Abstract
The Ca2+-sensing receptor (CaSR) detects small changes in extracellular calcium (Ca2+e) concentration ([Ca2+]e) and transduces the signal into modulation of various signaling pathways. Ca2+-induced relaxation of isolated phenylephrine-contracted mesenteric arteries is mediated by the CaSR of the perivascular nerve. Elucidation of the regulatory mechanisms involved in vascular CaSR signaling may provide insights into the physiologic functions of the receptor and identify targets for the development of new treatments for cardiovascular pathologies such as hypertension. Protein kinase Cα (PKCα) is a critical regulator of multiple signaling pathways and can phosphorylate the CaSR leading to receptor desensitization. In this study, we used automated wire myography to investigate the effects of CaSR mutation and small-interfering RNA downregulation of PKCα on CaSR-mediated relaxation of phenylephrine-contracted mesenteric arteries from aged Dahl salt-sensitive (SS) rats on a low-salt diet. The data showed minimal relaxation responses of arteries to Ca2+e in wild-type (SS) and CaSR mutant (SS-Casrem1Mcwi) rats. Mutation of the CaSR gene had no significant effect on relaxation. PKCα expression was similar in wild-type and mutant rats, and small-interfering RNA downregulation of PKCα and/or inhibition of PKC with the Ca2+-sensitive Gӧ 6976 resulted in a >80% increase in relaxation. Significant differences in EC50 values were observed between treated and untreated controls (P < 0.05 analysis of variance). The results indicate that PKCα plays an important role in the regulation of CaSR-mediated relaxation of mesenteric arteries, and its downregulation or pharmacological inhibition may lead to an increased Ca2+ sensitivity of the receptor and reversal of age-related changes in vascular tone.
SIGNIFICANCE STATEMENT
G protein-coupled CaSR signaling leads to the regulation of vascular tone and may, therefore, play a vital role in blood pressure regulation. The receptor has several PKC phosphorylation sites in the C-terminal intracellular tail that mediate desensitization. We have previously shown that activation of the CaSR in neuronal cells leads to PKC phosphorylation, indicating that protein kinase C is an important regulator of CaSR function. Therefore, PKC in the CaSR signaling pathway in mesenteric arteries is a potential target for the development of new therapeutic approaches to treat hypertension and age-related vascular dysfunction. The present studies show that small-interfering RNA downregulation of PKCα and pharmacological inhibition of PKC enhanced CaSR-mediated relaxation of phenylephrine-contracted mesenteric arteries from aged Dahl salt-sensitive rats.
Introduction
An important component of many physiologic functions, such as contraction of skeletal and smooth muscle cells, neuronal transmission, hormone secretion, and regulation of gene control, is the precise control of intracellular Ca2+. Availability of Ca2+i and availability in organs that participate in cardiovascular control is a crucial factor in the maintenance and regulation of blood pressure (Hatton and McCarron, 1994; McCarron and Reusser, 1999). Small changes in extracellular Ca2+ concentration ([Ca2+]e) induce changes in parathyroid hormone secretion through activation of the Ca2+-sensing receptor (CaSR) in parathyroid glands to cause subsequent calcium absorption or elimination (Hannan et al., 2016). The CaSR has also been shown to regulate Ca2+i levels independently of parathyroid hormone activity (Kos et al., 2003; Kantham et al., 2009). The CaSR plays an important role in blood vessel tone and blood pressure control as it is involved in mediating Ca2+-induced vascular reactivity (Schepelmann et al., 2016). Previous studies from our laboratory indicated that a mechanism by which CaSR may regulate blood pressure involves Ca2+-induced activation of perivascular sensory nerve CaSR signaling in the mesenteric artery network that leads to activation of the endocannabinoid system and subsequent vascular relaxation (Bukoski, 2001; Bukoski et al., 2001; Awumey et al., 2008). This transduction of changes in [Ca2+]e into changes in cell signaling makes the CaSR a promising target for therapeutic drug design. Elucidation of the regulatory mechanisms of CaSR-mediated signal transduction may not only provide insights into the physiology of the receptor but may also help identify its critical downstream signaling proteins as potential drug targets for treating diseases associated with poor blood pressure regulation.
The CaSR, unlike other G protein-coupled receptors (GPCRs), has a large N-terminal extracellular domain, with glycosylation sites, that binds Ca2+ with low affinity (Pin et al., 2005); a seven-transmembrane, hydrophobic domain that binds allosteric modulators; as well as a C-terminal intracellular tail containing multiple phosphorylation sites (Ruat et al., 1995; Ferry et al., 2000). The CaSR couples to several Gα subunits leading to mediation of Gβγ signaling (Neves et al., 2002) and regulation of numerous second messengers such as inositol-1,4,5-trisphosphate, Ca2+i, cAMP, and phosphatidic acid (Ward, 2004). We have previously shown that activation of the CaSR leads to Gαq coupling and generation of inositol 1,4,5-trisphosphate and diacylglycerol that is metabolized to arachidonic acid and subsequent generation of epoxyeicosatrienoic acids (EETs) and glycated epoxyeicosatrienoic acids (GEETs) that cause vascular relaxation (Awumey et al., 2008).
Agonist-induced activation of CaSR is typically followed by initiation of signaling cascades that result in rapid signal reduction, leading to functional desensitization of the receptor. Receptor phosphorylation by GPCR kinases (GRKs) and/or second messenger-dependent protein kinases such as protein kinase C (PKC) is an early step in functional desensitization (Conigrave and Ward, 2013). GRKs can also mediate signal attenuation by direct binding to the activated form of Gα proteins, independently of receptor phosphorylation (Penela et al., 2003), and binding of β-arrestins to uncouple from G proteins (Hannan et al., 2018; Luttrell et al., 2018). An association of the receptor-β-arrestin complex with clathrin also initiates receptor internalization that contributes to functional desensitization (Lefkowitz and Shenoy, 2005). We have previously reported that activation of PKCα and phosphorylation plays an important role in suppressing CaSR-mediated Ca2+i mobilization and in reduced receptor activity (Sesay et al., 2015). Furthermore, PKCα may indirectly mediate CaSR functional desensitization by modulating the activity of GRK2 (Krasel et al., 2001). In the current study, we investigated the role of PKCα in Ca2+e-induced activation of the CaSR and relaxation of mesenteric arteries from Dahl salt-sensitive hypertensive rats. Experiments were conducted with isolated arteries from aged wild-type salt-sensitive (SS) and mutant SS-Casrem1Mcwi (with only one functional Casr allele) rats that were maintained on a low-salt (0.1% NaCl) diet.
