Abstract
Background
Low-grade glioma is grade I-II glioma. Immunotherapy is a promising way of tumor killing. Research on immune molecular mechanisms in low-grade gliomas and discovery of new immune checkpoints for low-grade gliomas are of great importance.
Methods
Gene sequencing data and clinical data of low-grade glioma were downloaded from TCGA database. Prognosis related lncRNAs were identified by Cox regression and their possible functions were found by gene enrichment set analysis.
Results
A total of 529 low-grade glioma samples and 5 non-tumor brain tissue samples are obtained from the TCGA database. Two hundred forty-seven immune-associated lncRNAs are screened. Cox regression showed that 16 immune-related lncRNAs are associated with low-grade glioma prognosis, and 7 lncRNAs are independent risk factors. Gene set enrichment analysis suggests that these molecules are enriched in extracellular region, sequence-specific DNA binding, neuropeptide signaling pathway, transcriptional misregulation in cancer, cytokine-cytokine receptor interaction, protein digestion and absorption, chemokine signaling pathway, etc.
Conclusion
The identification of immune-related lncRNA may provide new targets for the research of the molecular mechanisms and treatment of low-grade glioma.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-6032-3) contains supplementary material, which is available to authorized users.
Keywords: Low-grade glioma, lncRNA, Immune, Prognosis
Background
Low-grade glioma is grade I-II glioma, the main components of which are oligodendroglioma and astrocytoma. Prognosis of low-grade glioma is better than high-grade glioma, suggesting that the pathogenesis of low-grade glioma and high-grade glioma is different [1]. The current treatment of low-grade gliomas still tends to be based on surgically based comprehensive treatment [2, 3]. Immunotherapy is a new way of killing tumors. Among them, blockers for the PD-1/PD-L1 pathway have achieved great success in melanoma [4, 5]. Although glioma immunotherapy has a long history, the effect is unsatisfactory [6]. Therefore, the study of immune molecular mechanisms for low-grade gliomas and the discovery of new immune checkpoints are important for the treatment of low-grade gliomas. Long non-coding RNA (lncRNA) is a kind of non-coding RNA of more than 200 nucleotides in length, which is involved in epigenetic regulation, alternative splicing, post-transcriptional regulation and other gene regulation methods in gliomas [7]. This study identified immune-related lncRNAs in low-grade gliomas and explored the relationship between these immune-related lncRNAs and the prognosis of low-grade gliomas.
Methods
Acquisition of low-grade glioma expression data
Low-grade glioma non-tumor brain tissue RNA-Seq data (level 3) and clinical data were downloaded from the TCGA (https://cancergenome.nih.gov/) database. “edgeR” package of R software was used to normalize the whole dataset and obtain the differentially expressed genes. |log2FC| > 2 and false discovery rate (FDR) < 0.05 were used as threshold. All of these data were retrieved from TCGA database which are open to the public under guidelines, so it is confirmed that all informed consent was achieved.
Immune-associated lncRNAs
Immune regulatory factor list was downloaded from the InnateDB database (www.innatedb.com). Correlation between the molecules was calculated. lncRNAs with correlation coefficient > 0.7 and P < 0.05 were used for further analysis.
Cox regression
Univariate Cox regression was performed on immune-related lncRNA and clinical survival data to identify prognostic-related lncRNAs (Efron approximation was used). Stepwise regression multivariate Cox analysis was performed to establish a risk score. The risk score is expressed as: risk score = βgene1 × Expressiongene1 + βgene2 × Expressiongene2 + βgene3 × Expressiongene3 + ... + βgenen × Expressiongenen. Kaplan-Meier survival curve based on risk scores was drew.
Gene set enrichment analysis
GO (gene ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis of low-grade glioma immune-related lncRNAs was performed on the DAVID website (https://david.ncifcrf.gov/) to explore potential biological pathways that immune-related lncRNA may be involved in.
Statistical analysis
R software 3.6.0 was used to conduct all statistical analyses in this study. P < 0.05 was considered statistically different. The Pearson correlation test analyzes the correlation between molecules.
