Significance
Optic neuritis, an inflammatory disease of the optic nerve, leads to death of retinal ganglion cells (RGC) and impaired vision. Both LXRα and LXRβ are expressed in the retina and optic nerve. The present study reveals that deletion of LXRβ from the mouse genome leads to loss of RGC. Immunostaining of LXRβ knockout mouse eyes showed that loss of AQP4 expression and activation of microglia in the optic nerve precedes the loss of RGC. The conclusion is that RGC loss is secondary to optic nerve degeneration and that LXRβ is a promising target for the treatment of retinal neurodegenerative diseases.
Keywords: retinal degeneration, optic neuritis, aquaporin 4, β amyloid, nuclear receptor
Abstract
The retina is an extension of the brain. Like the brain, neurodegeneration of the retina occurs with age and is the cause of several retinal diseases including optic neuritis, macular degeneration, and glaucoma. Liver X receptors (LXRs) are expressed in the brain where they play a key role in maintenance of cerebrospinal fluid and the health of dopaminergic neurons. Herein, we report that LXRs are expressed in the retina and optic nerve and that loss of LXRβ, but not LXRα, leads to loss of ganglion cells in the retina. In the retina of LXRβ−/− mice, there is an increase in amyloid A4 and deposition of beta-amyloid (Aβ) aggregates but no change in the level of apoptosis or autophagy in the ganglion cells and no activation of microglia or astrocytes. However, in the optic nerve there is a loss of aquaporin 4 (AQP4) in astrocytes and an increase in activation of microglia. Since loss of AQP4 and microglial activation in the optic nerve precedes the loss of ganglion cells, and accumulation of Aβ in the retina, the cause of the neuronal loss appears to be optic nerve degeneration. In patients with optic neuritis there are frequently AQP4 autoantibodies which block the function of AQP4. LXRβ−/− mouse is another model of optic neuritis in which AQP4 antibodies are not detectable, but AQP4 function is lost because of reduction in its expression.
Neuromyelitis optica is an inflammatory disease of the central nervous system (CNS) that selectively affects the optic nerves and spinal cord (1). This disorder is a well-known cause of optic neuritis, an inflammatory disease of the optic nerve, which leads to death of retinal ganglion cells (RGCs) and even severe visual loss (2). Aquaporin 4 (AQP4) water channel antibody (AQP4-IgG) is the biomarker of optic neuritis (3, 4). It is thought that autoantibodies against AQP4 inhibit the activity of this water channel. Thus, a functional AQP4 is essential for the maintenance of the function of the optic nerve.
Liver X receptor α (LXRα) and LXRβ are members of the nuclear receptor supergene family of ligand-activated transcription factors (5, 6). Both LXRs play crucial roles in lipid metabolism, glucose homeostasis, inflammation (7–9), CNS functions (10–12), and water transport (13, 14). LXRα−/−β−/− mice showed retinal vascular injury and diabetic retinopathy (15). LXRα and LXRβ have been detected in the RGCs and have been reported to protect against retinal degeneration (16–19). However, the functions of LXRs in the eye have not been fully investigated.
Beta-amyloid (Aβ), the protein which is deposited in the brain of patients with Alzheimer’s disease (AD), has been implicated in the development of optical disorders (20, 21). Indeed, retinal dysfunction such as loss of RGCs and reduced thickness of the retinal nerve fiber layer are observed in AD patients (22, 23). In addition, in mice overexpressing amyloid precursor protein (APP), the loss of LXRβ in the brain results in deposition of Aβ aggregates (24). Thus, it appears that one common dysfunction between AD and retinal degeneration is disruption of LXRβ signaling.
In the present study, we confirm that both LXRα and LXRβ are expressed in the retina and optic nerve and that loss of LXRβ leads to loss of ganglion cells and accumulation of Aβ in the retina. AQP4 expression decreased in the optic nerve, but no AQP4 antibodies were detected in the serum of LXRβ−/− mice. These data suggest that the cause of the neuronal loss in the retina of LXRβ−/− mice is due to optic nerve degeneration and that optic neuritis in these mice is caused by loss of AQP4 expression.
Results
Loss of Ganglion Cells in the Retina of LXRβ−/− Mice.
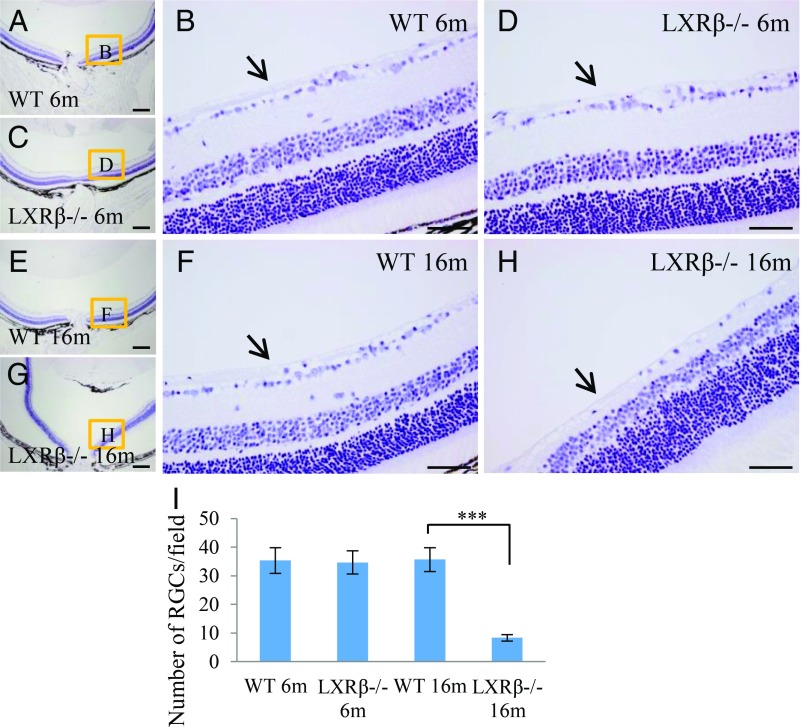
To evaluate morphological alterations in the retina of LXRβ−/− mice, sections of eyes were stained with hematoxylin. In 6-mo-old WT and LXRβ−/− mice ganglion cells are normal and the nerve fiber layer (arrow) is visible. There is no detectable difference between WT and LXRβ−/− mice at this age (Fig. 1 A–D). At 16 mo of age, ganglion cells are still normal and the nerve fiber layer is still visible in WT mice (Fig. 1 E and F), but in LXRβ−/− mice at this age, the number of ganglion cells is markedly reduced as is the nerve fiber layer (Fig. 1 G–I). The loss of ganglion cells from the retina in 16-mo-old LXRβ−/− mouse eye is very clear upon NeuN staining (SI Appendix, Fig. S1).
