Abstract
Background
Human skin is an appropriate environment for the growth of different types of microbes that may inhabit the skin as commensal flora. This study aims at identifying the diversity of skin microbiota in healthy Saudi population. In this study, 80 Saudi subjects of both males and females, from different habitat, and different ages (elderly and young), were recruited to determine the aerobic bacterial flora from their three skin sites; hand, scalp and foot. A single colony obtained from aerobic culture was identified using Biomérieux VITEK® 2 system. For those not being identified by VITEK® 2 system, the identification was conducted using 16 s rRNA sequence.
Results
Thirty-three bacterial species were isolated from males, whilst 24 species were isolated from females. Micrococci are the predominant organisms, followed by Staphylococci, Pantoea species, and lastly Enterococcus faecium. Acinetobacter baumannii, Enterococcus faecalis, and Klebsiella pneumoniae were only found in elder subjects, while Pseudomonas aeruginosa was isolated from the young only. The number of bacterial isolates in the elders was higher that of the young. The average number of flora was larger in foot, then hand and lastly scalp.
Conclusion
Here we show the difference in the number of cultivable bacteria across age and gender that may result in the variety of local skin infection. This study paves the way to further investigation in the aspect of in-depth metagenomics analysis and host-pathogen interaction.
Electronic supplementary material
The online version of this article (10.1186/s12866-019-1569-5) contains supplementary material, which is available to authorized users.
Keywords: Microbiota, Skin, Aerobic bacteria, Staphylococci, Diversity
Background
Skin microbiota introduces the entire pool of microbes, which comprise bacteria, archaebacteria, fungi, virus and mites [1]. The variation of skin microbiota is seen in different parts of the body and between individuals [2]. Microbes that colonise skin could be influenced by skin microenvironment, such as moisture, sebaceous environment, by intrinsic features such as the age and gender, and by extrinsic features such as clothing, hygiene, humidity and occupation [3].
Previously, the microbes found on skin are often considered pathogenic organisms or symbiotic organisms. Nevertheless, recent data suggested that skin microbes, in healthy skin, play a role in host defence [4]. The chemical and physical factors of the skin could be the main reason for the adaptation of unique groups of microorganisms to inhabit specific region of the skin [5]. The arrangement of the skin surface differs due to regional variety in skin anatomy; culture-based studies indicated that these regions are identified to support distinct groups of microorganisms [2]. Features specific to the host, such as age, sex and location, also affected the diversity of the bacteria on the skin. Age has a great influence on the microenvironment of the skin and, consequently, on the colonising microbiota. The commence of microbiota colonisation in human remains under the argument. The colonisation can occurs directly after birth, either through vaginal delivery or in the minutes after birth by caesarean section, or it happens in utero [6, 7]. Previous studies demonstrated that healthy microbiota of the skin varies depending on microenvironment of the skin. Moreover, other individual factors, such as age and gender, have an impact on skin microbiota [8]. In Saudi Arabia there are very limited researches about the human microbiota. We have compiled three studies, one of which was on microbiota collected from the reproductive tracts of women [9], and the other two on gut microbiota [10, 11]. but, according to our knowledge this is the first work regarding the aerobic skin flora of Saudis. One of the main goals of this study is to identify bacterial fingerprint for Saudi community and to study the relation of these flora microbiota to diseases.
Results
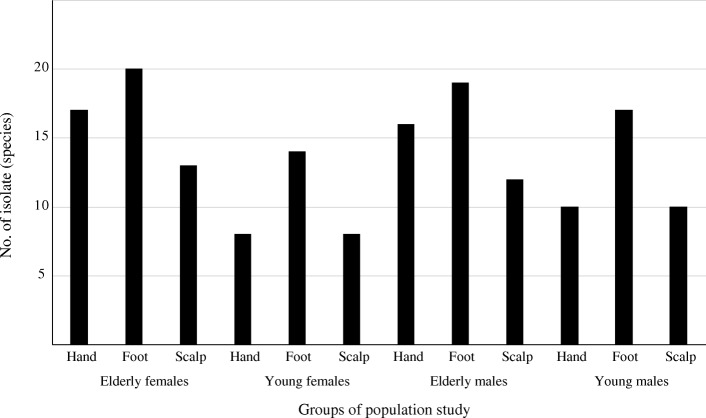
Eighty Saudi subjects from both sex groups (males and females), different habitat, and different ages (elder and young), were screened to determine the aerobic bacterial flora from their three skin sites; hand, scalp and foot (Additional file 1: Table S1). Biochemical tests were performed to isolate the bacteria along with molecular techniques. The results of all isolates that were identified using 16 s rRNA sequencing were in Genus Micrococcus. Thirty-three bacterial species were isolated from males, whilst 24 species were isolated from females. The sites and the total numbers of bacteria isolated from the different sites of subjects are illustrated in Fig. 1. The majority of bacteria were isolated from foot, followed by the hand and scalp. A one – factor ANOVA showed the significant difference of the amount of culturable bacteria from three different sites (scalp, hand and foot) (p < 0.05).
