Abstract
Background
Nisin, an effective natural food preservative, is an antimicrobial peptide produced by Lactococcus lactis. Although it has been mainly studied and developed as a potential alternative for antibiotics, other pharmacological effects of the nisin including cytotoxic and anti-tumor activity have been attracted many attentions.
Objectives
Here, we aimed to evaluate in vitro cytotoxic activity of the nisin against breast cancer cells.
Materials and Methods
The effect of temperature, pH, and chemical composition of the medium on the yield of nisin production was evaluated. As well, the anti-proliferative effect of nisin against a breast cancer cell line (i. e., MCF-7) and a non-cancerous cell line (i.e, HUVEC) was determined using MTT assay. Furthermore, the potential of the synergistic effect of the nisin on the doxorubicin cytotoxicity was evaluated.
Results
The optimum culture condition for the nisin production by L. lactis was found to be MRS medium (pH 6.3) supplemented with the tryptone and incubation at 30 °C. MTT assay results indicate that nisin exhibits a high and selective cytotoxicity against MCF-7 cell line with IC50 value of 5 μM. Furthermore, a combination of nisin and doxorubicin at sub-inhibitory concentrations were more cytotoxic compared to either of drugs alone.
Conclusion
It could be suggested that nisin, either alone or in combination with other chemotherapeutic agents, could be a potential therapeutic for the breast cancer cells.
Keywords: Antimicrobial peptide; Breast cancer; Cytotoxicity; Nisin, Lactococcus lactis
1. Background
Breast cancer is the most common cancer among women worldwide. Approximately, 12% of U.S. women will develop invasive breast cancer during their lifetime. Breast cancer is the second leading cause of death of cancer in women after lung cancer (1). Different therapeutic strategies, including surgery, radiotherapy, chemotherapy, hormone therapy, and biological therapy are currently used for the treatment of breast cancer (2). However, the discovery and development of novel alternative or synergistic anticancer agents are still required to improve the efficacy and reduction of the side effects. A large number of potential anticancer agents are under examination in preclinical and clinical studies (3), among them anticancer agents derived from the natural sources such as plants or bacteria have gained an increasing attention (4, 5).
Bacteriocins are bacterial proteinaceous toxins, usually peptides, with antimicrobial activity against other closely-related bacterial strains (6). Nowadays, bacteriocins are used in the food industry (as preservatives) (7), agriculture (as inhibitors of the plant and animal pathogens) (8) and medicine (9). Among bacteriocins, nisin is the only one approved by FDA as “Generally Regarded as Safe” (GRAS) compound for food applications (10). Nisin is a polycyclic peptide composed of 34 amino acids, which is produced by Lactococcus lactis (11). For industrial and large-scale applications, the chemical synthesis of this peptide is not cost-effective and nisin is mostly produced by the L. lactis using cheap and natural cultivation substrates (12). However, the high yield of bacteriocins production usually needs an optimized culture condition (e.g., the chemical composition of the media and physical parameters such as temperature and pH) (13).
Nisin has different pharmacological effects including antimicrobial/biofilm, immuno-modulatory, and anticancer. Unlike other bacteriocins, nisin has effective antibacterial activity against a broad spectrum of Gram-positive and Gram-negative bacteria (14). However, nisin exhibited lower antibacterial activity against certain Gram-negative bacteria (15). Because of its unique antimicrobial properties, it can inhibit the growth of drug-resistant bacterial strains and biofilm formation (16). Furthermore, as a cationic and amphiphilic peptide, nisin shares similar physicochemical properties with host defense peptides which regulate innate immune response (17). Both in vitro and in vivo studies have shown that nisin can modulate innate immune system by inducing secretion of the chemokines and suppressing lipopolysaccharide stimulated cytokines (18). The cytotoxic and antitumor properties of nisin were explored in head and neck squamous cell carcinoma (HNSCC) in vitro and in vivo (19). Recently, Preet et al. have reported the synergistic effect of the combination of nisin and doxorubicin against murine skin carcinogenesis (20).