Fig. 1.
Loss of ganglion cells in LXRβ−/− mice. The ganglion cells appear normal in both 6-mo-old WT and LXRβ−/− mice, and the nerve layer (arrow) is visible in both (A–D). In 16-mo-old WT mouse eyes, the ganglion cells are still normal, and the nerve fiber layer is visible (E and F). In 16-mo-old LXRβ−/− mouse eyes, the number of ganglion cells is markedly reduced (***P < 0.001) as is the nerve fiber layer (G–I). n = 4. (Scale bars: A, C, E, and G, 200 μm; B, D, F, and H, 50 μm.)
Expression of LXRα and LXRβ in the Retina and Optic Nerve.
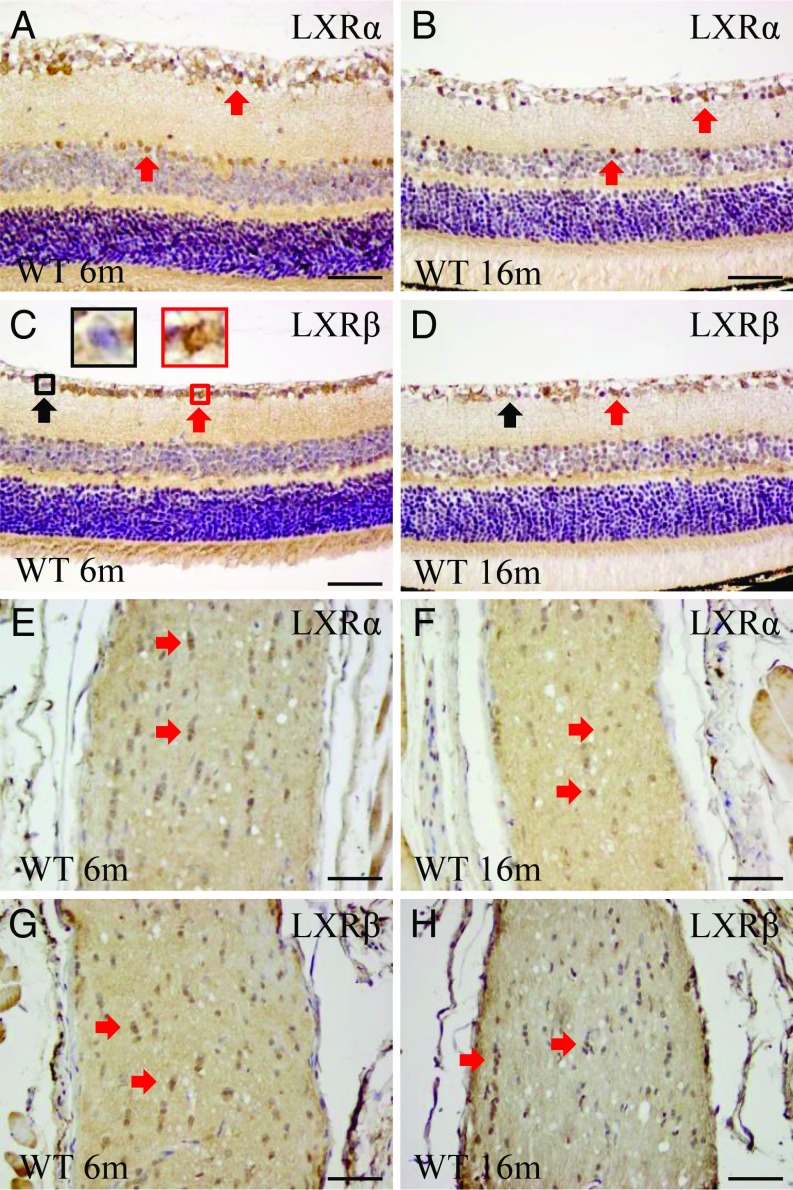
To detect expression of LXRs in the retina and optic nerve of WT mice, we used our in-house antibodies which have been well characterized for staining LXRs in the CNS (14, 23–25). We also used the commercially available LXR antibodies. We found that ganglion cells express both LXRα (cytoplasm) and LXRβ (nucleus). In the retina, LXRα is expressed in the ganglion and amacrine cells but not in bipolar cells (Fig. 2 A and B). The number of LXRα-positive amacrine cells is reduced with age. LXRβ is expressed in the ganglion cells, but there are fewer LXRβ-positive than LXRα-positive amacrine cells. In addition, some bipolar cells express LXRβ (Fig. 2 C and D). In the optic nerve, small glial cells express both LXRα and LXRβ (Fig. 2 E–H).
Fig. 2.
Expression of LXRα and LXRβ in the retina and optic nerve. In the retina, LXRα is expressed in the ganglion and amacrine cells. There is no expression in the bipolar cells (A and B). LXRβ is expressed in the ganglion cells but its expression in amacrine cells is more limited than LXRα. Some bipolar cells express LXRβ (C and D). Small cells in the optic nerve express both LXRα and LXRβ (E–H). n = 4. (Scale bars: A–H, 50 μm.)
Glial Cells in the Retina of LXRβ−/− Mice.
