Significance
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that results from the loss of midbrain dopamine neurons. While the cause of PD is unknown, male sex is one of the strongest risk factors. Traditionally, sex differences in PD have been attributed to the neuroprotective actions of estrogen in females. Here we show that the Y-chromosome gene, SRY, is up-regulated in animal and cell culture models of PD, and that suppressing SRY expression in male rats diminishes neurodegeneration and motor deficits in preclinical PD models. Taken together, our findings suggest that SRY up-regulation might be a potential pathogenic mechanism of male PD, and that SRY inhibition may be a novel male-specific strategy to slow or halt the progression of PD.
Keywords: brain sex differences, sex chromosome, transcription factor, inflammation, neuroprotection
Abstract
Parkinson’s disease (PD) is a debilitating neurodegenerative disorder caused by the loss of midbrain dopamine (DA) neurons. While the cause of DA cell loss in PD is unknown, male sex is a strong risk factor. Aside from the protective actions of sex hormones in females, emerging evidence suggests that sex-chromosome genes contribute to the male bias in PD. We previously showed that the Y-chromosome gene, SRY, directly regulates adult brain function in males independent of gonadal hormone influence. SRY protein colocalizes with DA neurons in the male substantia nigra, where it regulates DA biosynthesis and voluntary movement. Here we demonstrate that nigral SRY expression is highly and persistently up-regulated in animal and human cell culture models of PD. Remarkably, lowering nigral SRY expression with antisense oligonucleotides in male rats diminished motor deficits and nigral DA cell loss in 6-hydroxydopamine (6-OHDA)-induced and rotenone-induced rat models of PD. The protective effect of the SRY antisense oligonucleotides was associated with male-specific attenuation of DNA damage, mitochondrial degradation, and neuroinflammation in the toxin-induced rat models of PD. Moreover, reducing nigral SRY expression diminished or removed the male bias in nigrostriatal degeneration, mitochondrial degradation, DNA damage, and neuroinflammation in the 6-OHDA rat model of PD, suggesting that SRY directly contributes to the sex differences in PD. These findings demonstrate that SRY directs a previously unrecognized male-specific mechanism of DA cell death and suggests that suppressing nigral Sry synthesis represents a sex-specific strategy to slow or prevent DA cell loss in PD.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized primarily by the inability to initiate and maintain voluntary movement. Motor symptoms of PD emerge when the loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNc) exceeds 70%. While the cause of this DA cell loss is unknown, male sex is a known significant risk factor for PD (1–4). Compared with women, men have a 2-fold greater risk of developing PD and a 1.4- to 3.7-fold higher prevalence of PD (1–4). Males with PD also show an earlier age of onset (3, 5), faster rate of disease progression (5) and greater dopaminergic denervation (6). Animal models of PD reproduce these sex differences, as male rodents or nonhuman primates are more susceptible than females to toxin-induced dopaminergic degeneration (7–10).
The prevailing view is that sex differences in PD arise solely from the neuroprotective actions of estrogen in females (11–15). However, emerging evidence suggests that sex chromosome genes also contribute to the inherent sex differences in healthy and diseased DA systems alike (16–18). For instance, embryonic midbrain cells develop more DA neurons when the cultures are composed of XY cells rather than XX cells (16, 19). Microarray analyses of single DA neurons from human SNc sections have revealed higher expression levels of PD pathogenesis genes (e.g., α-synuclein, PINK-1) (20) and lower expression levels of genes involved in oxidative phosphorylation and synaptic transmission in the SNc of males compared with females (21). Thus, male DA cells have intrinsic sex differences that may influence their pattern of gene expression, predisposing males to the development of PD. Here we describe a pathway for male-specific DA cell loss involving the Y-chromosome gene Sex-determining Region Y (SRY).
SRY encodes a transcriptional activator that initiates male sex determination by directing the development of the embryonic bipotential gonads to testes rather than ovaries (22, 23). In most mammals, SRY is found as a single locus; however, multiple loci of SRY are expressed in the rat that code for proteins with altered amino acid sequences (24). SRY is also expressed in nonreproductive tissues of adult males such as the heart, adrenal glands, kidneys, and brain (25, 26). In the male brain, SRY protein is expressed in DA-abundant regions, such as the SNc and ventral tegmental area (27, 28), where it colocalizes with DA neurons (28). Furthermore, SRY enhances in vitro transcription of DA biosynthesis and the catabolic enzymes tyrosine hydroxylase (TH) and monoamine oxidase-A (MAOA) (28, 29), and regulates voluntary movement in male rats (27). Given the presence and function of SRY in male DA neurons, we hypothesized that dysregulation of SRY may contribute to male susceptibility to PD. In the present study, we investigated whether SRY expression is dysregulated and, if so, whether SRY dysregulation contributes to male-specific DA cell loss in experimental PD.
Results
SRY Expression Is Aberrantly Up-Regulated in Both In Vivo and In Vitro Models of PD.