Other group have evaluated the cytotoxic effect of the nisin on the intestinal epithelial cells in vitro and have reported a mild cytotoxic effect (IC50 = 90 μM) (21). Anti-proliferative and apoptotic effect of nisin on colon cancer cells (SW480) has been recently evaluated (22). Ahmadi et al. assessed the mechanism of the apoptotic effect of nisin on the colon adenocarcinoma cell line by real-Time PCR and western blotting method. They found that nisin provokes intrinsic pathway of apoptosis and increases the bax/bcl-2 ratio in the cancer cells (22).
The evidence from these studies suggests that nisin may be a good potential anti-cancer agent. However, to the best of our Knowledge, no study has the evaluated cytotoxic effect of the combination of nisin and doxorubicin against breast cancer cells.
2. Objectives
Considering a large number of nisin potentials for therapeutic applications, various strategies for the production of this peptide at suitable amounts and low costs were proposed. Here, we studied the effects of some physical parameters (temperature and pH) and chemical composition of MRS medium on the yield of the nisin production by L. lactis. Furthermore, the present study has evaluated the potential anticancer activity of nisin against a breast cancer cell line (MCF7) and a non-cancerous cell line (HUVEC).
3. Materials and Methods
3.1. Chemicals
The chemicals used in cell culture assays were obtained from Gibco (Scotland) via local vendors. Other chemicals and reagents used in this work were of analytical grade and were purchased from Merck (Darmstadt, Germany).
3.2. Microorganisms
Nisin producer microorganism, L. lactis subsp. Lactis (PTCC 1336) and Escherichia coli (PTCC 1330), as an indicator microorganism, were obtained from the Persian Type Culture Collection, Iran, Tehran. L. lactis and E. coli were subcultured on trypticase soy yeast extract and nutrient agar plates and were incubated at 30 and 37 °C, respectively.
3.3. Optimization of the Medium, pH, and Temperature for Nisin Production
A single colony of L. lactis was inoculated into 5 mL of de Man, Rogosa and Sharpe (MRS) medium and incubated at 37 °C and 100 rpm for 16 h. This sample was subcultured twice on the same medium to prepare the pre-culture. The pre-culture was used to inoculate (10% v⁄v) 10 mL of the fresh medium and incubated for 48 h. The next day, 5 mL of the fresh medium was added to each culture and incubated overnight. For optimization of nisin production, cultivations were performed under different pH (3.85, 4.7, 5.65, 6.3 or 7.5), temperatures (25, 30 or 37 °C) and different supplements (triptone, casein, yeast extract, lactose, sucrose or Tween 80) were added to the MRS medium. At the final cultivation time, cell density (OD600) and pH of each sample were measured.
3.4. Recovery of Nisin by pH-Mediated Cell Adsorption-Desorption
The samples were acidified with HCl to pH 2, following to boiling for 10 min to bring nisin into the aqueous phase. Then, each sample was centrifuged at 7,500 ×g for 10 min and the supernatant the cell-free supernatant (CFS) was collected. To prepare inactivated CSF (iCSF), after extraction of nisin by setting pH to 2, the pH was adjusted to 11, following to heating at 63 °C for 30 min. This alkaline heating led to rapid inactivation of nisin. The pH of CFS and iCSF were adjusted to 6 and then the supernatants were filter-sterilized through a 0.22 μm membrane filter.
3.5. Indirect Determination of the Nisin Concentration by Antimicrobial Activity
The agar diffusion bioassay is a highly sensitive (less than 0.0125 μg.mL-1), simple and cost-effective method widely used for the quantification of nisin (23). The titers of the produced nisin in the culture broth were measured indirectly by disc diffusion method (24). Briefly, sterile paper discs (6 mm diameter) were placed on the Mueller-Hinton agar (MHA) plates inoculated with E. coli. Fifty μL of the standard nisin (Sigma, USA), CFS or iCSF (negative control) were added to the paper discs and incubated overnight at 37 °C. The same solvent (MRS) was used to dissolve the powder of standard nisin and to dilute the known (standard nisin) and unknown samples (CFS). The next day, the diameter of inhibition zone was measured to the nearest 0.1 mm by the means of a ruler. A calibration curve was plotted based on the relationship between standard nisin concentration and zone of inhibition diameter. The concentrations of nisin in the CSFs were calculated from a linear equation of the calibration curve.
