Key Points
Biliary epithelial cells secrete Th17-polarizing cytokines.
Biliary epithelial cells chemoattract AhR+CCR6+CD4 T cells.
Th17 cells promote local Th17 expansion and bile duct proliferation.
Abstract
There is no effective treatment for autoimmune biliary diseases. Therefore, understanding their immunopathology is crucial. The biliary epithelial cells (BEC), expressing TLR-4, are constantly exposed to gut microbes and bacterial wall LPS, and in settings of inflammation, the immune infiltrate is dense within the peribiliary region of human liver. By dual immunohistochemistry, we affirm human intrahepatic T cell infiltrate includes CCR6+CD4+ and AhR+CD4+ T cells with potential for plasticity to Th17 phenotype. Mechanistically, we demonstrate that Th1 and Th17 inflammatory cytokines and LPS enhance human primary BEC release of the CCR6 ligand CCL20 and BEC secretion of Th17-polarizing cytokines IL-6 and IL-1β. Cell culture assays with human BEC secretome showed that secretome polarizes CD4 T cells toward a Th17 phenotype and supports the survival of Th17 cells. BEC secretome did not promote Th1 cell generation. Additionally, we give evidence for a mutually beneficial feedback of the type 17 cell infiltrate on BEC, showing that treatment with type 17 cytokines increases BEC proliferation, as monitored by Ki67 and activation of JAK2-STAT3 signaling. This study identifies human BEC as active players in determining the nature of the intrahepatic immune microenvironment. In settings of inflammation and/or infection, biliary epithelium establishes a prominent peribiliary type 17 infiltrate via recruitment and retention and enhances polarization of intrahepatic CD4 cells toward Th17 cells via type 17 cytokines, and, reciprocally, Th17 cells promote BEC proliferation for biliary regeneration. Altogether, we provide new insight into cross-talk between Th17 lymphocytes and human primary biliary epithelium in biliary regenerative pathologies.
Introduction
The autoimmune biliary diseases, including primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), account for a significant proportion of patients with chronic liver disease who develop fibrosis and cirrhosis (1). Currently, there are no effective treatments for these conditions. To develop suitable therapies, it is necessary to understand the pathological mechanisms that underlie their development.
IL-17–secreting Th cells (Th17 cells) differentiate from naive CD4 T cells in response to specific combinations of cytokines that include TGF-β and IL-6 (2–5). Th17 cells play critical roles in the immune defense response against pathogens and contribute to the pathogenesis of inflammatory diseases in both mice and humans (6–8). They are involved in the pathogenesis of autoimmune diseases like psoriasis, multiple sclerosis, and rheumatoid arthritis (9–12) and have been implicated in autoimmune liver diseases (8, 13). However, recent findings indicate a possible immune protective role for Th17 cells within certain tissue sites and/or microenvironments (14). For example, anti–IL-17A therapy (secukinumab) is effective in the treatment of psoriasis; however, it leads to exacerbation of Crohn disease in a clinical trial (15). Consistent with this, populations of IL-17–expressing cells that are nonpathogenic are now being characterized (14, 16, 17).
We previously reported Th17 cell accumulation and localization around bile ducts (8). Although periductal IL-17 production has been shown to contribute to the pathogenesis of cholangiopathies (18), the importance of the nonpathogenic IL-17 cell populations in biliary protection and restraining the progression of biliary disease toward end-stage liver disease has not been formally addressed. In addition, gut microbes have been shown to direct the differentiation of Th17 cells in the small intestine (19). For example, Th17 cell proliferation is promoted by segmented filamentous bacteria, whereas Bacteroides fragilis, a gut commensal bacterium, dampens IL-17 production (17). Our previous work also demonstrated that bacteria-exposed biliary epithelial cells (BEC) can activate mucosal-associated invariant T cells (20), leading to the hypothesis that bile ducts stimulated by bacteria may be able to attract and induce the differentiation of other infiltrating CD4 T cells.
In this study, we investigated the mechanisms by which inflamed BEC induce functional Th17 generation in the peribiliary hepatic microenvironment and the feedback effect on BEC survival and proliferation. We detected increased production of the CCR6 ligand, chemokine CCL20, by BEC under inflamed conditions and found that BEC secretome induced by Th17 cytokines induced the differentiation of CD4 T cells toward a Th17 phenotype and their survival. Taken together, our data suggest that paracrine signaling networks in the hepatic microenvironment lead to the recruitment and persistence of Th17 cells and Th17 differentiation in human liver diseases. Furthermore, we identify an important relationship wherein the Th17 cytokines generated by the peribiliary Th17 population support biliary repair.
Materials and Methods
Use of human tissue
All studies using human tissues were reviewed and approved by an appropriate institutional ethics review committee. Ethical permission for the use of human liver tissue for research was granted by the local research ethics committee (approval no. CA/5192).
Isolation of primary human BEC
Primary human BEC were isolated from explanted liver disease tissue or from normal donor liver surplus to transplant requirements, as previously described (21, 22), obtaining cultures that are >95% positive for the epithelial marker CK18 and the marker CK19, specific to BEC of the liver but negative for the endothelial protein CD31 and fibroblast marker CD90/Thy-1 (22).
Briefly, liver (∼30 g) was finely diced and incubated with collagenase type 1A (Sigma-Aldrich, St. Louis, MO). The digest was layered onto a 33 and 77% Percoll gradient and centrifuged at 500 × g for 30 min. The interface layer was collected, washed three times in PBS, and incubated with the cholangiocyte-specific mAb to Human Epithelial Antigen 125 (Progen, Heidelberg, Germany). BEC were positively selected by incubating with anti-mouse IgG1-coated Dynabeads (Invitrogen, Carlsbad, CA) followed by magnetic separation. The cells were cultured in a 1:1 mix of DMEM and Hams F12 medium supplemented with heat-inactivated human serum (10% v/v; TCS Biosciences, Buckingham, U.K.), penicillin, streptomycin (100 μg/ml) and glutamine (2 mM; Life Technologies), hepatocyte growth factor (10 ng/ml; Peprotech, London, U.K.), epidermal growth factor (10 ng/ml; Peprotech), cholera toxin (10 ng/ml; Sigma-Aldrich), tri-iodo-thyronine (2 nM; Sigma-Aldrich), hydrocortisone (2 μg/ml; Queen Elizabeth Hospital, Birmingham, U.K.), and insulin (0.124 μ/ml; Queen Elizabeth Hospital). Cells were grown in 25-cm2 tissue culture flasks coated with rat tail collagen with regular medium exchanges until they became a confluent monolayer, then expanded into 75-cm2 tissue culture flasks coated with rat tail collagen. Cells were used between passages two and six to ensure phenotypic stability.
Isolation of liver-infiltrating lymphocytes
Fresh isolation of human liver-infiltrating lymphocytes from various diseased explanted livers was carried out for ex vivo phenotyping (21). Briefly, resected liver tissue was diced into 5-mm3 cubes and placed for 5 min at 260 rpm in a Stomacher 400 circulator (Seward, U.K.). Mechanical digestion was applied to preserve chemokine receptors, resultant homogenized tissue was filtered through a fine gauze mesh, and lymphocytes were separated by layering the suspension over a Lympholyte density gradient (Cedarlane) and centrifugation at 800 × g for 30 min.