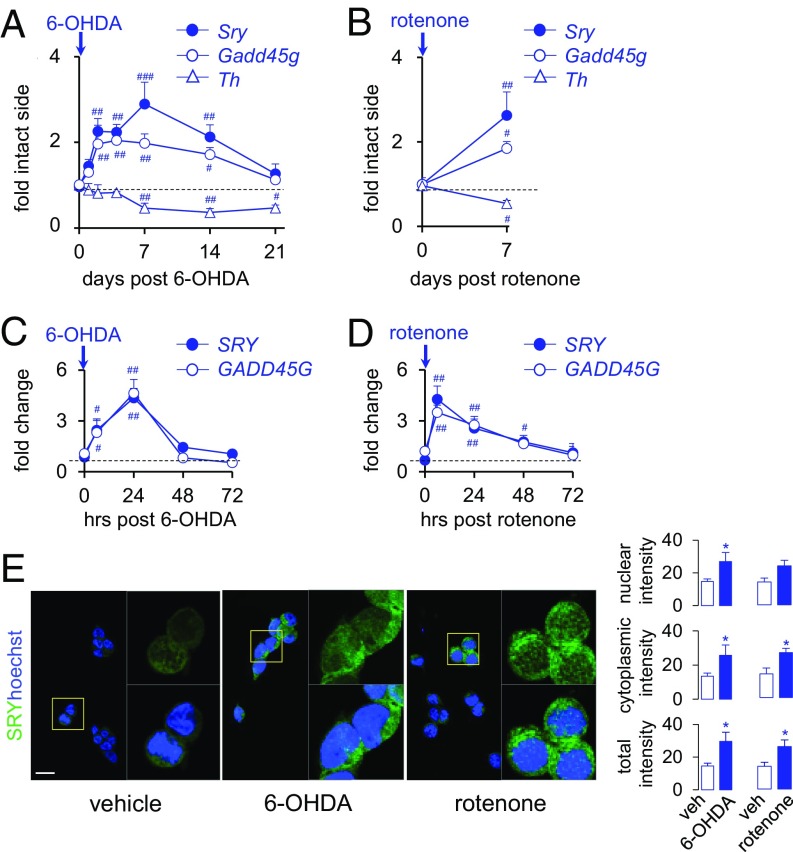
To examine whether SRY expression is altered in response to DA cell injury, we assessed SRY expression in animal and cell culture models of PD. In the 6-hydroxydopamine (6-OHDA) rat model of PD, nigral Sry mRNA expression was elevated from day 2 to day 14 after 6-OHDA injection in male rats, with the peak effect seen at day 7 (289% vs. day 0; F(6,42) = 8.65, P < 0.01) (Fig. 1A). The up-regulation in Sry expression was paralleled by an increase in nigral Gadd45g mRNA (a marker of DNA damage and regulator of Sry) from day 2 to day 14 (Fig. 1A), which is consistent with our previous in vitro findings (30). Nigral Sry up-regulation occurred before the decline in Th expression at day 7, showing that Sry up-regulation occurs before and during DA cell loss in males. Similarly, acute injection of the mitochondrial and DA toxin rotenone elevated nigral Sry expression (+255%; F(5,4) = 1.08) and Gadd45g expression (+192%; F(5,4) = 2.47) at 7 d postinjection in male rats (Fig. 1B). Consistent with the in vivo findings, human SRY and GADD45G mRNA expression was markedly elevated at 6 to 24 h after exposure to 6-OHDA (Fig. 1C) or rotenone (Fig. 1D) in the human male cell line M17. Moreover, the average nuclear, cytosolic, and total immunofluorescence intensities of human SRY protein were increased at 24 h after exposure to 6-OHDA or rotenone (Fig. 1E). Collectively, these results show that Sry expression is aberrantly up-regulated in both in vivo and in vitro preclinical models of PD, suggesting a role for SRY in cellular events underlying DA cell loss in males.
Fig. 1.
SRY expression is up-regulated in experimental models of PD. (A and B) Time courses of nigral Sry, Gadd45g, and Th mRNA expression (as fold change of the intact side relative to Tbp1) at 0 to 21 d following a single intranigral 6-OHDA (A) or rotenone (B) injection in male rats (n = 5/group). #P < 0.05; ##P < 0.01; ###P < 0.001 vs. d 0, 1-way ANOVA. The dashed line represents baseline levels. (C and D) Time course of SRY or GADD45G mRNA expression (as fold change of 0 h relative to β2M) at 0 to 72 h after treatment with 6-OHDA (C) or rotenone (D) in human M17 cells (n = 5/group). #P < 0.05, ##P < 0.01 vs. 0 h, 1-way ANOVA. (E) Effect of vehicle, 6-OHDA, or rotenone treatment on SRY protein (green) or DAPI (blue) expression at 24 h posttreatment in human M17 cells (n = 4/group). *P < 0.05 vs. vehicle, unpaired t test. (Scale bar: 10 µm.)
Suppressing Nigral SRY Up-Regulation Diminishes Motor Deficits and Nigrostriatal Degeneration in Toxin-Induced Rat Models of PD.
To determine the role of SRY up-regulation in injured male DA neurons, we assessed the effect of reducing nigral Sry expression, via intranigral Sry antisense oligonucleotide (ASO) infusion, on motor and nigrostriatal degeneration in toxin-induced rat models of PD. As reported previously (27), repeated ASO infusion in healthy male rats reduced motor function in the limb use and rotarod tests (SI Appendix, Fig. S1 A and B). In contrast, motor function in female rates was unaffected by ASO infusion (SI Appendix, Fig. S1 C and D). ASO infusion in male rats reduced the expression levels of nigral Sry mRNA, but not of the Sry homologs Sox3 and Sox6 (SI Appendix, Fig. S1E). ASO-mediated reduction of motor function in male rats was also associated with reductions in nigral Th, Maoa, dopa decarboxylase (Ddc), and dopamine D2 receptor (Drd2) mRNA expression (SI Appendix, Fig. S1E), nigral TH-positive neurons (SI Appendix, Fig. S1F), as well as striatal DA and 3,4-dihydroxyphenylacetic acid (DOPAC) content (SI Appendix, Fig. S1H). However, ASO infusion did not affect the number of neutral red-positive neurons in the SNc, indicating that the reduction of TH-positive neurons in males is due to reduced TH expression rather than neuronal cell loss. In support of this idea, ASO-induced reduction in motor function was transient and reversible, with limb use returning to baseline levels at 7 d after the last ASO infusion (SI Appendix, Fig. S1I).