3.6. Tricine-SDS-PAGE
CFSs were analyzed by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tricine-SDS-PAGE) as described previously (25). For sample preparation, 5 μL of a 5X gel loading buffer (0.25 M tris-HCl, pH 6.8, 5 % glycerol, 5 % 2-mercaptoethanol, 3 % SDS, and 0.2 mg.mL-1 bromophenol blue) was added to 20 μL of CSF and were heated to 95 °C for 5 min. Samples were then loaded on a tricine gel consisting of a stacking gel containing 4% acrylamide, a spacer gel containing 10% acrylamide, and a separation gel containing 16.5% acrylamide. The gel was run at 80 V for 3 h and stained with Coomassie Blue R-250. The concentration of nisin in the sample was also measured by densitometry analysis of acrylamide gels using TL120 software (Nonlinear Inc, Durham NC, USA). Bovine serum albumin samples with known concentrations were used as standards to estimate nisin concentration on gels.
3.7. Cell Culture
The human breast carcinoma cell line MCF-7 and human umbilical vein endothelial cells (HUVEC) were obtained from the Pasture Institute of Iran, Tehran. Cells were grown in the Roswell Park Memorial Institute (RPMI) 1,640 medium supplemented with 10 % (v/v) fetal bovine serum (FBS), 100 IU.mL-1 penicillin and 100 μg.mL-1 streptomycin in a humidified atmosphere containing 5 % CO2 at 37 °C.
3.8. Cytotoxicity Assays
The cytotoxic effect of nisin was evaluated against MCF-7 (a cancerous cell) and HUVEC (a normal cell) cells by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay as described previously (26). In brief, log-phase cells were seeded (1 × 104/well) in 96-well cell culture plates and incubated for overnight. The next day, 20 μL of the prepared concentrations of the produced nisin and commercial nisin (0.39–25 μg.mL-1) was added to each well and incubated for a further 48 h. The standard nisin (Sigma, USA) was dissolved in MRS medium and phosphate buffer saline to evaluate the effect of medium or other interfering materials on cytotoxicity of nisin. Every plate that also had cells treated with MRS medium, PBS and iCSF as negative controls. Cells treated with doxorubicin were used as the positive control. Furthermore, cells were incubated with the combined concentrations of the doxorubicin and nisin (10 μg.mL-1). To evaluate cell viability, cells were incubated with MTT at 37 °C for 3 h. Then, dimethyl sulfoxide (DMSO) was added to dissolve MTT-formazan crystals and the absorbance was measured at 570 nm by a plate reader. The percentage of cell viability was determined according to the following equation:
% Cell survival = Absorbance in drug treated wells / Absorbance in negative control well * 100
3.9. Cell Morphology Analysis
Changes in the cell morphology were evaluated to determine the effect of nisin on cell lysis and apoptosis. The morphology of cells was observed before and after each treatment using an optical microscope (200 × magnification, Nikon, Japan).
3.10. Statistical Analysis
Each assay was repeated three times to ensure reproducibility of the results. All data are expressed as the mean ± standard deviation. Comparisons between two groups were analyzed by Student’s t-test, and those between multiple groups were evaluated by analysis of variance (one-way ANOVA) followed by Tukey’s HSD post Hoc test to evaluate intergroup significance using SPSS version 16. The p values <0.05 were considered significant.
4. Results
4.1. Optimization of the Medium, pH, and Temperature for Nisin Production
The effect of different pH of the medium (3.85, 4.7, 5.65, 6.3 or 7.5) on nisin production was evaluated and the maximum production of nisin occurred when the pH of 6.3 was used (Fig. 1A). The effect of cultivation temperature (25, 30 or 37 °C) on the titer of nisin was investigated and the maximum nisin production was observed when culture was incubated at 30 °C (Fig. 1B). The effect of different supplements (tryptone, casein, yeast extract, lactose, sucrose or Tween 80) on nisin production was evaluated and more nisin production was observed when tryptone, casein or Tween 80 was added to MRS medium (Fig. 1C). Under optimum cultural conditions, using MRS medium (pH 6.3) supplemented with tryptone and incubation at 30 °C, the titer of 1100 IU.mL-1 was obtained for nisin (based on the standard curve of nisin, Supplementary Fig. 1).
Figure 1.