Fig. 1.
A bar chart illustrates the numbers of cultivable bacteria isolated from hands, hair and feet of elder female, young female, elder male, and young male
There was a significant effect of age and gender on the number of bacteria isolated for the four groups. By conducting post – hoc analysis, the mean numbers of culturable bacteria isolated from elder subjects (2.39 ± 0.99) was higher than young’s (1.82 ± 1.12) at the p < 0.05 level. The higher number of culturable isolates was found in old females (2.6 ± 0.94), in comparison with young females (1.45 ± 1.05). However, pairwise comparison of other pairs did not reveal significant difference. Taken together, these results suggested that the number of culturable aerobic bacteria varied by site, age and gender. Specifically, the foot of old females contained the highest number of culturable aerobic bacteria.
Out of 43 species identified, 19 species were isolated from male only and 10 species were from female only. The organisms isolated from both were listed in Table 1. Micrococcus spp. are the predominant organisms, followed by Staphylococcus spp., and Pantoea spp. Acinetobacter baumannii, Enterococcus faecalis, and Klebsiella pneumoniae were exclusively isolated from elder subjects, while Pseudomonas aeruginosa was isolated from the young only. The number of isolates obtained from the elderly were more than that of the young. Bacteria isolated only from elderly males represented 56% of total bacteria isolated from males (Additional file 1: Table S3) and bacteria isolated from elderly females represented 62.5% of total bacteria isolated from females (Additional file 1: Table S4).
Table 1.
A total number of bacteria commonly isolated from study population
| Isolate species | Elderly females | Young females | Elderly males | Young males |
|---|---|---|---|---|
| Acinetobacter baumannii | 1 | – | 2 | – |
| Enterococcus faecalis | 1 | – | 1 | – |
| Enterococcus faecium | – | 1 | 1 | – |
| Klebsiella pneumoniae | 1 | – | 1 | – |
| Kocuria kristinae | 4 | 1 | 2 | – |
| Micrococcus spp. | 9 | 12 | 11 | 15 |
| Pantoea spp | 3 | 2 | 5 | – |
| Pseudomonas aeruginosa | – | 1 | – | 2 |
| Pseudomonas luteola | 1 | – | 2 | 2 |
| Sphingomonas paucimobilis | 1 | – | 2 | 2 |
| Staphylococcus epidermidis | 4 | 4 | 1 | 1 |
| Staphylococcus haemolyticus | 11 | – | – | 2 |
| Staphylococcus hominis | 2 | 2 | 1 | 1 |
| Staphylococcus warneri | 3 | 1 | 1 | – |
| Total | 41 | 24 | 30 | 25 |
Discussion
The aerobic skin microbiota from three different sites of skin; scalp, hand and foot were investigated in 80 Saudi subjects live in Riyadh in a diversification of age and gender. There is a marked variation at the level of species, as some species were isolated exclusively from certain site and not isolated from others, however, other species have been isolated from two or even the three sites. The average number of flora was larger in foot, then hand and lastly scalp. There was also no significant difference between male and female subjects in the average number of overall detected bacteria. This finding was contrary to previous studies done by Ying et al. and Haro et al. which proved the differences in the bacterial community structure significantly related to gender [12, 13]. Interestingly, the mean numbers isolated only from elder subjects was significantly higher (p < 0.05) than young’s, (Additional file 1: Tables S3 and S4). At this outcome, a preceding study carried out by Leyden et al. reinforces our findings that the quantitative levels of resident aerobic and anaerobic bacteria of the face show a characteristic age-related pattern [14]. Furthermore, studies reported that the age of the individual was found to influence the bacterial flora [15, 16]. It is important to note here that these previous findings apply only to ours, regarding elder women, but intriguingly, multiple comparison detect no significant difference between elder males, young males and young females.