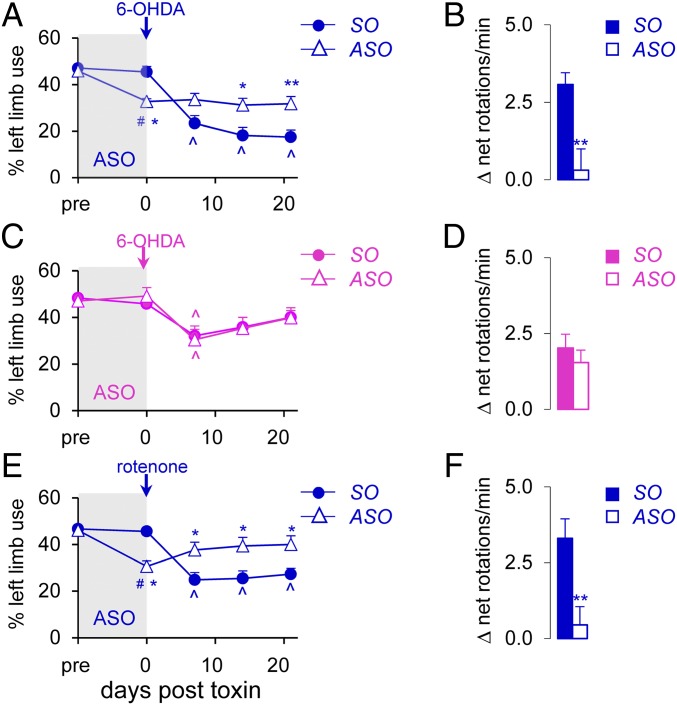
We next assessed the effect of ASO infusion in toxin-induced rat models of PD. Fig. 2A shows that previous ASO infusion in male rats led to the expected reduction in limb use at day 0. Remarkably, previous ASO infusion prevented the 6-OHDA–induced deficits in limb use observed in the sense oligonucleotide (SO)-infused group (interaction: F(4,200) = 9.81, P < 0.0001; time effect: F(4,200) = 26.18, P < 0.0001; treatment effect: F(1,200) = 7.24, P < 0.01, day 7), which persisted for another 14 d (days 14 and 21; P < 0.05 vs. SO) (Fig. 2A). In support of this, amphetamine-induced rotations were markedly reduced in the ASO-infused group (P < 0.01 vs. SO; Fig. 2B).
Fig. 2.
Nigral Sry ASO infusion mitigates 6-OHDA–induced and rotenone-induced motor deficits in male, but not female, rats. ASO (or SO) was infused before 6-OHDA injection in male (A and B) and female (C and D) rats and before rotenone injection in male rats (E and F). Toxin-induced motor deficits were assessed by limb use asymmetry (A, C, and E) and the amphetamine-induced rotation test (B, D, and F) (n ≥12/group). *P < 0.05, **P < 0.01 vs. SO; #P < 0.05, ##P < 0.01 vs. pre; ^P < 0.05 vs. d 0, 2-way ANOVA. Pre, before ASO infusion.
To ensure that ASO infusion was truly neuroprotective and not just inhibiting the uptake of the toxin, we tested the effect of ASO infusion at 8 h after 6-OHDA injection and found that this also diminished motor deficits in the limb use test (interaction: F(3,88) = 1.87, P = 0.14; time effect: F(3,88) = 25.49, P < 0.0001; treatment effect: F(1,88) = 29.23, P < 0.0001, days 14 and 21, P < 0.01 vs. SO) (SI Appendix, Fig. S2A) and rotation test (P < 0.01 vs. SO) in male rats (SI Appendix, Fig. S2B). In contrast, female rats showed no differences in 6-OHDA–induced motor deficits between the ASO-infused and SO-infused groups (Fig. 2 C and D), confirming that the protective effect of ASO infusion is male-specific. Suppressing Sry synthesis led to even greater protection in the rotenone-induced rat model of PD, as previous ASO infusion in male rats reversed rotenone-induced motor deficits in the limb use test (interaction: F(4,100) = 10.80, P < 0.0001; time effect: F(4,100) = 10.49, P < 0.0001; treatment effect: F(1,100) = 7.77, P < 0.01, days 7 to 21, P < 0.05 vs. SO; Fig. 2E) and the rotation test (P < 0.01 vs. SO; Fig. 2F).