Materials and Methods
Materials.
Dulbecco’s modified Eagle’s medium (DMEM; cat. no. 11960-044), heat inactivated fetal bovine serum (cat. no. 1614-071), penicillin/streptomycin (cat. no. 15070-063), Opti-MEM I reduced serum medium (cat. no. 31985-062), and Halt Protease and Phosphatase Inhibitor Cocktail (cat. no. 78425) were obtained from Invitrogen (Carlsbad, CA). Gö 6976 (cat. no. 365253) was obtained from EMD biosciences (La Jolla, CA). PKCα small-interfering RNA (siRNA; cat. no. sc-108089), siRNA transfection reagent (cat. no. sc-29528), siRNA transfection medium (cat. no. sc-36868) as well as mouse monoclonal PKCα (H-7) antibody specific for an epitope mapping between amino acids 645–672 at the C-terminus of PKCα of human origin (cat. no. sc-8393), mouse monoclonal CaSR (6D4) antibody raised against amino acids 15–29 of CaSR of rat origin (cat. no. sc-47741), mouse monoclonal GAPDH (6C5) antibody raised against GAPDH purified from muscle of rabbit origin (cat. no. sc-32233), and m-IgGκ BP-HRP (cat. no. sc-516102), a purified recombinant mouse IgGκ light-chain binding protein conjugated to horseradish peroxidase were from Santa Cruz Biotechnology (Santa Cruz, CA).
Animals.
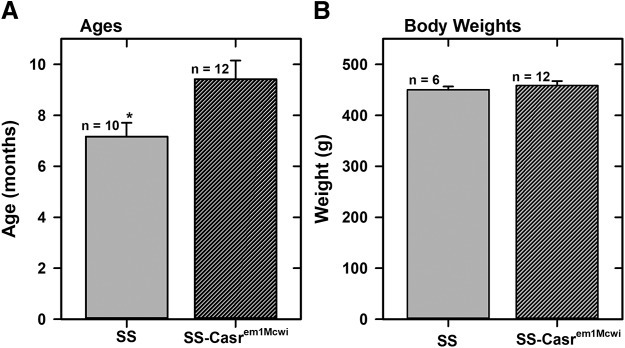
The animal studies have been carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of North Carolina Central University. Dahl SS wild-type and mutants (SS-Casrem1Mcwi) rats were maintained in the Animal Research Center at the Julius L. Chambers Biomedical/Biotechnology Research Institute at constant temperature and humidity and provided with 0.1% NaCl (cat. no. TD.7034) rodent chow (Envigo Tekland Diets, Madison, WI) and water ad libitum. Animals used were approximately 8 months old and of weight, 450–500 g (Fig. 1).
Fig. 1.
Average ages and body weights of animals. (A) Average ages of SS and SS-Casrem1Mcwi rats. (B) Average body weights of SS and SS-Casrem1Mcwi rats. Data are means (±S.E.M.) of 10 SS and 12 SS-CasremiMcwi animals.
Generation of Mutant SS-Casrem1Mcwi Rats.
The SS-Casrem1Mcwi mutant rat was generated from SS/JrHsdMcwi parental strain (ID 61499) in collaboration with the Gene Editing Rat Resource Center at the Medical College of Wisconsin (Milwaukee, WI) in Round 2 of a competitive process in March 2014. The mutant rats were produced by injecting a clustered regulatory interspaced short palindromic repeat (CRISPR) targeting the Casr (RGD Gene ID: 2277) sequence, GTACTTCCGCTGGAACTGGG, into SS/JrHsdMcwi rat embryos. The mutation region (RGSC 5.0/rns; chromosome 11: 70,329,456-70,329,457) is a 1-bp insertion resulting in a frame-shift in exon 4 of the Casr. The target site and the insertion of a T is shown below:
GTACTTCCGCTGGAACTGGG Casr CRISPR target (PAM in italics).
GTACTTCCGCTGGAACTTGGG Casr-m1 1-bp insertion mutation in exon 4 results in predicted frame-shift.
Founder animals were genotyped by the Cel-1 assay and confirmed by Sanger sequencing. The founders were backcrossed to the parental strain, and subsequent litters were identified by fluorescent genotyping. We received heterozygous pairs for breeding on April 4, 2015 and obtained offspring that were used to establish a colony for our studies.
Protein Extraction and Western Blot Analysis.
Total proteins were extracted from mesenteric arteries isolated from SS and SS-Casrem1Mcwi mutant rats, under isoflurane anesthesia, and analyzed for expression of PKCα and CaSR proteins by Western blotting. After trimming fat from the arteries, tissues were pooled from three animals each to obtain enough protein for assay. Proteins were also isolated from younger animals (<3 months old) for analysis to determine if there were any changes in the expression levels of the CaSR and PKCα owing to aging. Isolated arteries were also incubated with PKCα siRNA or with DMEM alone (control) for 24 hours and stored at −80°C until used for protein extraction. Tissues were homogenized in ice-cold Tris buffer (10 mM Tris, pH 7.5, 0.25 M sucrose and 3 mM MgCl2) with freshly added Halt Protease and Phosphatase Inhibitor Cocktail. Homogenates were centrifuged at 800g for 10 minutes, and the supernatant fractions separated using SDS-PAGE. Separated proteins were transferred onto polyvinylidene fluoride membranes, blocked with nonfat milk (Biorad, Hercules, CA), and then blotted with antibodies followed by incubation with m-IgGκ BP-HRP (1:5000 dilution) and visualization by enhanced chemiluminescence (ECL). Densitometry analysis of protein bands was done using Licor Image Studio software.
Vessel Isolation.
Mesenteric arteries were dissected from rats under deep anesthesia with isoflurane and sacrificed by open-chest cardiac puncture as previously described (Awumey et al., 2008; Bridges et al., 2011). The small intestine and all the associated vessels were removed in block and placed in cold physiologic salt solution (PSS; millimolars: 115 NaCl, 4.7 KCl, 1.4 MgSO4⋅7H2O, 5 NaHCO3, 1.1 Na2HPO4, 1.2 KH2PO4, 1.0 CaCl2, 20 HEPES, and 5 glucose; pH 7.4). Branches I and II mesenteric arteries were carefully dissected from the surrounding fat leaving a portion of the omental membrane attached to the vessel. Isolated vessels were then cut into 2-mm segments for wire myography.
siRNA Downregulation of PKCα in Mesenteric Arteries.
