were HBcAb-positive, and only two (1.5%) were HBV-negative (p 0.006).
Comparison between HBcAb-positive patients with and without HBsAb
In Table 3, the differences between the groups of HBcAb-positive patients with and without HBsAb are evaluated. While no other significant differences were found between these two groups of subjects, among HBcAb-positive patients, HIV VR was found in a significantly higher number of patients without HBsAb than in those with HBsAb (12 [35.3%] vs 6 [12.2%], p = 0.01).
Table 3.
Comparison between HBcAb/HBsAb-positive vs. HBcAb-positive/HBsAb-negative.
| Study population | HBcAb+/HBsAb+ (n = 49) | HBcAb+/HBsAb−. (n = 36) | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 48 (39–55) | 47 (39–55) | 0,71 |
| Duration of cART, months, median (IQR) | 70 (34–96,5) | 56 (34–88) | 0,73 |
| AST, UI/L, median (IQR) | 21 (17–28) | 25 (20–45) | 0,04 |
| ALT, UI/L, median (IQR) | 31 (26–42) | 33 (22,5–53) | 0,93 |
| Platelet count, 10^6/µL, median (IQR) | 192 (127–226) | 176 (119–238) | 0,91 |
| Flair of transaminases, median (IQR) | 30 (61%) | 26 (72%) | 0,35 |
| HCV-RNA-positive, n. (%) | 0 (0) | 1 (2,7%) | nc |
| Baseline CD4+, cell/mmc, median (IQR) | 193 (83–363) | 159 (58–280) | 0,25 |
| Pre-cART CD4+ cell/mmc, median (IQR) | 180 (68–302) | 159 (45–243) | 0,31 |
| Pre-cART HIV cp/mL median (IQR) | 102765 (41600–198989) | 355996 (49091–405087) | 0,24 |
| Undetectability at 6th month, median (IQR) | 32 (65,3%) | 18 (51,4%) | 0,26 |
| AIDS-related events, Yes/No, n. (%) | 17 (34,7%) | 18 (50%) | 0,15 |
| Non-AIDS related events, Yes/No, n. (%) | 31 (63,2%) | 17 (47,2%) | 0,14 |
| Antiretroviral drugs, n. (%): | |||
| -Lamivudine | 7 (19,4%) | 7 (14,3%) | 0,36 |
| -Tenofovir/TAF | 37 (75,5%) | 25 (69,4%) | 0,35 |
| -No anti-HBV agent | 5 (10,2%) | 4 (11,1%) | NC |
| >1 year without anti-HBV agent, n. (%) | 7 (14,3%) | 5 (14,3%) | NC |
| HIV VR, median (IQR) | 6 (12,2%) | 12 (35,3%) | 0,01 |
| HIV VB, median (IQR) | 34 (69,4%) | 28 (82,3%) | 0,18 |
IDU: injecting drug users; cART: combined antiretroviral therapy; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TAF: Tenofovir alafenamide; VR: virological rebound; VB: HIV viral blips.
HIV virological response to cART in HBcAb-positive and -negative patients
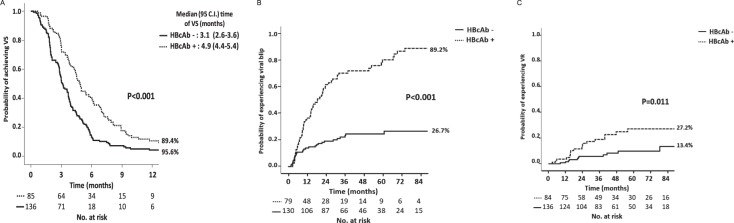
KM estimates were produced to analyze the ability to reach the VS after the start of first-line cART and the risk of incurring VR after virological success in the 2 groups of HBcAb-positive and -negative HIV patients. In Fig. 2, Kaplan-Meier curves show that, by 12 months after cART start, HBcAb-positive patients had a longer median time and a lower probability of achieving VS compared to HBcAb-negative patients (89.4% vs 95.6%, p < 0.001) (Panel A). Concerning the probability of experiencing a VB, Kaplan-Meier estimates showed that by the 84th month after the achievement of VS, HBcAb-positive patients had a higher probability of experiencing a VB compared to HBcAb-negative patients (26.7% vs 89.2%, p < 0.001) (Fig. 2, Panel B).
Figure 2.