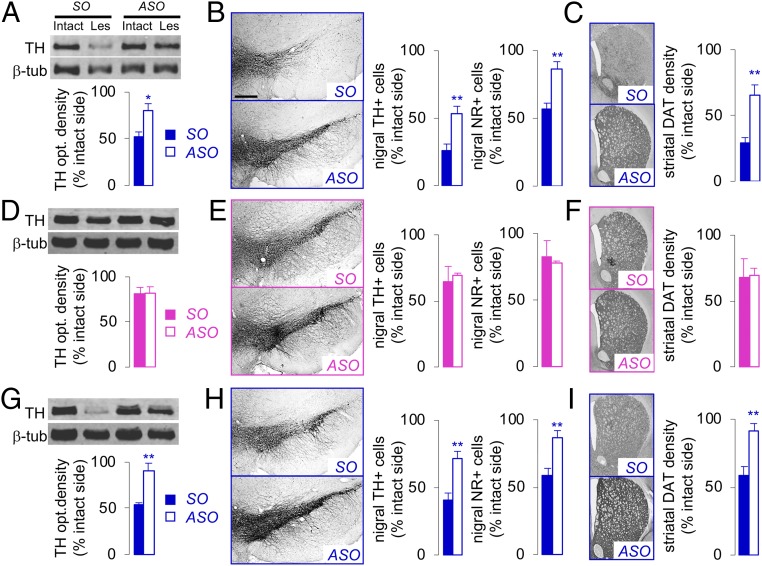
Postmortem analysis of 6-OHDA–injected male rats revealed a 50% to 75% loss in nigral TH protein expression (SO; Fig. 3A), TH and neutral red (NR)-positive cells (SO; Fig. 3B), and striatal DA terminals (SO; Fig. 3C). In line with the motor behavior, previous ASO infusion in male rats alleviated the 6-OHDA–induced loss in nigrostriatal DA cell bodies and terminals (P < 0.01 vs. SO; Fig. 3 A–C). Similarly, ASO infusion following 6-OHDA injection in male rats significantly diminished the 6-OHDA–induced nigrostriatal degeneration (P < 0.01 vs. SO; SI Appendix, Fig. S2 C–E). In contrast, there were no significant differences in nigrostriatal degeneration between the SO and ASO groups in 6-OHDA–injected female rats (Fig. 3 D–F). Rotenone-injected male rats showed a 45% to 55% loss of nigrostriatal cell bodies and terminals (SO; Fig. 3 G–I), which was prevented by previous ASO infusion in male rats (P < 0.01 vs. SO; Fig. 3 G–I). Taken together, these results indicate a divergent role for Sry in healthy and injured male SNc.
Fig. 3.
Nigral Sry ASO infusion mitigates 6-OHDA–induced and rotenone-induced nigrostriatal degeneration in male, but not female, rats. ASO (or SO) was infused before 6-OHDA injection in male (A–C) and female (D–F) rats or a single rotenone injection in male rats (G–I). Toxin-induced nigrostriatal degeneration was assessed by nigral TH protein expression (as optical density) in the intact and toxin-lesioned (Les) side (A, D, and G), total number of TH-positive (TH+) cells or neutral red-positive (NR+) cells (B, E, and H), and striatal DAT density (C, F, and I) as percentage of the intact side (n ≥12/group). *P < 0.05, **P < 0.01 vs. SO, unpaired t test. (Scale bar: 400 µm.)
The foregoing findings suggest a divergent role for SRY during physiological and pathophysiological conditions. In the healthy male SNc, SRY positively regulates DA biosynthesis and motor function; however, in the injured male SNc, aberrant SRY up-regulation is detrimental, exacerbating the degeneration of DA neurons and, consequently, motor function in males.
Protective Effect of Sry Reduction Is Mediated by Broad Suppression of Mitochondrial Degradation, DNA Damage, and Neuroinflammation in Male Rats.
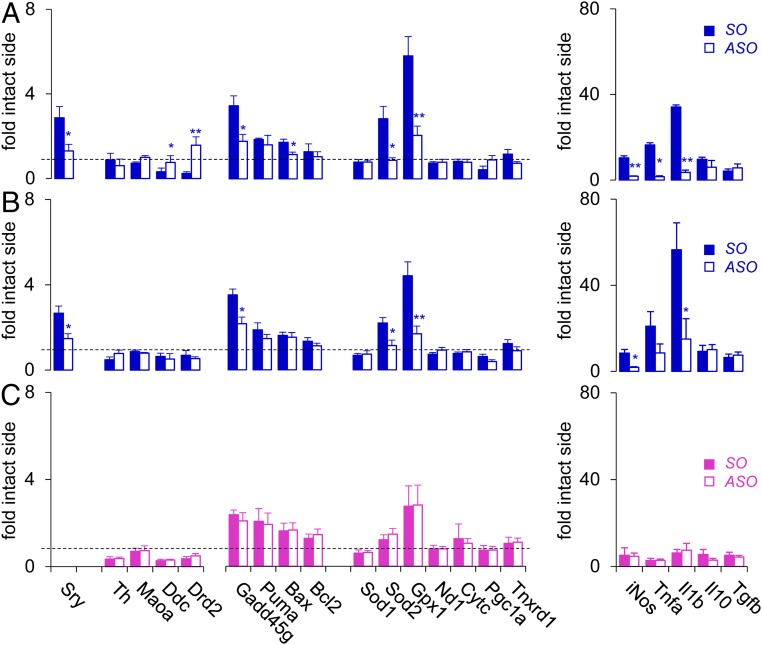
Given that the suppression of nigral Sry synthesis is protective in both 6-OHDA–induced and rotenone-induced rat models of PD, the detrimental effect of Sry up-regulation may be mediated by a common downstream mechanism shared by the 2 toxins. To identify potential downstream targets of Sry in the injured male SNc, we assessed the transcriptional profile of key genes involved in PD pathogenesis at 2 d after 6-OHDA or rotenone injection following ASO infusion. In particular, we focused on PD pathogenesis pathways that are sex-biased, such as mitochondrial dysfunction and oxidative stress (31, 32), apoptosis (33), and neuroinflammation (9, 10, 34). Quantitative polymerase chain reaction (qRT-PCR) analysis revealed that both 6-OHDA and rotenone increased nigral Sry mRNA expression in male rats (2.9- and 2.7-fold of intact side, respectively), which was suppressed with previous ASO infusion (1.3- and 1.4-fold; Fig. 4 A and B). In parallel, 6-OHDA and rotenone induced up-regulation of genes involved in DNA damage (Gadd45g), oxidative stress, and mitochondrial function (Sod2 and Gpx1), which was blocked or attenuated with ASO infusion in male rats (Fig. 4 A and B). In particular, 6-OHDA and rotenone injection in male rats induced robust increases in proinflammatory genes iNos (10.3- and 8.5-fold, respectively) and Il1b (34.1- and 56.5-fold), which were diminished with ASO infusion (Fig. 4 A and B). In contrast, no significant between-group differences in gene expression were observed in 6-OHDA–injected female rats (Fig. 4C).