Cytokine stimulation
To study proliferation, apoptosis, and necrosis of BEC, primary BECs were cultured for 24 h in BEC media supplemented with the following: culture medium only (control), IL-17 (50 ng/ml), IL-22 (20 ng/ml), TNF-α (10 ng/ml), IFN-γ (100 ng/ml), or TNF-α (10 ng/ml) + IFN-γ (100 ng/ml) (all from Peprotech).
Cell death and proliferation assays
Cell death after stimulation with recombinant human Th17-related cytokines (IL-17, IL-22, TNF-α, IFN-γ, or TNF-α + IFN-γ in combination for 24 h) was assessed by using Annexin V to detect apoptosis and 7-AAD for necrotic cell detection. Confluent monolayers were trypsinized and resuspended in 1 × binding buffer (0.1 M HEPES [pH 7.4], 1.4 M NaCl, 25 mM CaCl2). Annexin V and 7-AAD were added to each sample and incubated on ice in the dark for 15 min and then fixed in 2% paraformaldehyde before analysis on a Dako Cytomation CyAn flow cytometer. Data were analyzed using Summit software version 4. Proliferation after stimulation with recombinant human Th17-related cytokines was measured using flow cytometry and in situ Ki67 staining. For in situ analysis of Ki67, confluent monolayers of cells grown in 24-well plates were fixed in methanol for 10 min at room temperature, washed in PBS, and then stained. Endogenous peroxidase activity was blocked using 0.3% H2O2 in methanol for 5 min. After washing wells with PBS, Ag retrieval was performed using proteinase K (30 mg/ml; Qiagen) for 30 min at 37°C. The wells were washed twice in PBS and blocked with 10% horse serum for 20 min, after which the primary Ab (anti-Ki67 Ab; SP6, ab1667; Abcam) was incubated for 1 h. Excess Ab was removed by washing using PBS, and the secondary ImmPRESS (Vector Labs) reagent was added for 30 min. The wells were washed in PBS, and the AEC substrate (Vector Labs) was added for 30 min. The reaction was stopped using distilled water, and the cells were counterstained using Mayers hematoxylin. The counterstain was developed in tap water, and the samples were left in tap water at 4°C until they were counted using a light microscope. Three random fields of each sample were counted.
BEC cytokine and chemokine secretion
BEC were plated in rat tail collagen–coated 24-well plates at a density of 65,000 cells per well and allowed to adhere overnight. Adherent cells were then treated with untreated culture medium or medium containing IL-17 (50 ng/ml; Miltenyi Biotec), TNF-α (10 ng/ml; Peprotech) + IFN-γ (10 ng/ml; Peprotech), or LPS (1 μg/ml; Sigma-Aldrich) for 24 h. Culture volumes of 300 μl/well were used. Cell supernatants were analyzed by Luminex (Bio-Rad), according to the manufacturer’s instructions to measure the concentrations of IL-1β, IL-6, TGF-β1, and CCL20. All measurements were performed in duplicate for each experiment.
Western blotting
JAK2, STAT3, and phospho-STAT3 expression by BECs was examined by Western blotting. For Western immunoblotting studies, BEC were lysed at the end of the relevant experimental period using Nonidet P-40 lysis buffer (20 mM TRIS-HCl [pH 8], 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, 2 mM EDTA [all from Sigma-Aldrich]). Protein concentration was determined by Bradford protein assay, and 25 μg of protein was resolved on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (Hybond; Amersham Biosciences). The blotted membrane was blocked for 1 h at room temperature in TBS (pH 7.4)/Tween 0.1% (Sigma-Aldrich) containing 5% (w/v) BSA (A9418; Sigma-Aldrich). All primary Ab incubations were performed overnight at 4°C in TBS/Tween 0.1% containing 5% BSA (w/v). The incubation steps were followed by three washing steps of 5 min with TBS containing 0.1% Tween. All primary Abs were used at a dilution of 1:1000, as per manufacturer’s instructions. Specific primary Abs used included JAK2 (3230), STAT3 (4904), and phospho-STAT3 (9131) (all from New England Biolabs).
Binding of specific mAb was detected with an HRP-conjugated anti-rabbit IgG at a dilution of 1:2500 for 1 h (New England Biolabs). Protein bands were visualized using the ECL detection system (Amersham Biosciences) followed by exposure of the membranes to Hyperfilm-ECL (Amersham Biosciences). Equality of protein loading was checked by immunoblotting for β-actin (Sigma-Aldrich) (dilution 1:20000). All Western immunoblots were performed at least three times from different liver preparations of BEC.
Coculture of Th17 and CD4 T cells in BEC supernatant
Th17 cells were freshly isolated using the IL-17 Secretion Assay, according to manufacturer’s instructions (Miltenyi Biotec). Th17 cells were analyzed for viability using the MTT assay over 2 wk in culture supernatants generated by cytokine-stimulated BEC or Th17-polarizing cytokines. Total CD4 T cells were isolated by magnetic negative isolation (Mojosort Human CD4+ T cell isolation kit; BioLegend) and cultured for 7 d in culture supernatants generated by cytokine-stimulated BEC, activated with anti-CD3/anti-CD28 Dynabeads (Invitrogen) at a ratio of eight cells per one bead. Lineage changes toward Th17 or Th1 were explored based on cytokine production. To examine cytokine profiles, cells were stimulated for 5 h with PMA (50 ng/ml) and ionomycin (1 μM). Cytokine release was blocked with brefeldin A (10 μg/ml) during the final 4 h of stimulation. Cells were then stained using e506 viability dye (eBioscience), fixed in 3% formaldehyde, and stained in 0.1% saponin solution with fluorochrome-conjugated Abs against CD3 (SK7; BD Bioscience), CD4 (RPA-T4; eBioscience), and cytokines TNF-α (MAb11; eBioscience), IFN-γ (4S.B3; eBioscience), IL-17 (CZ8-23G1; Miltenyi Biotec), and IL-10 (JES3-9D7; BioLegend). Cells were read using a Dako CyAn ADP flow cytometer, and the data were analyzed using FlowJo V10 software, according to the gating strategy shown in Supplemental Fig. 1.
Flow cytometry analysis of intrahepatic CCR6-expressing cells
Surface marker expression by intrahepatic lymphocyte subsets was analyzed by flow cytometry, as described previously (20).