(A) KM estimates of the odds of VS (virological success, HIV < 50 copies/mL) according to HBcAb serostatus; (B) KM estimates of VB (viral blips) after the achievement of VS according to HBcAb serostatus; (C) KM estimates of the odds of experiencing VR (virological rebound) after the achievement of VS according to HBcAb serostatus.
Finally, concerning VR, in Fig. 2 panel C, it is shown that by the 84th month after the achievement of VS, HBcAb-positive patients had a higher probability of experiencing a VR compared to HBV-negative patients (13.4% vs 27.2%, p = 0.011). Patients who experienced VR had a higher number of blips (median number of VB 2 [IQR 1–3]) compared to those who did not experience VR (median number of VB 0 [IQR 0–1]) (p < 0.001 Mann-Whitney test) (data not shown).
Therefore, Kaplan Meier estimates showed that antiHBc-positive patients exhibited less virological control from the beginning of cART, including a delayed VS and subsequently more frequent occurrence of VB and VR.
Table 4 shows the adjusted hazard ratio (AHR) of achieving VS and experiencing VR after the start of cART. The following potential confounders were considered: age, AST levels, HCV coinfection, pre-cART plasma HIV-RNA, pre-cART CD4 count, anti-HBV treatment, risk factor for HIV acquisition, calendar year, and type of cART. HBcAb positivity, either with or without HBsAb, was associated with a lower AHR of achieving VS (AHR 0.63, CI 95% 0.46–0.87, p = 0.004) and an increased risk of VR after cART beginning (AHR 2.52, CI 95% 1.09–5.80, p = 0.030). After adjustment for potential confounders, HBcAb-positive status was confirmed to be a risk factor for lower HIV virological control after the initiation of first-line cART. Moreover, patients with a high pre-cART HIV viral load had a lower AHR of achieving VS (HIV-RNA > 100.000 and <500.000 cp/mL, AHR 0.68, CI 95% 0.47–0.98, p = 0.038; and HIV-RNA > 500.000 cp/mL AHR 0.40, CI 95% 0.25–0.66, p < 0.001), and patients with a pre-cART HIV-RNA > 500.000 cp/mL had a higher AHR of VR than was found in those with a lower level of viremia (AHR 4.99, CI95% 1.42–17.55, p = 0.012). The presence of integrase inhibitors (INIs) plus 2 nucleos(t)ide reverse transcriptase inhibitors (NRTIs) in the cART regimen was associated with a higher AHR of achieving VS (AHR 1.85, CI 95% 1.20–2.85, p = 0.006) as was cART initiation in a more recent calendar year (AHR 1.11, CI 95% 1.05–1.18, p < 0.001).
Table 4.
Cox regression models for estimated factors with predictive impact on VS and VR.
| Risk of achieving VS | Risk of experiencing VR | |||||||
|---|---|---|---|---|---|---|---|---|
| AHRa | 95.0% C.I. | P value | AHRa | 95.0% C.I. | P value | |||
| Lower | Upper | Lower | Upper | |||||
| Age (per 5 years higher) | 1.05 | 0.98 | 1.12 | 0.143 | 0.85 | 0.71 | 1.01 | 0.057 |
| AST elevated x2N | 1.05 | 0.42 | 2.62 | 0.914 | 2.04 | 0.21 | 20.12 | 0.540 |
| HCV coinfection | 1.16 | 0.57 | 2.36 | 0.684 | 2.48 | 0.40 | 15.17 | 0.327 |
| Plasma HIV-RNA at therapy start (copies/mL) | ||||||||
| <100,000b | 1 | |||||||
| 100,000–500,000 | 0.68 | 0.47 | 0.98 | 0.038 | 2.04 | 0.74 | 5.63 | 0.166 |
| >500,000 | 0.40 | 0.25 | 0.66 | <0.001 | 4.99 | 1.42 | 17.55 | 0.012 |
| CD4 cell count at cART start (cells/mm 3 ) | ||||||||
| <200b | 1 | |||||||
| 201–350 | 1.20 | 0.83 | 1.74 | 0.331 | 0.24 | 0.08 | 0.78 | 0.017 |
| 351–500 | 1.07 | 0.67 | 1.71 | 0.767 | 0.18 | 0.02 | 1.47 | 0.110 |
| >500 | 1.41 | 0.82 | 2.45 | 0.216 | 0.00 | 0.00 | ND | 0.973 |
| Drug abuser as risk factor | 0.83 | 0.51 | 1.35 | 0.457 | 1.02 | 0.28 | 3.72 | 0.980 |
| Anti-HBc+ | 0.63 | 0.46 | 0.87 | 0.004 | 2.52 | 1.09 | 5.80 | 0.030 |
| Anti-HBV drug included in cART | ||||||||
| None b | 1 | |||||||
| TDF | 1.43 | 0.74 | 2.75 | 0.290 | 0.79 | 0.16 | 4.02 | 0.778 |
| 3TC | 1.83 | 0.94 | 3.54 | 0.074 | 0.80 | 0.16 | 3.99 | 0.783 |