To induce PKCα knockdown, isolated mesenteric arteries were incubated with PKCα siRNA for 24 hours. A solution containing 130 nM PKCα siRNA was mixed with transfection reagent in transfection medium and incubated at room temperature for 30 minutes to allow complex formation. Mesenteric arteries were then incubated at 37°C in 5% CO2 for 6 hours, after which media was replaced with fresh DMEM containing 10% fetal bovine serum and antibiotics, and the incubation continued 24 hours before use in protein extraction or assay of reactivity to Ca2+ by wire myography.
Wire Myography.
Isometric force generation in mesenteric artery segments was determined as previously described (Awumey et al., 2008; Bridges et al., 2011). Stainless steel wires (40-μm in diameter) were inserted through the lumens of 2-mm long artery segments and mounted in a small-vessel Mulvany-Halpern 510A Auto Dual Wire Myograph (DMT-USA, Marietta, GA). Segments were maintained in PSS containing 1 mM CaCl2 and 100 μM ascorbic acid and gassed with a mixture of 95% air and 5% CO2. After a 30-minute equilibration at 37°C, vessels were normalized by stepwise stretching using pre-established normalization protocols. The parameters used for normalization were as follows: target transmural pressure, 13.3 kPa (100 mm Hg); time, 60 seconds; IC1/IC100 = 0.9 (IC100 − internal circumference corresponding to target pressure, IC1 − internal circumference of normalized vessel); eyepiece calibration, 2*Δ (mm/division). The normalization procedure defines the lumen diameter (d100) that the artery would have in vivo when relaxed and under a transmural pressure of 100 mm Hg (Halpern et al., 1978; Angus and Wright, 2000). The mounted vessels were then challenged with 5 μM phenylephrine (PE) until reproducible contractions were observed. Active force development of ≥10 mN was viewed as ideal for the experiment to proceed. Relaxation was assessed by addition of cumulative concentrations of Ca2+ to precontracted vessels. When an inhibitor was used, vessels were preincubated with the PKC inhibitor Gӧ 6976 for 5 minutes prior to addition of PE and was present throughout the relaxation assays.
Statistical Analysis.
[Ca2+]e-response curves and bar graphs were analyzed using Sigma Plot 14 software (SYSTAT, Port Richmond, CA). EC50 values were determined by fitting data to sigmoid curves in the program. Comparisons between groups were determined by one-way analysis of variance (ANOVA) with a difference of P < 0.05 considered significant. Power of performed test with α = 0.050:1.000.
Results
Genotyping of Mutant SS-Casrem1Mcwi Rats.
Male and female SS-Casrem1Mcwi rats, from our colony, were randomly bred on a 0.3% NaCl diet (TD.95027; Tekland), and DNA extracts from tail samples of offspring were genotyped using a Custom TaqMan SNP Genotyping Assay, on a fee-for-service basis by the Molecular Genetics Core of the Duke Molecular Physiology Institute (Durham, NC). The probe sequences and the reporter dyes (in brackets) used are as follows:
Reporter Sequence 1- CCACCCAAGTTCCAG (VIC dye).
Reporter Sequence 2- CCACCCAGTTCCAG (FAM dye).
Most of the offspring from the breeding were wild-type SS (D/D) and heterozygous SS-Casrem1Mcwi mutant (I/D) animals, confirming a single-allele mutation in the Casr (Supplemental Fig. 1). Thus, the breeding protocol we established has allowed us to maintain a colony of heterozygous breeder pairs in sufficient numbers to generate wild-type and age-matched heterozygous mutants in adequate numbers continuously for our studies. Figure 1 shows the ages and weights of SS wild-type and SS-Casrem1Mcwi heterozygous animals used in the present studies. The ages of the animals used were about 8 to 9 months but the weights were similar.
Effects of CaSR and PKCα Downregulation on Protein Expression.
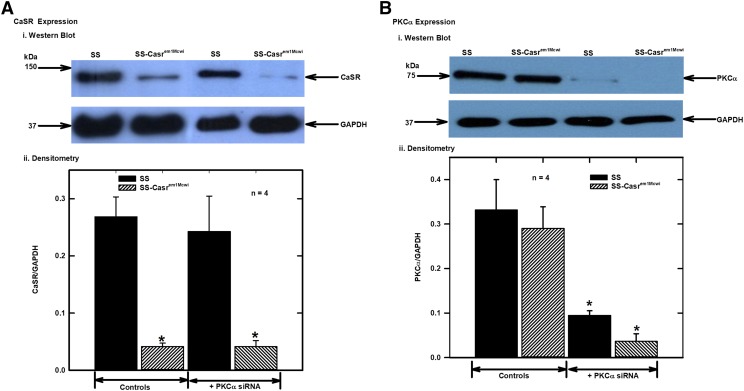
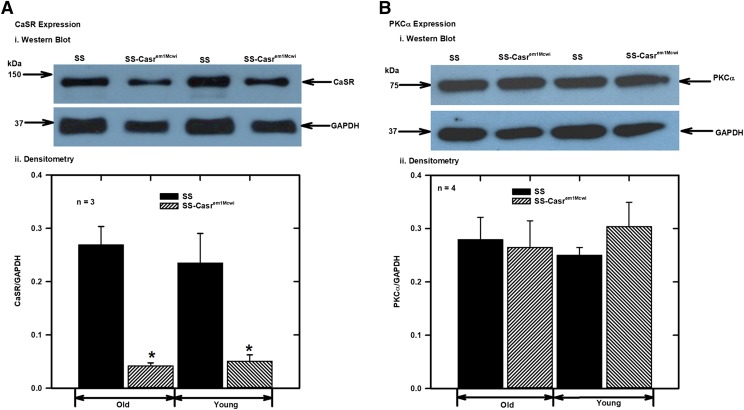
To confirm knockdown of CaSR and PKCα expression, proteins extracted from isolated mesenteric arteries (control and those incubated with PKCα siRNA) were analyzed by Western blotting with CaSR and PKCα antibodies. The results showed significant reduction in the expression of the CaSR in the mesenteric arteries from SS-Casrem1Mcwi rats compared with the SS (Fig. 2A), and downregulation of expression of PKCα protein following PKCα siRNA treatment of the vessels (Fig. 2B). Western blotting also showed that there were no significant differences in CaSR and PKCα expression between young (<3 months old) rats compared with the aged rats used in this study (Fig. 3).