Results
Differentially expressed lncRNAs in low-grade glioma
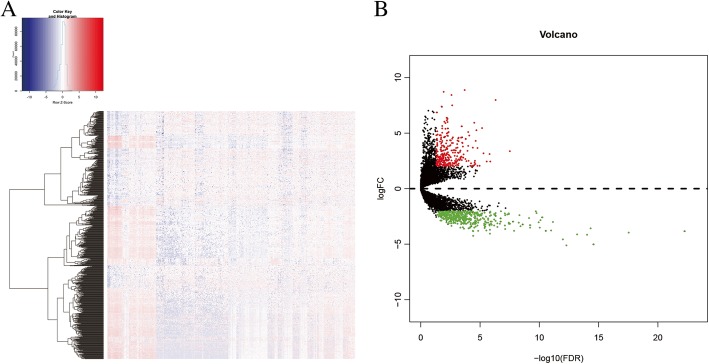
A total of 529 low-grade glioma samples and 5 non-tumor brain tissue samples were obtained from the TCGA database. The median age of diagnosis was 41.2 years (14.4–87.1 years). Among them, there are 282 males and 227 females. Three hundred eighty-four patients survived and 125 died at the point of the last follow-up. By contrasting the tumor samples and normal samples, 717 glioblastomas differentially expressed lncRNAs were screened with the threshold of |log2FC| > 2 and FDR < 0.05. Among them, 295 lncRNAs expression were up-regulated and 422 lncRNAs expression were down-regulated. The heatmap and volcano map of differentially expressed lncRNAs are shown in Fig. 1.
Fig. 1.
Differential expression lncRNAs. a Heatmap of differential expression lncRNAs. b Volcano plot of differential expression lncRNAs
Immune-associated lncRNAs in low-grade glioma
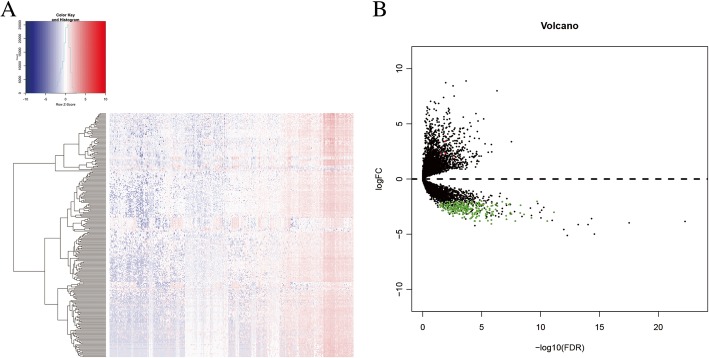
The list of immunoregulatory genes was downloaded from the InnateDB database, and we extracted the immunomodulatory genes. Interestingly, we identified more down-regulated immuno-related lncRNAs (242 lncRNAs) than up-regulated immune-related lncRNAs (5 lncRNAs). The heatmap and volcano map of the immune-related lncRNAs in low-grade glioma are shown in Fig. 2.
Fig. 2.
Differential expression immune-associated lncRNAs. a Heatmap of differential expression immune-associated lncRNAs. b Volcano plot of differential expression immune-associated lncRNAs
Cox regression
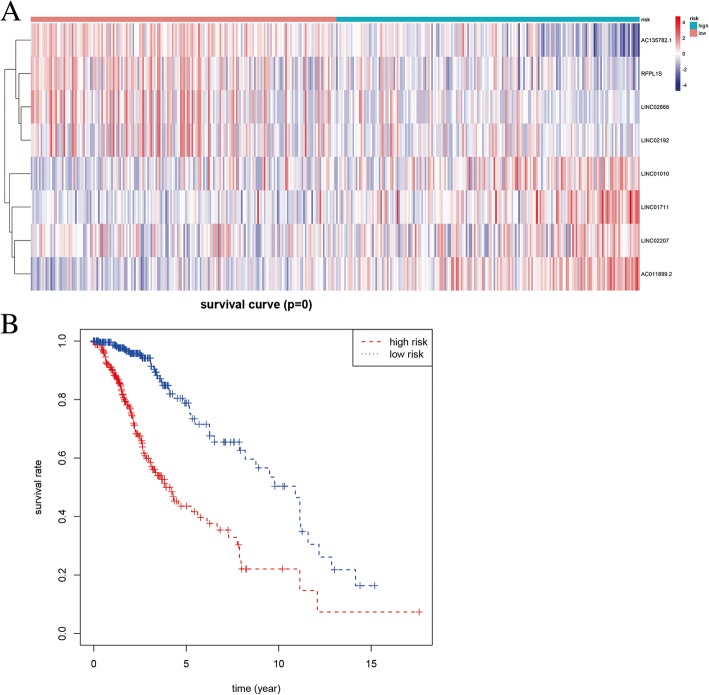
We used “caret” package of R language to divide the glioma samples into training cohort and validation cohort by the ratio of 7:3. The expression matrix of 247 immune-related lncRNAs were fused with survival data, and univariate Cox regression was used to analyze the prognostic risk factors of low-grade glioma in the training cohort first. A total of 16 lncRNAs were identified as prognostic risk factors. Stepwise regression multivariate Cox regression was performed to establish the risk score. Eight lncRNAs entered the risk scoring model, risk score = 0.281 * ExpressionLINC01010–0.271 * Expression AC135782.1 + 0.214* Expression LINC01711–0.196* Expression RFPL1S - 0.262* Expression LINC02668–0.143* Expression LINC02207 + 0.289* Expression AC011899.2 + 0.319 * Expression LINC02192. Among the 8 lncRNAs, 7 immune-related lncRNAs were independent prognostic