Fig. 4.
Reducing nigral Sry expression in male rats alleviates toxin-induced elevation of PD pathogenesis genes. Effect of ASO (or SO) infusion on expression of nigral genes (fold-intact side) involved in DA machinery, DNA damage, oxidative stress and mitochondrial function, and inflammation at 2 d after injection of 6-OHDA (A) or rotenone (B) in male rats or injection of 6-OHDA in female rats (C) (n ≥5/group). *P < 0.05, **P < 0.01 vs. SO, unpaired t test. The dashed line represents baseline levels.
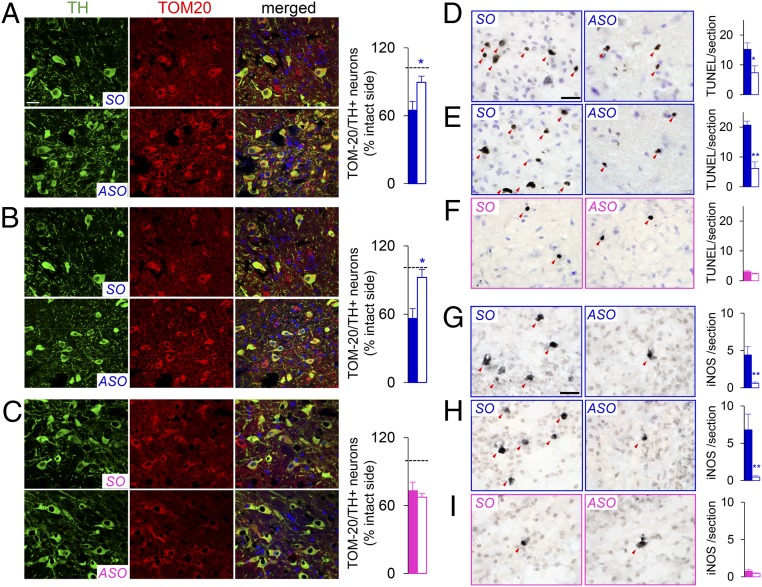
We next assessed the protein expression of key markers involved in mitochondrial function, apoptosis, and neuroinflammation. Co-immunofluorescence staining for TOM-20 (a marker of mitochondrial integrity) and TH revealed marked losses in the number of TOM-20 and TH copositive neurons following 6-OHDA or rotenone injection, which was reversed with ASO infusion in male rats (P < 0.05, SO vs. ASO; Fig. 5 A and B). However, there were no significant differences in the number of TOM-20 and TH copositive neurons between the SO and ASO groups in the 6-OHDA–treated female rats (Fig. 5C). 6-OHDA or rotenone injection in male rats induced significant increases in the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive neurons in the SNc, which was attenuated by ASO infusion (P < 0.05 and < 0.01, respectively, SO vs. ASO; Fig. 5 D and E). Similarly, 6-OHDA–induced and rotenone-induced increases in inducible NO synthase (iNOS)-positive neurons in male rats (SO; Fig. 5 G and H) were abolished by ASO infusion (P < 0.01, SO vs. ASO; Fig. 5 G and H). In contrast, there were no between-group differences in the number of TUNEL-positive or iNOS-positive neurons in the 6-OHDA–lesioned female rats (Fig. 5 F and I). Collectively, these findings demonstrate that reducing nigral Sry expression exerts male-specific neuroprotection in preclinical models of PD by inhibiting a breadth of cellular events underlying PD pathogenesis, such as DNA damage, mitochondrial dysfunction, and neuroinflammation.
Fig. 5.
Reducing nigral Sry expression in male rats alleviates toxin-induced mitochondrial degradation, DNA damage, and neuroinflammation. Effect of ASO infusion on nigral TH (green) and TOM-20 (red) copositive neurons (A–C), nigral TUNEL-positive cells (D–F), and nigral iNOS-positive cells (G–I) at 2 d after injection of 6-OHDA (A, D, and G) or rotenone (B, E, and H) in male rats or injection of 6-OHDA in female rats (C, F, and I). Arrows represent TUNEL-positive or iNOS-positive cells in the SNc (n = 7/group). *P < 0.05, **P < 0.01 vs. SO, unpaired t test. (Scale bar: 20 µm.)
In keeping with previous studies (7, 35, 36), we show that acute 6-OHDA injection led to greater motor deficits and nigrostriatal degeneration, as well as higher numbers of TUNEL- and iNOS-positive neurons in male rats compared with female rats (male SO 6-OHDA vs. female SO 6-OHDA; SI Appendix, Table S1). Moreover, reducing Sry expression in 6-OHDA male rats reversed or reduced the male-bias in toxin-induced motor deficits, nigrostriatal degeneration, and TUNEL and iNOS expression (male ASO 6-OHDA vs. female SO 6-OHDA; SI Appendix, Table S1), suggesting that Sry directly contributes to sex differences in experimental models of PD.