Out of 43 diverse organisms, Micrococci and Staphylococci are the most frequently isolated organisms. Congruently, Somerville investigated the normal flora of the skin in different age groups. It was found that coagulase negative staphylococci and micrococci were found in, virtually, all skin sites in each person [15]. With few exceptions, the bacteria isolated in this work are well documented as human pathogens in healthy and immunocompromised individuals and some are reported as emergent pathogens. Interestingly, several well-known species, including E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp., were observed as skin flora in this study. These species might be environmental organisms or hospital-associated organisms. It is believed that close contact can provide the transmission of skin colonisers; however, there is no strong evidence indicating skin infection caused by skin flora.
By investigating Genus Enterobacter, E. cloacae, a well-known nosocomial pathogen, was identified from the skin culture [17]. Enterococci are opportunistic pathogens in individuals with serious diseases whose immune systems are compromised and in patients who have been hospitalised for prolonged periods or who have received broad-spectrum antimicrobial therapy [18]. There were three species of Enterococci identified: E. faecalis, E. faecium, and E. casseliflavus. E. faecalis and E. faecium were obtained from both males and females. Nevertheless, E. casseliflavus was found from hands and feet in male. Even though the species was reported associated with biliary tract disease in human, the infection caused by E. casseliflavus remained unknown [19, 20].
Coagulase-negative staphylococci (CoNS) becomes more important in nosocomial infections [21]. In exception of S. lentus, an animal pathogens which was recently reported to cause peritonitis in human [22], all CoNS were known to cause human infections. For example, S. capitis was reported to be associated with septic arthritis in prosthetic joint [23]. S. gallinarum was found associated with traumatic endophthalmitis after iron nail injury [24]. Interestingly, S. saprophyticus, which is a common skin commensal that is associated with urinary tract infection in women, was absent in the present study [25].
For unusual species, Escherichia hermannii and Klebsiella oxytoca were isolated from male elderly subjects only. The former was mainly found in environment. However, it was also recovered from clinical samples (i.e. purulent wound and discharge) and it was found as both mixed and sole infectious isolates. The latter is considered an opportunistic pathogen that carries drug-resistance genes [26, 27].
According to our findings, there are species most likely to be found in less frequency in the healthy subjects. Notwithstanding, it is possible that low abundance skin microbes (< 1%) can be recovered by the culture [28]. Hence, further study using molecular investigation, such as metagenomic approach, should be applied to determine broader, especially, uncultivable species.
Conclusion
Our results suggested that, bacterial diversity of flora is different across age and gender. The number of flora seen in elderly is more than that of the young. The foot contains the highest number of flora, followed by the hand and the scalp, respectively. Indeed, this study provides a preliminary step for researches on the skin microbiota of Saudis, however its findings do not reflect the final data. Therefore, the metagenomic analyses of skin flora, especially anaerobes, higher sample size and more cofactors variables will be considered in the future. The finding will pave the way for the study of association between flora and systemic diseases.
Methods
Study population
Eighty Saudi adults from Riyadh were enrolled in this study (Additional file 1: Table S1). They were divided into four groups according to their gender and age; elder females, elder males, young females and young males whose average ages were 62, 51, 20 and 21, respectively.
Study area and sample collection
The study was conducted in the Princess Nourah bint Abdulrahman University and King Saud University Research Center in Riyadh. The samples were collected by sterile cotton swabs from most susceptible skin areas that contact with exogenous environmental factors and more susceptible pollution such as (hand, foot, and scalp), then were kept in 4 °C for less than 2 h. The sample collection process in this study was conducted for research purpose only.
Identification of isolates
Swaps were inoculated on blood agar, tryptic Soy agar and MacConkey agar and incubated aerobically overnight at 37 °C. The bacteria were identified biochemically using Biomérieux VITEK® 2 system.
Molecular identification
Bacteria that weren’t recognized by Biomérieux VITEK® 2 system were identified by 16 s rRNA. DNA was extracted and purified using a QIAGEN DNeasy Blood & Tissue kit according to the manufacturer’s instructions. 16 s rRNA universal primers (Additional file 1: Table S2) were used and PCR products were purified using ExoSAP-IT (Usb. Affymetrix, Inc). The PCR products were treated with ExoSAP-IT, and then subjected to 16 s rRNA sequencing (MOLECULE-ON, New Zealand).
