Key Points
PGE2 induces immune exhaustion and promotes disease progression of BLV infection.
Dual blockade of PGE2 and PD-L1 invigorates antiviral immune response in cattle.
Abstract
Bovine leukemia virus (BLV) infection is a chronic viral infection of cattle and endemic in many countries, including Japan. Our previous study demonstrated that PGE2, a product of cyclooxygenase (COX) 2, suppresses Th1 responses in cattle and contributes to the progression of Johne disease, a chronic bacterial infection in cattle. However, little information is available on the association of PGE2 with chronic viral infection. Thus, we analyzed the changes in plasma PGE2 concentration during BLV infection and its effects on proviral load, viral gene transcription, Th1 responses, and disease progression. Both COX2 expression by PBMCs and plasma PGE2 concentration were higher in the infected cattle compared with uninfected cattle, and plasma PGE2 concentration was positively correlated with the proviral load. BLV Ag exposure also directly enhanced PGE2 production by PBMCs. Transcription of BLV genes was activated via PGE2 receptors EP2 and EP4, further suggesting that PGE2 contributes to disease progression. In contrast, inhibition of PGE2 production using a COX-2 inhibitor activated BLV-specific Th1 responses in vitro, as evidenced by enhanced T cell proliferation and Th1 cytokine production, and reduced BLV proviral load in vivo. Combined treatment with the COX-2 inhibitor meloxicam and anti-programmed death-ligand 1 Ab significantly reduced the BLV proviral load, suggesting a potential as a novel control method against BLV infection. Further studies using a larger number of animals are required to support the efficacy of this treatment for clinical application.
Introduction
Prostaglandin E2 is an inflammatory mediator produced by cyclooxygenase (COX) enzymes (COX-1 and COX-2) from arachidonic acid (1). COX-1 is a constitutive enzyme expressed in many tissues and involves in a multitude of physiological processes (2), whereas COX-2 is an inducible enzyme regulated by inflammatory cytokines via the activation of NF-κB (3). The level of PGE2 is regulated not only by its synthesis but also by its degradation. 15-HydroxyPG dehydrogenase (15-PGDH), encoded by the HPGD gene, is a key enzyme for PGE2 inactivation (4, 5). PGE2 suppresses the activity of immune cells, such as T cells (especially Th1 cells), NK cells, and dendritic cells, via EP2 and EP4 receptors, thereby contributing to immune evasion during chronic infection (6, 7). By contrast, by reducing PGE2 synthesis, COX-2 inhibitors activate immune responses in vitro and in vivo (8, 9). Thus, COX-2 inhibition is considered a potential immunotherapy for chronic infections.
Bovine leukemia virus (BLV), which is a member of the genus of Deltaretrovirus, causes enzootic bovine leukosis (EBL) and is closely related to human T cell leukemia virus type 1 (10). This virus generally infects B cells of cattle, and most BLV-infected cattle are asymptomatic carriers of the virus or aleukemic (AL). However, ∼30% of the infected cattle show persistent lymphocytosis (PL), characterized by nonmalignant polyclonal expansion of infected B cells in peripheral blood. Less than 5% of the infected cattle develop EBL, characterized by fatal lymphoma or lymphosarcoma after extended latency periods of 5–10 y (11). BLV infection is highly prevalent in many countries, including Japan, and several reports have demonstrated that the seroprevalence of BLV in Japanese cattle is increasing because of the lack of an effective treatment or vaccine (12). Thus, the development of a new control method for BLV infection is required to guarantee the continued supply of livestock and livestock production.
The Th1 response is critical for the control of BLV infection. During BLV infection, suppression of both CD4+ T cell proliferation and cytotoxic immune response against BLV Ags is associated with disease progression (13, 14). To develop a novel strategy for effective control of BLV infection, our recent studies have examined the detailed mechanisms of immune dysfunction in BLV infection. Upregulation of immunoinhibitory molecules, such as programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), lymphocyte activation gene 3 (LAG-3), T cell Ig domain and mucin domain-3 (Tim-3), and CTL Ag-4 (CTLA-4), was shown to suppress BLV-specific Th1 responses and promote disease progression (15–19). Alternatively, Ab-mediated blockade of the PD-1/PD-L1 pathway significantly reduced the proviral load in BLV-infected cattle (20, 21), suggesting that therapy targeting immunoinhibitory molecules, such as PD-1 and PD-L1, may be a novel control strategy against BLV infection. However, it is still unknown how these molecules are upregulated during BLV infection.
Our previous report has shown that PGE2 inhibits the Th1 response and induces the expression of PD-L1 in cattle (22). In addition, we showed that PGE2 is associated with the progression of Johne disease, a bovine chronic infection caused by Mycobacterium avium subsp. paratuberculosis, and that a COX-2 inhibitor can activate Th1 responses in cattle with Johne disease. Furthermore, the combined treatment with an anti–PD-L1 Ab and a COX-2 inhibitor strongly enhanced M. avium subsp. paratuberculosis–specific Th1 responses in vitro (22).
However, few studies are available on the association of PGE2 with BLV infection (23). Therefore, in this study, we analyzed the kinetics of PGE2 in BLV infection, the association of PGE2 with disease progression, and the antiviral effects of a COX-2 inhibitor in vitro and in vivo. Finally, we also tested whether a combined blockade of the PGE2 and PD-1/PD-L1 pathways in vitro and in vivo can enhance antiviral effects during BLV infection.
Materials and Methods
Cell preparation
Blood samples of BLV-infected and uninfected cattle were obtained from several farmers and veterinarians in Hokkaido, Japan. Infection was confirmed by detection of the provirus using nested PCR targeting the viral long terminal repeat (16) and by detection of the anti-BLV Ab using a commercial ELISA (JNC, Tokyo, Japan). The leukocyte numbers in BLV-infected cattle were counted using a Celltac α MEK-6450 automatic hematology analyzer (Nihon Kohden, Tokyo, Japan), and animals were classified as AL or PL as described previously (24). PBMCs derived from these blood samples were purified by density gradient centrifugation on Percoll (GE Healthcare, Buckinghamshire, U.K.) and cultured in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated FCS (Thermo Fisher Scientific, Waltham, MA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (Thermo Fisher Scientific). All cell cultures were grown in 96-well plates (Corning, Corning, NY) in 200 μl of medium.
As described previously with slight modifications (18, 21), CD14+ and CD21+ cells were freshly isolated from bovine PBMCs using BD IMagnet Cell Separation System (BD Biosciences, San Jose, CA) or autoMACS Pro (Miltenyi Biotec, Bergisch Gladbach, Germany), and the following Abs: anti-bovine CD14 mAb (CAM36A; Washington State University mAb Center, Pullman, WA) and anti-bovine CD21 mAb (GB25A; Washington State University mAb Center). CD14 was used as a marker of monocytes and CD21 was used as that of B cells. The purity of each cell population was confirmed using FACSVerse (BD Biosciences). Only highly purified cells (>90%) were used for expression analysis by quantitative real-time PCR (qPCR) as described below.
qPCR
Total RNA was extracted from total PBMCs and each subpopulation (CD14+ and CD21+) using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. cDNA was synthesized from the total RNA using PrimeScript Reverse Transcriptase (Takara Bio, Otsu, Japan) according to the manufacturer’s instructions. To measure the mRNA expression levels of COX2, EP4, EP2 (25), HPGD (26), and IFN-γ in total PBMCs and each subpopulation, qPCR was performed using a thermal cycler (LightCycler 480 System II; Roche Diagnostic, Mannheim, Germany) with SYBR Premix DimerEraser (Takara Bio), following the manufacturer’s instructions. Internal control genes were β-actin (ACTB) and GAPDH. All primer sequences for PCR are listed in Table I. To examine whether the expression levels of viral genes gp51, G4, and R3 are increased by PGE2 signaling, the PBMCs of BLV-infected cattle were incubated in the presence of PGE2 (1 μM; Cayman Chemical, Ann Arbor, MI), EP2 agonist [1 μg/ml, Butaprost (free acid), Cayman Chemical] or EP4 agonist (1 μg/ml, Rivenprost; Cayman Chemical) at 37°C under 5% CO2 for 3 d and collected, and the expression levels were quantified by qPCR as described above. To measure the proviral loads of samples from BLV-infected cattle, qPCR was performed as previously described, with slight modifications (27). Briefly, genomic DNA was extracted from the PBMCs of BLV-infected cattle using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI). The DNA concentration was measured by UV absorbance at 260 nm using a NanoDrop 8000 Spectrophotometer (Thermo Fisher Scientific). Amplification was conducted in a reaction mixture containing 5 μl of 2× Cycleave PCR Reaction Mix SP (Takara Bio), 0.5 μl of Probe/Primer Mix for BLV (Takara Bio), 1 μl of template DNA, and 3.5 μl of RNase-free distilled water (Takara Bio) using a LightCycler 480 System II. The PCR condition was 95°C for 10 s, followed by amplification of the template for 55 cycles at 95°C for 5 s and 64°C for 30 s. Serial dilutions of BLV Positive Control (Takara Bio) were used to generate calibration curves to determine the BLV provirus copy number. Each DNA sample was tested in triplicate, and the reported values are the mean number of copies per 50 ng of genomic DNA.