Discussion
In this study, we provide evidence that SRY, a Y-chromosome gene present exclusively in males, directs a novel genetic mechanism for DA cell loss in males. We show that nigral Sry expression is aberrantly up-regulated in human cell culture and animal models of PD. Moreover, reducing Sry expression in male rats diminishes or prevents 6-OHDA–induced or rotenone-induced motor deficits and nigrostriatal degeneration. Importantly, the protective effect of ASO infusion was absent in female rats (which do not express Sry), demonstrating neuroprotection mediated by a male-specific, and thereby sex-specific, gene.
The sex differences in clinical PD are reproduced in experimental PD, as male rats and primates are more susceptible than their female counterparts to toxin-induced nigrostriatal degeneration and motor deficits (7, 8, 10, 35). Along these lines, males exhibit greater apoptosis (33), neuroinflammation (9, 10, 34), and oxidative stress (31, 32, 37) than females in experimental models of PD and neurodegeneration. Given the symptomatic and neuroprotective effects of estrogen in females (7, 12, 13, 15), sex hormones have been considered the sole drivers of sex differences in susceptibility to PD. Interestingly, the effect of estrogen on adult male DA neurons is minimal if not harmful (7, 38), suggesting that the vulnerability of male DA neurons might not be explained by lower estrogen levels. Here we show that suppressing Sry synthesis removed or diminished the male susceptibility to mitochondrial degradation, apoptosis, and inflammation in the 6-OHDA–treated male rats. Moreover, reducing Sry up-regulation in 6-OHDA–treated male rats alleviated the motor and nigrostriatal deficits to levels observed in the 6-OHDA–treated females. Taken together, these results demonstrate that, in addition to the protective effect of estrogen in females, aberrant nigral Sry up-regulation in males contributes to the male bias in experimental PD, supporting the concept that factors encoded by the sex chromosome genes, as well as sex hormones, contribute to brain sex differences in health and disease (18).
Our findings also demonstrate that reducing Sry expression exerts distinct actions in healthy and Parkinsonian male rats, suggesting a divergent role for SRY in both healthy and injured male SNc. In healthy male rats, reducing Sry expression transiently reduced nigrostriatal DA biosynthesis and motor function, indicating that the nigrostriatal DA system is uniquely regulated by SRY in males (27). SRY may be required for the maintenance of adult DA neurons, playing a role similar to nigral transcription factors such as Pitx3 (39) and Sox6 (40) or as an alternate mechanism to estrogen in females. In response to nigral injury, however, GADD45G is rapidly up-regulated (30), stimulating the p38-MAPK pathway in both sexes. In males, this leads to an increase in Sry expression, perhaps as a compensatory response to initial stages of nigral injury. Ultimately, the up-regulation of Sry is detrimental, exacerbating mitochondrial degradation, apoptosis, and neuroinflammation and, consequently, loss of the remaining DA neurons in the injured male SNc.
Interestingly, we previously showed that reducing SRY expression in 6-OHDA–treated M17 cells exacerbates oxidative stress and diminishes cell viability (30), suggesting that SRY up-regulation is protective in the in vitro PD model. There may be several explanations for the conflicting findings from the in vitro and in vivo studies. One major difference between the 2 studies is that the in vitro system lacks the network of cells that surround the DA neurons, namely the supporting microglia, the immune cells of the brain. After nigral injury, the infiltration of activated microglial cells and consequent release of proinflammatory mediators is thought to underlie the progression of DA cell loss in PD (41, 42). We observed that 6-OHDA or rotenone injection in male rats led to marked elevation of proinflammatory mediators Tnfa, Il1b, and iNOS, which was abolished with Sry ASO infusion. These results strongly suggest that the deleterious effect of Sry up-regulation in the in vivo rat PD models likely involves a proinflammatory response mediated by activated microglial cells, which is absent in a single cell line in vitro PD model. Another difference is the time course of SRY up-regulation between the 2 studies. Specifically, SRY up-regulation occurs earlier and is of shorter duration in vitro (6 to 24 h after 6-OHDA), compared with the in vivo rat model (2 to 14 d), which may have different consequences on DA cell viability, from protective to detrimental.
While our study links cellular pathways involved in DA cell loss with SRY up-regulation, the exact molecular mechanisms by which SRY up-regulation exacerbates DA cell loss remain to be determined. In contrast to the embryonic gonads, where nuclear SRY activates SOX9 transcription to initiate the development of testes (43), the presence of SRY protein in both the nucleus and cytoplasm of neurons (28) suggests a role for SRY beyond a classical transcription factor in the male brain. In human and rodent neuronal cells, SRY activates TH (28, 44) and MAOA (29) transcription, as well as the expression of DA biosynthesis genes, including DRD2, AADC, and DBH (28, 44). Thus, SRY up-regulation may drive dysregulation of DA biosynthesis and metabolism and consequently increase DA turnover and oxidative stress in male DA neurons. Alternatively, the toxin-induced elevation of nigral SRY may induce dysregulation of a much broader gene expression response and/or additional interactions with cytosolic and mitochondrial proteins involved in cell survival, energy metabolism, and inflammation. Indeed, chromatin immunoprecipitation studies revealed that SRY regulates a wide spectrum of target genes, including those involved in mitochondrial function (e.g., glutathione peroxidase, nitric oxide synthase, mitochondrial membrane proteins), inflammation (e.g., interleukin, tumor necrosis factor), and apoptosis (e.g., Parp-1) in embryonic day 11.5 mouse gonads (45, 46), the time point at which SRY is maximally expressed. Further investigation of the downstream targets activated by SRY during nigrostriatal degeneration in male DA neurons by genome-wide approaches, such as RNA and SRY chromatin immunoprecipitation sequencing, will reveal the precise mechanism(s) underlying the toxic up-regulation of SRY in male PD and pathways that can be modulated as a sex-specific neuroprotective strategy for PD.