Fig. 2.
Analysis of expression of CaSR and PKCα in control and PKCα siRNA-treated mesenteric arteries from SS and SS-Casrem1Mcwi rats. Animals were maintained on 0.1% NaCl diet for 8 months and protein extracts from control siRNA-treated mesenteric arteries were analyzed with monoclonal antibodies. CaSR, PKCα and GAPDH bands were analyzed from the separated proteins in the same samples. After separation, proteins were transferred onto membranes and cut into three strips for blotting with monoclonal antibodies to the CaSR (150 kDa), PKCα (80 kDa) and GPADH (37 kDa). (A) (i) Representative CaSR Western blot, and (ii) bar chart showing densitometric analysis of protein bands from four different Western blot experiments as in (i). Values plotted are means (±S.E.M.) of four experiments. (B) (i) Representative PKCα Western blot, and (ii) bar charts showing densitometric analysis of protein bands from four different Western blot experiments as in (i). Values plotted are means (±S.E.M.) Protein bands were normalized to GAPDH as the loading standard. (*P < 0.05 vs. control; ANOVA followed by multiple comparisons using the Holm-Sidak method).
Fig. 3.
CaSR and PKCα expression in mesenteric arteries from old (≈ 8 months) and young (≤3 months) SS and SS-Casrem1Mcwi rats. Protein extracts from mesenteric arteries were analyzed with monoclonal antibodies to compare expression levels in young and old rats. CaSR, PKCα, and GAPDH bands were analyzed from the separated proteins in the same samples. After separation, proteins were transferred onto membranes and the latter cut into three strips for blotting with monoclonal antibodies to the CaSR (150 kDa), PKCα (80 kDa), and GPADH (37 kDa). (A) (i) Representative CaSR Western blot; (ii) bar charts showing densitometric analysis of protein bands from four separate experiments as in (i). Values plotted are means (±S.E.M.); (B) (i) Western blot of PKCα; (ii) bar charts showing densitometric analysis of protein bands from four separate experiments as in (i). Values plotted are means (±S.E.M.). Protein bands were normalized to GAPDH as the loading standard. (*P < 0.05 vs. control; ANOVA followed by multiple comparisons using the Holm-Sidak method).
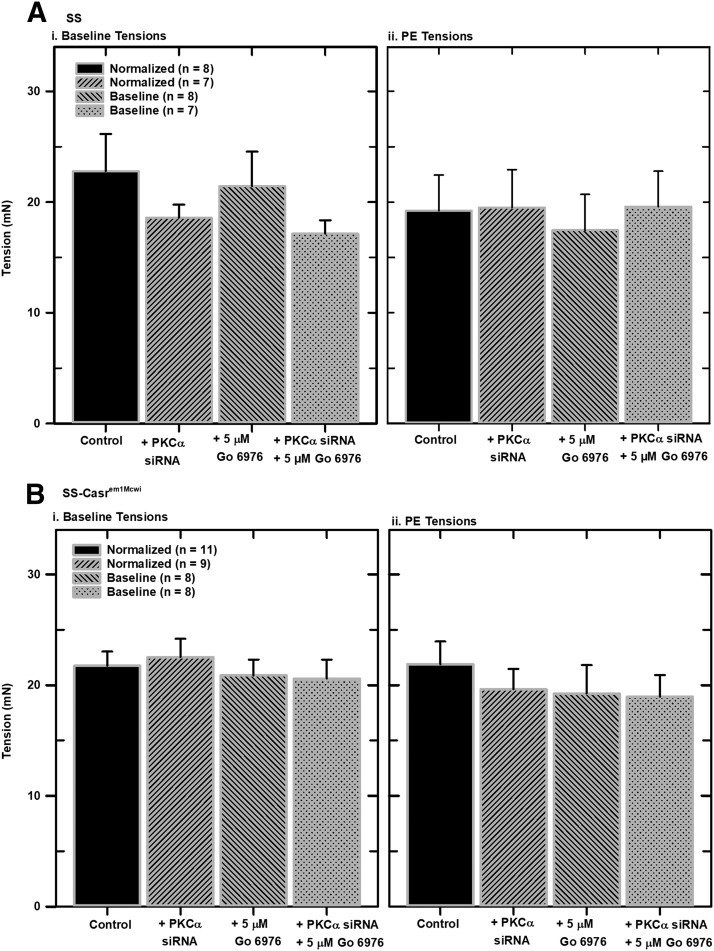
Normalization of Mounted Mesenteric Arteries.
It is important to set a baseline condition in myography studies of isolated vessels in order to acquire reproducible results. To do this, vessels equilibrated in PSS were stretched in a stepwise manner with increasing tension over time until they developed passive tension (Angus and Wright, 2000). Normalization of isolated mesenteric artery segments from SS and SS-Casrem1Mcwi rats were similar (see Supplemental Data). PKCα downregulation did not have any effect on the slopes of the normalization curves. Figure 4 shows resting tensions, after normalization, and tensions resulting from treatment of vessels with 5 μM PE, in SS and SS-Casrem1Mcwi, respectively. There were no significant differences in resting tensions and PE tensions resulting from either CaSR or PKCα downregulation. No significant changes in PE tensions resulted from treatment of mounted vessels with the PKC inhibitor Gö 6976.
Fig. 4.
Effects of CaSR and PKCα downregulation on normalized and phenylephrine tensions in mounted mesenteric arteries from SS and SS-Casrem1Mcwi rats. Segments (2-mm) of isolated mesenteric arteries were mounted in a wire myograph chamber in PSS medium (containing 1 mM Ca2+ and 100 μM ascorbic acid), equilibrated at 37°C, and gassed with a mixture of 95% air and 5% CO2. Vessel segments were normalized by step-wise increases in passive force until a resting tension (RT) was achieved. Tensions resulting from the addition of PE were also determined. (A) Normalized resting tensions from SS and SS-Casrem1Mcwi rats. (B) PE tensions (active force from PE addition minus resting tensions) from SS and SS-Casrem1Mcwi rats). Statistical analysis of the data showed no significant differences.