Table I. Sequences of primers used in this study.
| Gene | Primer Sequence (5′-3′) |
|---|---|
| IFN-γ | Forward: 5′-ATA ACC AGG TCA TTC AAA GG-3′ |
| Reverse: 5′-ATT CTG ACT TCT CTT CCG CT-3′ | |
| EP4 | Forward: 5′-GTG ACC ATC GCC ACC TAC TT-3′ |
| Reverse: 5′-CTC ATC GCA CAG ATG ATG CT-3′ | |
| EP2 | Forward: 5′-CTC TGC TGT CGG GTT TCA TTA-3′ |
| Reverse: 5′-CTA CCC TCC TCA AAG GTC AAT C-3′ | |
| HPGD | Forward: 5′-GAA TCT CGA AGC AGG TGT CA-3′ |
| Reverse: 5′-CCA GCT TTC CAA AGT GGT CT-3′ | |
| COX2 | Forward: 5′-ACG TTT TCT CGT GAA GCC CT-3′ |
| Reverse: 5′-TCT ACC AGA AGG GCG GGA TA-3′ | |
| gp51 | Forward: 5′-ACC TTT CTG TGC CAA GTC-3′ |
| Reverse: 5′-ATC GGG GCT CGC AAT CAT A-3′ | |
| G4 | Forward: 5′-TTC GGC GCC CAG CCA CAT C-3′ |
| Reverse: 5′-GTC GTT ATC AGG TAA TGG ATC CCG A-3′ | |
| R3 | Forward: 5′-GAT CAT CAG ATG GGT CCT GAT GAA C-3′ |
| Reverse: 5′-GCT GCT GGA TGT GGC TGG AAT GTC-3′ | |
| Foxp3 | Forward: 5′-CAC AAC CTG AGC CTG CAC AA-3′ |
| Reverse: 5′-TCT TGC GGA ACT CAA ACT CAT C-3′ | |
| TGF-β1 | Forward: 5′-CTG CTG AGG CTC AAG TTA AAA GTG-3′ |
| Reverse: 5′-CAG CCG GTT GCT GAG GTA G-3′ | |
| ACTB | Forward: 5′-TCT TCC AGC CTT CCT TCC TG-3′ |
| Reverse: 5′-ACC GTG TTG GCG TAG AGG TC-3′ | |
| GAPDH | Forward: 5′-GGC GTG AAC CAC GAG AAG TAT AA-3′ |
| Reverse: 5′-CCC TCC ACG ATG CCA AAG T-3′ |
Quantitation of PGE2 by ELISA
The concentration of PGE2 was measured in plasma or serum samples from BLV-infected and uninfected cattle using the PGE2 Express ELISA Kit (Cayman Chemical) as described previously (22). To assess whether the BLV Ag promotes PGE2 production, PBMCs (4 × 105 cells per well) from infected and uninfected cattle were incubated with the BLV Ag (fetal lamb kidney[FLK]–BLV; 2% heat-inactivated culture supernatant of FLK–BLV cells) at 37°C under 5% CO2 for 6 d. Culture supernatants were collected, and the concentration of PGE2 was measured by ELISA. To compare PGE2 production, the total PBMCs, CD14+ cells and CD21+ cells (5 × 105 cells per well) were incubated separately at 37°C under 5% CO2 for 3 d, and the culture supernatant was collected for measurement of PGE2 concentration by ELISA as described above.
Immunohistochemical assay of PD-L1 and PGE2
Immunohistochemical assays were performed as previously described, with modifications (22). Briefly, sections of superficial cervical lymph nodes from healthy BLV-uninfected cattle (Holstein, female, 75-mo old) and periocular mass lesions from EBL cattle (Holstein, female, 24-mo old) were subjected to immunohistochemical staining for PGE2 and PD-L1 using an anti-PGE2 polyclonal Ab (ab2318; Abcam, Cambridge, U.K.) and anti–PD-L1 mAb (6C11-3A11, Rat IgG2a; 22). Cattle exhibiting tumors were diagnosed with EBL as described previously (28, 29). Additionally, the tumor mass lesion was confirmed as B cell lymphoma by immunohistochemical staining with an anti-CD20 polyclonal Ab (PA5-16701; Thermo Fisher Scientific; data not shown).
In vitro analysis of immunostimulation by COX-2 inhibition
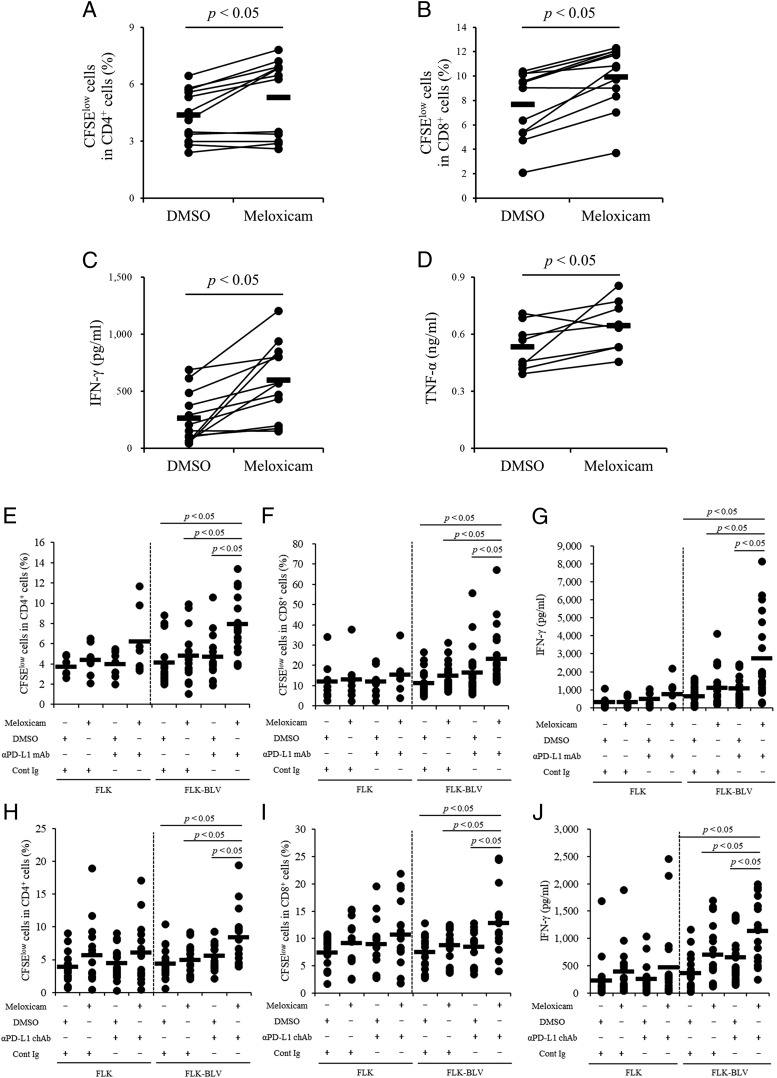
To evaluate the immunostimulatory effects of COX-2 inhibition, PBMCs (4 × 105 cells per well) from BLV-infected cattle were labeled with 0.2 μM CFSE and incubated with 1 μM meloxicam (Sigma-Aldrich) or DMSO as a vehicle control (Nacalai Tesque, Kyoto, Japan) at 37°C under 5% CO2 for 6 d in the presence of FLK–BLV. After incubation, the concentrations of IFN-γ and TNF-α in the medium were measured by ELISA, and cell proliferation was analyzed by FACS based on CFSE staining strength as described previously (22).
In vitro analysis of immunostimulation by combined COX-2 inhibition and PD-L1 blockade
To examine the immunostimulatory effects of combined COX-2 inhibition plus anti–PD-L1 Abs treatment in vitro, PBMCs (4 × 105 cells per well) from BLV-infected cattle were CFSE labeled and incubated with 1 μM meloxicam and 10 μg/ml anti–PD-L1 Ab in the presence of FLK–BLV for 6 d. DMSO, rat IgG (Sigma-Aldrich), or bovine IgG (Sigma-Aldrich) was used as negative control. Heat-inactivated culture supernatant of FLK cells was used as a negative control Ag. Two forms of anti–PD-L1 Ab, namely anti–PD-L1 mAb (4G12) with the C region of rat IgG2a and Igκ (30) and anti–PD-L1 chimeric Ab (chAb; Boch4G12) with the C region of bovine IgG1 (mutated to reduce Ab-dependent cellular toxicity), and Igλ (21) were used to evaluate the combination effects. After 6 d, the concentration of IFN-γ was measured by ELISA, and cell proliferation was analyzed by FACS analysis of CFSE labeling as described above.