Data analysis
The data were analysed using R software. Descriptive statistics were performed for quantitative variables; the results were expressed as mean ± standard deviation in each group. One-Sample Kolmogorov-Smirnov and Levene statistic were carried out to test the distribution normality and Homogeneity of bacteria’s numbers respectively. We used one-way ANOVA to test the difference between the four groups (elder males, elder females, young males and young females). Because the variances of bacteria’s numbers among the three skin sites were unequal, we adopted Welch’s ANOVA, followed by Tukey test (to compare the means between groups). The mean difference is significant at the 0.05 level.
Additional file
Table S1. The summary of demographic data of healthy participants. Table S2. The DNA sequence of PCR primers for 16 s rDNA sequence in this study. Table S3. Species and numbers of bacterial flora isolated from males. Table S4. Species and numbers of bacteria isolated from females. (DOCX 28 kb)
Acknowledgements
The authors would like to thank all of the staffs of Princess Nourah bint Abdulrahman University and King Saud University Research Center for their help in sample collection.
Abbreviations
- ANOVA
Analysis of variance
- CoNS
Coagulase-negative Staphylococci
- PCR
Polymerase chain reaction
- rRNA
Ribosomal RNA
Authors’ contributions
AS planed and conducted most of the study. AS and PP performed data analysis and wrote the manuscript. AS and SAM collected the samples. AS and AAB carried out DNA extraction. SAR helped to draft the manuscript. All authors read and approved the manuscript.
Funding
This research was funded by Deanship of Scientific Research at Princess Nourah bint Abdulrahman University (Grant number 012508-F08). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional file.
Ethics approval and consent to participate
The techniques used in the study were in accordance with national regulations that govern the protection of human subjects, rules and regulations of the Government of Saudi Arabia, the Princess Nourah bint Abdulrahman Institutional Review Board Policies and procedures, and the ICH Good Clinical Practice guidelines. The protocol of this study was approved by Princess Nourah bint Abdulrahman University IRB with IRB Registration Number with KACS’I, KSA: H-01-R-059 and written informed consent from all the participants in this study was obtained. Urine sample was obtained with written informed consent of the participant. All participants recruited in this study were older than 16 years old.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashwag Shami, Email: ayshami@pnu.edu.sa.
Samiah Al-Mijalli, Email: dr.samiah10@hotmail.com.
Pisut Pongchaikul, Email: pisut.pongchaikul@gmail.com.
Ahmed Al-Barrag, Email: albarrak@ksu.edu.sa.
Samah AbduRahim, Email: samahawad90@yahoo.com.
References
- 1.Kong HH. Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med. 2011;17(6):320–328. doi: 10.1016/j.molmed.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson ELCA, Whittier S, Lai L, Lyle CT, Della Latta P. Differences in skin flora between inpatients and chronically ill outpatients. Heart Lung. 2000;29(4):7. doi: 10.1067/mhl.2000.108324. [DOI] [PubMed] [Google Scholar]
- 4.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158(3):442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 2001;6(3):170–174. doi: 10.1046/j.0022-202x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes. 2017;12:15. doi: 10.1111/ijpo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibagaki N, Suda W, Clavaud C, Bastien P, Takayasu L, Iioka E, Kurokawa R, Yamashita N, Hattori Y, Shindo C, et al. Aging-related changes in the diversity of women's skin microbiomes associated with oral bacteria. Sci Rep. 2017;7(1):10567. doi: 10.1038/s41598-017-10834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Rezaki A, Aldahmash W, Alwassel S, Abotalib Z. Assessment of the Microbiome collected from the reproductive tracts of women from Saudi Arabia and its potential influence on infertility. Adv Reprod Sci. 2017;05(02):23–32. doi: 10.4236/arsci.2017.52003. [DOI] [Google Scholar]