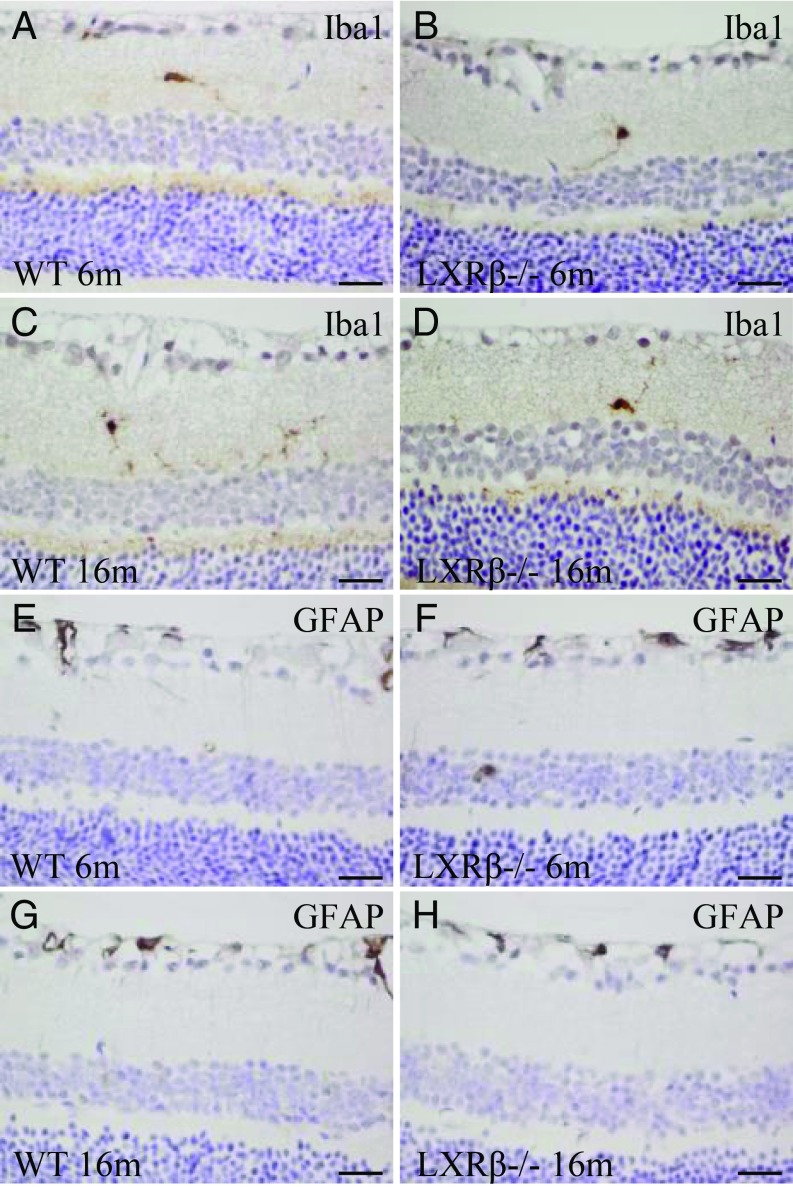
There were very few microglia (Iba1-positive cells) in the retina of 6-mo-old WT or LXRβ−/− mice (Fig. 3 A and B). The number and activation of microglia did not change with age (Fig. 3 C and D). GFAP (astrocyte marker) was expressed mainly in the nerve fiber layer and around the blood vessels. There was no detectable difference between WT and LXRβ−/− mice at either 6- or 16-mo of age mice in expression of GFAP-positive astrocytes in the retina (Fig. 3 E–H). There were no significant differences in the activation of glial cells or in inflammatory mediators including NF-κB, IL-1β, IL-6, and iNOS in the retina between WT and LXRβ−/− mice (SI Appendix, Fig. S2).
Fig. 3.
Microglia and astrocytes in the retina of LXRβ−/− mice. There were few microglial (Iba1-positive) cells in the retina of 6-mo-old WT or LXRβ−/− mice (A and B), and there was no increase in the number of these cells in16-mo-old mice of either genotype (C and D). At either 6 mo (E and F) or 16 mo (G and H) of age, there were few astrocytes in the retina, and there was no detectable difference between WT and LXRβ−/− mouse retinas. n = 4. (Scale bars: A–H, 20 μm.)
Accumulation of Aβ in the Retina of LXRβ−/− Mice.
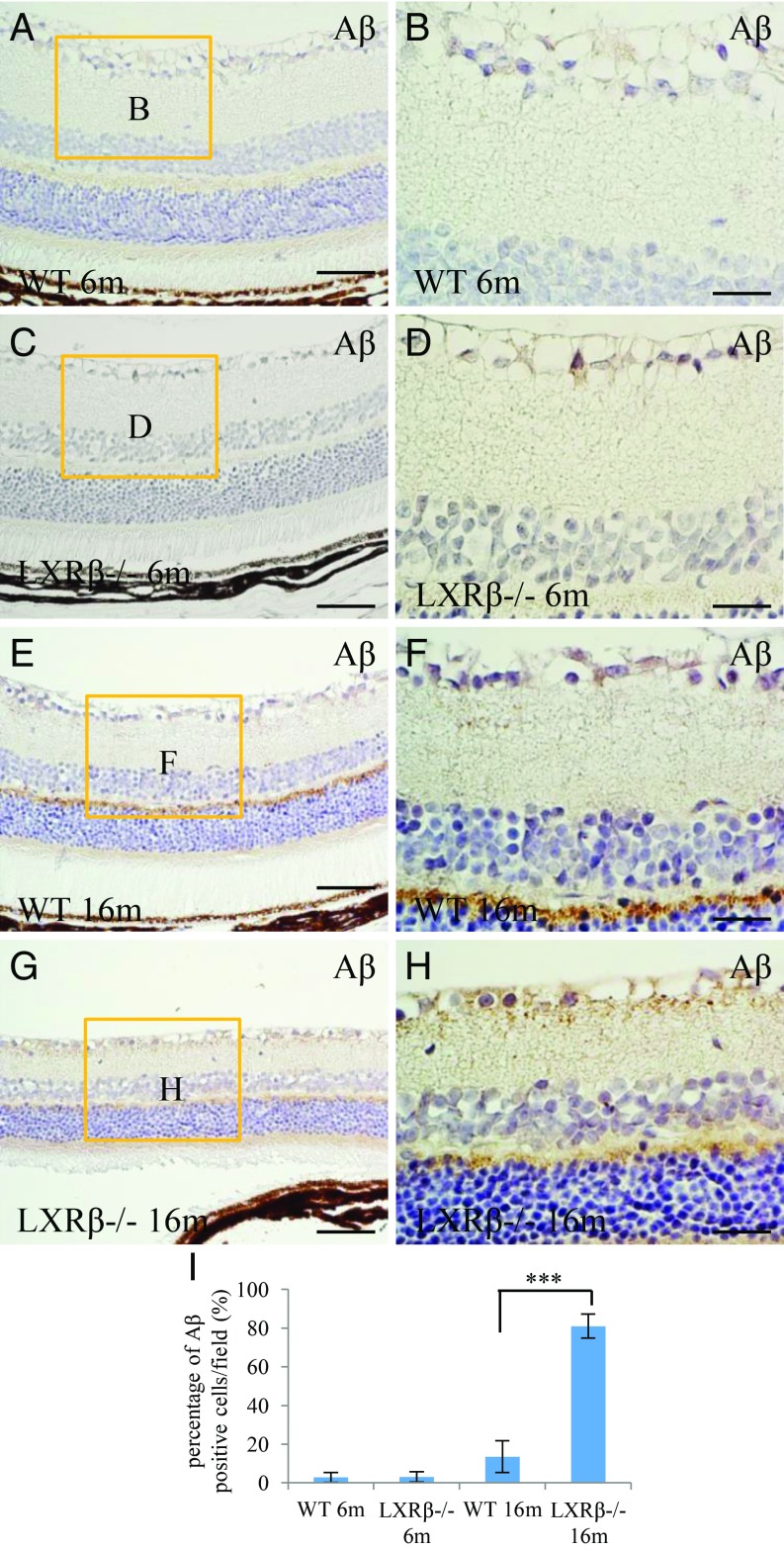