| Calendar year of cART start | 1.11 | 1.05 | 1.18 | <0.001 | 1.19 | 0.99 | 1.43 | 0.063 |
| Type of cART | ||||||||
| PIb + 2 NRTIsb | 1 | |||||||
| NNRTI + 2 NRTI | 1.17 | 0.84 | 1.64 | 0.356 | 2.74 | 0.98 | 7.61 | 0.054 |
| INI + 2 NRTI | 1.85 | 1.20 | 2.85 | 0.006 | 1.38 | 0.38 | 5.06 | 0.627 |
| Other | 1.25 | 0.54 | 2.91 | 0.606 | 2.19 | 0.40 | 12.00 | 0.365 |
aAdjusted for age, AST levels, HCV coinfection, pre-ART plasma HIV-RNA, pre-ART CD4 count, anti-HBV treatment, risk factor, calendar year, and type of ART. bReference (dummy). Bold variables were significantly associated with virological response (p < 0.05). VS: virological success; VR: virological rebound; 3TC: lamivudine; AST: alanine aminotransferase; ART: antiretroviral therapy; INI: integrase inhibitor; NRTI: nucleos(t)ide reverse transcriptase inhibitor; NNRTI: Non-NRTI; PIb: ritonavir/cobicistat boosted protease inhibitor.
Discussion
In our cohort of HIV-positive patients who started cART, the presence of serological markers of a past HBV infection was significantly correlated with a delay in achieving HIV viremia undetectability and the appearance of HIV VR after the start of cART (AHR 2.52, CI 95% 1.09–5.80, p = 0.030 and AHR 0.63, CI 95% 0.46–0.87, p 0.004, respectively). Moreover, significantly higher numbers of AIDS and non-AIDS-related events were found in the group of HIV/HBcAb-positive patients than in HIV mono-infected individuals.
In our population, 41% of the 231 patients included in this study had an ongoing or resolved HBV infection. Slightly lower rates of HBV-resolved infection or CHB were found in two American cohorts, which reported values of 23% and 35% in HIV seroconverters and HIV patients on cART, respectively11,12.
CHB in HIV-positive subjects has been demonstrated to worsen the prognosis of both infections, and a higher risk of mortality was recently observed in patients coinfected with CHB and an HBV DNA viral load > 2000 UI/ml13. Very little is known about the influence of resolved HBV infection on the course of HIV infection. From the results of a longitudinal study performed in a large cohort of HIV-negative and -positive patients7, the authors concluded that the isolated presence of HBcAb was a sign of an impaired immune response in patients with an HIV infection.
In our study cohort, HBcAb+/HBsAb+/−-positive subjects presented flares of transaminases more frequently than was found in HBV-negative patients (65.9% vs 36%, p < 0.0001), suggesting the potentially transient reactivation of HBV. Hepatitis flares have been widely documented in HBcAb-positive patients during immunosuppressive therapy7, whereas the association of liver diseases with signs of resolved HBV infection in coinfected HIV people is difficult to ascertain as few studies have assessed the association between liver damage and both isolated anti-HBc patterns and occult HBV infections14. In the Veterans Aging Cohort Study15, the presence of HBcAb was associated with more advanced hepatic fibrosis in HIV-HCV coinfected patients, while Morsica and his colleagues9 showed that 21% of HBcAb-positive HIV-coinfected patients in the Italian national cohort (ICONA) presented detectable HBV-DNA associated with an increase in transaminase levels.
An increased number of AIDS and non-AIDS events were documented in the follow-up of our study cohort, which is associated with ongoing or resolved HBV infections. A similar result was reported by Chun HM and his collaborators12, who observed that the risk of AIDS and death was high in both CHB and resolved HBV subjects from a cohort of 2536 HIV-positive cART recipients. Similarly, HBV coinfection negatively impacted patient survival in a group of HIV seroconverters, among whom the rates of AIDS and death were significantly higher in CHB patients and increased, although not significantly, in patients with resolved HBV infections11.