In conclusion, our study provides compelling evidence that dysregulation of the Y-chromosome gene, SRY, directs a novel male-specific mechanism of DA cell death. In addition to the established protective effect of sex hormones in females, the detrimental effect of SRY up-regulation in males may also contribute to the male bias in PD, supporting the notion that the cause and progression of PD are mechanistically different between males and females (20, 21). While the current study provides evidence that SRY up-regulation is detrimental in experimental PD, we do not have any evidence that this occurs in men with PD. Thus, assessing SRY expression in postmortem brains of male PD patients, as well as aging male brain samples (as a surrogate for early stages of nigral degeneration) will be critical for validating SRY as a clinically relevant target for PD. Since normalization of SRY expression appears to be important for the protection of male DA neurons, the development of human SRY inhibitors could lead to novel disease-modifying therapeutics for PD in males.
Materials and Methods
Detailed information on the materials and methods used in this study is provided in SI Appendix.
Human Cell Culture Model of PD.
Human XY neuroblastoma BE (2)-M17 (M17) cells (CRL-2267; American Type Culture Collection), which express endogenous human SRY and intact DA machinery, were maintained as described previously (28, 30). M17 cells were plated onto 6-well plates and then treated with vehicle, 6-OHDA (20 µM) or rotenone (100 µM). Cells were extracted for RNA at 0 to 72 h posttreatment or seeded at 24 h and processed for immunocytochemistry.
Animals.
All animal procedures were approved by the Monash University Animal Ethics Committee.
Repeated Sry ASO Infusions.
Sry expression in healthy or DA toxin-treated male rats was reduced by repeated intranigral infusions of Sry ASO or control SO. The Sry ASO used was a mixture of 3 distinct ASOs directed against rat Sry mRNA added in equal proportions, as described previously (27).
Toxin-Induced Rat Models of PD.
To assess the regulation of Sry expression in preclinical rat PD models, nigral gene expression was assessed by qRT-PCR at various time points following a single intranigral injection of 6-OHDA or rotenone in male rats. To assess the function of Sry in preclinical rat PD models, male rats were infused daily with Sry ASO (or SO) for 10 d, followed by a single intranigral injection of 6-OHDA or rotenone. A group of female rats were infused daily with Sry ASO (or SO) for 10 d, followed by a single injection of 6-OHDA to account for any nonspecific effects. Motor function was assessed by the limb use asymmetry and amphetamine-induced rotation tests. At the end of the behavioral tests, all rat brains were isolated and processed for measurement of nigrostriatal gene and protein expression.
qRT-PCR.
Total RNA from the rat SNc was isolated using TRI reagent (Sigma-Aldrich) and real-time quantification of mRNA measured as described previously (28, 30) using the primers listed in SI Appendix, Table S2.
Striatal DA and DOPAC Measurements.
The striata was isolated and levels of DA and DOPAC were measured, as described previously (47).
TH and Dopamine Reuptake Transporter Immunohistochemistry and Stereology.
TH and dopamine reuptake transporter (DAT) immunohistochemistry was performed on rat SNc and rat striatal sections, respectively, as described previously (47). TH-immunoreactive and neutral-red positive neurons or DAT-immunoreactive terminals were quantified stereologically on regularly spaced sections covering the whole SNc or striatum, as described previously (47).
Statistical Analysis.
Motor behavior studies of the treatment groups across the days of testing were analyzed by 2-way analysis of variance (ANOVA) and Tukey’s post hoc test. Histological and biochemical studies were analyzed using the 2-tailed unpaired Student t test or 1-way ANOVA, where appropriate.
Supplementary Material
Acknowledgments
We thank Peter J. Fuller and Janelle Ryan for their critical appraisal of the manuscript. Support for this work was provided by the Victorian Government’s Operational Infrastructure Support Program, Australian National Health and Medical Research Council (NHMRC) Project Grant 1029401 (to J.L.), National Institutes of Health Grant R01 075046 (to E.V. and V.R.H.), an NHMRC Fellowship (to V.R.H.), Cass Foundation Grant SM/08/2053 (to J.L.), a Rebecca L. Cooper Foundation grant (J.L.), and Helen McPherson Smith Trust Project Grant 5965 (to J.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900406116/-/DCSupplemental.