Detection of bilirubin in liver supernatants
Liver supernatants were collected by preparing 1 g of liver tissues in RPMI. Acetonitrile (ACN), methanol (MeOH), and HPLC-mass spectrometry (MS) quality water (H2O) were obtained from Fisher Scientific (U.K.). MS quality formic acid and low binding tubes were obtained from Sigma-Aldrich (U.K.). Metabolites were extracted, applying a monophasic extraction protocol in a randomized order to produce (same number of samples) extractions. For each sample, 50 μl of liver supernatants was transferred to an Eppendorf tube (Eppendorf, Cambridge, U.K.). Into each tube at room temperature, 100% methanol was added in a ratio of 4:1 to precipitate the proteins. Samples were centrifuged at 14,000 × g for 15 min at 4°C, and 150 μl of the supernatants was then transferred into separate glass HPLC vials for ultra-HPLC (UHPLC)-MS analysis. Remaining aliquots of the extraction solutions were collected into a single pooled quality control (QC) sample of which 200 μl was transferred to a new HPLC glass vial and analyzed as described for the biological samples. UHPLC-MS was used to analyze the metabolome of liver supernatant extracts. Analysis was performed, applying an Ultimate3000 RSLC UHPLC system coupled to an electrospray Q-Exactive Focus mass spectrometer operating positive and negative ion modes. Five microliters of each extracted sample was injected onto a Hypersil Gold C18 reversed phase column (100 × 2.1 mm 1.9 μm) at a flow rate of 300 μl/min. A gradient elution was applied, as previously described (23). All samples were analyzed in a random order in one analytical batch, with 10 QC samples analyzed at the start of the analytical batch, after every five biological samples, and with two QC samples analyzed at the end of the analytical batch. Two blank samples were also analyzed. Data preprocessing and analysis was performed with UHPLC-MS raw data files (.RAW) converted to an mzML format by using the MS Convert software. Data deconvolution was then performed using XCMS to provide a two-dimensional matrix of chromatographic peak responses in which each peak was defined by the m/z ratio and retention time. This two-dimensional matrix was exported as a.csv for data analysis. Metabolites were annotated applying PUTMEDID_LCMS (24). Data filtering was performed to remove all metabolite features with >30% missing values for all samples and a relative SD of >20% for QC samples analyzed from injection nine onwards (25).
Immunohistochemical analysis of the distribution of CCR6+CD4 and AhR+CD4 T cells in normal and diseased liver tissues
Paraffin-embedded human liver sections were dual stained for CCR6+CD4 and AhR+CD4 T cells using a sequential staining protocol on the Leica Bond Max automated staining system. All sections were pretreated with the Epitope Retrieval Solution 1, heating to 95°C for 20 min, and peroxide activity was blocked using Leica Peroxide block for 5 min. Development of the first Ag stained (CCR6 [clone 53103; R&D Systems]) or aryl hydrocarbon receptor (AhR) (clone MAI-514; Invitrogen) was performed using DAB Solution for 10 min, and nuclei were detected with hematoxylin staining. A second epitope retrieval phase involving incubation at 95°C in Epitope Retrieval Solution 2 was included before staining of CD4 (ab133616; Abcam) and its subsequent development with Leica Mixed Red Refine Detection for 15 min and a second staining with hematoxylin. At the end of staining, slides were cover slipped in Invitrogen Diamond Antifade Aqueous Mountant and left to dry for 24 h before scanning at ×20 on the Perkin Elmer Vectra imaging system. The numbers and distributions of CCR6+CD4 or AhR+ CD4 cells from 10 representative fields were quantified using the inForm analysis software (PerkinElmer). Patterns of cell organization visible from hematoxylin staining enabled biliary structures within the tissues to be identified and, thus, the distributions of CCR6+CD4 and AhR+CD4 cells to be expressed relative to the biliary epithelium.
Statistical analysis
Effects of treatment were assessed by one-way ANOVA using Friedman nonparametric test with Dunn multiple comparisons post hoc analysis to compare the effects of each treatment to untreated control or by Wilcoxon matched pairs tests, applying the Bonferroni correction for multiple comparisons. Effects of disease, cell type, and tissue on cell distribution were examined by two-way ANOVA. Tests used are described in the accompanying figure legends. Analysis was performed using GraphPad Prism 5.0 software (GraphPad software, San Diego, CA). Statistical significance was defined as a p value <0.05.
Results
Bacterial products and type 17 cytokines lead to the secretion of Th17-polarizing cytokines from biliary epithelium
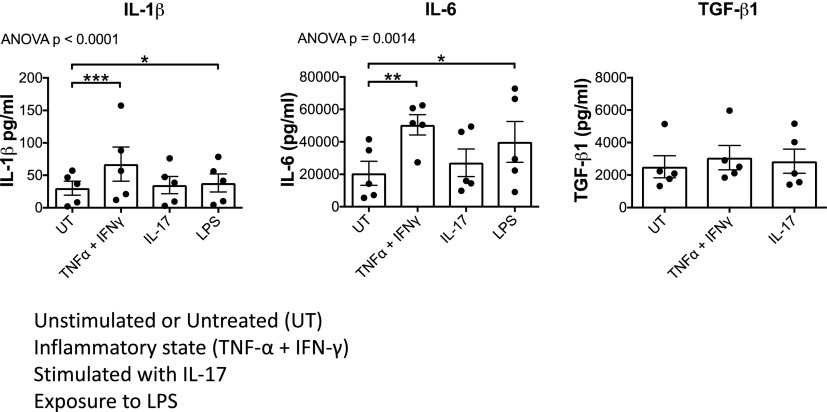
We and others have shown that BEC express both IL-17A receptor (8, 26) and TLR-4 (27). This suggests they may respond to IL-17 and bacterial products by secreting Th17-polarizing cytokines that maintain local Th17 responses. Thus, we measured the concentrations of Th17-polarizing cytokines IL-1β, IL-6, and TGF-β in the supernatants of BEC cultures treated with IL-17, LPS, and TNFα + IFN-γ and identified a significant effect of treatment on IL-1β and IL-6 secretion by BEC (one-way ANOVA). Compared with basal levels, we observed significant increases in the secretion of both IL-1β and IL-6 with both LPS and TNF-α + IFN-γ stimulations (Fig. 1, Supplemental Fig. 2).
FIGURE 1.
BEC secrete IL-1β, IL-6, and TGF-β1 on exposure to type 17 cytokines and LPS. BEC were stimulated with type 17–related cytokines (TNF-α + IFN-γ or IL-17) or LPS for 24 h, and Th17-polarizing cytokines IL-1β, IL-6, and TGF-β1 were measured in their supernatants. Effects of treatment were assessed by one-way ANOVA using Friedman nonparametric test with Dunn multiple comparisons post hoc tests (significant comparisons indicated by asterisks) comparing the effects of each treatment to untreated control (UT). Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0. 001.
CCR6-expressing intrahepatic CD4 lymphocytes reside in the peribiliary region
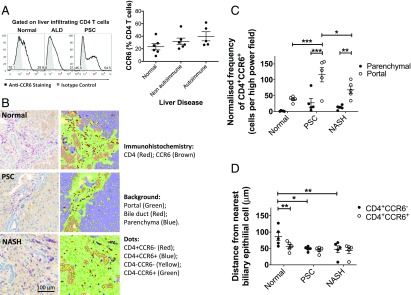
The CCR6 chemokine receptor defines the Th17 lineage, and both blood and tissue Th17 cells express CCR6 (8, 28). We previously reported that there is a high level of functional CCR6 expression on liver-infiltrating Th17 cells in inflamed human livers (8, 28). Our observation that IL-17 cells are present at a higher frequency around bile ducts led us to quantify the frequency of CCR6 expression among human liver CD4 T cells across different liver diseases. A total of 20–62% of human intrahepatic CD4 T cells from diseased livers, both autoimmune (PSC and PBC) and nonautoimmune (alcoholic liver disease and nonalcoholic steatohepatitis), expressed the CCR6 chemokine receptor in contrast to 8–45% of CD4 T cells from normal liver, with the highest frequencies observed on the CD4 T cells from PSC/PBC livers (Fig. 2A). To complement studies of intracellular IL-17 distribution in inflamed human livers, we applied immunohistochemistry to examine the localization of CCR6+CD4 T cells in liver tissue (Fig. 2B). CCR6+CD4 T cells were found mostly in the portal tracts associated with inflamed bile ducts (Fig. 2B, 2C). Numbers of CCR6+CD4 T cells were significantly increased in the portal regions of PSC liver tissue compared with normal liver tissue (Fig. 2C). In normal liver, the CCR6+CD4 T cells resided significantly closer to biliary epithelium than their counterparts (Fig. 2D); the same trend was also seen in diseased livers (Fig. 2D).