The deposition of Aβ in plaques is a definitive feature of AD, and Aβ has been reported to be implicated in the development of optical disorders. At 6 mo of age, there was no Aβ deposition in the retina of either WT or LXRβ−/− mice (Fig. 4 A–D). In the 16-mo-old WT mouse retina (Fig. 4E), there was Aβ deposition in the outer plexiform layer, but not around the RGCs. In the LXRβ−/− mouse retina (Fig. 4G), there was pronounced staining around the RGCs. Aβ deposits could be occasionally found in WT mice, but the deposits were smaller than those in LXRβ−/−mice (Fig. 4 E–I). There was also an up-regulation of amyloid A4 in LXRβ−/− mice. The accumulation of amyloid A4 increased with age as shown in SI Appendix, Fig. S3.
Fig. 4.
Accumulation of Aβ in the retina of LXRβ−/− mice. There were only occasional Aβ deposits in the retina of 6-mo-old WT or LXRβ−/− mice (A–D). However, in 16-mo-old mice there was a large amount of Aβ deposition in and around the ganglion cells in the retina of LXRβ−/− mice but very little in the WT mice at this age. (***P < 0.001) (E–I). n = 4. (Scale bars: A, C, E, and G, 50 μm; B, D, F, and H, 20 μm.)
Despite the presence of Aβ deposits, no changes in apoptosis (TUNEL-positive) or cleaved-caspase 3-positive RGCs (SI Appendix, Fig. S4) were detectable, and there was no change in autophagy (LC3B and Beclin 1 staining) (SI Appendix, Fig. S5).
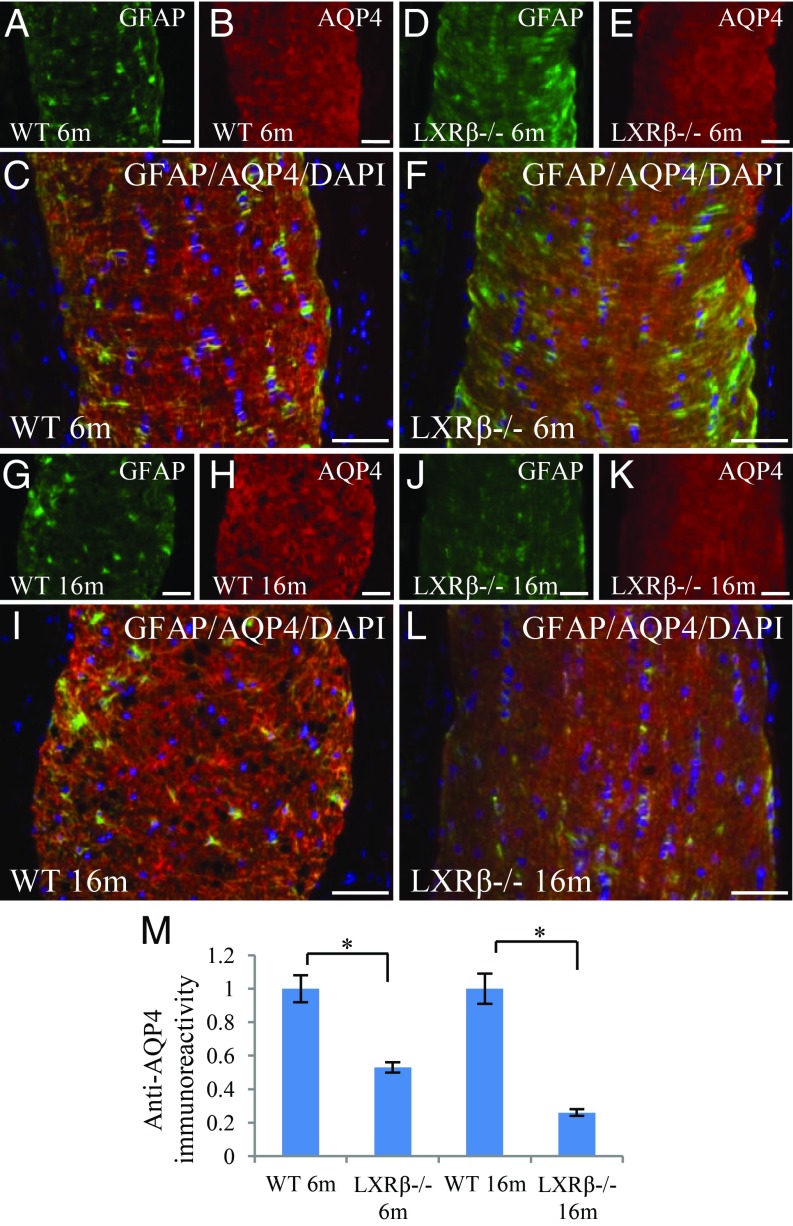
Loss of AQP4 in Astrocytes in the Optic Nerve of LXRβ−/− Mice.