Optimization of the culture conditions for production of nisin by L. lactis. A) The effect of growth temperature , B) The effect of medium pH and C) The effect of medium supplements
4.2. Tricine-SDS-PAGE of the Partially Purified Nisin
The apparent molecular weight of bacteriocin produced by L. lactis was estimated by tricine–SDS–PAGE. As shown in Figure 2, the envisioned molecular weight of the partially purified nisin was approximately 3.5 kDa, which was located at the same locus of commercial nisin (Sigma, USA) in tricine-SDS-PAGE.
Figure 2.
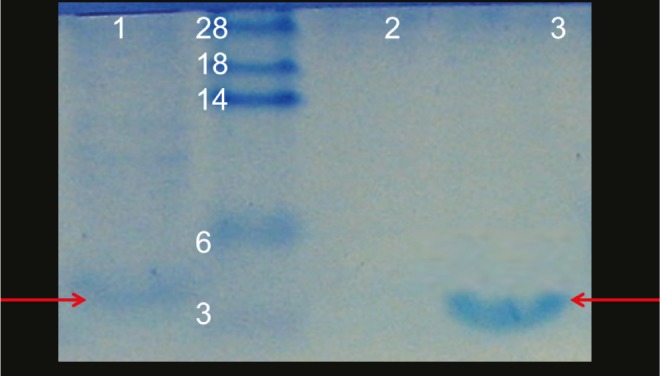
Tricine-SDS-PAGE analysis. Lanes 1: Cell free supernatant from L. lactis; 2: negative control; 3: standard nisin (Sigma, USA). Nisin (3.5 kDa) is denoted by the arrows
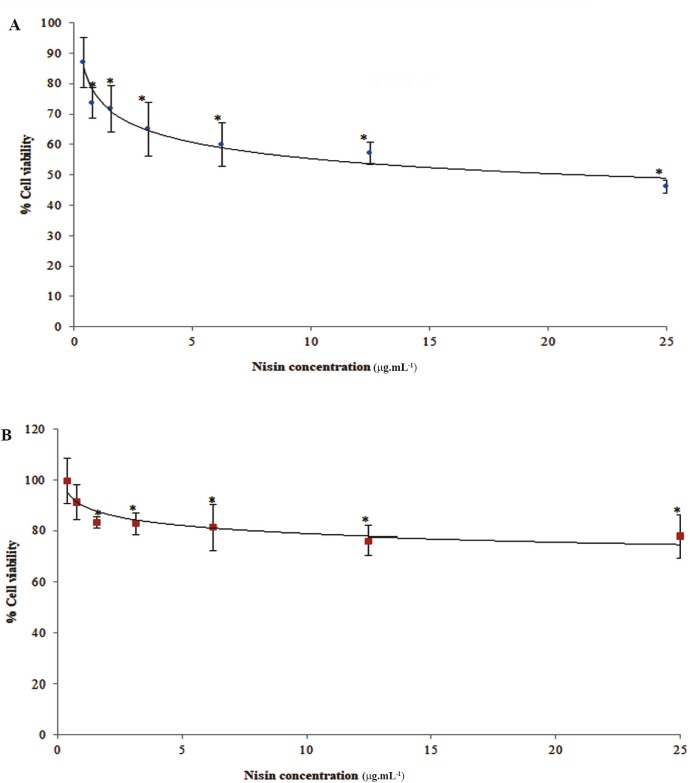
4.3. In vitro Cytotoxicity of Nisin
The cytotoxicity of nisin against MCF-7 and HUVEC cells was evaluated using MTT assay. The viability of MCF-7 cells decreased from 87 to 46% in a concentration-dependent of nisin from 0.39 to 25 μg.mL-1. The survival of MCF-7 cells was significantly inhibited by nisin and the IC50 value of 17 μg.mL-1 was found (Fig. 3A). Anti-prolifratory effect of produced nisin on MCF-7 cell line was very close to commercial nisin dissolved in MRS medium (Supplementary Fig. 2). However, it had a little more cytotoxic effect (supplementary Fig. 2) in comparison with standard nisin dissolved in PBS which could be attributed to interfering substances presenting in MRS medium or better solubility of nisin in MRS (pH 5.5).
Figure 3.
The effects of nisin on cell viability as determined by MTT assay. A) MCF-7 and B) HUVEC. Cells were incubated for 48 h with different concentrations (0.39–25 μg.mL-1) of nisin. The vertical bars indicate the standard deviations (n = 4). Asterisks indicate the means, which were significantly different (p < 0.05) from the control (untreated cells).
