Abstract
In contrast to Andean natives, high-altitude Tibetans present with a lower hemoglobin concentration that correlates with reproductive success and exercise capacity. Decades of physiological and genomic research have assumed that the lower hemoglobin concentration in Himalayan natives results from a blunted erythropoietic response to hypoxia (i.e., no increase in total hemoglobin mass). In contrast, herein we test the hypothesis that the lower hemoglobin concentration is the result of greater plasma volume, rather than an absence of increased hemoglobin production. We assessed hemoglobin mass, plasma volume and blood volume in lowlanders at sea level, lowlanders acclimatized to high altitude, Himalayan Sherpa, and Andean Quechua, and explored the functional relevance of volumetric hematological measures to exercise capacity. Hemoglobin mass was highest in Andeans, but also was elevated in Sherpa compared with lowlanders. Sherpa demonstrated a larger plasma volume than Andeans, resulting in a comparable total blood volume at a lower hemoglobin concentration. Hemoglobin mass was positively related to exercise capacity in lowlanders at sea level and in Sherpa at high altitude, but not in Andean natives. Collectively, our findings demonstrate a unique adaptation in Sherpa that reorientates attention away from hemoglobin concentration and toward a paradigm where hemoglobin mass and plasma volume may represent phenotypes with adaptive significance at high altitude.
Keywords: hypoxia, altitude, hemoglobin, Tibetans, Andeans
Hemoglobin concentration is a defining feature in the differential manifestations of adaptation between high-altitude Andeans and Tibetans (1). Tibetans demonstrate a hemoglobin concentration that would be comparable to sea level residents (1); the lower concentration is associated with greater reproductive success (2) and exercise capacity (3). Genome scans of polymorphisms have detected strong selective sweep signals in Tibetans at 2 loci, EGLIN1 and EPAS 1 (4, 5). However, a recent study of Tibetans found no clear evidence for polygenic adaptation for low hemoglobin concentration (2), questioning whether hemoglobin concentration is truly the target of selection, or merely a consequence or component of the true adaptive trait, as proposed previously (6). The study of Jeong et al. (2) found polygenic adaptation for a lower heart rate in Tibetan females that was also associated with greater reproductive success (2). However, it is unlikely that heart rate, per se, is the target for natural selection; rather, it is more likely that hematological adaptation or the downstream effect on cardiovascular function and cardiorespiratory capacity has been selected.
It has been widely assumed that the lower hemoglobin concentration in Tibetans is achieved via the absence of a significant erythropoietic response to hypoxia (1, 2). However, this oversimplification disregards the equally important contribution of plasma volume in the regulation of hematocrit (7). A larger plasma volume would decrease hemoglobin concentration and could decrease heart rate by mediating a larger stroke volume, but the importance of these volumetric measures has been largely ignored. Therefore, we sought to test the hypothesis that the lower hemoglobin concentration is a consequence of a larger hemoglobin mass and a greater plasma volume. Such a phenomenon would enable Tibetans to maximize total oxygen carrying capacity of the blood without the detrimental effect of a high viscosity on microcirculatory blood flow (8).
Results
Four separate populations were recruited: lowland natives 1) close to sea level (n = 16, 27 ± 6 y) and 2) after 10 ± 5 d at 5,050 m (n = 20, 30 ± 8 y), 3) Sherpa highland natives from the Khumbu Valley (5,050 m), Nepal (n = 20, 25 ± 7 y), and 4) Andean Quechua natives from Cerro de Pasco (4,340 m), Peru (n = 19, 32 ± 9 y; n = 7 with chronic mountain sickness) (Table 1).
Table 1.
Participant characteristics
| LL (244 m) | LL (5,050 m) | Sherpa | Andeans | ANOVA | |
| Height (cm) | 179 ± 5 | 177 ± 8 | 168 ± 5*,† | 161 ± 5*,†,‡ | P < 0.001 |
| Mass (kg) | 77 ± 10 | 71 ± 8 | 65 ± 11* | 67 ± 12* | P = 0.001 |
| Body mass index | 24.2 ± 2.6 | 22.7 ± 1.7 | 23.1 ± 3.4 | 25.7 ± 3.9†,‡ | P = 0.003 |
| SpO2 (%) | 98 ± 1 | 87 ± 2* | 87 ± 4* | 83 ± 7*,† | P = 0.003 |
| Hematocrit (%) | 47 ± 3 | 50 ± 3 | 49 ± 3 | 61 ± 8*,†,‡ | P < 0.001 |
| Peak VO2 (mL⋅kg−1⋅min−1) | 47.9 ± 5.5 | 32.0 ± 5.4 | 32.5 ± 6.6 | 36.3 ± 7.8 | P = 0.034 |
LL, lowlanders; VO2, volume of oxygen.
Vs. LL at 244 m.
Vs. LL at 5,050 m.
Vs. Sherpa.