Optic neuritis is characterized by the presence of antibodies to the water channel AQP4. AQP4 expression was abundant at the end feet and branches of astrocytes (GFAP-positive) in the optic nerve of 6-mo-old WT mice (Fig. 5 A–C). However, in 6-mo-old LXRβ−/− mice, although there was no significant change in the expression of GFAP, there was a marked decrease in AQP4 expression (Fig. 5 D–F). AQP4 was strongly expressed in the optic nerve of 16-mo-old WT mice, where it was colocalized with GFAP in astrocytes (Fig. 5 G–I). However, immunoreactivity of both AQP4 and GFAP was markedly reduced in the optic nerve of 16-mo-old LXRβ−/− mice (Fig. 5 J–M).
Fig. 5.
AQP4 in astrocytes in the optic nerve. In 6-mo-old WT mice, GFAP (green) and AQP4 (red) were strongly expressed at the feet and branches of astrocytes in the optic nerve (A–C). AQP4 expression was lower in the optic nerve of 6-mo-old LXRβ−/− mice (D–F). In 16-mo-old mice, GFAP and AQP4 were still strongly expressed in the optic nerve of WT mice (G–I), but their expression was reduced in the optic nerve of LXRβ−/− mice (*P < 0.05) (J–M). GFAP (green) was colocalized with AQP4 (red); the colocalized color is orange. The nuclei are counterstained with DAPI (C, F, I, and L). n = 4. (Scale bars: A–L, 50 μm.)
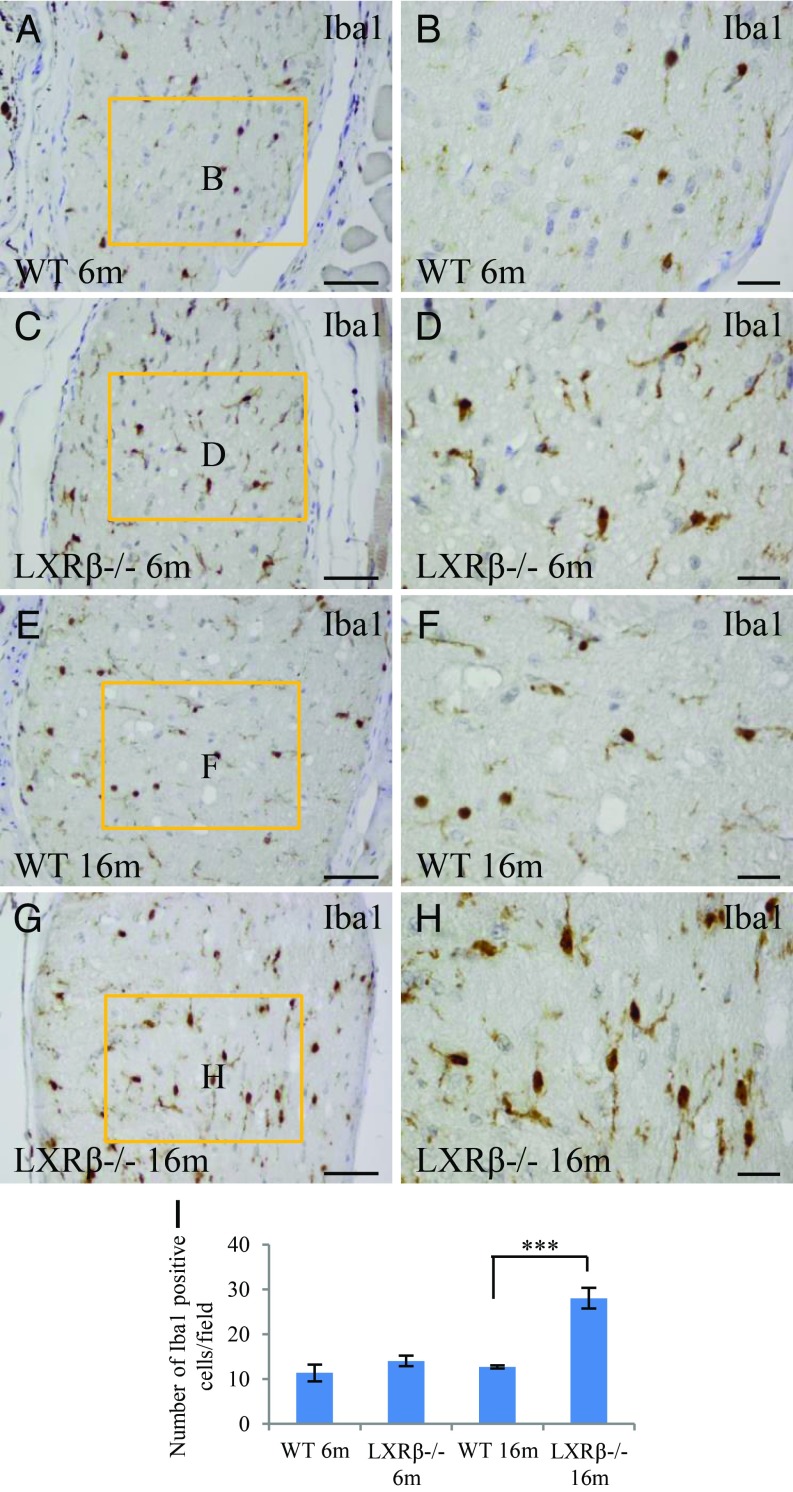
Increased Activation of Microglia in the Optic Nerve of LXRβ−/− Mice.
Resting microglia are small cells with long and thin ramified processes, while activated microglia are characterized by larger cell bodies and poorly ramified, short, and thick processes. Based on morphology, the few microglia detected in the retina of 6- and 16-mo-old WT mice were resting microglia (Fig. 6 A, B, E, and F). However, in the optic nerve of 6- and 16-mo-old LXRβ−/− mice, there were more microglia and these were activated (Fig. 6 C, D, G, and H).
Fig. 6.
Increased activation of microglia in the optic nerve of LXRβ−/− mice. There were Iba1-positive resting microglial cells in the optic nerve of 6-mo-old WT mice (A and B). The Ibal-1–positive microglial cells in the optic nerve of 6-mo-old LXRβ−/− mice are ameboid in shape, indicating that they are activated (C and D). In 16-mo-old mice, the number of active Iba1-positive microglial cells was higher in LXRβ−/− than in WT mice (***P < 0.001) (E–I). n = 4. (Scale bars: A, C, E, and G, 50 μm; B, D, F, and H, 20 μm.)