Evaluation of COX-2 inhibitor administration for reducing viral load in BLV-infected cattle
To examine the antiviral efficacy of COX-2 inhibition in vivo, three BLV-infected cattle (Table II) were administered 0.5 mg/kg meloxicam (Metacam; Boehringer Ingelheim, Ingelheim, Germany) by s.c. injection once weekly for a total of nine doses (animals #1 and #2) or a total of five doses (animal #3). Animals #1–3 were launched for this experiment and kept separately at the three different locations: in a private dairy farm (Shibecha, Hokkaido, Japan), a biosafety level I animal facility at the Animal Research Center, Agricultural Research Department, Hokkaido Research Organization (Shintoku, Hokkaido, Japan), and an animal facility at the Faculty of Veterinary Medicine, Hokkaido University (Sapporo, Hokkaido, Japan). These animal experiments were approved by the Ethics Committee of the Animal Research Center, Agricultural Research Department, Hokkaido Research Organization, and the Ethics Committee of the Faculty of Veterinary Medicine, Hokkaido University. Peripheral blood samples were collected from these cattle at least once per week after first administration.
Table II. Cattle used in clinical studies.
| Cattle | #1 | #2 | #3 (Clinical Study of the COX-2 Inhibitor) | #4 | #5 | #6 | #3 (Clinical Study of the Combined Treatment) |
|---|---|---|---|---|---|---|---|
| Age | 43 mo old | 96 mo old | 47 mo old | 19 mo old | 13 mo old | 76 mo old | 52 mo old |
| Breed | Holstein | Holstein | Holstein | Holstein | Holstein | Holstein | Holstein |
| Sex | Female | Female | Female | Female | Female | Female | Female |
| Body weight | 749 kg | 736 kg | 759 kg | 433 kg | 295 kg | 799 kg | 799 kg |
| Inoculation dose (Boch4G12) | Saline 50 ml, i.v. | 1 mg/kg, i.v. | 1 mg/kg, i.v. | 1 mg/kg, i.v. | |||
| Administration dosage (Metacam) | 0.5 mg/kg, once a week, total of nine times, s.c. | 0.5 mg/kg, once a week, total of nine times, s.c. | 0.5 mg/kg, once a week, total of five times, s.c. | 0.5 mg/kg, once a week, total of three times, s.c. |
Inoculation of the anti–PD-L1 chAb in BLV-infected cattle
To examine the antiviral efficacy of anti–PD-L1 chAb (Boch4G12; 21) in vivo, two cattle naturally infected with BLV (Table II) were inoculated i.v. with 1 mg/kg purified Boch4G12 (animal #5) or saline (as a control, animal #4). These animals were kept in a private dairy farm in Hokkaido. Peripheral blood samples were collected from both animals at least once per week after inoculation for measurement of proviral load as described.
Evaluation of combined COX-2 inhibition and PD-L1 blockade for reducing viral load in infected cattle
To investigate the in vivo antiviral effects of the combined treatment compared with monotreatment of anti–PD-L1 chAb, two BLV-infected cattle (animals #3 and #6) were inoculated with 1 mg/kg Boch4G12 (21), and animal #3 was also coadministered 0.5 mg/kg meloxicam (Metacam) by s.c. injection three times at weekly intervals (Table II). These animals were kept in an animal facility at the Faculty of Veterinary Medicine, Hokkaido University (Sapporo, Hokkaido, Japan). This animal experiment was approved by the Ethics Committee of the Faculty of Veterinary Medicine, Hokkaido University. Animal #3 was also used for the clinical study of meloxicam treatment alone and inoculated with Boch4G12 after a long interval (118 d) from the final administration of meloxicam. Peripheral blood samples were then collected from these cattle at least once per week after the inoculation of Boch4G12, and proviral loads were evaluated using qPCR as described.
Statistics
Differences were identified using the Dunnett test, Wilcoxon signed-rank test, and Steel–Dwass test. Correlation was analyzed using the Spearman correlation. A p value <0.05 was considered statistically significant. In clinical tests, we performed the quantitation of proviral load by qPCR in triplicate. Using the value obtained from the experiments, we performed statistical analysis using a Dunnett test to test whether the proviral load at each point was significantly different from that at day 0.
Results
PGE2 concentration changes with disease progression in BLV-infected cattle
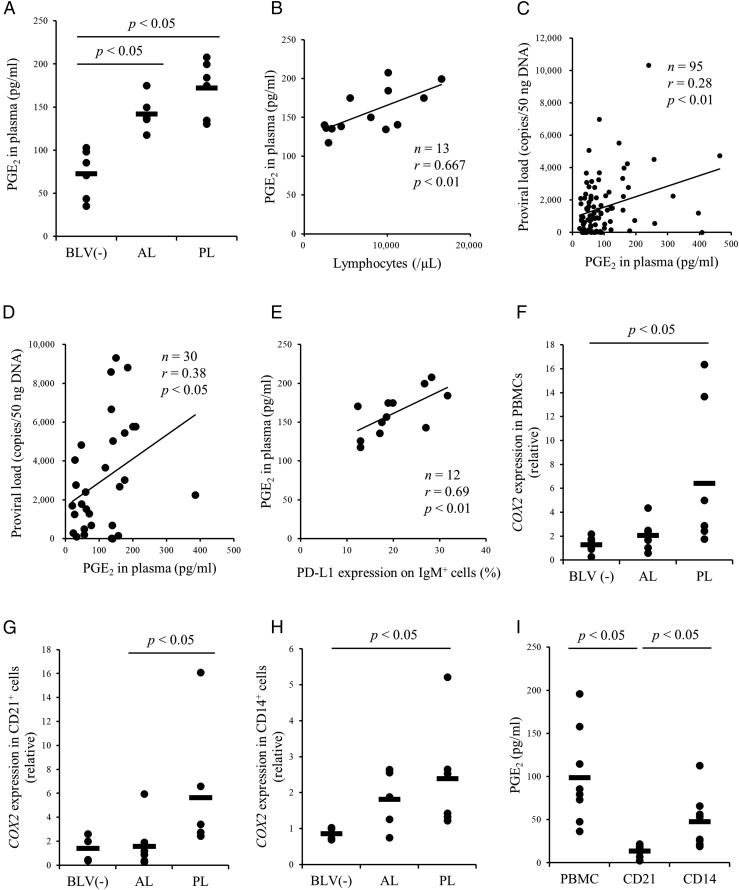
Our previous report demonstrated an association between blood PGE2 concentration and the progression of Johne disease, a chronic bacterial infection of cattle (22). Next, to investigate whether this immune dysfunction is specific to Johne disease or commonly occurred in bovine chronic infections, we focused on a chronic viral infection of cattle, BLV infection, which is known to show T cell dysfunction during the disease progression (14–16). To examine whether PGE2 is also associated with the progression of BLV infection, the plasma PGE2 concentration was compared among uninfected, AL, and PL cattle by ELISA. The plasma concentration of PGE2 was significantly higher in BLV-infected cattle compared with BLV-uninfected cattle (Fig. 1A). In addition, the plasma concentration of PGE2 was positively correlated with the number of lymphocytes and BLV proviral load (Fig. 1B–D). Plasma PGE2 concentration was also positively correlated with PD-L1 expression in IgM+ B cells (Fig. 1E). Thus, PGE2 production appears to increase with disease progression.
FIGURE 1.
Kinetic analysis of PGE2 in cattle infected with BLV. (A) Plasma PGE2 concentration in cattle uninfected (n = 6) or infected with BLV (AL, n = 7; PL, n = 6) were determined using ELISA. (B–E) Positive correlation between the lymphocyte number (n = 13), proviral load (C: Japanese black cattle and first filial of Japanese black/Holstein cattle, n = 95; D: Holstein cattle, n = 30) or the percentages of PD-L1+ cells in IgM+ cells and plasma PGE2 concentration in cattle infected with BLV. (F–H) Quantification of COX2 mRNA expression in PBMCs [BLV (−), n = 7; AL, n = 7; PL, n = 7] and subpopulations of CD21+ [BLV (−), n = 4; AL, n = 7; PL, n = 6) and CD14+ [BLV (−), n = 4; AL, n = 5; PL, n = 6) cells derived from BLV-uninfected and BLV-infected cattle by qPCR. (I) Quantitation of PGE2 production in PBMCs, CD21+ cells, and CD14+ cells by ELISA (n = 8). Each symbol represents PGE2 concentration or relative expression level of each animal; pooled data from several experiments were analyzed by the Steel–Dwass test (A and F–I). Correlation statistic was analyzed using the Spearman correlation (B–E). BLV (−), BLV-uninfected cattle.