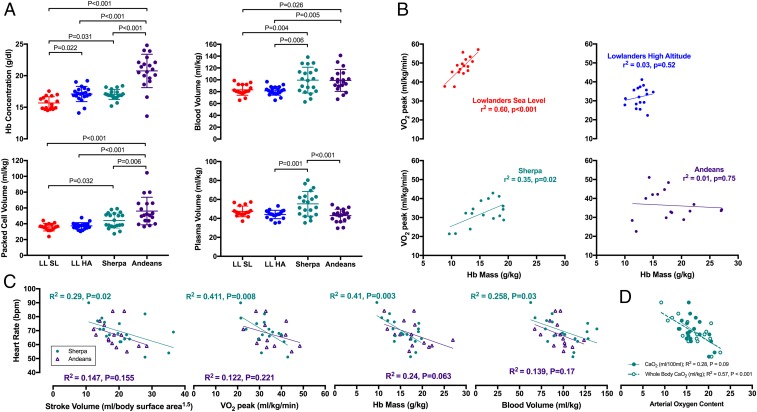
Sherpa and Andeans demonstrated a higher packed cell volume compared with lowlanders at sea level (Fig. 1A). Despite a much higher packed cell volume in Andeans, blood volume was comparable to Sherpa (P = 0.99) due to a larger plasma volume in the Sherpa. Hemoglobin concentration was not related to maximal oxygen consumption (VO2 peak) in any of the 4 groups examined. In contrast, hemoglobin mass was significantly related to VO2 peak in lowlanders at sea level, but not following acclimatization to high altitude (Fig. 1B). Sherpa also demonstrated a significant positive relationship between hemoglobin mass and VO2 peak at high altitude; however, this relationship was not present in Andean natives.
Fig. 1.
Hematological and cardiovascular variables demonstrating (A) between-group differences in blood volume, (B) the relationship between hemoglobin mass and exercise capacity, (C) factors related to the lower heart rate in Sherpa and Andeans, and (D) the different relationships between absolute (milliliters per kilogram) and relative (milliliters per 100 mL) oxygen content.
Heart rate was inversely related to stroke volume, VO2 peak, hemoglobin mass, and blood volume in Sherpa but not Andeans (Fig. 1C). Arterial oxygen content per 100 mL was weakly and nonsignificantly correlated to heart rate, whereas whole-body oxygen content showed a strong inverse relationship in Sherpa (R2 = 0.57, P < 0.001; Fig. 1D), but not in Andeans (R2 = 0.23, P = 0.16).
Discussion
Evidence of Erythrocytosis in High-Altitude Sherpa Is Masked by Plasma Volume Expansion.
The extensive search for the genetic origins of the lower hemoglobin mass has focused on hypoxia-inducible factor pathway genes (9). The widespread underlying assumption has been that the lower hemoglobin concentration in Tibetans is due to an absence of hypoxia-induced erythrocytosis. Herein, we demonstrate this is not the case, as Sherpa have a larger hemoglobin mass than lowlanders, but a considerably larger plasma volume. The result is an equivalent blood volume to Andeans but at a lower hemoglobin concentration. This difference is critically important, as it means Sherpa are able to benefit from the increased oxygen carrying capacity that comes from an expansion of their hemoglobin mass, but are not restricted by an increase in blood viscosity that hinders microcirculatory blood flow (8). Such hematological changes may have important implications for health, female fecundity, and, ultimately, selection. The focus on hemoglobin concentration as a target of natural selection for the last 2 decades has overlooked the regulatory role of the kidney as the “critmeter” (7). Here, hematocrit is regulated through the control of erythropoiesis and water retention and secretion via a feedback loop based on renal tissue oxygen pressure. Therefore, differences in renal physiology may exist to enable a higher renal oxygen pressure, potentially achieved via 1) lower levels of preglomerular arterio-venous shunting, 2) more efficient oxygen offloading, or 3) a lower oxygen demand from the kidney achieved via a metabolic adaptation, as seen in other tissues in Sherpa (10). Consequently, our understanding of how hemoglobin concentration is differentially regulated in high-altitude populations requires realignment.
Functional Consequences of Hemoglobin Mass in High-Altitude Natives.
The positive relationship between hemoglobin mass and exercise capacity in Sherpa at high altitude conflicts with the current understanding of oxygen transport in hypoxia, given that the convective elements are thought to have diminished importance with increasing altitude (11). We propose that this may only be the case when examined in isolation from other unique elements of adaptive physiology in Sherpa that include minimal hypoxic pulmonary vasoconstriction (12), higher lung diffusing capacity (13), capillary density, microcirculatory flow (14), and faster oxygen unloading rate (15). Collectively, these adaptations could shift the balance back toward the convectional components, as seen at sea level. These observations further highlight the oversimplification of searching for the genetic origins for one component of an integrative process such as oxygen transport.
Resting Heart Rate in Sherpa; Role of Hemoglobin Mass.
The inverse relationships observed between VO2 peak, stroke volume, blood volume, and hemoglobin mass suggest the lower heart rate to be a consequence of cardiorespiratory capacity and/or hematological acclimatization rather than an adaptive phenotype in itself. That heart rate showed the strongest relationship with whole-body oxygen carrying capacity—calculated from hemoglobin mass and not concentration—further emphasizes the importance of considering volumetric variables when investigating the adaptive phenotype. In future, it will be important to determine whether specific phenotypes related to the maintenance of an optimized hematological composition have been selected (16), especially in the context of pregnancy, due to the significance for reproductive success.