A series of potential factors have been shown to form the basis of the HBV and HIV interaction and to result in the acceleration of both infection courses16. HIV exerts direct activity on hepatocytes and Kupffer cells, thereby contributing to liver inflammation. Moreover, the rate of microbial translocation was higher in HIV/HBV-coinfected individuals than in HBV mono-infected individuals17. Microbial translocation has been associated with ongoing immune dysfunction and contributes to the persistence of chronic immune activation and inflammation, which have been associated with the development of comorbidities in HIV-infected persons, even those receiving effective cART18,19.
In our study cohort, virological control was worse in HIV/HBcAb/HBsAb+/− patients than in HIV mono-infected subjects in a multivariate analysis, and the status of the resolved HBV coinfection was strongly correlated with HIV VB onset in HIV monoinfected people (89.2% vs 26.7%, p < 0.001).
The appearance of VB is commonly described in cART-treated HIV patients20, and some authors have found a correlation between VB and subsequent VR21–23. In our study, we found that the median VB was higher in patients with VR than in those without VR. The origin of VB remains uncertain, but an increased HIV replication rate has been correlated with the presence of other infections and with certain vaccinations24,25. A number of viral coinfections, when present in HIV-positive subjects, seem to contribute to unfavorable outcomes. Cytomegalovirus coinfection was associated with HIV disease progression26,27, and hepatitis C virus is a leading cause of non-AIDS-related mortality among HIV-coinfected patients28.
HBV DNA is less rarely detected among patients with HIV and those with isolated anti-HBc (OBI patients), with its rate reported to vary from 0.6% to 89% in several studies29, and is related to a poor immune status8. Therefore, it is possible to argue that occasional, although limited, HBV replications may also contribute to the appearance of HIV viremia during the course of cART.
Before any conclusions can be drawn, some of the limitations of our study need to be discussed. First, the lack of HBV-DNA data in our population was a major limitation. Currently, no shared indications are used in the monitoring of HBV viremia in coinfected patients with resolved HBV infections, but it may be interesting to ascertain whether poor control of HIV viremia during cART is correlated with the simultaneous presence of HBV-DNA in patients with resolved HBV infections. Second, all of the evaluated patients were included from a single institution, and this might reduce the generalizability of our conclusions. Third, the retrospective nature of this study limits the power of the obtained results.
In conclusion, during the course of cART, an HBcAb-positive status was a risk factor delayed achievement of HIV < 50 copies/mL and the appearance of VR in our cohort of HIV/HBV-coinfected patients. Further studies are needed to assess how HBV-resolved infections exert a harmful effect on HIV and how they might reduce HIV replication control.
Acknowledgements
No additional financial support was provided for this study. The results of this work were partially presented at the HIV Drug Therapy Conference, Glasgow, 28–31 October, Scottish Event Campus, Glasgow, UK, Poster Control Number 4602.
Author Contributions
V.M. conceived and designed the study. V. M., C.C. and L.S. contributed to the analysis and interpretation of the data. G.M., E.T., C.S., L.F.B., N.C. and M.D.M. carried out data acquisition. D.A. carried out the statistical analysis. V.M. and L.S. carried out the drafting of the manuscript. V.S., R.S., M.A. and L.S. carried out critical article revision.