To identify the major cell type producing PGE2 in the blood of BLV-infected cattle, the mRNA expression levels of COX2 were measured in total PBMCs, isolated CD21+ cells, and isolated CD14+ cells using qPCR (Table I). Consistent with plasma PGE2 measurements, COX2 was significantly upregulated in PL cattle compared with AL and uninfected cattle (Fig. 1F–H). The production and release of PGE2 were significantly higher in CD14+ cell cultures than CD21+ cell cultures from infected cattle (Fig. 1I), suggesting that CD14+ cells are the predominant source of PGE2 in peripheral blood. Immunohistochemical analysis also revealed strong expression of PGE2 and PD-L1 in B cell lymphoma tissues of EBL cattle but very weak expression in the healthy lymph nodes of BLV-uninfected cattle (Supplemental Fig. 1).
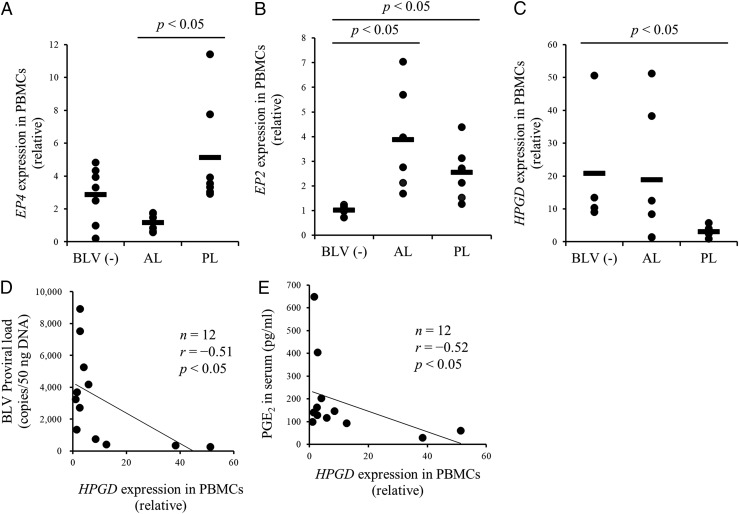
Finally, we measured the mRNA expression of EP4, EP2, and HPGD in PBMCs from BLV-infected and -uninfected cattle (Table I), and found that the expression of EP4 and EP2 was upregulated in PL cattle (Fig. 2A, 2B). By contrast, the mRNA expression of the PGE2 catabolic enzyme gene HPGD was downregulated in PL cattle and was negatively associated with both BLV proviral load and serum PGE2 concentration (Fig. 2C–E). Taken together, PGE2 production and signaling are positively associated with disease progression in BLV-infected cattle. We then examined whether elevated PGE2 actually drives disease progression by immune inhibition, particularly suppression of Th1 cell responses, thereby allowing for increased viral replication and gene expression.
FIGURE 2.
Quantification of EP4, EP2, and HPGD mRNA expression in cattle infected with BLV. (A–C) Quantification of EP4 [BLV (−), n = 7; AL, n = 7; PL, n = 7), EP2 [BLV (−), n = 6; AL, n = 6; PL, n = 7), and HPGD [BLV (−), n = 4; AL, n = 6; PL, n = 6) mRNA expression in PBMCs of BLV-uninfected and BLV-infected cattle by qPCR. (D and E) Negative correlation between proviral load or the serum concentration of PGE2 and HPGD expression in PBMCs of cattle infected with BLV (n = 12). Each symbol represents relative expression level of each animal; pooled data from several experiments were analyzed by the Steel–Dwass test (A–C). Correlation statistic was analyzed using the Spearman correlation (D and E). BLV (−), BLV-uninfected cattle.
Upregulation of PGE2 production by BLV Ag
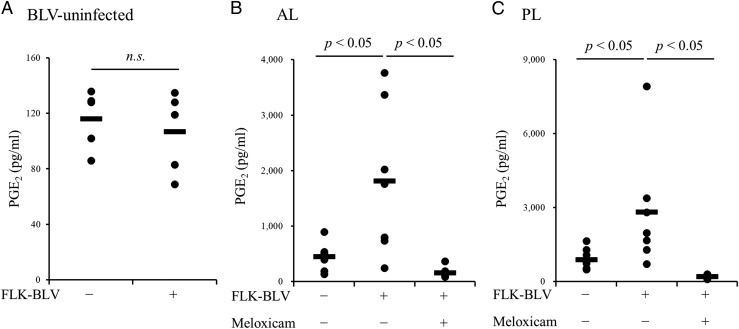
The capacity of BLV to evoke PGE2 production was examined in PBMC cultures from BLV-infected and uninfected cattle using the viral Ag FLK–BLV. FLK–BLV exposure for 6 d significantly upregulated PGE2 production by PBMCs from infected AL and PL cattle (Fig. 3B, 3C) but not by PBMCs from uninfected cattle (Fig. 3A). This induced PGE2 production was inhibited by the COX-2 inhibitor meloxicam (Fig. 3B, 3C). Collectively, these results showed that the COX-2/PGE2 pathway was activated by the stimulation of BLV Ag in BLV-infected cattle.
FIGURE 3.
Upregulation on PGE2 production by FLK–BLV stimulation. (A–C) PBMCs of BLV-uninfected (A) and BLV-infected (B, AL; C, PL) cattle were incubated with FLK–BLV. The concentration of PGE2 in culture supernatants was determined by ELISA (A, n = 5; B, n = 7; C, n = 7). Symbols represent mean from single-culture wells of different individuals; pooled data from several experiments were analyzed by the Wilcoxon signed-rank test (A) or the Steel–Dwass test (B and C).
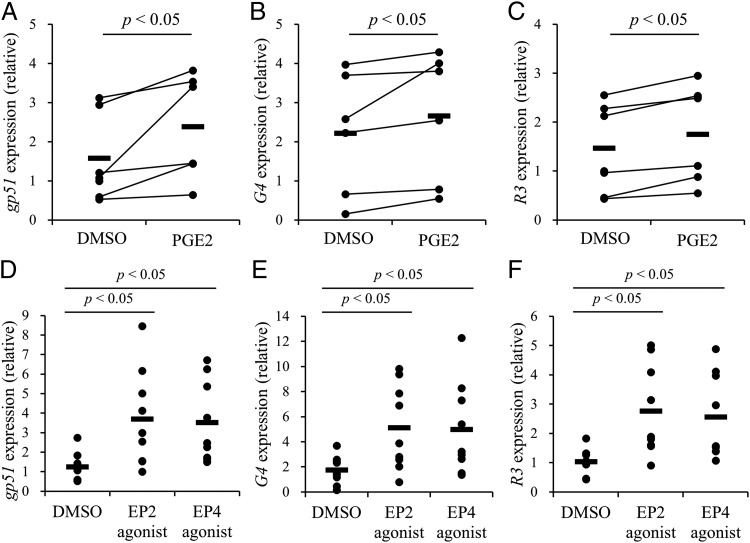
Upregulation of gp51, G4, and R3 viral genes by PGE2
A previous report demonstrated that PGE2 can induce the expression of BLV viral genes such as tax (23). To examine PGE2-induced viral expression in PBMCs, cells from BLV-infected cattle were cultured in the presence of PGE2 for 3 d. Indeed, the mRNA expression levels of env (gp51), G4, and R3 were facilitated by PGE2 as measured by qPCR (Fig. 4A–C, Table I). To investigate the transduction mechanism, PBMCs of BLV-infected cattle were cultured with EP2 or EP4 agonist, both of which induced the expression of these three viral genes (Fig. 4D–F). Thus, the expression of BLV viral genes is regulated by PGE2 via its endogenous receptors EP2 and EP4.
FIGURE 4.
Upregulation on the gene expression of gp51, G4, and R3 by PGE2. (A–F) PBMCs from BLV-infected cattle were incubated with PGE2, EP2 agonist, or EP4 agonist, and the gene expression of gp51, G4, and R3 was quantitated by qPCR (A–C, n = 6; D–F, n = 9). Symbols represent mean from single-culture wells of different individuals; pooled data from several experiments were analyzed by the Wilcoxon signed-rank test (A–C) and the Steel–Dwass test (D–F).
Activation of BLV-specific Th1 responses by the COX-2 inhibitor meloxicam in vitro
In humans, COX-2 inhibitors activate immune responses in vitro and in vivo (8, 9), and we previously reported that meloxicam activates Th1 responses of cattle in vitro (22). To examine whether meloxicam activates the BLV-specific Th1 responses, PBMCs from BLV-infected cattle were cultured in the presence of FLK–BLV plus meloxicam or vehicle, and T cell proliferation was measured according to CFSE labeling (low is indicative of proliferation) by flow cytometry and Th1 cytokine production by ELISA (Fig. 5). The gating strategy and representative plots for CFSE staining are shown in Supplemental Fig. 2. The proliferation rates of both CD4+ and CD8+ cell populations were upregulated by meloxicam relative to vehicle (Fig. 5A, 5B), and the production levels of both IFN-γ and TNF-α were enhanced by meloxicam (Fig. 5C, 5D), consistent with the activation of BLV-specific Th1 responses.