- 10.Yasir M, Angelakis E, Bibi F, Azhar EI, Bachar D, Lagier JC, Gaborit B, Hassan AM, Jiman-Fatani AA, Alshali KZ, et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr Diabetes. 2015;5:e153. doi: 10.1038/nutd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelakis E, Yasir M, Bachar D, Azhar EI, Lagier JC, Bibi F, Jiman-Fatani AA, Alawi M, Bakarman MA, Robert C, et al. Gut microbiome and dietary patterns in different Saudi populations and monkeys. Sci Rep. 2016;6:32191. doi: 10.1038/srep32191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying S, Zeng DN, Chi L, Tan Y, Galzote C, Cardona C, Lax S, Gilbert J, Quan ZX. The influence of age and gender on skin-associated microbial communities in urban and rural Human populations. PLoS One. 2015;10(10):e0141842. doi: 10.1371/journal.pone.0141842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, Quintana-Navarro GM, Landa BB, Navas-Cortés JA, Tena-Sempere M, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11(5):e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Age-related changes in the resident bacterial flora of the human face. J Invest Dermatol. 1975;65(4):379–381. doi: 10.1111/1523-1747.ep12607630. [DOI] [PubMed] [Google Scholar]
- 15.Somerville DA. The normal flora of the skin in different age groups. Br J Dermatol. 1969;81(4):248–258. doi: 10.1111/j.1365-2133.1969.tb13976.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison LM, Morris JA, Telford DR, Brown SM, Jones K. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol. 1999;25(1–2):19–28. doi: 10.1111/j.1574-695X.1999.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 17.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davin-Regli A, Pages JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392. doi: 10.3389/fmicb.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo-Rojas G, Mazari-Hiriart M, Ponce de Leon S, Amieva-Fernandez RI, Agis-Juarez RA, Huebner J, Lopez-Vidal Y. Comparison of enterococcus faecium and enterococcus faecalis strains isolated from water and clinical samples: antimicrobial susceptibility and genetic relationships. PLoS One. 2013;8(4):e59491. doi: 10.1371/journal.pone.0059491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SH, Lee SO, Kim TH, Chung JW, Choo EJ, Kwak YG, Kim MN, Kim YS, Woo JH, Ryu J, et al. Clinical features and outcomes of bacteremia caused by enterococcus casseliflavus and enterococcus gallinarum: analysis of 56 cases. Clin Infect Dis. 2004;38(1):53–61. doi: 10.1086/380452. [DOI] [PubMed] [Google Scholar]
- 21.Shields BE, Tschetter AJ, Wanat KA. Staphylococcus simulans: an emerging cutaneous pathogen. JAAD Case Rep. 2016;2(6):428–429. doi: 10.1016/j.jdcr.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera M, Dominguez MD, Mendiola NR, Roso GR, Quereda C. Staphylococcus lentus peritonitis: a case report. Perit Dial Int. 2014;34(4):469–470. doi: 10.3747/pdi.2012.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tevell S, Hellmark B, Nilsdotter-Augustinsson A, Soderquist B. Staphylococcus capitis isolated from prosthetic joint infections. Eur J Clin Microbiol Infect Dis. 2017;36(1):115–122. doi: 10.1007/s10096-016-2777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibra NK, Jalali S, Reddy AK, Narayanan R, Agarwal R. Traumatic endophthalmitis caused by Staphylococcus gallinarum. J Med Microbiol. 2010;59(Pt 3):365–366. doi: 10.1099/jmm.0.011551-0. [DOI] [PubMed] [Google Scholar]
- 25.M C . Medical laboratory manual for tropical countries. Cambridge: Butterworth & Co Ltd (pub); 2008. [Google Scholar]
- 26.Poulou A, Dimitroulia E, Markou F, Tsakris A. Escherichia hermannii as the sole isolate from a patient with purulent conjunctivitis. J Clin Microbiol. 2008;46(11):3848–3849. doi: 10.1128/JCM.01119-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsakris A, Poulou A, Markou F, Pitiriga V, Piperaki E-T, Kristo I, Pournaras S. Dissemination of clinical isolates of Klebsiella oxytoca harboring CMY-31, VIM-1, and a new OXY-2-type variant in the community. Antimicrob Agents Chemother. 2011;55(7):3164–3168. doi: 10.1128/AAC.00102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YE, Tsao H. The skin microbiome: current perspectives and future challenges. J Am Acad Dermatol. 2013;69(1):143–155. doi: 10.1016/j.jaad.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The summary of demographic data of healthy participants. Table S2. The DNA sequence of PCR primers for 16 s rDNA sequence in this study. Table S3. Species and numbers of bacterial flora isolated from males. Table S4. Species and numbers of bacteria isolated from females. (DOCX 28 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional file.