Nisin exhibited a lower level of cytotoxicity for a normal cell line, HUVEC, with an IC50 value of 64 μg.mL-1 (Fig. 3B). This four-fold difference in nisin cytotoxicity against MCF-7 over HUVEC cells was shown to be statistically significant in MTT assay (p < 0.05).
In addition, MCF-7 and HUVEC cells were treated with the combined concentrations of doxorubicin and nisin. It was shown that co-incubation with nisin increases the cytotoxicity of the doxorubicin (Fig. 4). This synergic effect was more significant in the lower concentration of doxorubicin and against MCF-7. Approximately, three-fold higher cytotoxicity was observed when MCF-7 cells were incubated with a combination of nisin (10 μg.mL-1) and doxorubicin (6 μg.mL-1) compared to doxorubicin alone (p < 0.01).
Figure 4.
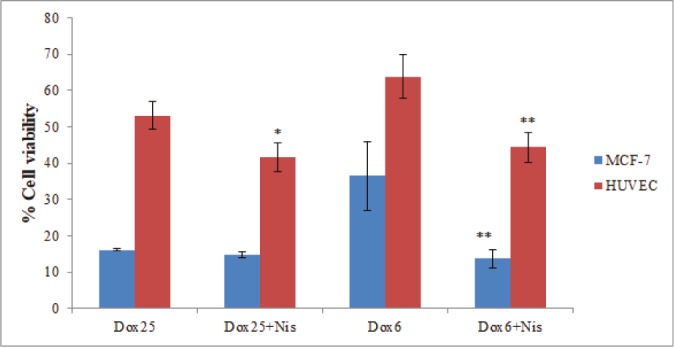
Cell viability of MCF-7 and HUVEC after 48 h of incubation with the combinations of nisin (10 μg.mL-1) and doxorubicin (6 or 25 μg.mL-1). The vertical bars indicate the standard deviations (n = 4). Asterisks indicate the means which were significantly different (p < 0.05) from the control (cells were incubated with doxorubicin alone).
4.4. Morphological Analysis
Changes in cell morphology were evaluated using an optical microscope. The morphological analysis showed that compared to the control or untreated cells (Fig. 5A), treatment of cells with nisin (0.39–25 μg.mL-1) led to apoptosis (Fig. 5B), but not cell lysis. However, nisin in combination with doxorubicin results in a significant cell lysis (Fig. 5C).
Figure 5.
The morphological analysis of the MCF-7 cells using optical microscopy. A) untreated cells, B) cells treated with doxorubicin (6 μg.mL-1), and C) cells treated with a combination of nisin (10 μg.mL-1) and doxorubicin (6 μg.mL-1).
5. Discussion
Like other bacteriocins, culture conditions such as cultivation temperature, pH, and composition of the medium could influence the yield of nisin production. Depending on the bacterial strains and medium which was used, different additives, optimization of the pH, and cultivation temperatures can be applied. In this study, the maximum production of nisin was acquired when MRS medium with pH of 6.3 was used. The initial pH of the medium significantly influences the L. lactis growth rate; a lactic acid producer bacterium, as well as the titer of nisin production. However, the optimum pH for the nisin production was lower than the optimum pH for bacterial growth (data were not shown). The maximum nisin production was observed when L. lactis was cultivated at 30 °C. Similar to our results, other groups have found the optimal production of nisin when L. lactis was cultured in a lactose-based medium with pH 6 at 31 °C (27).
Another critical factor influencing the production of nisin is the medium compositions. The effect of different supplements (tryptone, casein, yeast extract, lactose, sucrose, or Tween 80) on nisin production was evaluated and a higher amount of nisin production was observed when tryptone, casein or Tween 80 was added to the MRS medium. We found that additional nitrogen source such as tryptone and casein have more influence on nisin production compared with that of carbon source supplements (sucrose and lactose). It could be explained based on strict and specific nutritional requirements of the lactic acid bacteria for their growth (28).