Effects of siRNA Downregulation of PKCα and Pharmacological Inhibition of PKC in Mesenteric Arteries Increased Ca2+- Induced Relaxation.
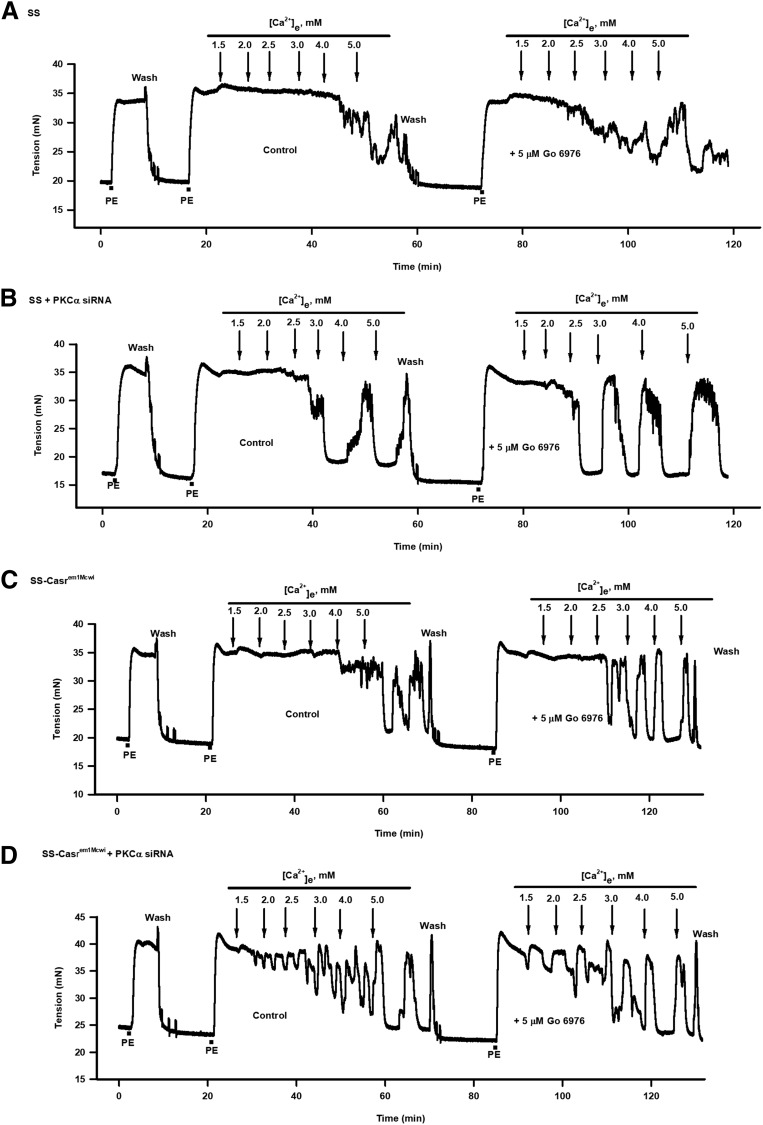
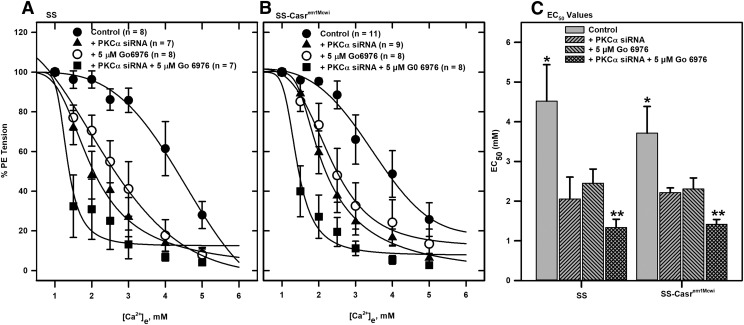
Arteries from SS and SS-Casrem1Mcwi animals that had PKCα downregulated or PKC inhibited showed significant differences in responses to cumulative addition of Ca2+ compared with controls (Fig. 5). Tension changes in these vessels indicate that the nontransfected (control) PE-contracted tissues showed minimal response to Ca2+e, with relaxation starting after addition of high [Ca2+]e (Fig. 5, A and C). However, pretreatment of vessels with 5 μM Gö 6976 resulted in increased responses to Ca2+e. These increased responses were also observed in PKCα siRNA-treated vessels, which showed responses to much lower [Ca2+]e compared with the nontransfected tissues (Fig. 5, B and D). This effect was enhanced in tissues in which PKCα was downregulated and treated with 5 μM Gö 6976. The [Ca2+]e-response curves from both sets of tissues also indicate the effect of PKCα knockdown and/or PKC inhibition on relaxation response (Fig. 6, A and B). Response curves in PKCα-downregulated arteries were shifted to the left compared with the nontransfected tissues, with combined downregulation and inhibition shifting the response curves further to the left. EC50 values for the Ca2+ relaxation responses were derived by fitting sigmoid curves to the data (Fig. 6C). No significant differences in vessel responses to Ca2+e were observed between the SS and SS-Casrem1Mcwi tissues regardless of treatment.
Fig. 5.
Effect of downregulation of CaSR and PKCα, and pharmacological inhibition of PKC on Ca2+-induced relaxation of PE-contracted mesenteric arteries. Force tracings showing the effect of cumulative additions of Ca2+e to PE-contracted mesenteric arteries, from SS and SS-Casrem1Mcwi rats, in the absence or presence of 5 μM Gö 6976 on relaxation. (A) SS control; (B) SS + PKCα siRNA; (C) SS-Casrem1Mcwi control; (D) SS-Casrem1Mcwi + PKCα siRNA. PKCα siRNA downregulation and PKC inhibition by Gö 6976 resulted in increased relaxation responses to cumulative additions of Ca2+e.
Fig. 6.
Effects of downregulation of CaSR and PKCα and inhibition of PKC on [Ca2+]e-response curves derived from force tracings in Fig. 5. [Ca2+]e-response curves were generated from tension data obtained in arteries from (A) SS and (B) SS-Casrem1Mcwi rats. (C) Bar charts showing EC50 values determined from response data fitted to sigmoid curves *Significantly different from treated groups; **Significantly different from PKCα siRNA-treated and Go 6976-treated groups (P < 0.05; ANOVA followed by multiple comparisons using the Holm-Sidak method).