FIGURE 5.
Activation of BLV-specific responses by the combination of meloxicam and anti–PD-L1 Ab. (A–D) PBMCs from BLV-infected cattle (A–C, n = 12; D, n = 8) were cultured with meloxicam in the presence of FLK–BLV. CD4+ (A) and CD8+ (B) cell proliferations were assayed by flow cytometry. IFN-γ (C) and TNF-α (D) productions were determined by ELISA. (E–G) PBMCs from BLV-infected cattle were incubated with meloxicam and anti–PD-L1 mAb in the presence of FLK or FLK–BLV. CD4+ (E: FLK, n = 7; FLK–BLV, n = 15) and CD8+ (F: FLK, n = 8; FLK–BLV, n = 24) cell proliferations were assayed by flow cytometry. IFN-γ production was determined by ELISA (G, FLK, n = 7; FLK–BLV, n = 20). (H–J) PBMCs from BLV-infected cattle were incubated with meloxicam and anti–PD-L1 chAb in the presence of FLK or FLK–BLV (H and I, n = 16; J, n = 18). CD4+ (H) and CD8+ (I) cell proliferations were assayed by flow cytometry. IFN-γ production was determined by ELISA (J). Symbols represent mean from single-culture wells of different individuals; pooled data from several experiments were analyzed by the Wilcoxon signed-rank test (A–D) and the Steel–Dwass test (E–J). Cont Ig, rat IgG (for E–G), or bovine IgG (for H–J); αPD-L1 chAb, anti–PD-L1 chAb (Boch4G12); αPD-L1 mAb: anti–PD-L1 mAb (4G12).
Activation of BLV-specific T cell responses in vitro by combined COX-2 inhibition and anti–PD-L1 Ab treatment
We then evaluated the immunomodulatory effects of combined COX-2 inhibition plus anti–PD-L1 Ab treatment on the BLV-specific Th1 cell response in vitro. The proliferation rates of CD4+ and CD8+ cells were significantly enhanced by combination treatment with meloxicam and anti–PD-L1 mAb (4G12) in the presence of FLK–BLV compared with the other treatment groups (Fig. 5E, 5F). The production of IFN-γ was also upregulated by combined treatment in the presence of FLK–BLV (Fig. 5G), and the stimulation of FLK, a negative control Ag, had no effect in either group (Fig. 5E–G). In addition, combined treatment with meloxicam and anti–PD-L1 chAb Boch4G12 significantly increased the proliferation of CD4+ and CD8+ cells and the production of IFN-γ compared with the other treatment groups (Fig. 5H–J). Collectively, these results demonstrate enhancement of BLV-specific Th1 responses by combined COX2 inhibitor and anti–PD-L1 Ab treatment in vitro.
Antiviral effects of COX-2 inhibition in BLV-infected cattle
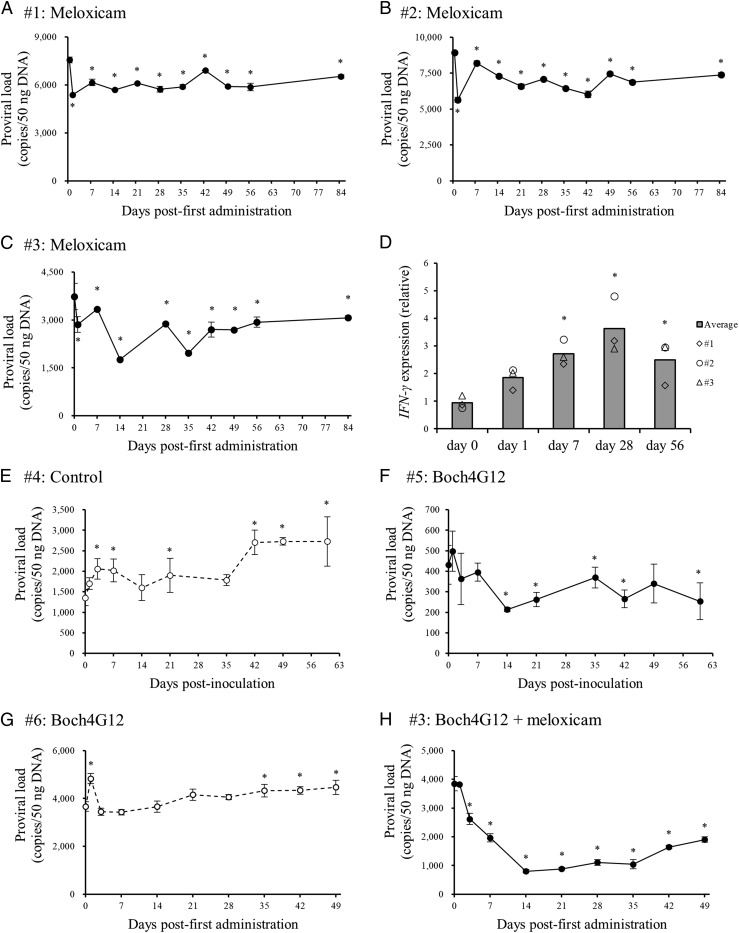
Three cattle naturally infected with BLV (animal #1–3) were administered 0.5 mg/kg meloxicam by s.c. injection once a week (Table II), and BLV proviral load was measured periodically from PBMCs. Consistent with antiviral efficacy, the proviral load fell significantly during the observation periods (Fig. 6A–C). To confirm whether Th1 cell responses were activated by the administration of meloxicam, the mRNA expression levels of IFN-γ were measured in PBMCs at several times points (days 0, 1, 7, 21, and 56) by qPCR (Table I). Expression was significantly higher on days 7, 21, and 56 compared with day 0 (Fig. 6D), indicating that COX-2 inhibitor administration induced sustained Th1-mediated antiviral effects in these BLV-infected cattle.
FIGURE 6.
Clinical tests using BLV-infected cattle. (A–C) BLV-infected cattle were administered 0.5 mg/kg of meloxicam, s.c. (D) The expression of IFN-γ at days 0, 1, 7, 28, and 56 was quantitated by qPCR in triplicate. (E and F) BLV-infected cattle were inoculated with 1 mg/kg of the purified anti–PD-L1 chAb (Boch4G12, animal #5) or saline (control, animal #4), i.v. (G and H) BLV-infected cattle (animals #3 and #6) were inoculated with 1 mg/kg of the purified Boch4G12, i.v. In addition, animal #3 was administered 0.5 mg/kg meloxicam once a week (total of three times) s.c. Proviral loads of these cattle were measured by qPCR in triplicate, and data are presented mean ± SD (A–C and E–H). Statistical significance was determined by the Dunnett test (A–H). *p < 0.05 versus day 0.
In vivo antiviral effects of anti–PD-L1 chAb, Boch4G12 inoculation
Our previous report clearly demonstrated the antiviral effects of anti–PD-L1 chAb Boch4G12 in experimentally BLV-infected calf (21). In the current study, we examined the antiviral effects of anti–PD-L1 chAb Boch4G12 in naturally infected cattle (Table II). Animal #4 was inoculated with saline (as a negative control) and animal #5 was inoculated with 1 mg/kg Boch4G12, and peripheral blood samples were then collected periodically to measure the BLV proviral load in PBMCs. In animal #5, the proviral load decreased significantly after inoculation, and the viral load in animal #4 continued to rise (Fig. 6E, 6F). Therefore, Boch4G12 has antiviral effects on naturally infected BLV cattle in vivo.
Antiviral efficacy of combined COX-2 inhibitor and anti–PD-L1 Ab treatment
Finally, we examined the antiviral efficacy of combined treatment compared with Boch4G12 treatment alone in two BLV-infected cattle (animals #3 and #6, Table II) with high proviral load (>2000 copies/50 ng DNA). Peripheral blood samples were collected from these cattle at least once per week for the measurement of BLV proviral load following treatment. Proviral load was not reduced in animal #6 by Boch4G12 treatment, but animal #3 exhibited significantly reduced viral load (nearly 80% at day 14) following combined treatment (Fig. 6G, 6H). Therefore, these findings demonstrate the potential of combined COX-2 inhibition plus anti–PD-L1 treatment as a new control strategy of BLV infection.
Discussion
In the advanced stages of BLV infection, BLV-specific Th1 responses are downregulated, leading to further disease progression and possible EBL (31). Several previous studies have demonstrated that loss of BLV-specific Th1 responses is mediated by upregulation of immunoinhibitory molecules such as PD-1 and PD-L1, as well as by expansion of regulatory T cells (15, 16, 24). Similarly, our previous study revealed that PGE2 suppresses Th1 responses, such as T cell proliferation and Th1 cytokine production, and induces the expression of PD-L1 (22). In addition, the expression levels of TGF-β1 and Foxp3, a key cytokine and transcription factor for the development of regulatory T cells, were increased in cultured bovine PBMCs by PGE2 treatment (Supplemental Fig. 3). In the current study, we found that the production of PGE2 increased in parallel with the progression of BLV infection. The expression of EP4 and EP2, PGE2 receptors associated with immune dysfunction (32), was also upregulated in the late stage of BLV infection, and the catabolic enzyme 15-PGDH was downregulated. Thus, PGE2 signaling may be markedly enhanced in late stage BLV infection, which, combined with the induction of PD-L1 and regulatory T cells via EP4 and EP2, would dramatically reduce BLV-specific Th1 responses.