The produced nisin under optimum cultural conditions was partially purified using pH-mediated cell adsorption-desorption method to extract cell-bound nisin. Because of its basic nature (pI > 9) nisin has more solubility under acidic pH. At neutral pH, produced nisin is associated with cell membrane but when pH decreased it desorbs and release into the medium. Zhang et al. also used a pH-based extraction method to purify pentocin 31-1 produced by Lactobacillus pentosus (29). They effectively purified this bacteriocin with a high increase in the specific activity after adsorption at pH 4.5 and release at pH 7.0.
Nisin is a safe and food grade bacteriocin which has growing potential application in medicine. There is some evidence suggesting a potential anticancer effect of nisin. In the present study, cytotoxic activity of nisin against MCF-7 and HUVEC cells were evaluated using MTT assay. Interestingly, this effect was cell type dependent and a lower toxicity was observed for HUVEC, a normal cell line. Similar results were also reported by Joo et al. They observed that in contrast to HNSCC cell line, normal oral keratinocytes were not significantly affected by nisin treatment (80 μg.mL-1) (30).
The selectivity of nisin for cancer cells could be explained based on membrane differences. Tumor cells usually carry a net negative charge as they contain more anionic molecules such as phosphatidylserine in the cell membrane (31, 32). In contrast, normal cells have an overall neutral charge as their membrane compound mainly composed of zwitterionic lipids (e.g., sphingomyelin, phosphatidylethanolamine, and phosphatidylcholine) (33). As an antimicrobial peptide, nisin has both cationic and hydrophobic characteristics and can interact with both the negatively charged heads and the lipophilic tail of cell membrane phospholipids (34). The electrostatic interactions between cationic nisin and anionic membrane of cancer cells may play a major role in the selective cytotoxicity of this peptide.
The exact mechanism of anti-cancer effect of nisin remains to be explained. Some studies have suggested several mechanisms including cell membrane disruption and pore formation, altering the level of intracellular ions (e.g., calcium), changing the transmembrane potential, induction of cell cycle arrest, and apoptosis (30, 35). Recently it was demonstrated that nisin induces calcium-dependent apoptosis of the HNSCC cells via calpain but independent of caspase-3 cleavage (19). We found that nisin has a high cytotoxic effect (IC50 = 17 μg.mL-1 or 5 μM) against MCF-7, a caspase-3-deficient cell line (36). However, a previous study which evaluated the cytotoxicity of the nisin against human colorectal cell lines reported a higher IC50 (21). It could be hypothesized that caspase-3 negative cancer cells are more sensitive to the anticancer effect of nisin. However, further studies are still required to explore the exact nisin mode of action.
Interestingly, nisin can significantly enhance the cytotoxic effect (approximately three folds) of an anticancer drug, doxorubicin. This peptide could increase uptake of doxorubicin by cancer cells via destabilization of the cellular membrane or pore-formation. In the present study, morphological analysis has revealed that combination of nisin and doxorubicin results in the cell disruption and lysis. In agreement with our results, other groups have reported the synergistic cytotoxic effect of the combination of doxorubicin and α-helical peptides against HeLa and HepG2 cell lines (37). They evaluated the possible mechanism of this synergy using scanning electron and fluorescence microscopy. They observed that co-administration of doxorubicin and α-helical peptides enhanced cellular uptake of doxorubicin as a result of altering membrane integrity, cavity formation, and loss of microvilli (37). Many other antimicrobial peptides have exhibited anticancer effects. However, nisin because of its unique mode of action and safety has attracted many attentions. In addition to the cytotoxic agent, nisin can act as an immuno-modulator agent so co-administration of nisin with other chemotherapeutic agents might improve patient response and decrease adverse inflammatory effects.
In conclusion, our study reported the effective cytotoxic effects of nisin against MCF-7, a breast cancer cell line. Furthermore, nisin exhibited a selective toxicity against cancerous cells in comparison to HUVEC, a normal cell line. Most importantly, a combination of nisin with doxorubicin led to synergetic anticancer effect. It could be suggested that a combination of nisin and doxorubicin at sub-inhibitory concentrations can be a potential treatment for the breast cancer, thereby reducing side effects of anticancer drug and improving its therapeutic index; however more studies including in vitro and in vivo studies need to be performed.
Acknowledgment
This work was supported by the Isfahan University of Medical Sciences (grant no. 394995)
Footnotes
Spplementary Data
This paper includes two supplementary files containing the standard curve of nisin (File 1) and the antiprofilatory effect of standard nisin dissolved in MRS or BPS on MCF-7 cells which are appeared on the web site of the IJB journal (http://www.ijbiotech.com).