Discussion
The [Ca2+]e is maintained within a narrow range (1.1–1.4 mM) in humans (Hurwitz, 1996), therefore the CaSR must be able to detect small changes in this concentration for receptor activation to be possible. In fact, the CaSR has been shown to detect small changes in Ca2+e (Brown and MacLeod, 2001) and small increases in interstitial fluid Ca2+ ([Ca2+]ISF) that are within the physiologic range (Breitwieser, 2008). In situ microdialysis studies that measured [Ca2+]ISF in the duodenal submucosa and kidney cortex, two tissues that are essential for the regulation of peripheral resistance and BP, showed dynamic changes as a function of the gut lumen [Ca2+]. Increasing gut [Ca2+] from 0 to 6 mM increased the [Ca2+]ISF from 1.1 to 1.9 mM (Mupanomunda et al., 1998, 1999, 2000), which is in the range observed to activate the perivascular CaSR expressed in human embryonic kidney (HEK) cells (Awumey et al., 2007), and relaxed isolated mesenteric arteries (Awumey et al., 2008). We have also shown that the CaSR detects decreases in [Ca2+]e and an increase in the action potential duration through activation of the nonselective cation channel, which in turn attenuates the impact on release probability at neocortical terminals (Chen et al., 2010). The latter suggests that the CaSR provides presynaptic feedback to alter brain excitability in response to Ca2+e. We also found that in HEK-CaSR.EGFP cells expressing the dorsal root ganglia CaSR-EGFP fusion protein, desensitization of the receptor occurs with repeated, prolonged stimulation (Awumey et al., 2007), further suggesting that an understanding of the regulation of this receptor will clarify its role under both normal and hypertensive conditions. Studies have shown that small changes in [Ca2+]ISF are enough to activate the CaSR and contribute to vasodilator synthesis under normal conditions (Mupanomunda et al., 1999, 2000; Bukoski, 2001). Other studies have also revealed that a high-salt diet reduced renal [Ca2+]ISF and increases systolic BP in Dahl salt-sensitive rats (Palmer et al., 2003; Eley et al., 2008).
The mesenteric arterial network contributes significantly to total peripheral resistance, as it receives about 25% of cardiac output, and so this system is very important for overall regulation of blood pressure (Christensen and Mulvany, 1993). CaSR, and its downstream signaling protein complements, has been shown to play crucial roles in this system and as such is involved in blood pressure regulation (Bukoski et al., 1997; Bukoski, 1998; Wang and Bukoski, 1998; Awumey et al., 2008). Given the key role played by the mesenteric artery CaSR in controlling Ca2+i, the regulation of its function has important consequences for the entire animal physiology.
PKCα is a critical component of various signaling mechanisms that regulate vascular contractility. It has been shown that PKCα mediates L-type voltage-gated Ca2+ channel control of Ca2+ entry (Navedo et al., 2005; Potts et al., 2012). PKCα is also involved in increasing Ca2+-sensitizing pathways in the contractile myofilaments coupled to α1-adrenergic arterial vasoconstriction (Villalba et al., 2007; Kitazawa and Kitazawa, 2012). Conversely, as we have shown in a previous study, PKCα is also involved in CaSR-signaling mechanisms that reduce Ca2+ mobilization (Sesay et al., 2015). This mechanism involves Gӧ 6976 inhibition of [Ca2+]e-induced phosphorylation and translocation of PKCα to the plasma membrane.
In this study we examined the role played by PKCα in decreasing CaSR-mediated relaxation of mesenteric arteries, as well as investigating the effects of reduced expression of CaSR. We performed these studies using isolated arteries from wild-type male SS and SS-Casrem1Mcwi mutant rats, which have only one functional Casr allele. It is well established that aging results in myriad changes in vascular structure, and such remodeling (Xu et al., 2017) affects endothelial-dependent and -independent relaxations (Muller-Delp et al., 2002; Prisby et al., 2007; Zicha et al., 2012) and Ca2+ homeostasis, although the latter has mainly been shown to occur via an endothelium-dependent mechanism with acetylcholine as the vasodilator agent. We have observed in our laboratory that there is significant decrease in relaxation response of PE-contracted mesenteric arteries to Ca2+e as animals get older. However, we did not observe any significant differences in expression levels of CaSR or PKCα in young and old animals (Fig. 3), which suggests that changes in function or activity of these proteins owing to aging contributed to decreased Ca2+-induced relaxation of phenylephrine-contracted mesenteric arteries.
To accurately and reproducibly conduct mechanical studies on isolated vessels, it is very important to normalize mounted vessel segments to establish standard baseline tension and determine tissue viability. Furthermore, stepwise stretching of the vessel, until a lumen diameter is defined that is comparable to transmural pressure in vivo, is important, as this determines vessel activity and sensitivity to various treatments. Our results show that siRNA downregulation of PKCα did not affect basal tensions of both SS and SS-Casrem1Mcwi vessels. There was also no effect of PKCα downregulation on PE tension levels. This is consistent with previous studies showing that PKCα is not directly involved in α1-adrenergic-mediated vasoconstriction (Ohanian et al., 1996). It should also be noted that even though there was reduced expression of CaSR in the arteries from the SS-Casrem1Mcwi animals, this did not result in any significant difference in basal or PE tensions.
As we have previously established, the Ca2+e relaxation response in mesenteric arteries is a result of CaSR activity (Awumey et al., 2008, 2013). In these studies, we showed that Ca2+e relaxation of precontracted arteries involved CaSR-mediated increase in intracellular [Ca2+] that leads to the generation of cytochrome P450-derived metabolites of the endocannabinoids 2-arachidonoylglycerol and anandamide. Furthermore, endothelial nitric oxide synthase knockout in mice upregulates CaSR expression in mesenteric arteries.