risk factors for low-grade glioma. The results of the univariate and multivariate Cox regression models are shown in Table 1. The 8-lncRNAs heatmap involved in constructing the risk scoring model are shown in Fig. 3a. According to the median value of the risk score, low-grade glioma patients were divided into high-risk group and low-risk group in the training cohort. We found that the overall survival time of patients in the high-risk group was much lower than that in the low-risk group (as shown in Fig. 3b).
Table 1.
Univariate analysis and multivariate analysis of immune-associated lncRNAs
| lncRNA | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| LINC01010 | 1.018 | 0.003 | 1.325 | 0.001 |
| AC135782.1 | 0.995 | 0.030 | 0.763 | 0.002 |
| LINC01711 | 1.001 | 0.041 | 1.238 | 0.001 |
| RFPL1S | 1.000 | 0.029 | 0.822 | 0.029 |
| LINC02668 | 0.980 | 0.023 | 0.770 | 0.010 |
| LINC02207 | 1.014 | 0.024 | 0.867 | 0.109 |
| AC011899.2 | 1.004 | < 0.001 | 1.335 | 0.003 |
| LINC02192 | 0.991 | 0.037 | 1.375 | < 0.001 |
Fig. 3.
Survival-associated immune mRNAs. a Heatmap of survival-associated immune lncRNAs. b Kaplan–Meier survival curves for survival-associated immune lncRNAs
The predicting performance of the 8-lncRNAs model was calculate in both training cohort and validation cohort by the area under ROC (Receiver operating characteristic) curve (AUC). The ROC curve had a 3-year survival AUC area of 0.845 and a 5-year survival AUC area of 0.746 in the training cohort while The ROC curve had a 3-year survival AUC area of 0.810 and a 5-year survival AUC area of 0.738 in the training cohort, as shown in Additional file 1: Figure S1.
Gene set enrichment analysis
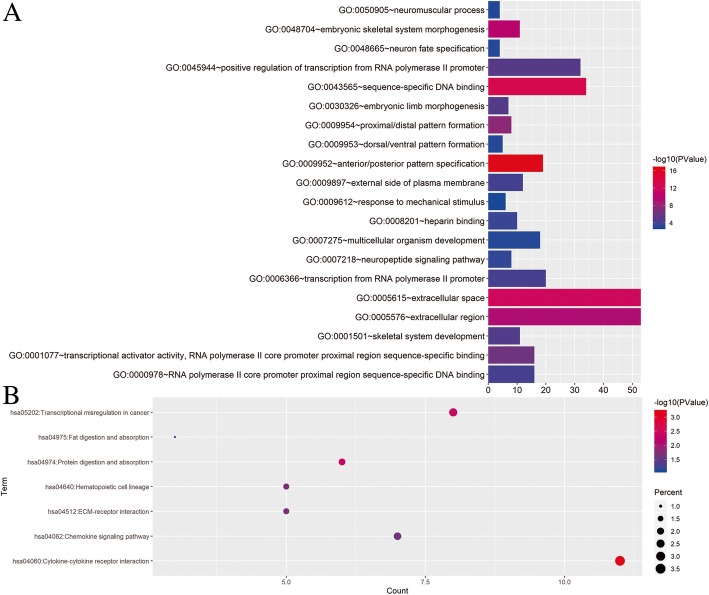
GO and KEGG enrichment analysis were performed on the differentially expressed gene sets of the above high-risk and low-risk groups. The results are shown in Fig. 4. Taking P < 0.05 as the statistical threshold, GO enrichment analysis indicated that the genes were enriched in extracellular region, sequence-specific DNA binding, neuropeptide signaling pathway, etc. KEGG enrichment analysis suggested that these genes were involved in transcriptional misregulation in cancer, cytokine-cytokine receptor interaction, protein digestion and absorption, chemokine signaling pathway, etc. These enriched items may help scientists and doctors determine the directions of further research of the mechanisms by which immune-related lncRNAs affecting glioma.