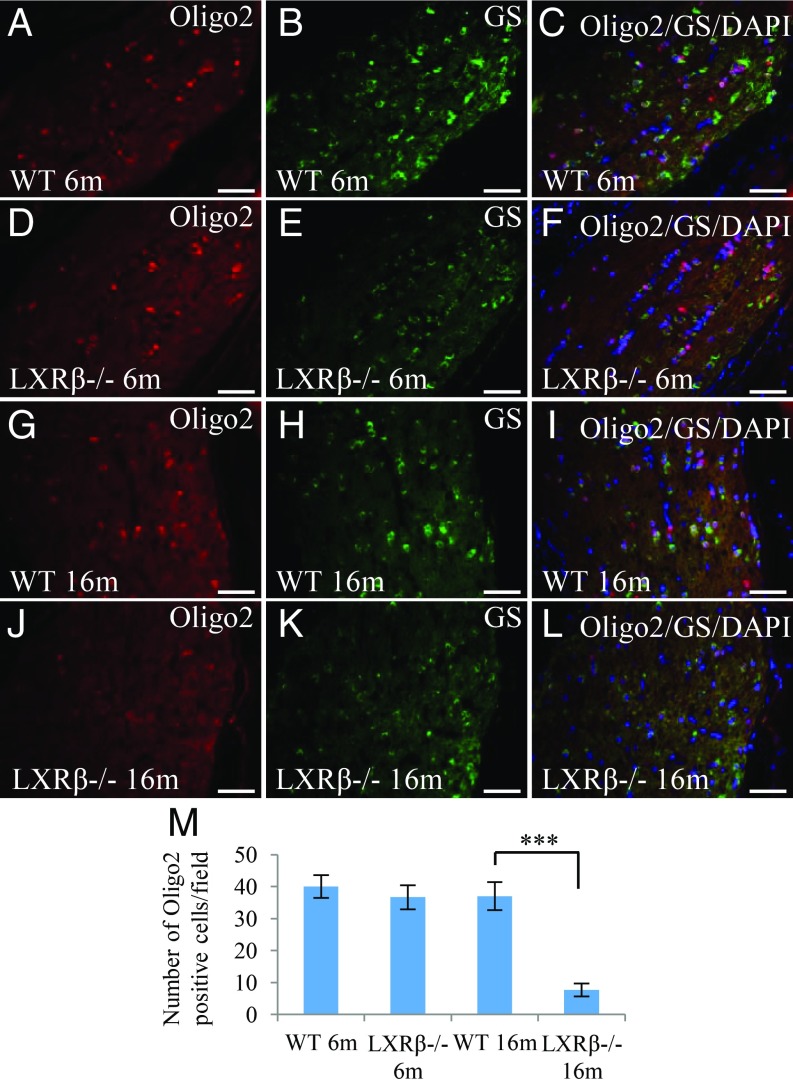
Loss of Oligodendrocytes in the Optic Nerve of LXRβ−/− Mice.
At 6 mo of age, there were numerous oligodendrocytes (Oligo2-positive) in the optic nerve of both WT and LXRβ−/− mice (Fig. 7 A and D). However, in 6-mo-old LXRβ−/− mice staining for glutamine synthetase (GS), a marker for functional oligodendrocytes, was lower than that in WT mice indicating dysfunction of oligodendrocyte maturation in the optic nerve (Fig. 7 A–F). Oligodendrocyte number as well as oligodendrocyte impairment increased with age, as indicated by the reduction in Oligo2 and GS staining in 16-mo-old LXRβ−/− mice (Fig. 7 G–M).
Fig. 7.
Loss of oligodendrocytes in the optic nerve of LXRβ−/− mice. There were Oligo2-positive (red) cells and GS-positive (green) oligodendrocyte cells in the optic nerve of 6-mo-old WT mice (A–C). Although there was no difference in the number of Oligo2-positive cells in the optic nerve of 6-mo-old LXRβ−/− mice, there were fewer GS-positive oligodendrocytes (D–F). In 16-mo-old mice, there were fewer Oligo2-positive and GS-positive oligodendrocytes (***P < 0.001) in the optic nerve of 16-mo-old LXRβ−/− mice (G–M). n = 4. (Scale bars: A–L, 50 μm.)
Discussion
In the present study, we found that there is inflammation of the optic nerve and loss of ganglion cells from the retina with age in LXRβ−/− mice. This was accompanied by deposition of Aβ in the retina, increase in activated microglia, and decrease in oligodendrocytes and glutamine synthetase in the optic nerve. No AQP4 antibodies were detected in the LXRβ−/− mice, but there was loss of AQP4 expression in the optic nerve.
In brain, both LXRα and LXRβ are expressed in the nuclei of glial cells of both cortex and substantia nigra (11), but not in neurons. Neurons in the paraventricular nucleus (PVN) express both LXRβ and LXRα. In addition, LXRβ expression in PVN is higher than that of LXRα. In retina, ganglion cells express both LXRα and LXRβ, and LXRα is expressed in the cytoplasm. Cytosolic expression of nuclear receptors is not unusual. It could indicate a lack of a specific ligand or the inability of the receptor to be transported into the nucleus or the exit of the receptor from the nucleus. This is a matter that is presently under investigation.
Death by apoptosis causes loss of ganglion cells (18). No increase in the level of apoptosis was detected in the present study, but, since apoptotic cells are rapidly cleared, it cannot be ruled out as a cause of death in the ganglion cells of LXRβ−/− mice as only two time points were examined: 6 and 16 mo of age.
Aβ is a pathogenic peptide associated with AD. Increasing evidence suggests that there are similarities between AD and retinal degeneration. It has been demonstrated that Aβ-related amyloidosis also occurs during several ocular diseases including Alzheimer’s-related optic neuropathy, glaucoma, and age-related macular degeneration (25–27). As other researchers have pointed out, understanding optic neuropathy is the key to broadly understanding the pathogenesis of neurodegenerative disorders and ultimately to developing outcome measures to evaluate therapies (28). Genetic loss of either LXRα or LXRβ in APP/PS1 transgenic mice results in increased amyloid plaque load (24), and activation of LXR with an agonist protects against retinal damage by inhibiting Aβ accumulation (18, 21). In the present study, we have shown that Aβ is a likely mediator of ganglion cell death and that it could be a potential target for therapeutic intervention in both the eye and the brain. These results provide a general mechanism for the role of LXR efficacy in chronic diseases such as AD and retinal degeneration.