The observed effect of downregulation of PKCα and/or pharmacological inhibition of PKC on mesenteric artery relaxation confirms that PKCα plays an important role in the regulation of CaSR activity, similar to our observation in a previous study (Sesay et al., 2015). It has also been suggested previously that receptor phosphorylation by PKC regulates attenuation of CaSR signaling via a mechanism involving β-arrestin binding to PKC-phosphorylated CaSR and subsequent desensitization to Ca2+ (Lorenz et al., 2007). Our results indicate that PKCα mediates relaxation of phenylephrine-contracted mesenteric arteries in response to cumulative addition of Ca2+e. Both siRNA downregulation of PKCα and pharmacological inhibition of PKC resulted in significant increases in Ca2+e-induced relaxation of isolated, PE-constricted mesenteric arteries, and, in effect, reversal of the effects of aging on vasodilation in these vessels. PKCα downregulation resulted in increased responsiveness of arteries to Ca2+e as illustrated by the reduction in EC50 values. The proposed mechanism for such a process is outlined in Fig. 7. It should also be noted that there were no significant differences in responses to Ca2+e between mesenteric arteries from SS and SS-Casrem1Mcwi animals (EC50 values were similar), indicating that changes in receptor function are the key factor. The current study only involved the use of animals on a low-salt diet, and a future direction would be to investigate whether CaSR plays a more significant role in Ca2+e-induced vasodilation when there is exposure to a high-salt diet by comparing responses in tissues from SS and SS-Casrem1Mcwi rats.
Fig. 7.
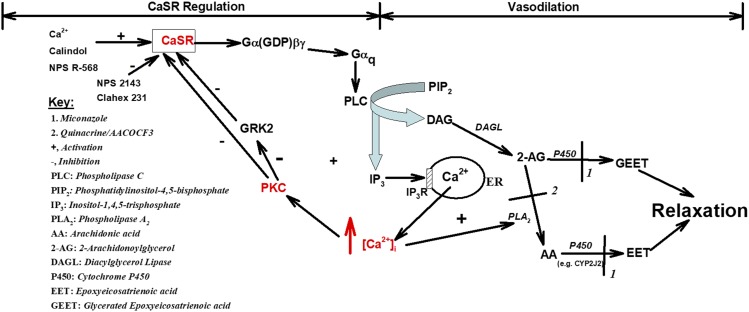
Proposed mechanism of CaSR-mediated signaling and mesenteric artery relaxation. Mechanism of CaSR-mediated vasodilation and its regulation by PKC. This signaling pathway uses published data from our laboratory (Awumey et al., 2008) and the present studies that focused on PKC and Ca2+i. Cytochrome P450 metabolites EET and GEET play a role in CaSR-mediated mesenteric artery relaxation.
In conclusion, our results confirm that PKCα plays an important regulatory role on CaSR signaling in the context of Ca2+-induced vasodilation. Reduction in PKCα activity along with increased exposure to Ca2+ could help counteract some of the reduction in vasodilation that occurs because of aging, suggesting a possible direction for combined diet and drug therapeutic studies for the management of hypertension and its related cardiovascular pathologies in aging.
Abbreviations
- ANOVA
analysis of variance
- CaSR
Ca2+-sensing receptor
- Ca2+e
extracellular Ca2+
- [Ca2+]e
extracellular Ca2+ concentration
- DMEM
Dulbecco’s modified Eagle’s medium
- EET
epoxyeicosatrienoic acid
- GEET
glycerated epoxyeicosatrienoic acid
- Gö 6976
5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile
- GRK
GPCR kinase
- PE
phenylephrine
- PKC
protein kinase C
- PSS
physiologic salt solution
- siRNA
small-interfering RNA
- SS
salt sensitive
Authorship Contributions
Participated in research design: Odutola, Awumey.
Performed data analysis: Odutola, Bridges, Awumey.
Wrote or contributed to the writing of the manuscript: Odutola, Awumey.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant SC1HL136279] and National Institute of Minority Health and Health Disparities [Grant 1U54MD012392].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Angus JA, Wright CE. (2000) Techniques to study the pharmacodynamics of isolated large and small blood vessels. J Pharmacol Toxicol Methods 44:395–407. [DOI] [PubMed] [Google Scholar]
- Awumey EM, Bridges LE, Williams CL, Diz DI. (2013) Nitric-oxide synthase knockout modulates Ca2+-sensing receptor expression and signaling in mouse mesenteric arteries. J Pharmacol Exp Ther 346:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awumey EM, Hill SK, Diz DI, Bukoski RD. (2008) Cytochrome P-450 metabolites of 2-arachidonoylglycerol play a role in Ca2+-induced relaxation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 294:H2363–H2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awumey EM, Howlett AC, Putney JW, Jr, Diz DI, Bukoski RD. (2007) Ca(2+) mobilization through dorsal root ganglion Ca(2+)-sensing receptor stably expressed in HEK293 cells. Am J Physiol Cell Physiol 292:C1895–C1905. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. (2008) Extracellular calcium as an integrator of tissue function. Int J Biochem Cell Biol 40:1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges LE, Williams CL, Pointer MA, Awumey EM. (2011) Mesenteric artery contraction and relaxation studies using automated wire myography. J Vis Exp (55 pii 3119 doi:):10.3791/3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81:239–297. [DOI] [PubMed] [Google Scholar]
- Bukoski RD. (1998) The perivascular sensory nerve Ca2+ receptor and blood pressure regulation: a hypothesis. Am J Hypertens 11:1117–1123. [DOI] [PubMed] [Google Scholar]
- Bukoski RD. (2001) Dietary Ca(2+) and blood pressure: evidence that Ca(2+)-sensing receptor activated, sensory nerve dilator activity couples changes in interstitial Ca(2+) with vascular tone. Nephrol Dial Transplant 16:218–221. [DOI] [PubMed] [Google Scholar]
- Bukoski RD, Bian K, Wang Y, Mupanomunda M. (1997) Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension 30:1431–1439. [DOI] [PubMed] [Google Scholar]
- Bukoski RD, Shearin S, Jackson WF, Pamarthi MF. (2001) Inhibition of Ca2+-induced relaxation by oxidized tungsten wires and paratungstate. J Pharmacol Exp Ther 299:343–350. [PubMed] [Google Scholar]
- Chen W, Bergsman JB, Wang X, Gilkey G, Pierpoint CR, Daniel EA, Awumey EM, Dauban P, Dodd RH, Ruat M, et al. (2010) Presynaptic external calcium signaling involves the calcium-sensing receptor in neocortical nerve terminals. PLoS One 5:e8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KL, Mulvany MJ. (1993) Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res 30:73–79. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Ward DT. (2013) Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 27:315–331. [DOI] [PubMed] [Google Scholar]
- Eley SL, Allen CM, Williams CL, Bukoski RD, Pointer MA. (2008) Action of thiazide on renal interstitial calcium. Am J Hypertens 21:814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry S, Traiffort E, Stinnakre J, Ruat M. (2000) Developmental and adult expression of rat calcium-sensing receptor transcripts in neurons and oligodendrocytes. Eur J Neurosci 12:872–884. [DOI] [PubMed] [Google Scholar]