Competing Interests
The authors declare they have no conflicts of interest related to this manuscript. However, MA has received founds for attending symposia, speaking, grant research support, consultancy and advisory board membership from ABBOT, BRISTOL, GILEAD, MERCK, JANSENN CILAG, PFIZER, ROCHE, and ViiV HEALTHCARE. LS has received founds for attending symposia, speaking, and grant research support from GILEAD, MERCK, JANSENN CILAG, PFIZER, and ViiV HEALTHCARE.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pinchoff, J. et al. Impact of hepatitis B on mortality and specific causes of death in adults with and without HIV co-infection in NYC, 2000–2011. Epidemiol Infect, 1–11 (2016) [DOI] [PMC free article] [PubMed]
- 2.Rajbhandari R, Jun T, Khalili H, Chung RT, Ananthakrishnan AN. HBV/HIV coinfection is associated with poorer outcomes in hospitalized patients with HBV or HIV. J Viral Hepatitis. 2016;23:820–829. doi: 10.1111/jvh.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandeler G, Gsponer T, Bihl F, Bernasconi E, Cavassini M, Kovari H, Schmid P, Battegay M, Calmy A, Egger M, Furrer H, Rauch A, Swiss HIV. Cohort Study. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013;208:1454–8. doi: 10.1093/infdis/jit351. [DOI] [PubMed] [Google Scholar]
- 4.Thio CL, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/S0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu & European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–244.e3. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Chang JJ, Mohtashemi N, Bhattacharya D. Significance and Management of Isolated Hepatitis B Core Antibody (Anti-HBc) in HIV and HCV: Strategies in the DAA Era. Curr HIV/AIDS Rep. 2018;15:172–181. doi: 10.1007/s11904-018-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morsica G, et al. Occult hepatitis B virus infection in a cohort of HIV-positive patients: correlation with hepatitis C virus coinfection, virological and immunological features. Infection. 2009;37:445–449. doi: 10.1007/s15010-008-8194-9. [DOI] [PubMed] [Google Scholar]
- 10.Bivigou-Mboumba B, et al. Hepatitis B infection among HIV infected individuals in Gabon: Occult hepatitis B enhances HBV DNA prevalence. PLoS ONE. 2018;13:e0190592. doi: 10.1371/journal.pone.0190592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun HM, et al. Hepatitis B virus coinfection negatively impacts HIV outcomes in HIV seroconverters. J. Infect. Dis. 2012;205:185–193. doi: 10.1093/infdis/jir720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun HM, et al. HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J. Acquir. Immune Defic. Syndr. 2014;66:197–205. doi: 10.1097/QAI.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouamé G-M, et al. Higher Mortality Despite Early Antiretroviral Therapy in Human Immunodeficiency Virus and Hepatitis B Virus (HBV)-Coinfected Patients With High HBV Replication. Clin. Infect. Dis. 2018;66:112–120. doi: 10.1093/cid/cix747. [DOI] [PubMed] [Google Scholar]
- 14.Witt MD, et al. Predictors of the isolated hepatitis B core antibody pattern in HIV-infected and -uninfected men in the multicenter AIDS cohort study. Clin. Infect. Dis. 2013;56:606–612. doi: 10.1093/cid/cis908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya D, et al. Isolated Hepatitis B Core Antibody is Associated With Advanced Hepatic Fibrosis in HIV/HCV Infection But Not in HIV Infection Alone. J. Acquir. Immune Defic. Syndr. 2016;72:e14–17. doi: 10.1097/QAI.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh KP, et al. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017;31:2035–2052. doi: 10.1097/QAD.0000000000001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 18.Debes JD, Bohjanen PR, Boonstra A. Mechanisms of Accelerated Liver Fibrosis Progression during HIV Infection. J Clin Transl Hepatol. 2016;4:328–335. doi: 10.14218/JCTH.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 20.Young J, et al. Transient detectable viremia and the risk of viral rebound in patients from the Swiss HIV Cohort Study. BMC Infect. Dis. 2015;15:382. doi: 10.1186/s12879-015-1120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore AL, et al. Raised viral load in patients with viral suppression on highly active antiretroviral therapy: transient increase or treatment failure? AIDS. 2002;16:615–618. doi: 10.1097/00002030-200203080-00013. [DOI] [PubMed] [Google Scholar]
- 22.Masquelier B, et al. Intermittent viremia during first-line, protease inhibitors-containing therapy: significance and relationship with drug resistance. J. Clin. Virol. 2005;33:75–78. doi: 10.1016/j.jcv.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J InfectDis. 2012;205:1230–8. doi: 10.1093/infdis/jis104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones LE, Perelson AS. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with HAART. Bull. Math. Biol. 2005;67:1227–1251. doi: 10.1016/j.bulm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Günthard HF, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J. Infect. Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 26.Grzywacz M, et al. Response of asymptomatic cytomegalovirus viraemia to oral ganciclovir 3 g/day or 6 g/day in HIV-infected patients. J. Med. Virol. 1999;59:323–328. doi: 10.1002/(SICI)1096-9071(199911)59:3<323::AID-JMV11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Lichtner M, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J. Infect. Dis. 2015;211:178–186. doi: 10.1093/infdis/jiu417. [DOI] [PubMed] [Google Scholar]
- 28.Kovari H, et al. Hepatitis C Infection and the Risk of Non-Liver-Related Morbidity and Mortality in HIV-Infected Persons in the Swiss HIV Cohort Study. Clin. Infect. Dis. 2017;64:490–497. doi: 10.1093/cid/ciw809. [DOI] [PubMed] [Google Scholar]
- 29.Tsui JI, et al. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin. Infect. Dis. 2007;45:736–740. doi: 10.1086/520989. [DOI] [PMC free article] [PubMed] [Google Scholar]