Fig. 4.
Functional enrichment analysis. a GO biological process enrichment results. b KEGG biological process enrichment results
Discussion
Targeted therapy for immune checkpoints is one method of tumor immunotherapy. Immune regulation against immune checkpoints can lead to tumor cell death by providing immune response signals to T cells [8]. Classical tumor immune checkpoints include PD-1, PD-L1, PD-L2 and CTLA-4. So far, ipilimumab (CTLA-4 blocking antibody), and Pembrolizumab and Nivolumab (PD-1 blocking antibody) have been approved by the FDA. Satisfactory results are presented in the treatment of melanoma [9]. However, there are no effective immune checkpoints for the treatment of glioma [10]. For instance, immunohistochemistry experiments show that PD-L1 appears to be highly expressed only in grade IV gliomas [11]. There is a strong need to screen and research of new immune checkpoints for low-grade glioma.
SCHLAP1 is one of the low-grade glioma immune-related lncRNAs we screened. It has been reported that SCHLAP1 is up-regulated in prostate cancer compared with benign prostatic hyperplasia and normal tissue [12–16]. SCHLAP1 promotes proliferation and metastasis of prostate cancer by targeting miR-198 and promoting MAPK1 pathway [17]. In bladder cancer, SCHLAP1 acts as a pro-oncogene, and silencing SCHLAP1 induces proliferation of bladder cancer cells, promotes apoptosis, and inhibits cell migration [18]. In addition, CALML3-AS1 is also one of the low-grade glioma immune-related lncRNAs we screened, and it has been reported to inhibit microRNA-4316 in bladder cancer, thereby upregulating ZBTB2 and promoting tumorigenesis of bladder cancer [19].
Conclusion
This study identified 247 immune-related lncRNAs in low-grade glioma. Cox regression analysis showed that 16 lncRNAs were associated with prognosis in patients with low-grade glioma, and 7 lncRNAs were independent prognostic risk factors. Gene set enrichment analysis revealed that these immune-related lncRNAs may be involved in functions such as extracellular region, sequence-specific DNA binding, neuropeptide signaling pathway, transcriptional misregulation in cancer, cytokine-cytokine receptor interaction, protein digestion and absorption, chemokine signaling pathway, etc. The identification of immune-related lncRNA may provide new targets for the research of the molecular mechanisms and treatment of low-grade glioma.
Additional file
Figure S1. Evaluation of prognostic performance of the model. (A) ROC curves of training cohort. (B) ROC curves of validation cohort. (TIF 696 kb)
Acknowledgements
Not applicable.
Abbreviations
- AUC
The area under ROC curve
- GO
Gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- lncRNA
Long non-coding RNA
- ROC
Receiver operating characteristic
Authors’ contributions
Conceived and designed the study: YM. Performed the data analysis: XL. Checked the data: XL and YM. Obtained the original data from the database: XL. Wrote the paper: XL. Critical review of the manuscript: YM. All authors have read and approved the manuscript.
Funding
Not applicable.
Availability of data and materials
This study obtained open data from the TCGA database. (https://cancergenome.nih.gov/).