References
- 1.U.S. Breast Cancer Statistics. Breastcancer.org. 2016. avaible from: http://www.breastcancer.org/symptoms/understand_bc/statistics [DOI] [PubMed]
- 2.Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013:1–17. doi: 10.1093/annonc/mdt284 [DOI] [PubMed] [Google Scholar]
- 3.Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F. Management of locally advanced breast cancer [mdash] perspectives and future directions. Nat Rev Clin Oncol. 2015;12(3):147–62. doi: 10.1038/nrclinonc.2015.13 [DOI] [PubMed] [Google Scholar]
- 4.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100(1):72–79. doi: 10.1016/j. jep.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 5.Mukhtar E, Adhami VM, Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther. 2014;13(2):275–284. doi: 10.1158/1535-7163.MCT-13-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daw MA, Falkiner FR. Bacteriocins: nature, function and structure. Micron. 1996;27(6):467–479. doi: 10.1016/S0968-4328(96)00028-5 [DOI] [PubMed] [Google Scholar]
- 7.O’sullivan L, Ross R, Hill C. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie. 2002;84(5):593–604. doi: 10.1016/S0300-9084(02)01457-8 [DOI] [PubMed] [Google Scholar]
- 8.Svetoch E, Stern N. Bacteriocins to control Campylobacter spp. in poultry-a review. Poult Sci. 2010;89(8):1763–1768. doi: 10.3382/ps.2010-00659 [DOI] [PubMed] [Google Scholar]
- 9.Sang Y, Blecha F. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev. 2008;9(02):227–235. doi: 10.1017/S1466252308001497 [DOI] [PubMed] [Google Scholar]
- 10.Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71(1):1–20. doi: 10.1016/S0168-1605(01)00560-8 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez J. Review: Antimicrobial spectrum, structure, properties and mode of action of nisin, a bacteriocin produced by Lactococcus lactis. Food Sci Technol Int. 1996;2(2):61–68. doi: 10.1177/108201329600200202 [Google Scholar]
- 12.Ongey EL, Neubauer P. Lanthipeptides: chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production. Microb Cell Fact. 2016; 15: 1–16. doi: 10.1186/s12934-016-0502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parente E, Ricciardi A. Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl Microbiol Biotechnol. 1999;52(5):628–638. doi: 10.1007/s002530051570 [DOI] [PubMed] [Google Scholar]
- 14.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55. doi: 10.1124/pr.55.1.2 [DOI] [PubMed] [Google Scholar]
- 15.Helander IM, Mattila-Sandholm T. Permeability barrier of the Gram-negative bacterial outer membrane with special reference to nisin. Int J Food Microbiol. 2000;60(2):153–161. doi: 10.1124/pr.55.1.2 [DOI] [PubMed] [Google Scholar]
- 16.Qi X, Poernomo G, Wang K, Chen Y, Chan-Park MB, Xu R, et al. Covalent immobilization of nisin on multi-walled carbon nanotubes: superior antimicrobial and anti-biofilm properties. Nanoscale. 2011;3(4):1874–1880. doi: 10.1039/C1NR10024F [DOI] [PubMed] [Google Scholar]
- 17.Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16(5):321–334. doi: 10.1038/nri.2016.29 [DOI] [PubMed] [Google Scholar]
- 18.Kindrachuk J, Jenssen H, Elliott M, Nijnik A, Magrangeas-Janot L, Pasupuleti M, et al. Manipulation of innate immunity by a bacterial secreted peptide: lantibiotic nisin Z is selectively immunomodulatory. Innate Immun. 2013;19(3):315–327. doi: 10.1177/1753425912461456 [DOI] [PubMed] [Google Scholar]
- 19.Kamarajan P, Hayami T, Matte B, Liu Y, Danciu T, Ramamoorthy A, et al. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PloS one. 2015;10(7):1–20. doi: 10.1371/journal.pone.0131008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preet S, Pandey S, Saini N, Koul A, Rishi P. Nisin Augments Doxorubicin Permeabilization and Ameliorates Signaling Cascade during Skin Carcinogenesis. Transl Med. 2015;6(161). doi: 10.4172/2161-1025.1000161. [Google Scholar]