Optic neuritis is a type of retinal degeneration initiated by degeneration of the optic nerve. It is an autoimmune inflammatory disease characterized by the presence of antibodies to AQP4. Both the optic nerve and spinal cord are affected. AQP4 IgG binds to AQP4 on the surface of astrocytes and disrupts its polarized expression, causing cellular cytotoxicity and inflammation (1). AQP4 is expressed in astrocytes throughout the CNS, including brain, spinal cord, and optic nerve. In the brain, AQP4 is expressed on perivascular end feet of astrocytes at the border between the brain parenchyma and the cerebrospinal fluid or blood (29). We have reported that in LXRα−/−β−/− mice AQP4 expression is increased in white matter surrounding the lateral ventricles (14). Expression of AQP4 regulates substances moving in and out of the brain (30).
AQP4 is expressed in Müller cells in the retina and in fibrous astrocytes in the optic nerve (31, 32). In LXRβ−/− mice, no AQP4 antibodies were detected, but there was loss of AQP4 expression in the optic nerve. We conclude that, in addition to the presence of AQP4 antibodies, which affect the function of AQP4, optic neuritis can be caused by loss of expression of AQP4. This year Saadane et al. (33) showed that inactivation of CYP46, which is responsible for the synthesis of 24-hydroxy cholesterol, caused a retinal phenotype typical of diabetic retinopathy. In that study LXR expression was higher than in WT mice, and expression of LXR-induced genes was normal. However, LXR-repressed genes (proinflammatory genes) were overexpressed. AQP4 was not down-regulated but was phosphorylated. It is not yet clear whether phosphorylation of AQP4 inactivates it as it does other aquaporins (34).
Inflammation is associated with many neurodegenerative diseases, including AD, amyotrophic lateral sclerosis, multiple sclerosis, and retinal neurodegeneration (35, 36). Microglia play a key role in neuroinflammation. LXR agonists exhibit an antiinflammatory effect by inhibiting activation of microglia and astrocytes in the CNS (11, 37, 38). LXR modulates retinal disorders partially through inhibition of inflammation (15, 16, 18, 27). In the present study, although there was no significant change in the activation of glial cells or in the expression of inflammatory mediators in the retina, we found a significant increase in the number of activated microglia in the optic nerve of LXRβ−/− mice. Optic nerve inflammation contributes to optic nerve degeneration. GS catalyzes the condensation of glutamate and ammonia to form glutamine (39). GS immunoreactivity in oligodendrocytes has been previously demonstrated in the optic nerve and optic chiasm (40, 41). GS is involved in the function of oligodendrocytes in rat brain, optic nerve, and retina (42). In LXRβ−/− mice, there was decreased expression of Oligo2 and GS in the optic nerve, indicating that there is optic nerve degeneration of LXRβ−/− mice.
In this study, global knockout mice were used, and developmental effects cannot be ruled out. The use of cell-type–specific and inducible knockout will facilitate the identification of the cells in the retina, the functions of which are changed by loss of LXRβ, and the understanding of how LXRβ exerts a protective effect. To this end we have created mice in which loxP sites have been inserted around the LXRβ gene, and these mice will permit cell-specific knockout of LXR.
In summary, we have shown that loss of LXRβ−/− in mice results in loss of RGCs in the retina with age. From the temporal changes occurring in the eye, it appears to be the increase in inflammation and decrease in AQP4 expression in the optic nerve. Our study highlights the protective effect of LXRβ in the eye. Therefore, LXRβ is a potential target for the treatment of retinal neurodegenerative diseases. Further characterization of the role of LXRβ in the eye could lead to new insights into the etiology and treatment of some retinal disorders.
Materials and Methods
Animals.
Female C57BL/6 WT and LXRβ−/− mice were used for experiments. LXRβ−/− mice on the C57BL/6 genetic background have been described (43). Mice were housed in a room of standard temperature (22 ± 1 °C) with a regular 12-h light, 12-h dark cycle and given free access to water and standard rodent chow. All animal experiments were carried out according to the standards and recommendations set forth in the National Research Council’s 1996 Guide for the Care and Use of Laboratory Animals (44) and in accordance with the University of Houston guidelines on the use of laboratory animals.
Immunohistochemistry.
Paraffin-embedded sections were deparaffinized in xylene, rehydrated through grade alcohol, and processed for antigen retrieval in 10 mM citrate buffer (pH 6.0) for 12–15 min at 97 °C with pretreatment module. Sections were incubated in 0.3% H2O2 for 30 min at room temperature to quench endogenous peroxidase. To block nonspecific binding, sections were incubated in 3% BSA for 30 min, and then a biotin blocking system (Dako) was used to block endogenous biotin. Sections were then incubated overnight with antibodies specific for LXRα and LXRβ (1:1,000; made in our laboratory), LXRα (1:500; Novus), LXRβ (1:500; Abcam), LXRα/β (1:200; Life Span Biosciences), Iba1 (1:1,000; Abcam), GFAP (1:1,000; Santa Cruz Biotechnology), caspase3 (1:100; Zymed Laboratories), LC3B (1:600; Abcam), Beclin1 (1:500; Abcam), NF-κB (1; 200; Abcam), IL-1β (1:100; Abcam), IL-6 (1:100; Santa Cruz Biotechnology), iNOS (1:100; Abcam), Amyloid A4 (1:40; Santa Cruz Biotechnology), and Aβ (1:40; Immuno-Biological Laboratories). Primary antibodies were replaced with BSA as negative controls. Sections were incubated with biotinylated secondary antibody (1:200) for 1 h at room temperature, and then the Vectastain Avidin-Biotin Complex kit (Vector Laboratories) was used according to the manufacturer’s instruction, followed by 3,3-diaminobenzidine staining (Thermo Fisher Scientific) and counterstaining with Mayer’s hematoxylin (Sigma-Aldrich).
For immunofluorescence, quenching of endogenous peroxidase and blocking of endogenous biotin were omitted. Sections were incubated with anti-Oligo2, anti-GS, anti-GFAP, anti-AQP4, anti-TUNEL, and anti-NeuN. Primary antibodies were detected with fluorescent secondary antibodies. Sections were later counterstained with Vectashield mounting medium containing DAPI (Vector Laboratories) to label nuclei.