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shengjing Hospital of China Medical University and conformed to the provisions of the Declaration of Helsinki. Data of our present study was downloaded from an open database TCGA, so there was no informed consent from participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olar A, Sulman EP. Molecular markers in low-grade glioma-toward tumor reclassification. Semin Radiat Oncol. 2015;25(3):155–163. doi: 10.1016/j.semradonc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol. 2015;125(3):503–530. doi: 10.1007/s11060-015-1867-1. [DOI] [PubMed] [Google Scholar]
- 3.Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G, Solheim O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. Jama. 2012;308(18):1881–1888. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 4.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 5.Wan MT, Ming ME. Nivolumab versus ipilimumab in the treatment of advanced melanoma: a critical appraisal: ORIGINAL ARTICLE: Wolchok JD, Chiarion-Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345–56. Br J Dermatol. 2018;179(2):296–300. doi: 10.1111/bjd.16785. [DOI] [PubMed] [Google Scholar]
- 6.McGranahan T, Therkelsen KE, Ahmad S, Nagpal S. Current state of immunotherapy for treatment of glioblastoma. Curr Treat Options in Oncol. 2019;20(3):24. doi: 10.1007/s11864-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rynkeviciene Ryte, Simiene Julija, Strainiene Egle, Stankevicius Vaidotas, Usinskiene Jurgita, Miseikyte Kaubriene Edita, Meskinyte Ingrida, Cicenas Jonas, Suziedelis Kestutis. Non-Coding RNAs in Glioma. Cancers. 2018;11(1):17. doi: 10.3390/cancers11010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romani M, Pistillo MP, Carosio R, Morabito A, Banelli B. Immune checkpoints and innovative therapies in glioblastoma. Front Oncol. 2018;8:464. doi: 10.3389/fonc.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen RQ, Liu F, Qiu XY, Chen XQ. The prognostic and therapeutic value of PD-L1 in glioma. Front Pharmacol. 2018;9:1503. doi: 10.3389/fphar.2018.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber ST, Hashimoto Y, Weathers SP, Xiu J, Gatalica Z, Verhaak RG, Zhou S, Fuller GN, Khasraw M, de Groot J, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol. 2016;18(10):1357–1366. doi: 10.1093/neuonc/now132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua MLK, Lo W, Pintilie M, Murgic J, Lalonde E, Bhandari V, Mahamud O, Gopalan A, Kweldam CF, van Leenders G, et al. A prostate Cancer “Nimbosus”: genomic instability and SChLAP1 dysregulation underpin aggression of Intraductal and cribriform subpathologies. Eur Urol. 2017;72(5):665–674. doi: 10.1016/j.eururo.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Cimadamore A, Gasparrini S, Mazzucchelli R, Doria A, Cheng L, Lopez-Beltran A, Santoni M, Scarpelli M, Montironi R. Long non-coding RNAs in prostate Cancer with emphasis on second chromosome locus associated with Prostate-1 expression. Front Oncol. 2017;7:305. doi: 10.3389/fonc.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehra R, Udager AM, Ahearn TU, Cao X, Feng FY, Loda M, Petimar JS, Kantoff P, Mucci LA, Chinnaiyan AM. Overexpression of the Long non-coding RNA SChLAP1 independently predicts lethal prostate Cancer. Eur Urol. 2016;70(4):549–552. doi: 10.1016/j.eururo.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouraviev V, Lee B, Patel V, Albala D, Johansen TE, Partin A, Ross A, Perera RJ. Clinical prospects of long noncoding RNAs as novel biomarkers and therapeutic targets in prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(1):14–20. doi: 10.1038/pcan.2015.48. [DOI] [PubMed] [Google Scholar]
- 16.Wang YH, Ji J, Wang BC, Chen H, Yang ZH, Wang K, Luo CL, Zhang WW, Wang FB, Zhang XL. Tumor-derived Exosomal Long noncoding RNAs as promising diagnostic biomarkers for prostate Cancer. Cell Physiol Biochem. 2018;46(2):532–545. doi: 10.1159/000488620. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Luo H, Xiao N, Duan J, Wang Z, Wang S. Long noncoding RNA SChLAP1 accelerates the proliferation and metastasis of prostate Cancer via targeting miR-198 and promoting the MAPK1 pathway. Oncol Res. 2018;26(1):131–143. doi: 10.3727/096504017X14944585873631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Shi Z, Nan Y, Li M. Inhibiting malignant phenotypes of the bladder cancer cells by silencing long noncoding RNA SChLAP1. Int Urol Nephrol. 2016;48(5):711–716. doi: 10.1007/s11255-016-1230-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Zu Y, Huang W, Chen H, Xie H, Yang Y. LncRNA CALML3-AS1 promotes tumorigenesis of bladder cancer via regulating ZBTB2 by suppression of microRNA-4316. Biochem Biophys Res Commun. 2018;504(1):171–176. doi: 10.1016/j.bbrc.2018.08.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Evaluation of prognostic performance of the model. (A) ROC curves of training cohort. (B) ROC curves of validation cohort. (TIF 696 kb)
Data Availability Statement
This study obtained open data from the TCGA database. (https://cancergenome.nih.gov/).