FIGURE 2.
Intrahepatic CD4+CCR6+ lymphocytes are present around bile ducts in the portal tract. (A) Expression of CCR6 by human intrahepatic CD3+CD4+ T cells from normal and diseased livers as determined by flow cytometry. Representative histograms (left) (black line = CCR6 staining; filled histograms = isotype matched control staining) and summary data (right) are shown. Data are mean ± SEM. (B) Localization of CD4+CCR6+ and CD4+CCR6− T cells in liver tissue examined by immunohistochemistry (IHC) on paraffin-embedded liver tissue sections from normal, PSC, and nonalcoholic steatohepatitis (NASH) livers. Overlay of tissue segmentation and cell phenotype generated in inForm is displayed on the right. (C) Frequency of CD4+CCR6+ cells in parenchymal and portal regions of diseased and normal liver sections (n = 5) determined by IHC analysis, normalized to the number of cells present in each tissue category per image. Significance was assessed by two-way ANOVA with Sidak correction (between locations [portal versus parenchyma]) and Tukey post hoc test. Significance interval indicated by asterisks (*). *p < 0.05, **p < 0.01, ***p < 0.001. Data are mean ± SEM. (D) Mean distance of CD4+CCR6+ and CD4+CCR6− cells from the nearest cell identified as bile ducts based on morphology by inForm IHC analysis of normal and diseased liver sections (n = 5). Significance was assessed by two-way ANOVA with Sidak (between cell types) and Tukey (between disease category) multiple comparisons post hoc tests (significant intervals indicated by asterisks [*]). Data are mean ± SEM. *p < 0.05, **p < 0.01.
AhR-expressing CD4 cells are concentrated in the portal tract, and the AhR ligand bilirubin is increased in the inflamed human liver
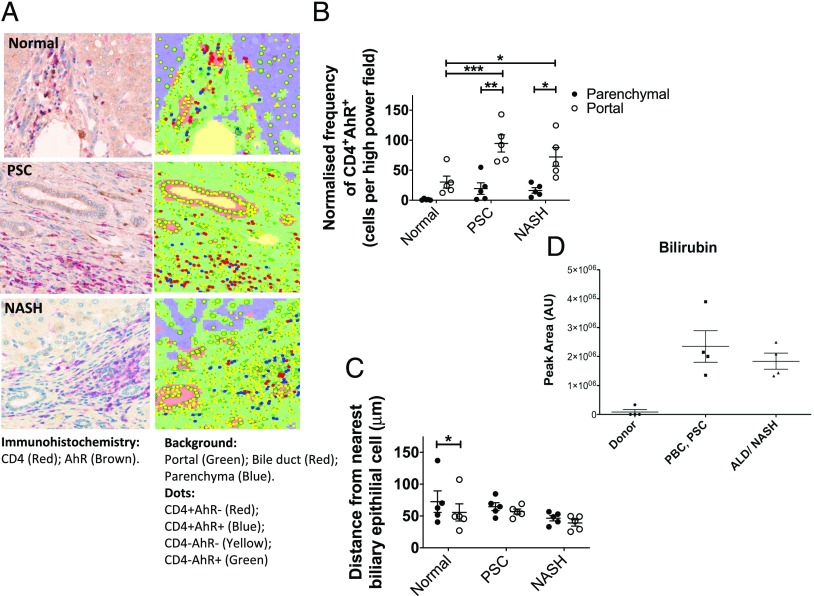
The AhR is a ligand-dependent transcription factor that, when bound by ligand, activates nuclear transcription factors that drive Th17 differentiation (29–32). Similar to CCR6, immunohistochemical dual staining for CD4 and AhR on diseased liver tissues demonstrated that AhR-expressing CD4 T cells are densely populated in the portal tracts (Fig. 3A, 3B) and that they are increased in diseased liver compared with normal liver. Furthermore, AhR+ CD4 T cells approached closer to biliary epithelium cells in diseased livers (Fig. 3C). Bilirubin is a recognized AhR ligand (30), and we found that this potential local ligand for AhR was present in significant quantities in inflamed human liver tissue (Fig. 3D). It is possible that bilirubin acts synergistically with BEC to drive establishment of a prominent Th17 population in the portal tracts of diseased livers.
FIGURE 3.
AhR-expressing intrahepatic CD4 T cells are present at high density around bile ducts, and AhR ligand, bilirubin, is present in inflamed human liver tissue. (A) Localization of CD4+AhR+ and CD4+AhR− T cells in liver tissue examined by immunohistochemistry on paraffin-embedded liver tissue sections from normal, PSC, and nonalcoholic steatohepatitis (NASH) livers. Overlay of tissue segmentation and cell phenotype generated in inForm is displayed on the right. Original magnification ×20. (B) Frequency of CD4+AhR+ cells in parenchymal and portal regions of diseased and normal liver sections (n = 5) determined by immunohistochemistry analysis, normalized to the number of cells present in each tissue category per image. Significance was assessed by two-way ANOVA with Sidak (between locations [portal versus parenchyma]) and Tukey (between disease category) multiple comparisons post hoc tests. Significance intervals are indicated by asterisks (*). *p < 0.05, **p < 0.01, ***p < 0.001. Data are mean ± SEM. (C) Mean distance of CD4+AhR+ and CD4+AhR− cells from the nearest cell identified based on morphology was assessed by two-way ANOVA with Sidak (between cell types) and Tukey (between disease category) multiple comparisons post hoc tests. Significance intervals are indicated by asterisks (*). *p < 0.05. Data are mean ± SEM. (D) Bilirubin levels in normal liver and inflamed diseased livers were determined by UHPLC-MS. All data were normalized against the total ion count, and the statistical analysis was performed in R applying Mann–Whitney U test. ALD, alcoholic liver disease.
LPS-exposed and inflamed BEC secrete CCR6 ligand CCL20
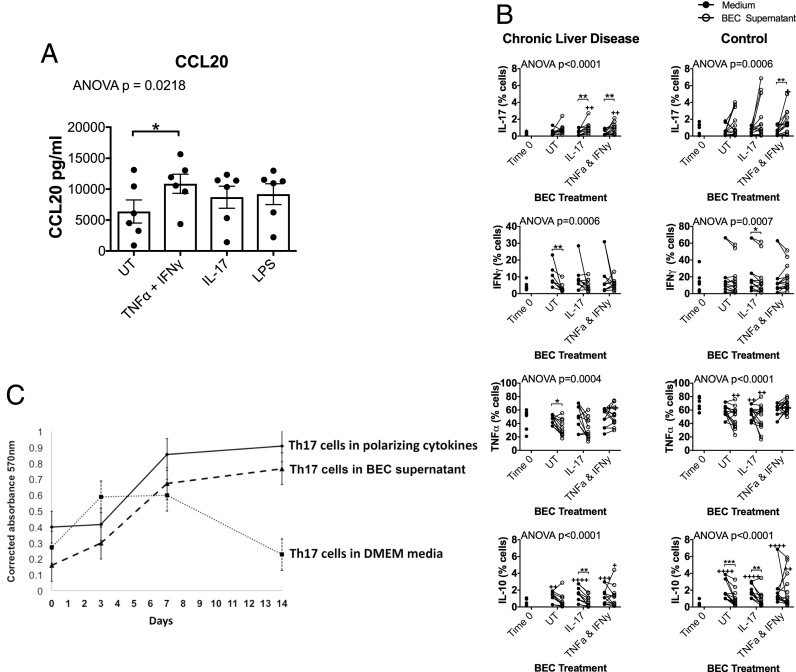
BEC are known to provide a primary defense barrier against microbes such as Gram-negative bacteria ascending from the gastrointestinal tract (33). TLR-4 recognizes Gram-negative bacteria and is expressed on human BECs (34). Therefore, we investigated how exposure to the TLR-4 ligand, LPS, affects production of the CCR6 ligand, CCL20, by BEC. We observed a trend toward increased CCL20 release by BEC with LPS (Fig. 4A, Supplemental Fig. 3A).