Monocytes are a major source of PGE2 production (33). In this study, we identified the major cell type producing PGE2 in peripheral blood of BLV-infected cattle by the measurement of COX2 expression and PGE2 production in CD21+ cells and CD14+ cells. As shown in Fig. 1, COX2 expression was elevated in total PBMCs, CD21+ cells, and CD14+ cells of PL cattle compared with AL and uninfected cattle. The production capacity of PGE2 was higher in CD14+ cells than in CD21+ cells, suggesting that CD14+ cells are the main source of PGE2. However, in the late stage of BLV infection, the number of circulating B lymphocytes is dramatically increased (34), and our immunohistochemical staining results clearly revealed PGE2 production by tumor B cells in cattle with EBL. Thus, B cells may also contribute to or act as the main source of PGE2 production in the late stage of BLV infection.
In humans, transcription of COX-2 is induced by inflammatory cytokines, TLR signaling, and Ag stimulation via NF-κB (35, 36). In the current study, stimulation of PBMCs from BLV-infected cattle with BLV Ag induced PGE2 production. Generally, in the PBMC culture of the infected animals, BLV-specific CD4+ T cells mainly secrete Th1 cytokines, including TNF-α, by FLK–BLV stimulation. TNF-α is a key cytokine to induce the COX2 expression via NF-κB (36), suggesting that the BLV Ag upregulates COX2 expression via TNF-α–NF-κB pathway in BLV-infected cattle. In contrast, T cells from uninfected cattle could not respond to BLV Ag stimulation because of no acquired immunological memory against BLV. Our previous paper has demonstrated the same phenomenon in M. avium subsp. paratuberculosis–infected cattle. We observed the upregulation of PGE2 production and PD-L1 expression by the culture of PBMCs from M. avium subsp. paratuberculosis–infected cattle in the presence of M. avium subsp. paratuberculosis Ag, but not by that of PBMCs from uninfected cattle (22). Therefore, the acquired immunity might be necessary for the phenomena. Furthermore, in this study, the expression of HPGD was downregulated in PL cattle and negatively correlated with the BLV proviral load and serum PGE2 concentration. Taken together, coordinate regulation of increased PGE2 production and decreased PGE2 degradation leads to elevated levels of PGE2 which, in turn, suppresses BLV-targeted Th1 responses.
The viral gene gp51 encodes an envelope glycoprotein essential for cell-to-cell infection (37–40), and Tax, R3, and G4 are nonstructural proteins involved in the activation of viral transcription and propagation (41–44). A previous report demonstrated that PGE2 promotes Tax expression in PBMCs isolated from BLV-infected cattle (23), and the current study showed that the expression levels of gp51, G4, and R4 were also upregulated by PGE2 via EP2 and EP4 signaling in PBMCs isolated from BLV-infected cattle. Binding of PGE2 to EP2 and EP4 increases intracellular cAMP production (7), which activates protein kinase A (PKA) and downstream transcription factors that regulate genes with cAMP-response element (CRE) sites in the promoter. The BLV–long terminal repeat has a CRE that induces transcription of BLV gene (45, 46), strongly suggesting that PGE2/EP2 and PGE2/EP4 facilitate BLV viral gene transcription through activation of cAMP/PKA/CRE signaling.
CD8+ cytotoxic T cells activated by Th1 cytokines play a critical role in the control of BLV infection. COX-2 inhibitors are known to activate immune responses, including T cell responses (8, 9). Our previous report demonstrated that a selective COX-2 inhibitor, meloxicam, induced Th1 responses in PBMC cultures from both healthy cattle and cattle infected with M. avium subsp. paratuberculosis (22). In this study, meloxicam also activated BLV-specific Th1 responses in vitro, and administration of Metacam (a veterinary formulation of meloxicam) activated Th1 responses and significantly decreased proviral load in BLV-infected cattle. These findings suggest that inhibition of PGE2 using COX-2 inhibitors is a potential treatment strategy against BLV infection. Indeed, possible benefits of the COX-2 inhibitor celecoxib on the immune function were reported in patients with HIV infection (9). However, in the veterinary field, the current study is the first to investigate the therapeutic effects of COX-2 inhibitors against chronic infections.
In the current study, BLV proviral load was not dramatically reduced by treatment with meloxicam alone, probably because of the upregulation of immunoinhibitory molecules. Proviral load was extremely high (>2000 copies/50 ng DNA) in the cattle (animals #1–3) receiving meloxicam alone, and the expression of immunoinhibitory molecules, such as PD-L1, LAG-3, CTLA-4, Tim-3, and Foxp3, is substantially higher in BLV-infected cattle with high proviral loads compared with infected cattle with low proviral loads (16, 18, 19, 47, 48). Additionally, the proportions of CD4+ and CD8+ cells coexpressing PD-1 and LAG-3 increase in the late stage of BLV infection (49). Thus, the dual blockade of PGE2 and other immunoinhibitory molecules may be required to enhance the antiviral effects in advanced BLV infections.
In a murine model of chronic infection, PGE2 signaling via EP2 and EP4, impaired survival and the function of CD8+ CTLs, and CTL function was restored, and viral control was improved by combined blockade of the PGE2 and PD-1/PD-L1 signaling pathways (50). Furthermore, our previous study showed that PGE2 regulates PD-L1 expression in cattle and that the combined treatment with a COX-2 inhibitor plus an anti–PD-L1 Ab improves Th1 responses in Johne disease (22). Therefore, in this study, we evaluated the immunostimulative effects of combination treatment and found significantly enhanced BLV-specific Th1 responses in vitro.
Next, we performed the clinical study using BLV-infected cattle with high proviral load to evaluate antiviral effects of the treatment. We also examined the effects of monotherapy and combined therapy on high proviral load in BLV-infected cattle. Monotherapy with anti–PD-L1 chAb had no impact on BLV-infected cattle with high proviral load (animal #6), but combined treatment of animal #3 with the COX-2 inhibitor plus anti–PD-L1 chAb substantially reduced BLV proviral load to below 2000 copies/50 ng DNA from day 7 to day 49 after the first injection. Previous studies have demonstrated that BLV proviral load is positively correlated with vertical and horizontal transmission risks, and BLV-infected cattle with a high proviral load (>2000 copies/50 ng DNA) are the major source of transmission within a herd (26, 51, 52). Therefore, our findings suggest that the cotargeting of PGE2 and PD-L1 in the most severely infected animals could be a novel method for herd BLV infection control. In this study, although the number of the infected animals used in clinical tests was limited, much attention was paid to obtaining accurate data from these experiments. First, environmental influence was removed in the clinical tests of this study as much as possible. The clinical test of the single treatment of anti–PD-L1 chAb (animals #4 and #5) or the combined treatment (animals #3 and #6) was designed to compare the antiviral effect of the treatments between two BLV-infected animals and was conducted in the same facility at the same day and time, respectively. In contrast, three animals (animals #1–3) were launched for the clinical test of the COX-2 inhibitor and were kept at the three different locations in Hokkaido. These animals were administered the inhibitor at the same time of day on different days using the same injection method by a particular veterinarian. During all of the clinical tests, the tested animals were kept at “loose housing barn” and fed on equivalent grass, hay, and pellet. Additionally, all of the tested animals were not milked to prevent the influence of sex hormones and other biological changes during lactation. Second, all of the blood samples were kept at 4°C immediately after blood collection and shipped to the laboratory of Hokkaido University because the clinical tests were conducted at three totally different locations in Hokkaido. Then, PBMCs were isolated from the blood samples in the laboratory of Hokkaido University on the day after the blood collection to prevent nonspecific effects on results of the analysis caused by methods of shipping and storage. Finally, blood samples were collected at least once per week, and qPCR was performed for the detection of BLV proviral load in triplicate to reduce experimental error. The result of these clinical tests requires confirmation in further clinical trials with larger numbers of the infected cattle from different herds or farms. To the best of our knowledge, no previous human or veterinary study has investigated the benefits of combining a COX-2 inhibitor with an Ab targeting immune checkpoint molecules in vivo. Thus, our findings may provide a novel strategy for control of chronic retrovirus infection in both humans and livestock.
Recently, several studies have demonstrated an association between PGE2 elevation and upregulated expression of other immunoinhibitory molecules, such as PD-1, in human cancer (53, 54). In addition, dual blockade of PGE2 and PD-1 improved tumor eradication in a mouse model (55). However, the associations between PGE2 and other immunoinhibitory molecules are generally unclear. Further study is warranted to investigate these associations using bovine chronic infection as a disease model.