Conclusion.
Collectively, our findings reorientate focus toward volumetric measures of hematological adaptation in humans at high altitude. Future biological models, polygenetic searches, and epidemiological studies should prioritize volumetric measures of hematological adaptation as candidates for natural selection.
Materials and Methods
All experimental procedures were approved by the University of British Columbia Medical Research Ethics Board and Universidad Peruana Cayetano Heredia. Experimental procedures were explained both in writing and verbally in the participants’ native language, and written informed consent was provided.
Participants.
Both groups were males of European descent who regularly engaged in physical activity and were free from cardiorespiratory disease and nonsmokers (Table 1). The low-altitude group were studied at 344 m, and acclimatized lowlanders participated at the Ev-K2-CNR Pyramid Research Laboratory (5,050 m), following 10 ± 5 d of acclimatization.
Twenty self-identified male Sherpa were assessed at 5,050 m. High-altitude male Andean natives were recruited from the town of Cerro de Pasco in Peru (4,340 m). Nine Sherpa and 2 Andeans were current smokers. All participants were born and permanently reside above 3,000 m. Chronic mountain sickness (CMS) was diagnosed using the “Qinghai CMS Questionnaire” and hemoglobin concentration. There was no difference in age between those with CMS and those without (31.7 ± 7.3 vs. 31.4 ± 9.3 y, P = 0.77). None of the high-altitude natives had nonaltitude-related symptoms of disease, as determined during a one-on-one medical interview.
Hematological and Cardiovascular Measures.
In all 4 groups, hemoglobin mass was determined using the modified carbon monoxide rebreathing method (17). Venous blood samples were acquired before and after rebreathing in the seated position via an indwelling catheter for the determination of hemoglobin concentration and the percentage of carboxyhemoglobin (ABL 90; Radiometer). Hematocrit was assessed in triplicate via centrifugation (Hawksley). Left ventricular stroke volume was determined from 2D echocardiographic images using the Simpsons biplane method (Vivid q; GE) (18).
Maximal Exercise Capacity.
An incremental step test (20 W/min) to exhaustion was performed in the semirecumbent position on an electronically braked cycle ergometer (Lode Angio; Lode). Habituation with the cycle ergometer was performed for the high-altitude populations, and breath-by-breath respiratory data were collected throughout (Oxycon Mobile; Carefusion).
Statistical Analysis.
Data were tested for normality, and a logarithmic transformation was performed where data were nonnormally distributed. A one-way analysis of variance was then conducted, with pairwise post hoc tests completed and a Tukey adjusted P value reported, with linear regression performed to determine relationships.
Footnotes
The authors declare no conflict of interest.
References
- 1.Beall C. M., Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. U.S.A. 104 (suppl. 1), 8655–8660 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong C., et al. , Detecting past and ongoing natural selection among ethnically Tibetan women at high altitude in Nepal. PLoS Genet. 14, e1007650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonson T. S., et al. , Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J. Physiol. 593, 3207–3218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonson T. S., et al. , Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Beall C. M., et al. , Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. U.S.A. 107, 11459–11464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storz J. F., Scott G. R., Cheviron Z. A., Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125–4136 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly S., Why is erythropoietin made in the kidney? The kidney functions as a ‘critmeter’ to regulate the hematocrit. Adv. Exp. Med. Biol. 543, 73–87 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Fan F. C., Chen R. Y., Schuessler G. B., Chien S., Effects of hematocrit variations on regional hemodynamics and oxygen transport in the dog. Am. J. Physiol. 238, H545–H522 (1980). [DOI] [PubMed] [Google Scholar]
- 9.Semenza G. L., Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horscroft J. A., et al. , Metabolic basis to Sherpa altitude adaptation. Proc. Natl. Acad. Sci. U.S.A. 114, 6382–6387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner P. D., A theoretical analysis of factors determining VO2 MAX at sea level and altitude. Respir. Physiol. 106, 329–343 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Groves B. M., et al. , Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J. Appl. Physiol. 74, 312–318 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Faoro V., et al. , Pulmonary circulation and gas exchange at exercise in Sherpas at high altitude. J. Appl. Physiol. 116, 919–926 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Gilbert-Kawai E., et al. , Sublingual microcirculatory blood flow and vessel density in Sherpas at high altitude. J. Appl. Physiol 122, 1011–1018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies T., et al. , Sustained vasomotor control of skin microcirculation in Sherpas versus altitude-naive lowlanders: Experimental evidence from Xtreme Everest 2. Exp. Physiol. 103, 1494–1504 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Moore L. G., Measuring high-altitude adaptation. J. Appl. Physiol. 123, 1371–1385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt W., Prommer N., The optimised CO-rebreathing method: A new tool to determine total haemoglobin mass routinely. Eur. J. Appl. Physiol. 95, 486–495 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Lang R. M., et al. , Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39.e14 (2015). [DOI] [PubMed] [Google Scholar]