FIGURE 4.
LPS-exposed and inflamed BEC secrete CCL20, and culture of CD4 T cells in BEC-conditioned media promotes CD4 differentiation toward Th17 and maintains the survival of Th17 cells. (A) BEC were stimulated with soluble recombinant human cytokines (TNF-α + IFN-γ or IL-17) or LPS for 24 h, and the supernatants were analyzed for CCL20 chemokine secretion (n = 7). Effects of treatment were assessed by one-way ANOVA using Friedman nonparametric test with Dunn multiple comparisons post hoc tests (significant comparisons indicated by asterisks [*]) comparing the effects of each treatment to untreated control (UT). *p < 0.05. Data are mean ± SEM. (B) BEC were treated with type 17 cytokines for 24 h, and their supernatants were collected. CD4 T cells from chronic autoimmune liver disease patients and hemochromatosis (control) liver disease patients were stimulated with anti-CD3/CD28 beads and cultured in the BEC supernatants or control (recombinant cytokine containing media), and their expression of IL-17, TNF-α, IFN-γ, and IL-10 was examined at day 0 and day 7 by flow cytometry. Summary data for multiple donors are presented. Lines link frequencies of cytokine expression by cells from a given donor when cultured for 7 d in BEC supernatants versus the respective medium control. Effects of culture and treatment were assessed by one-way ANOVA using Friedman nonparametric test. Differences in expression compared with ex vivo were identified by Dunn multiple comparisons post hoc tests comparing to time 0 (significant comparisons indicated by crosses). Differential effects of BEC-secreted products compared with control medium at 7 d were identified by individual Wilcoxon matched pairs tests, applying the Bonferroni correction for multiple comparisons (significant comparisons indicated by asterisks [*] over a linking bracket). */+p < 0.05, **/++p < 0.01, ***/+++p < 0.001, ++++p < 0.0001. (C) Th17 cell viability over 14 d when cultured in supernatant from untreated BEC cultures (dashed line) was monitored and compared over 14 d to the viability of Th17 cells cultured in control medium (dotted line) or Th17-polarizing medium (IMDM supplemented with IL-1β, IL-6, and TGF-β) (solid black line); n = 3.
In addition, when we considered how cytokines generated by cells of the portal immune infiltrate influence the release of CCL20 by BEC, consistent with our previous findings, we observed that Th1 cytokines (TNF-α and IFN-γ) induced the secretion of CCL20 by BEC. Treating BEC with IL-17 also tended to increase their release of CCL20 (Fig. 4A, Supplemental Fig. 3A). Of note, BEC themselves produce IL-17 and tended to increase IL-17 release under inflammatory (TNF-α and IFN-γ) conditions (Supplemental Fig. 3B).
BEC secretome promotes local Th17 generation from CD4 T cells and supports Th17 survival
Evidence of the ability of BEC supernatant to direct the differentiation of CD4 T cells toward a Th17 phenotype came from experiments in which the supernatants from BEC cultured for 24 h in blank culture medium or medium containing the recombinant cytokines IL-17 or TNF-α + IFN-γ were used to condition 7-d stimulations of blood-derived CD4 T cells activated with anti-CD3/CD28 beads. Medium containing the cytokines was used as treatment controls for the influence of BEC. Effects on CD4 T cells from autoimmune hepatitis (AIH) patients were compared with those on CD4 T cells from a control cohort with the nonimmune-mediated liver condition hemochromatosis (HFE). Although the cytokines alone (medium controls) did not lead to significant changes in the frequencies of CD4 T cells producing IL-17, IFN-γ, or TNF-α compared directly with ex vivo (time 0), they caused BEC to release factors that supported the differentiation of CD4 T cells to IL-17–producing cells. Supernatants from BEC stimulated with IL-17 or TNF-α + IFN-γ increased frequencies of IL-17 cells 2- to 3-fold compared with their respective control media at 7 d. (AIH patients: IL-17 treatment: 0.95% ± 0.20 versus 0.45% ± 0.1; TNF-α + IFN-γ treatment: 0.97% ± 0.18 versus 0.39% ± 0.10) (HFE control cohort: IL-17 treatment: 1.84% ± 0.60 versus 0.59% ± 0.11; TNF-α + IFN-γ treatment: 1.62% ± 0.34 versus 0.65% ± 0.14) (Fig. 4B, Supplemental Fig. 3C). All differences were significant, with the exception of the effects of IL-17 treatment on CD4 T cells from the HFE control cohort. There was a trend toward the support of increased IL-17+ CD4 T cell frequencies with culture in untreated BEC supernatant compared with control medium, but this was not statistically significant for cells from either AIH patients or controls.
Although there is a change in the frequencies of IFN-γ– and TNF-α–expressing cells with the different treatments, we did not identify any consistent polarizing effects of BEC on cells of these phenotypes for either AIH patient or HFE controls (Fig. 4B, Supplemental Fig. 4). Interestingly, however, we noted significantly reduced IFN-γ and TNF-α cell frequencies among AIH patients compared with HFE controls directly ex vivo (Fig. 4B).
We also considered the effect of BEC on frequencies of IL-10–expressing CD4 T cells. The culture of CD4 T cells over 7 d with anti-CD3/CD28–coated beads led to a significant increase in IL-10+ CD4 T cell frequencies in the absence of BEC supernatant. Culturing in supernatants from nonstimulated and IL-17-treated BEC prevented this rise (Fig. 4B).
Having observed BEC-dependent changes in IL-17 and IL-10 frequencies, we characterized the IL-17+ cells in greater detail. Almost all IL-17+ cells also expressed TNF-α (AIH: mean >75%; control: mean >95%). Less than 40% made IFN-γ, and <10% made IL-10. In accordance with the significant suppression in the induction of total IL-10+ cells with supernatant from IL-17–stimulated BEC, frequencies of IL-10+IL-17+ cells were significantly reduced under these conditions compared with IL-17 cytokine medium control conditions (Supplemental Fig. 4).
Next we explored how the BEC microenvironment can support the survival of the local Th17 population by culturing isolated Th17 cells in BEC supernatant or under Th17-polarizing cytokine conditions. We observed significant maintenance of Th17 cell viability over 2 wk with either BEC supernatant or Th17-polarizing cytokines compared with control (Fig. 4C).