Although BLV infection is endemic in many countries (12, 56–59), there are no effective therapies or vaccines. Therefore, a novel strategy is required for effective BLV infection control. Our current work is a pilot study to assess the potential efficacies of pharmacological COX-2 inhibition and combined COX-2 and PD-1/PD-L1 inhibition. Treatment with the COX-2 inhibitor meloxicam with or without anti–PD-L1 Ab demonstrated an antiviral activity in BLV-infected cattle. Additional experiments using larger numbers of infected cattle are required to confirm the efficacy of these novel treatments. Finally, better understanding of the molecular mechanisms underlying immune dysfunction by PGE2 and the associations with other immune dysfunctional pathways is essential for the development of improved antiviral and anti-bacterial treatments.
Supplementary Material
Acknowledgments
We are grateful to Dr. Hideyuki Takahashi, Dr. Yasuyuki Mori, and Dr. Tomio Ibayashi for valuable advice and discussions. We thank Enago for the English language review.
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, by Research Project for Improving Animal Disease Prevention Technologies to Combat Antimicrobial Resistance 2017–2021 FY, and by grants from the Project of the NARO, Bio-oriented Technology Research Advancement Institution (Research Program on Development of Innovative Technology 26058 BC [to S.K.] and Special Scheme Project on Regional Developing Strategy, Grant 16817557 [to S.K.]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The online version of this article contains supplemental material.
- AL
- aleukemic
- BLV
- bovine leukemia virus
- chAb
- chimeric Ab
- COX
- cyclooxygenase
- CRE
- cAMP-response element
- EBL
- enzootic bovine leukosis
- FLK
- fetal lamb kidney, LAG-3, lymphocyte activation gene 3
- PD-1
- programmed death 1
- PD-L1
- programmed death-ligand 1
- PL
- persistent lymphocytosis
- qPCR
- quantitative real-time PCR.
Disclosures
K.Y. and M.T. are employed by Fuso Pharmaceutical Industries, Ltd. Y. Sajiki, S.K., T.O., A.N., N.M., S.G., Y. Suzuki, S.M., and K.O. are authors of a patent application covering materials and techniques described in this paper (PCT/JP2018/27041). The other authors have no financial conflicts of interest.
References
- 1.Phipps R. P., Stein S. H., Roper R. L. 1991. A new view of prostaglandin E regulation of the immune response. Immunol. Today 12: 349–352. [DOI] [PubMed] [Google Scholar]
- 2.Morita I. 2002. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 68–69: 165–175. [DOI] [PubMed] [Google Scholar]
- 3.Subbaramaiah K., Telang N., Ramonetti J. T., Araki R., DeVito B., Weksler B. B., Dannenberg A. J. 1996. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 56: 4424–4429. [PubMed] [Google Scholar]
- 4.Ryu Y. M., Myung S. J., Park Y. S., Yang D. H., Song H. J., Jeong J. Y., Lee S. M., Song M., Kim D. H., Lee H. J., et al. 2013. Inhibition of 15-hydroxyprostaglandin dehydrogenase by Helicobacter pylori in human gastric carcinogenesis. Cancer Prev. Res. (Phila.) 6: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink S. P., Yamauchi M., Nishihara R., Jung S., Kuchiba A., Wu K., Cho E., Giovannucci E., Fuchs C. S., Ogino S., et al. 2014. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci. Transl. Med. 6: 233re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris S. G., Padilla J., Koumas L., Ray D., Phipps R. P. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23: 144–150. [DOI] [PubMed] [Google Scholar]
- 7.Kalinski P. 2012. Regulation of immune responses by prostaglandin E2. J. Immunol. 188: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolina M., Sharma S., Lin Y., Dohadwala M., Gardner B., Luo J., Zhu L., Kronenberg M., Miller P. W., Portanova J., et al. 2000. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J. Immunol. 164: 361–370. [DOI] [PubMed] [Google Scholar]
- 9.Pettersen F. O., Torheim E. A., Dahm A. E., Aaberge I. S., Lind A., Holm M., Aandahl E. M., Sandset P. M., Taskén K., Kvale D. 2011. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J. Virol. 85: 6557–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aida Y., Murakami H., Takahashi M., Takeshima S. N. 2013. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 4: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz I., Lévy D. 1994. Pathobiology of bovine leukemia virus. Vet. Res. 25: 521–536. [PubMed] [Google Scholar]
- 12.Murakami K., Kobayashi S., Konishi M., Kameyama K., Tsutsui T. 2013. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009-2011. J. Vet. Med. Sci. 75: 1123–1126. [DOI] [PubMed] [Google Scholar]
- 13.Orlik O., Splitter G. A. 1996. Progression to persistent lymphocytosis and tumor development in bovine leukemia virus (BLV)-infected cattle correlates with impaired proliferation of CD4+ T cells in response to gag- and env-encoded BLV proteins. J. Virol. 70: 7584–7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabeya H., Ohashi K., Onuma M. 2001. Host immune responses in the course of bovine leukemia virus infection. J. Vet. Med. Sci. 63: 703–708. [DOI] [PubMed] [Google Scholar]
- 15.Ikebuchi R., Konnai S., Sunden Y., Onuma M., Ohashi K. 2010. Molecular cloning and expression analysis of bovine programmed death-1. Microbiol. Immunol. 54: 291–298. [DOI] [PubMed] [Google Scholar]
- 16.Ikebuchi R., Konnai S., Shirai T., Sunden Y., Murata S., Onuma M., Ohashi K. 2011. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of anti-viral immune responses in vitro via PD-L1 blockade. Vet. Res. (Faisalabad) 42: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirai T., Konnai S., Ikebuchi R., Okagawa T., Suzuki S., Sunden Y., Onuma M., Murata S., Ohashi K. 2011. Molecular cloning of bovine lymphocyte activation gene-3 and its expression characteristics in bovine leukemia virus-infected cattle. Vet. Immunol. Immunopathol. 144: 462–467. [DOI] [PubMed] [Google Scholar]
- 18.Okagawa T., Konnai S., Ikebuchi R., Suzuki S., Shirai T., Sunden Y., Onuma M., Murata S., Ohashi K. 2012. Increased bovine Tim-3 and its ligand expressions during bovine leukemia virus infection. Vet. Res. 43: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S., Konnai S., Okagawa T., Ikebuchi R., Nishimori A., Kohara J., Mingala C. N., Murata S., Ohashi K. 2015. Increased expression of the regulatory T cell-associated marker CTLA-4 in bovine leukemia virus infection. Vet. Immunol. Immunopathol. 163: 115–124. [DOI] [PubMed] [Google Scholar]
- 20.Okagawa T., Konnai S., Nishimori A., Maekawa N., Ikebuchi R., Goto S., Nakajima C., Kohara J., Ogasawara S., Kato Y., et al. 2017. Anti-bovine programmed death-1 rat-bovine chimeric antibody for immunotherapy of bovine leukemia virus infection in cattle. Front. Immunol. 8: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimori A., Konnai S., Okagawa T., Maekawa N., Ikebuchi R., Goto S., Sajiki Y., Suzuki Y., Kohara J., Ogasawara S., et al. 2017. In vitro and in vivo antivirus activity of an anti-programmed death-ligand 1 (PD-L1) rat-bovine chimeric antibody against bovine leukemia virus infection. PLoS One 12: e0174916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajiki Y., Konnai S., Okagawa T., Nishimori A., Maekawa N., Goto S., Ikebuchi R., Nagata R., Kawaji S., Kagawa Y., et al. 2018. Prostaglandin E2 induction suppresses the Th1 immune responses in cattle with Johne’s disease. Infect. Immun. 86: e00910-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyeon D., Diaz F. J., Splitter G. A. 2000. Prostaglandin E(2) increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation by interleukin-2, interleukin-10, and bovine leukemia virus. J. Virol. 74: 5740–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohira K., Nakahara A., Konnai S., Okagawa T., Nishimori A., Maekawa N., Ikebuchi R., Kohara J., Murata S., Ohashi K. 2016. Bovine leukemia virus reduces anti-viral cytokine activities and NK cytotoxicity by inducing TGF-β secretion from regulatory T cells. Immun. Inflamm. Dis. 4: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontes P. K., Castilho A. C., Razza E. M., Ereno R. L., Satrapa R. A., Barros C. M. 2014. Prostaglandin receptors (EP2 and EP4) and angiotensin receptor (AGTR2) mRNA expression increases in the oviducts of Nelore cows submitted to ovarian superstimulation. Anim. Reprod. Sci. 151: 112–118. [DOI] [PubMed] [Google Scholar]
- 26.von Hof J., Sprekeler N., Schuler G., Boos A., Kowalewski M. P. 2017. Uterine and placental expression of HPGD in cows during pregnancy and release of fetal membranes. Prostaglandins Other Lipid Mediat. 128–129: 17–26. [DOI] [PubMed] [Google Scholar]