- 21.Maher S, McClean S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol. 2006;71(9):1289–1298. doi: 10.1016/j.bcp.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi S, Ghollasi M, Hosseini HM. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb Pathog. 2017;111:193–197. doi: 10.1016/j.micpath.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Pongtharangkul T, Demirci A. Evaluation of agar diffusion bioassay for nisin quantification. Appl Microbiol Biotechnol. 2004;65(3):268–272. doi: 10.1007/s00253-004-1579-5. [DOI] [PubMed] [Google Scholar]
- 24.Abedi D, Feizizadeh S, Akbari V, Jafarin-Dehkordi A. In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res Pharm Sci. 2013;8(4):260–268. [PMC free article] [PubMed] [Google Scholar]
- 25.Schägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2 [DOI] [PubMed] [Google Scholar]
- 26.Akbari V, Sadeghi HMM, Jafarian-Dehkordi A, Abedi D, Chou CP. Improved biological activity of a single chain antibody fragment against human epidermal growth factor receptor 2 (HER2) expressed in the periplasm of Escherichia coli. Protein Expr Purif. 2015;116:66–74. doi: 10.1016/j.pep.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 27.Meghrous J, Huot E, Quittelier M, Petitdemange H. Regulation of nisin biosynthesis by continuous cultures and by resting cell of Lactococcus lactis subsp. lactis. Res Microbiol. 1992;143(9):879–890. doi: 10.1016/0923-2508(92)90075-Y [DOI] [PubMed] [Google Scholar]
- 28.Hébert EM, Raya RR, Savoy de Giori G. Evaluation of minimal nutritional requirements of lactic acid bacteria used in functional foods. Walker JM, Spencer JFT, Spencer ALRd, Environmental Microbiology: Methods and Protocols,vol. 16. New Jersey, USA: Humana Press; 2004. pp. 139–148. doi: 10.1385/1-59259-765-3:139 [Google Scholar]
- 29.Zhang J, Liu G, Shang N, Cheng W, Chen S, Li P. Purification and partial amino acid sequence of pentocin 31-1, an anti-Listeria bacteriocin produced by Lactobacillus pentosus 31-1. J Food Prot. 2009;72(12):2524–1529. doi: 10.4315/0362-028X-72.12.2524 [DOI] [PubMed] [Google Scholar]
- 30.Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305. doi: 10.1002/cam4.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szachowicz-Petelska B, Dobrzyńska I, Skrodzka M, Darewicz B, Figaszewski ZA, Kudelski J. Phospholipid Composition and Electric Charge in Healthy and Cancerous Parts of Human Kidneys. J Membr Biol. 2013;246(5):421–425. doi: 10.1007/s00232-013-9554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobrzynska I, Skrzydlewska E, Figaszewski ZA. Changes in electric properties of human breast cancer cells. J Membr Biol. 2013;246(2):161–166. doi: 10.1007/s00232-012-9516-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaji-Hasegawa A, Tsujimoto M. Asymmetric distribution of phospholipids in biomembranes. Biol Pharm Bull. 2006;29(8):1547–1553. doi: 10.1248/bpb.29.1547 [DOI] [PubMed] [Google Scholar]
- 34.Hwang PM, Vogel HJ. Structure-function relationships of antimicrobial peptides. Biochem Cell Biol. 1998;76(2–3):235–246. doi: 10.1139/o98-026 [DOI] [PubMed] [Google Scholar]
- 35.Moll GN, Clark J, Chan WC, Bycroft BW, Roberts GC, Konings WN, et al. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179(1):135–140. doi: 10.1128/jb.179.1.135-140.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagawa S, Gu J, Honda T, McDonnell TJ, Swisher SG, Roth JA, et al. Deficiency of Caspase-3 in MCF7 Cells Blocks Bax-mediated Nuclear Fragmentation but not Cell Death. Clin Cancer Res. 2001;7(5):1474–1480. doi: 10.1186/bcr463 [PubMed] [Google Scholar]
- 37.Zhao J, Huang Y, Liu D, Chen Y. Two hits are better than one: synergistic anticancer activity of α-helical peptides and doxorubicin/epirubicin. Oncotarget. 2015;6(3):1769–1778. doi: 10.18632/oncotarget.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]