BEC proliferate in response to Th17-related cytokines
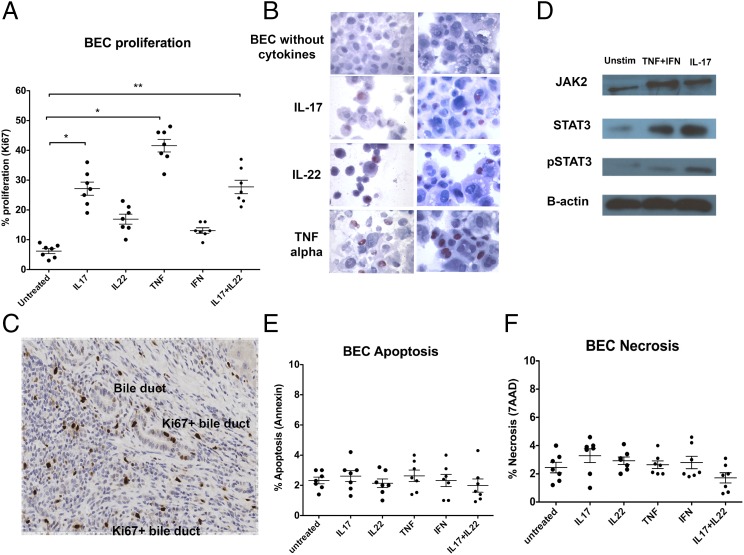
Next we investigated the possibility that Th17 cells around BEC feed back to the BEC to influence their proliferation and survival by studying the effect of Th17 cytokines IL-17, IL-22, and TNF-α on BEC proliferation, apoptosis, and necrosis. As indicated by Ki67 staining, BEC proliferated in response to Th17-cytokines (Fig. 5A, 5B). The strongest responses were seen with TNF-α and IL-17 treatments, followed by IL-22 (Fig. 5A, 5B). These results were consistent with our observation of Ki67+ (proliferating) cells within the biliary epithelium in inflamed human liver tissues by immunohistochemistry (Fig. 5C).
FIGURE 5.
Th17 cytokines lead to BEC proliferation via the STAT3 pathway. (A) BEC were stimulated for 24 h with rTh17-related cytokines IL-17, TNF-α, IFN-γ alone, or IL-17 and IL-22 in combination and then assessed for cell proliferation by flow cytometric analysis of Ki67 staining. (B) In situ Ki67 staining of cytospun BEC was performed to examine cell proliferation. (C) BEC proliferation on liver tissue sections was assessed by Ki67 staining (brown, DAB) (PSC liver). (D) BEC were stimulated with recombinant human Th17-related cytokines IL-17, TNF-α, and IFN-γ, and the expression/activation of intracellular signaling pathway components JAK2, STAT3, and pSTAT3 was analyzed by Western blotting. (E) Isolated primary BEC were stimulated with different Th17-related cytokines, and apoptosis and (F) necrosis of BEC was examined by Annexin and 7-AAD staining with flow cytometry. *p < 0.05, **p < 0.01, as compared with untreated controls.
Furthermore, we examined the effects of IL-17 and TNF-α + IFN-γ treatments on the expression of STAT3, JAK-2, and phosphorylation of STAT3 in BEC as this pathway is known to coordinate proliferation downstream of cytokine stimulation. In agreement with the induction of BEC proliferation by these cytokines, we noted upregulated expression and activation of these prosignaling components in BEC with treatment (Fig. 5D).
Finally, we evaluated the effects of these cytokines on BEC survival. We found no evidence of either apoptosis or necrosis of BECs on exposure to Th17 cytokines by Annexin V and 7-AAD staining (Fig. 5E, 5F).
Discussion
Th17 cells are critical players in the pathogenesis of human liver diseases (9, 13, 33, 35); however, in the gut–liver axis, they also act as a firewall against gut-derived infections (20), which ascend via the biliary tree. As such, they have a key antipathogenic role within the biliary environment. It is seen that Th17 cells are recruited to inflamed human liver and localize around the bile ducts (8, 33, 36), although the mechanisms by which they are recruited and maintained in the peribiliary environment are not fully known. Intrahepatic IL-17–secreting type 17 cells consist of not only Th17 cells (8) but also Tc17 cells (37), MAIT cells (20), and γδ T cells (38). There is minimal contribution of IL-17 by intrahepatic non-CD3 cells.
BEC participate in microbe-induced proinflammatory responses in human liver cholangiopathies (39). The common bile duct is continuously exposed to the gut flora in the human gastrointestinal tract, and the portal vein drains venous blood, enriched with endotoxins and bacterial products, from both the midgut and hindgut to the liver via the portal tracts. Expression of TLR (including TLR-4, the receptor for bacterial LPS) allows BEC to respond to Gram-negative bacteria, such as Escherichia coli (34). Although local antimicrobial responses are important for the maintenance of tissue homeostasis and control of ascending infection, inappropriate innate immune responses may contribute to the pathogenesis of biliary diseases, such as PSC (1). BEC secrete the biliary tropic chemokine CCL20 (8), which activates CCR6 on Th17 cells, and evidence suggests that this pathway is critical for the development of Th17 responses in the liver (28, 40) and gut (41). We now demonstrate that the secretion of CCL20 by BEC is increased under specific inflammatory conditions and in response to exposure to the bacterial product LPS. This enhanced local secretion of CCL20 may explain the concentration of CCR6-expressing cells around bile ducts and the localization and enrichment of IL-17–expressing cells in the portal tract of diseased livers.
The majority of intrahepatic CD4 cells in portal infiltrates localize around the bile ducts, particularly in biliary diseases in which BEC are the targets of immune-mediated tissue injury. BEC are highly active epithelial cells capable of secreting a range of proinflammatory and immune modulatory factors. Seminal studies in the skin have suggested that epithelial cells are able to modulate immune differentiation in response to evolving inflammation and tissue injury, a phenomenon that has been termed the epimmunome (42). We investigated whether BEC contribute to the epimmunome in the liver by promoting local Th17 immune responses. The crucial Th17-polarizing cytokines include IL-1β, IL-6, and TGF-β, all of which have been detected previously in the inflamed liver (3, 43–47). In this study, we demonstrate that BEC not only secrete Th17-polarizing cytokines IL-6, IL-1β, and TGF-β when they are exposed to type 17 cytokines or LPS, but they also have the capability to generate and maintain Th17 differentiation of other CD4 T cells. Our findings are consistent with a recent in vivo study suggesting that splenic CD4 T cells cocultured with liver nonparenchymal cells have increased IL-17 production (13). Thus, we provide evidence for a critical central role of the biliary epithelium in recruiting CCR6+ CD4 Th17 precursors to the portal tract by secreting CCL20.
The AhR is a ligand-dependent transcription factor (32) that is crucial for Th17 generation (32), and we now show that the majority of liver-infiltrating AhR+CD4 T cells, which have susceptibility to differentiate to the Th17 lineage, are located in the peribiliary region of portal tracts. Furthermore, the inflamed intrahepatic microenvironment is enriched with the AhR ligand bilirubin, which could work synergistically with the enhanced release of Th17-promoting cytokines by BEC in the inflamed/infected liver to support the establishment of a prominent portal Th17 population.