- 27.Mekata H., Sekiguchi S., Konnai S., Kirino Y., Horii Y., Norimine J. 2015. Horizontal transmission and phylogenetic analysis of bovine leukemia virus in two districts of Miyazaki, Japan. J. Vet. Med. Sci. 77: 1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikebuchi R., Konnai S., Okagawa T., Yokoyama K., Nakajima C., Suzuki Y., Murata S., Ohashi K. 2013. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet. Res. 44: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimori A., Konnai S., Okagawa T., Maekawa N., Goto S., Ikebuchi R., Nakahara A., Chiba Y., Ikeda M., Murata S., Ohashi K. 2017. Identification of an atypical enzootic bovine leukosis in Japan by using a novel classification of bovine leukemia based on immunophenotypic analysis. Clin. Vaccine Immunol. 24: e00067-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikebuchi R., Konnai S., Okagawa T., Yokoyama K., Nakajima C., Suzuki Y., Murata S., Ohashi K. 2014. Influence of PD-L1 cross-linking on cell death in PD-L1-expressing cell lines and bovine lymphocytes. Immunology 142: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konnai S., Murata S., Ohashi K. 2017. Immune exhaustion during chronic infections in cattle. J. Vet. Med. Sci. 79: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maślanka T., Spodniewska A., Barski D., Jasiecka A., Zuśka-Prot M., Ziółkowski H., Markiewicz W., Jaroszewski J. J. 2014. Prostaglandin E2 down-regulates the expression of CD25 on bovine T cells, and this effect is mediated through the EP4 receptor. Vet. Immunol. Immunopathol. 160: 192–200. [DOI] [PubMed] [Google Scholar]
- 33.Passwell J., Levanon M., Davidsohn J., Ramot B. 1983. Monocyte PGE2 secretion in Hodgkin’s disease and its relation to decreased cellular immunity. Clin. Exp. Immunol. 51: 61–68. [PMC free article] [PubMed] [Google Scholar]
- 34.Nieto Farias M. V., Souza F. N., Lendez P. A., Martínez-Cuesta L., Santos K. R., Della Libera A. M. M. P., Ceriani M. C., Dolcini G. L. 2018. Lymphocyte proliferation and apoptosis of lymphocyte subpopulations in bovine leukemia virus-infected dairy cows with high and low proviral load. Vet. Immunol. Immunopathol. 206: 41–48. [DOI] [PubMed] [Google Scholar]
- 35.Schmedtje J. F., Jr., Ji Y.-S., Liu W.-L., DuBois R. N., Runge M. S. 1997. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 272: 601–608. [DOI] [PubMed] [Google Scholar]
- 36.Jobin C., Morteau O., Han D. S., Balfour Sartor R. 1998. Specific NF-kappaB blockade selectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology 95: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston E. R., Powers M. A., Kidd L. C., Radke K. 1996. Peripheral blood mononuclear cells from sheep infected with a variant of bovine leukemia virus synthesize envelope glycoproteins but fail to induce syncytia in culture. J. Virol. 70: 6296–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatot J. S., Callebaut I., Mornon J. P., Portetelle D., Burny A., Kerkhofs P., Kettmann R., Willems L. 1998. Conservative mutations in the immunosuppressive region of the bovine leukemia virus transmembrane protein affect fusion but not infectivity in vivo. J. Biol. Chem. 273: 12870–12880. [DOI] [PubMed] [Google Scholar]
- 39.Derse D., Hill S. A., Lloyd P. A., Chung Hk, Morse B. A. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J. Virol. 75: 8461–8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Igakura T., Stinchcombe J. C., Goon P. K. C., Taylor G. P., Weber J. N., Griffiths G. M., Tanaka Y., Osame M., Bangham C. R. M. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299: 1713–1716. [DOI] [PubMed] [Google Scholar]
- 41.Derse D. 1987. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J. Virol. 61: 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Broeke A., Cleuter Y., Chen G., Portetelle D., Mammerickx M., Zagury D., Fouchard M., Coulombel L., Kettmann R., Burny A. 1988. Even transcriptionally competent proviruses are silent in bovine leukemia virus-induced sheep tumor cells. Proc. Natl. Acad. Sci. USA 85: 9263–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems L., Kettmann R., Dequiedt F., Portetelle D., Vonèche V., Cornil I., Kerkhofs P., Burny A., Mammerickx M. 1993. In vivo infection of sheep by bovine leukemia virus mutants. J. Virol. 67: 4078–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willems L., Kerkhofs P., Dequiedt F., Portetelle D., Mammerickx M., Burny A., Kettmann R. 1994. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc. Natl. Acad. Sci. USA 91: 11532–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willems L., Kettmann R., Chen G., Portetelle D., Burny A., Derse D. 1992. A cyclic AMP-responsive DNA-binding protein (CREB2) is a cellular transactivator of the bovine leukemia virus long terminal repeat. J. Virol. 66: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adam E., Kerkhofs P., Mammerickx M., Kettmann R., Burny A., Droogmans L., Willems L. 1994. Involvement of the cyclic AMP-responsive element binding protein in bovine leukemia virus expression in vivo. J. Virol. 68: 5845–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konnai S., Suzuki S., Shirai T., Ikebuchi R., Okagawa T., Sunden Y., Mingala C. N., Onuma M., Murata S., Ohashi K. 2013. Enhanced expression of LAG-3 on lymphocyte subpopulations from persistently lymphocytotic cattle infected with bovine leukemia virus. Comp. Immunol. Microbiol. Infect. Dis. 36: 63–69. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki S., Konnai S., Okagawa T., Ikebuchi R., Shirai T., Sunden Y., Mingala C. N., Murata S., Ohashi K. 2013. Expression analysis of Foxp3 in T cells from bovine leukemia virus infected cattle. Microbiol. Immunol. 57: 600–604. [DOI] [PubMed] [Google Scholar]
- 49.Okagawa T., Konnai S., Nishimori A., Maekawa N., Goto S., Ikebuchi R., Kohara J., Suzuki Y., Yamada S., Kato Y., et al. 2018. Cooperation of PD-1 and LAG-3 in the exhaustion of CD4+ and CD8+ T cells during bovine leukemia virus infection. Vet. Res. 49: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J. H., Perry C. J., Tsui Y. C., Staron M. M., Parish I. A., Dominguez C. X., Rosenberg D. W., Kaech S. M. 2015. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat. Med. 21: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mekata H., Sekiguchi S., Konnai S., Kirino Y., Honkawa K., Nonaka N., Horii Y., Norimine J. 2015. Evaluation of the natural perinatal transmission of bovine leukaemia virus. Vet. Rec. 176: 254. [DOI] [PubMed] [Google Scholar]
- 52.Sajiki Y., Konnai S., Nishimori A., Okagawa T., Maekawa N., Goto S., Nagano M., Kohara J., Kitano N., Takahashi T., et al. 2017. Intrauterine infection with bovine leukemia virus in pregnant dam with high viral load. J. Vet. Med. Sci. 79: 2036–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miao J., Lu X., Hu Y., Piao C., Wu X., Liu X., Huang C., Wang Y., Li D., Liu J. 2017. Prostaglandin E2 and PD-1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget 8: 89802–89810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J., Zhang L., Kang D., Yang D., Tang Y. 2018. Activation of PGE2/EP2 and PGE2/EP4 signaling pathways positively regulate the level of PD-1 in infiltrating CD8+ T cells in patients with lung cancer. Oncol. Lett. 15: 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zelenay S., van der Veen A. G., Böttcher J. P., Snelgrove K. J., Rogers N., Acton S. E., Chakravarty P., Girotti M. R., Marais R., Quezada S. A., et al. 2015. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.VanLeeuwen J. A., Forsythe L., Tiwari A., Chartier R. 2005. Seroprevalence of antibodies against bovine leukemia virus, bovine viral diarrhea virus, mycobacterium avium subspecies paratuberculosis, and neospora caninum in dairy cattle in Saskatchewan. Can. Vet. J. 46: 56–58. [PMC free article] [PubMed] [Google Scholar]
- 57.Benavides B. B., Cedeño Quevedo D. A., Serrano De La Cruz M. F. 2013. Epidemiological study of bovine leukemia virus in dairy cows in six herds in the municipality of Pasto, Nariño. Rev. Lasallista Investig. 10: 18–26. [Google Scholar]
- 58.Ruzina M. N., Andrianov B. V., Suprovich T. M., Sulimova G. E. 2013. [Specific genetic features of the Russian forms of bovine leukemia virus]. Genetika 49: 975–980. [DOI] [PubMed] [Google Scholar]
- 59.Lee E., Kim E. J., Ratthanophart J., Vitoonpong R., Kim B. H., Cho I. S., Song J. Y., Lee K. K., Shin Y. K. 2016. Molecular epidemiological and serological studies of bovine leukemia virus (BLV) infection in Thailand cattle. Infect. Genet. Evol. 41: 245–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.