- Halpern W, Mulvany MJ, Warshaw DM. (1978) Mechanical properties of smooth muscle cells in the walls of arterial resistance vessels. J Physiol 275:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan FM, Babinsky VN, Thakker RV. (2016) Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol 57:R127–R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan FM, Kallay E, Chang W, Brandi ML, Thakker RV. (2018) The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol 15:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DC, McCarron DA. (1994) Dietary calcium and blood pressure in experimental models of hypertension. A review. Hypertension 23:513–530. [DOI] [PubMed] [Google Scholar]
- Hurwitz S. (1996) Homeostatic control of plasma calcium concentration. Crit Rev Biochem Mol Biol 31:41–100. [DOI] [PubMed] [Google Scholar]
- Kantham L, Quinn SJ, Egbuna OI, Baxi K, Butters R, Pang JL, Pollak MR, Goltzman D, Brown EM. (2009) The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab 297:E915–E923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Kitazawa K. (2012) Size-dependent heterogeneity of contractile Ca2+ sensitization in rat arterial smooth muscle. J Physiol 590:5401–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. (2003) The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest 111:1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. (2001) Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J Biol Chem 276:1911–1915. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. (2005) Transduction of receptor signals by beta-arrestins. Science 308:512–517. [DOI] [PubMed] [Google Scholar]
- Lorenz S, Frenzel R, Paschke R, Breitwieser GE, Miedlich SU. (2007) Functional desensitization of the extracellular calcium-sensing receptor is regulated via distinct mechanisms: role of G protein-coupled receptor kinases, protein kinase C and β-arrestins. Endocrinology 148:2398–2404. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Wang J, Plouffe B, Smith JS, Yamani L, Kaur S, Jean-Charles PY, Gauthier C, Lee MH, Pani B, et al. (2018) Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal 11 (549 eaat 7650). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron DA, Reusser ME. (1999) Finding consensus in the dietary calcium-blood pressure debate. J Am Coll Nutr 18 (5 Suppl):398S–405S. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. (2002) Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283:H1662–H1672. [DOI] [PubMed] [Google Scholar]
- Mupanomunda MM, Ishioka N, Bukoski RD. (1999) Interstitial Ca2+ undergoes dynamic changes sufficient to stimulate nerve-dependent Ca2+-induced relaxation. Am J Physiol 276:H1035–H1042. [DOI] [PubMed] [Google Scholar]
- Mupanomunda MM, Tian B, Ishioka N, Bukoski RD. (2000) Renal interstitial Ca(2+). Am J Physiol Renal Physiol 278:F644–F649. [DOI] [PubMed] [Google Scholar]
- Mupanomunda MM, Wang Y, Bukoski RD. (1998) Effect of chronic sensory denervation on Ca(2+)-induced relaxation of isolated mesenteric resistance arteries. Am J Physiol 274:H1655–H1661. [DOI] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Votaw VS, Santana LF. (2005) Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA 102:11112–11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. (2002) G protein pathways. Science 296:1636–1639. [DOI] [PubMed] [Google Scholar]
- Ohanian V, Ohanian J, Shaw L, Scarth S, Parker PJ, Heagerty AM. (1996) Identification of protein kinase C isoforms in rat mesenteric small arteries and their possible role in agonist-induced contraction. Circ Res 78:806–812. [DOI] [PubMed] [Google Scholar]
- Palmer CE, Rudd MA, Bukoski RD. (2003) Renal interstitial Ca2+ during sodium loading of normotensive and Dahl-salt hypertensive rats. Am J Hypertens 16:771–776. [DOI] [PubMed] [Google Scholar]
- Penela P, Ribas C, Mayor F., Jr (2003) Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal 15:973–981. [DOI] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Liu J, Binet V, Goudet C, Rondard P, Prézeau L. (2005) Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J 272:2947–2955. [DOI] [PubMed] [Google Scholar]
- Potts LB, Ren Y, Lu G, Kuo E, Ngo E, Kuo L, Hein TW. (2012) Constriction of retinal arterioles to endothelin-1: requisite role of rho kinase independent of protein kinase C and L-type calcium channels. Invest Ophthalmol Vis Sci 53:2904–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, II, Donato AJ, Allen MR, Delp MD. (2007) Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res 22:1280–1288. [DOI] [PubMed] [Google Scholar]
- Ruat M, Molliver ME, Snowman AM, Snyder SH. (1995) Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci USA 92:3161–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepelmann M, Yarova PL, Lopez-Fernandez I, Davies TS, Brennan SC, Edwards PJ, Aggarwal A, Graça J, Rietdorf K, Matchkov V, et al. (2016) The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am J Physiol Cell Physiol 310:C193–C204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesay JS, Gyapong RN, Najafi LT, Kabler SL, Diz DI, Howlett AC, Awumey EM. (2015) Gαi/o-dependent Ca(2+) mobilization and Gαq-dependent PKCα regulation of Ca(2+)-sensing receptor-mediated responses in N18TG2 neuroblastoma cells. Neurochem Int 90:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba N, Stankevicius E, Garcia-Sacristán A, Simonsen U, Prieto D. (2007) Contribution of both Ca2+ entry and Ca2+ sensitization to the alpha1-adrenergic vasoconstriction of rat penile small arteries. Am J Physiol Heart Circ Physiol 292:H1157–H1169. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bukoski RD. (1998) Distribution of the perivascular nerve Ca2+ receptor in rat arteries. Br J Pharmacol 125:1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DT. (2004) Calcium receptor-mediated intracellular signalling. Cell Calcium 35:217–228. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, Xie L, Jin K. (2017) Age-related impairment of vascular structure and functions. Aging Dis 8:590–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha J, Dobešová Z, Vokurková M, Rauchová H, Hojná S, Kadlecová M, Behuliak M, Vaněčková I, Kuneš J. (2012) Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res 61 (Suppl 1):S35–S87. [DOI] [PubMed] [Google Scholar]