Our study provides evidence that BEC are active in the recruitment, de novo generation, and survival of a Th17 infiltrate around them. We have shown before that the IL-17 receptor is expressed on BEC (8) and now demonstrate that BEC do respond to IL-17 and other Th17 cytokines by activation of the JAK2–STAT3 pathway and proliferating, suggesting that in the liver, the peribiliary Th17 population in turn signals back to BEC not only to reinforce their release of CCL20 and Th17-polarizing cytokines but to also promote their proliferation and thus the regeneration of the biliary ductules in a disease context. This model is consistent with previous studies that have reported roles for Th17 cells in promoting the regeneration of tissues, including bone and hepatic tissue (48–50).
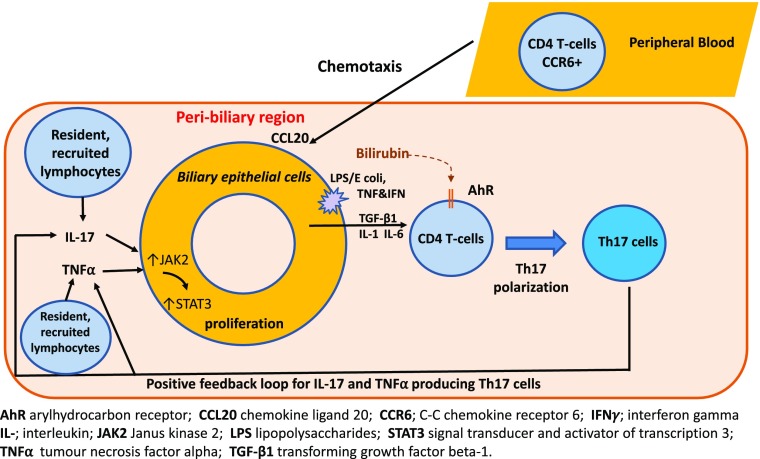
Overall, this study is an important example of the epi-immunome through which an epithelial response to injury in the portal tracts drives a paracrine loop, promoting the establishment and maintenance of a Th17 infiltrate (Fig. 6). Our findings provide insights into the role of the hepatic microenvironment together with lymphocyte-biliary epithelium cross-talk in governing Th17 fate and biliary proliferation in human liver diseases. Thus, our data suggest that Th17 cells may provide a protective mechanism against bile duct destruction, not only by combatting microbial infections but by promoting the epithelium to regenerate.
FIGURE 6.
An illustration of the bidirectional interaction between Th17 and BEC. Migrating bacteria from the gut express pathogen-associated molecular patterns (e.g., LPS) that trigger BEC activation via TLR4 signaling. The downstream cascade involves proinflammatory cytokines and chemokines, such as IL-1, IL-6, TGF-β1, and CCL20. IL-6 is both secreted by BEC and stimulates BEC proliferation via the JAK2/STAT3 signaling pathway in an autocrine manner. Importantly, although BEC undergo increased proliferation, this does not lead to increased apoptosis or necrosis. BEC-secreted CCL20 is responsible for chemotaxis and subsequent transmigration of lymphocytes that are CCR6+ (complementary receptor). The CD4+ lymphocytes undergo alterations in differentiation status, lineage stability, and function, depending on their respective subset. These cytokines act on BEC leading to BEC proliferation, which completes a self-sustaining positive feedback loop that propagates chronic peribiliary inflammation and biliary regeneration. This LPS-stimulated inflammatory cascade could be synergistically augmented by exposure to local bilirubin via the AhR present on lymphocytes.
Supplementary Material
Acknowledgments
We thank Gillian Muirhead for assistance with cell isolation, Dr. Nguyet Luu for assistance with flow sorting, and Charlotte Brewer and Kelly Hunter from the Human Biomaterial Resource Centre, University of Birmingham, U.K., for assistance with immunohistochemistry. We also thank medical, surgical, and anesthetic staff from University Hospitals Birmingham National Health Service Foundation Trust for help with tissue and blood acquirement and patients for providing the valuable samples that have made this research possible.
This work was supported by the Medical Research Council Clinician Scientist Award to Y.H.O. (G1002552), the Sir Jules Thorn Trust Biomedical Research Award to Y.H.O. (2018 recipient), the Sir Jules Thorn Trust, the National Institute for Health Research Birmingham Biomedical Research Centre, and the Queen Elizabeth Hospital Birmingham Charity.
The online version of this article contains supplemental material.
- AhR
- aryl hydrocarbon receptor
- AIH
- autoimmune hepatitis
- BEC
- biliary epithelial cell
- MS
- mass spectrometry
- PBC
- primary biliary cholangitis
- PSC
- primary sclerosing cholangitis
- QC
- quality control
- UHPLC
- ultra-HPLC.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hirschfield G. M., Karlsen T. H., Lindor K. D., Adams D. H. 2013. Primary sclerosing cholangitis. Lancet 382: 1587–1599. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M., Hocking R. J., Atkins C. J., Locksley R. M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189. [DOI] [PubMed] [Google Scholar]
- 4.Mangan P. R., Harrington L. E., O’Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441: 231–234. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 6.Lafdil F., Miller A. M., Ki S. H., Gao B. 2010. Th17 cells and their associated cytokines in liver diseases. Cell. Mol. Immunol. 7: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockinger B., Veldhoen M. 2007. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 19: 281–286. [DOI] [PubMed] [Google Scholar]
- 8.Oo Y. H., Banz V., Kavanagh D., Liaskou E., Withers D. R., Humphreys E., Reynolds G. M., Lee-Turner L., Kalia N., Hubscher S. G., et al. 2012. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J. Hepatol. 57: 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miossec P. 2009. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 11: 625–630. [DOI] [PubMed] [Google Scholar]
- 10.Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S., Saito S., Inoue K., Kamatani N., Gillespie M. T., et al. 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp T., Riedl E., Bangert C., Bowman E. P., Greisenegger E., Horowitz A., Kittler H., Blumenschein W. M., McClanahan T. K., Marbury T., et al. 2015. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 521: 222–226. [DOI] [PubMed] [Google Scholar]
- 13.Lan R. Y., Salunga T. L., Tsuneyama K., Lian Z. X., Yang G. X., Hsu W., Moritoki Y., Ansari A. A., Kemper C., Price J., et al. 2009. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J. Autoimmun. 32: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C., Yosef N., Gaublomme J., Wu C., Lee Y., Clish C. B., Kaminski J., Xiao S., Meyer Zu Horste G., Pawlak M., et al. 2015. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163: 1413–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueber W., Sands B. E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P. D., Wehkamp J., Feagan B. G., Yao M. D., Karczewski M., et al. Secukinumab in Crohn’s Disease Study Group 2012. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amatya N., Garg A. V., Gaffen S. L. 2017. IL-17 signaling: the Yin and the Yang. Trends Immunol. 38: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishi Y., Kondo T., Xiao S., Yosef N., Gaublomme J., Wu C., Wang C., Chihara N., Regev A., Joller N., Kuchroo V. K. 2016. Protein C receptor (PROCR) is a negative regulator of Th17 pathogenicity. J. Exp. Med. 213: 2489–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada K., Shimoda S., Sato Y., Isse K., Ikeda H., Nakanuma Y. 2009. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin. Exp. Immunol. 157: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov I. I., Frutos R. L., Manel N., Yoshinaga K., Rifkin D. B., Sartor R. B., Finlay B. B., Littman D. R. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffery H. C., van Wilgenburg B., Kurioka A., Parekh K., Stirling K., Roberts S., Dutton E. E., Hunter S., Geh D., Braitch M. K., et al. 2016. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol. 64: 1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oo Y. H., Weston C. J., Lalor P. F., Curbishley S. M., Withers D. R., Reynolds G. M., Shetty S., Harki J., Shaw J. C., Eksteen B., et al. 2010. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J. Immunol. 184: 2886–2898. [DOI] [PubMed] [Google Scholar]