References
- 1.Baldereschi M., et al. ; ILSA Working Group. Italian Longitudinal Study on Aging , Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. Neurology 55, 1358–1363 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Van Den Eeden S. K., et al. , Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 157, 1015–1022 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Wooten G. F., Currie L. J., Bovbjerg V. E., Lee J. K., Patrie J., Are men at greater risk for Parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 75, 637–639 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moisan F., et al. , Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J. Neurol. Neurosurg. Psychiatry 87, 952–957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haaxma C. A., et al. , Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 78, 819–824 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaasinen V., Joutsa J., Noponen T., Johansson J., Seppänen M., Effects of aging and gender on striatal and extrastriatal [123I]FP-CIT binding in Parkinson’s disease. Neurobiol. Aging 36, 1757–1763 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Murray H. E., et al. , Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: Differential actions of estrogen in males and females. Neuroscience 116, 213–222 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Leranth C., et al. , Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for Parkinson’s disease and memory. J. Neurosci. 20, 8604–8609 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q., Yang X., Zhang Y., Zhang L., Feng L., Chronic mild stress accelerates the progression of Parkinson’s disease in A53T alpha-synuclein transgenic mice. Exp. Neurol. 285, 61–71 (2016). [DOI] [PubMed] [Google Scholar]
- 10.De Miranda B. R., Fazzari M., Rocha E. M., Castro S., Greenamyre J. T., Sex differences in rotenone sensitivity reflect the male-to-female ratio in human Parkinson’s disease incidence. Toxicol. Sci. 170, 133–143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillies G. E., McArthur S., Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 62, 155–198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetti M. D., et al. , Hysterectomy, menopause, and estrogen use preceding Parkinson’s disease: An exploratory case-control study. Mov. Disord. 16, 830–837 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Bourque M., Dluzen D. E., Di Paolo T., Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson’s disease. Front. Neuroendocrinol. 33, 169–178 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Saunders-Pullman R., Estrogens and Parkinson disease: Neuroprotective, symptomatic, neither, or both? Endocrine 21, 81–87 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Saunders-Pullman R., et al. , The effect of estrogen replacement on early Parkinson’s disease. Neurology 52, 1417–1421 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Carruth L. L., Reisert I., Arnold A. P., Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 5, 933–934 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Davies W., Sex differences in attention deficit hyperactivity disorder: Candidate genetic and endocrine mechanisms. Front. Neuroendocrinol. 35, 331–346 (2014). [DOI] [PubMed] [Google Scholar]
- 18.McCarthy M. M., Arnold A. P., Reframing sexual differentiation of the brain. Nat. Neurosci. 14, 677–683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyer C., Pilgrim C., Reisert I., Dopamine content and metabolism in mesencephalic and diencephalic cell cultures: Sex differences and effects of sex steroids. J. Neurosci. 11, 1325–1333 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantuti-Castelvetri I., et al. , Effects of gender on nigral gene expression and Parkinson disease. Neurobiol. Dis. 26, 606–614 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simunovic F., Yi M., Wang Y., Stephens R., Sonntag K. C., Evidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One 5, e8856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harley V. R., et al. , DNA binding activity of recombinant SRY from normal males and XY females. Science 255, 453–456 (1992). [DOI] [PubMed] [Google Scholar]
- 23.Sinclair A. H., et al. , A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990). [DOI] [PubMed] [Google Scholar]
- 24.Prokop J. W., et al. , Analysis of Sry duplications on the Rattus norvegicus Y-chromosome. BMC Genomics 14, 792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loke H., Harley V., Lee J., Biological factors underlying sex differences in neurological disorders. Int. J. Biochem. Cell Biol. 65, 139–150 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Clépet C., et al. , The human SRY transcript. Hum. Mol. Genet. 2, 2007–2012 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Dewing P., et al. , Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 16, 415–420 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Czech D. P., et al. , The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J. Neurochem. 122, 260–271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J. B., Chen K., Li Y., Lau Y. F., Shih J. C., Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J. 23, 4029–4038 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czech D. P., et al. , Transient neuroprotection by SRY upregulation in dopamine cells following injury in males. Endocrinology 155, 2602–2612 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Pomatto L. C. D., et al. , The mitochondrial Lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr. Biol. 27, 1–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misiak M., Beyer C., Arnold S., Gender-specific role of mitochondria in the vulnerability of 6-hydroxydopamine-treated mesencephalic neurons. Biochim. Biophys. Acta 1797, 1178–1188 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Navarro J. A., et al. , Gender differences and estrogen effects in parkin null mice. J. Neurochem. 106, 2143–2157 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Villa A., Vegeto E., Poletti A., Maggi A., Estrogens, neuroinflammation, and neurodegeneration. Endocr. Rev. 37, 372–402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ookubo M., Yokoyama H., Kato H., Araki T., Gender differences on MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in C57BL/6 mice. Mol. Cell. Endocrinol. 311, 62–68 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Mitra S., et al. , Gender-specific brain regional variation of neurons, endogenous estrogen, neuroinflammation and glial cells during rotenone-induced mouse model of Parkinson’s disease. Neuroscience 292, 46–70 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Perez A. I., et al. , Renin angiotensin system and gender differences in dopaminergic degeneration. Mol. Neurodegener. 6, 58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McArthur S., Murray H. E., Dhankot A., Dexter D. T., Gillies G. E., Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J. Neurochem. 100, 678–692 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Kadkhodaei B., et al. , Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J. Neurosci. 29, 15923–15932 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panman L., et al. , Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 8, 1018–1025 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Hirsch E. C., Hunot S., Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 8, 382–397 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Johnson M. E., Stecher B., Labrie V., Brundin L., Brundin P., Triggers, facilitators, and aggravators: Redefining Parkinson’s disease pathogenesis. Trends Neurosci. 42, 4–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekido R., Lovell-Badge R., Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Milsted A., et al. , Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci. Lett. 369, 203–207 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Zheng M., Lau Y. F., The sex-determining factors SRY and SOX9 regulate similar target genes and promote testis cord formation during testicular differentiation. Cell Rep. 8, 723–733 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Li Y., Oh H. J., Lau Y. F., The poly(ADP-ribose) polymerase 1 interacts with Sry and modulates its biological functions. Mol. Cell. Endocrinol. 257-258, 35–46 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Lee J., et al. , Sprouting of dopamine terminals and altered dopamine release and uptake in Parkinsonian dyskinaesia. Brain 131, 1574–1587 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.