Data Analysis.
Data are expressed as mean ± SD. Statistical comparisons were done by using a one-way ANOVA followed by the Newman–Keuls post hoc test. P < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
This study was supported by the Brockman Foundation, Nova Nordisk Foundation, and the China Postdoctoral Science Foundation Grant 2018M643195 (to X.-y.S.). J.-Å.G. thanks the Robert A. Welch Foundation for a fellowship (E-0004).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904719116/-/DCSupplemental.
References
- 1.Levin M. H., Bennett J. L., Verkman A. S., Optic neuritis in neuromyelitis optica. Prog. Retin. Eye Res. 36, 159–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toosy A. T., Mason D. F., Miller D. H., Optic neuritis. Lancet Neurol. 13, 83–99 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Lennon V. A., et al. , A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R., IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apfel R., et al. , A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 14, 7025–7035 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teboul M., et al. , OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc. Natl. Acad. Sci. U.S.A. 92, 2096–2100 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutemberezi V., Guillemot-Legris O., Muccioli G. G., Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 64, 152–169 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Courtney R., Landreth G. E., LXR regulation of brain cholesterol: From development to disease. Trends Endocrinol. Metab. 27, 404–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maqdasy S., et al. , Once and for all, LXRα and LXRβ are gatekeepers of the endocrine system. Mol. Aspects Med. 49, 31–46 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Andersson S., Gustafsson N., Warner M., Gustafsson J. A., Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc. Natl. Acad. Sci. U.S.A. 102, 3857–3862 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai Y. B., Tan X. J., Wu W. F., Warner M., Gustafsson J. A., Liver X receptor β protects dopaminergic neurons in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 109, 13112–13117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y., et al. , Liver X receptor β regulates the development of the dentate gyrus and autistic-like behavior in the mouse. Proc. Natl. Acad. Sci. U.S.A. 115, E2725–E2733 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbi C., et al. , Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor β. Proc. Natl. Acad. Sci. U.S.A. 109, 3030–3034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y. B., et al. , Liver X receptors regulate cerebrospinal fluid production. Mol. Psychiatry 21, 844–856 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Hazra S., et al. , Liver X receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes. Diabetes 61, 3270–3279 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H., et al. , Activation of liver X receptor alleviates ocular inflammation in experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 55, 2795–2804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng W., et al. , Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One 7, e37926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng S., et al. , Activation of liver X receptor protects inner retinal damage induced by N-methyl-D-aspartate. Invest. Ophthalmol. Vis. Sci. 56, 1168–1180 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Hammer S. S., et al. , The mechanism of diabetic retinopathy pathogenesis unifying key lipid regulators, sirtuin 1 and liver X receptor. EBioMedicine 22, 181–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu K., et al. , Neurodegeneration of the retina in mouse models of Alzheimer’s disease: What can we learn from the retina? Age (Dordr.) 34, 633–649 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L., et al. , Targeting amyloid-beta in glaucoma treatment. Proc. Natl. Acad. Sci. U.S.A. 104, 13444–13449 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao Y., et al. , Visualization of focal thinning of the ganglion cell-inner plexiform layer in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 64, 1261–1273 (2018). [DOI] [PubMed] [Google Scholar]
- 23.La Morgia C., et al. , Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 79, 90–109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelcer N., et al. , Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proc. Natl. Acad. Sci. U.S.A. 104, 10601–10606 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinnon S. J., et al. , Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest. Ophthalmol. Vis. Sci. 43, 1077–1087 (2002). [PubMed] [Google Scholar]
- 26.Masuzzo A., Dinet V., Cavanagh C., Mascarelli F., Krantic S., Amyloidosis in retinal neurodegenerative diseases. Front. Neurol. 7, 127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei C., et al. , Amelioration of amyloid β-induced retinal inflammatory responses by a LXR agonist TO901317 is associated with inhibition of the NF-κB signaling and NLRP3 inflammasome. Neuroscience 360, 48–60 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Carelli V., La Morgia C., Ross-Cisneros F. N., Sadun A. A., Optic neuropathies: The tip of the neurodegeneration iceberg. Hum. Mol. Genet. 26, R139–R150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen S., et al. , Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 17, 171–180 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestre H., et al. , Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 7, e40070 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Patil R. V., Verkman A. S., Mildly abnormal retinal function in transgenic mice without Müller cell aquaporin-4 water channels. Invest. Ophthalmol. Vis. Sci. 43, 573–579 (2002). [PubMed] [Google Scholar]
- 32.Nagelhus E. A., et al. , Aquaporin-4 water channel protein in the rat retina and optic nerve: Polarized expression in Müller cells and fibrous astrocytes. J. Neurosci. 18, 2506–2519 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saadane A., et al. , Retinal vascular abnormalities and microglia activation in mice with deficiency in cytochrome P450 46a1-mediated cholesterol removal. Am. J. Pathol. 189, 405–425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche J. V., et al. , Phosphorylation of human aquaporin 2 (AQP2) allosterically controls its interaction with the lysosomal trafficking protein LIP5. J. Biol. Chem. 292, 14636–14648 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H., Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez A. I., et al. , The role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and glaucoma. Front. Aging Neurosci. 9, 214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang-Gandhi C. X., Drew P. D., Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 183, 50–59 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malek G., Lad E. M., Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 71, 4617–4636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bringmann A., et al. , Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem. Int. 54, 143–160 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Tansey F. A., Farooq M., Cammer W., Glutamine synthetase in oligodendrocytes and astrocytes: New biochemical and immunocytochemical evidence. J. Neurochem. 56, 266–272 (1991). [DOI] [PubMed] [Google Scholar]
- 41.Kawano J., Chemoarchitecture of glial fibrillary acidic protein (GFAP) and glutamine synthetase in the rat optic nerve: An immunohistochemical study. Okajimas Folia Anat. Jpn. 92, 11–30 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Rai N. K., et al. , Exposure to As, Cd and Pb-mixture impairs myelin and axon development in rat brain, optic nerve and retina. Toxicol. Appl. Pharmacol. 273, 242–258 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Alberti S., et al. , Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J. Clin. Invest. 107, 565–573 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.