- 22.Joplin R., Strain A. J., Neuberger J. M. 1989. Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell. Dev. Biol. 25: 1189–1192. [DOI] [PubMed] [Google Scholar]
- 23.Gehmlich K., Dodd M. S., Allwood J. W., Kelly M., Bellahcene M., Lad H. V., Stockenhuber A., Hooper C., Ashrafian H., Redwood C. S., et al. 2015. Changes in the cardiac metabolome caused by perhexiline treatment in a mouse model of hypertrophic cardiomyopathy. Mol. Biosyst. 11: 564–573. [DOI] [PubMed] [Google Scholar]
- 24.Brown M., Wedge D. C., Goodacre R., Kell D. B., Baker P. N., Kenny L. C., Mamas M. A., Neyses L., Dunn W. B. 2011. Automated workflows for accurate mass-based putative metabolite identification in LC/MS-derived metabolomic datasets. Bioinformatics 27: 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn W. B., Lin W., Broadhurst D., Begley P., Brown M., Zelena E., Vaughan A. A., Halsall A., Harding N., Knowles J. D., et al. 2015. Molecular phenotyping of a UK population: defining the human serum metabolome. Metabolomics 11: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lages C. S., Simmons J., Maddox A., Jones K., Karns R., Sheridan R., Shanmukhappa S. K., Mohanty S., Kofron M., Russo P., et al. 2017. The dendritic cell-T helper 17-macrophage axis controls cholangiocyte injury and disease progression in murine and human biliary atresia. Hepatology 65: 174–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X. M., O’Hara S. P., Nelson J. B., Splinter P. L., Small A. J., Tietz P. S., Limper A. H., LaRusso N. F. 2005. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol. 175: 7447–7456. [DOI] [PubMed] [Google Scholar]
- 28.Singh S. P., Zhang H. H., Foley J. F., Hedrick M. N., Farber J. M. 2008. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol. 180: 214–221. [DOI] [PubMed] [Google Scholar]
- 29.Bessede A., Gargaro M., Pallotta M. T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E. M., Macchiarulo A., Vacca C., et al. 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y. Y., Jeffery H. C., Hunter S., Bhogal R., Birtwistle J., Kaur Braitch M., Roberts S., Ming M., Hannah J., Thomas C., et al. 2016. Human intrahepatic regulatory T cells are functional, require IL-2 from effector cells for survival, and are susceptible to Fas ligand-mediated apoptosis. Hepatology 64: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veldhoen M., Hirota K., Christensen J., O’Garra A., Stockinger B. 2009. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veldhoen M., Hirota K., Westendorf A. M., Buer J., Dumoutier L., Renauld J. C., Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453: 106–109. [DOI] [PubMed] [Google Scholar]
- 33.Yang C. Y., Ma X., Tsuneyama K., Huang S., Takahashi T., Chalasani N. P., Bowlus C. L., Yang G. X., Leung P. S., Ansari A. A., et al. 2014. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology 59: 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang A. P., Migita K., Ito M., Takii Y., Daikoku M., Yokoyama T., Komori A., Nakamura M., Yatsuhashi H., Ishibashi H. 2005. Hepatic expression of toll-like receptor 4 in primary biliary cirrhosis. J. Autoimmun. 25: 85–91. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi P. J., Adams D. H. 2013. Mucosal immunity in liver autoimmunity: a comprehensive review. J. Autoimmun. 46: 97–111. [DOI] [PubMed] [Google Scholar]
- 36.Katt J., Schwinge D., Schoknecht T., Quaas A., Sobottka I., Burandt E., Becker C., Neurath M. F., Lohse A. W., Herkel J., Schramm C. 2013. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 58: 1084–1093. [DOI] [PubMed] [Google Scholar]
- 37.Kang Y. H., Seigel B., Bengsch B., Fleming V. M., Billerbeck E., Simmons R., Walker L., Willberg C. B., Barnes E. J., Bhagwanani A., et al. 2012. CD161(+)CD4(+) T cells are enriched in the liver during chronic hepatitis and associated with co-secretion of IL-22 and IFN-γ. Front. Immunol. 3: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajoriya N., Fergusson J. R., Leithead J. A., Klenerman P. 2014. Gamma delta T-lymphocytes in hepatitis C and chronic liver disease. Front. Immunol. 5: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada K., Nakanuma Y. 2012. Cholangiopathy with respect to biliary innate immunity. Int. J. Hepatol. 2012: 793569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Affò S., Rodrigo-Torres D., Blaya D., Morales-Ibanez O., Coll M., Millán C., Altamirano J., Arroyo V., Caballería J., Bataller R., et al. 2015. Chemokine receptor Ccr6 deficiency alters hepatic inflammatory cell recruitment and promotes liver inflammation and fibrosis. PLoS One 10: e0145147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C., Kang S. G., Lee J., Sun Z., Kim C. H. 2009. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swamy M., Jamora C., Havran W., Hayday A. 2010. Epithelial decision makers: in search of the ‘epimmunome’. Nat. Immunol. 11: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bueno-Topete M., Zepeda-Morales S., Garcia-Benavides L., Del Toro-Arreola S., Bastidas-Ramirez E., Fafutis-Morris M. 2015. Cholestatic liver fibrosis correlates with increased Il-17 and Tgfβ-2 expression. J. Hepatol. 62: S337. [DOI] [PubMed] [Google Scholar]
- 44.Cua D. J., Kastelein R. A. 2006. TGF-beta, a ‘double agent’ in the immune pathology war. Nat. Immunol. 7: 557–559. [DOI] [PubMed] [Google Scholar]
- 45.Hellerbrand C., Stefanovic B., Giordano F., Burchardt E. R., Brenner D. A. 1999. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J. Hepatol. 30: 77–87. [DOI] [PubMed] [Google Scholar]
- 46.Veldhoen M., Stockinger B. 2006. TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 27: 358–361. [DOI] [PubMed] [Google Scholar]
- 47.Yang L., Anderson D. E., Baecher-Allan C., Hastings W. D., Bettelli E., Oukka M., Kuchroo V. K., Hafler D. A. 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454: 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ono T., Okamoto K., Nakashima T., Nitta T., Hori S., Iwakura Y., Takayanagi H. 2016. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 7: 10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao R., Graffeo C.S., Gulati R., Jamal M., Narayan S., Zambirinis C.P., Barilla R., Deutsch M., Greco S.H., Ochi A., et al. 2014. Interleukin 17-producing γδT cells promote hepatic regeneration in mice. Gastroenterology 147: 473–484.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuya S., Kono H., Hara M., Hirayama K., Tsuchiya M., Fujii H. 2013. Interleukin-17A plays a pivotal role after partial hepatectomy in mice. J. Surg. Res. 184: 838–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.