Abstract
Objective
Immuno-compromised individuals with latent tuberculosis infection (LTBI) are at an increased risk for tuberculosis reactivation compared with the general population. The aim of this study was to determine the prevalence of latent tuberculosis infection among people living with human immunodeficiency virus (PLWH) and apparently healthy blood donors. Human Immunodeficiency Virus positive individuals and for the purpose of comparison apparently healthy blood donors were enrolled. Blood sample was collected and tested for LTBI using QuantiFeron-TB Gold In-Tube assay (QFT-GIT) and CD4+ T cell count was determined by using BD FACS count.
Results
The overall prevalence of LTBI regardless of HIV status was 46%. The prevalence of LTBI among PLWH was 44% and that of blood donors 48%. ART naïve HIV positive patients were three times more likely to have LTBI than patients under ART treatment (P = 0.04). Data also showed statistically significant negative association between previous or current preventive INH therapy and LTBI among HIV positive cases (P = 0.005). The proportion of LTBI was slightly lower among HIV positive individuals than apparently healthy blood donors. Nevertheless, HIV positive individuals should be screened for LTBI and take INH prophylaxis.
Keywords: Tuberculosis, Latent TB, HIV, Blood donor, QFT-GIT
Introduction
The risk for developing active TB among HIV positive individuals increased many folds even during anti-HIV treatment [1]. Moreover, HIV infection is among the most important risk factors for the progression of latent tuberculosis infection to active TB [2]. Latent tuberculosis infection (LTBI) is characterized by the presence of immune responses to previously acquired Mycobacterium tuberculosis infection without clinical evidence of active tuberculosis (TB). Persons with LTBI are not infectious and cannot transmit TB infection to others and have negative sputum tests [3].
The prevalence of LTBI has increased worldwide with marked variations in different regions. It is generally high in developing countries than the developed ones [4, 5]. The prevalence of LTBI among blood specimen collected from HIV patients in Italy was found 9.5% [6]. High LTBI (68.1%) among HIV positive cases was reported in Spain [4]. In Africa, higher prevalence of LTBI (69% and 76%) among HIV positive cases was reported in South Africa [7] and Tanzania [8], respectively. The prevalence of LTBI among apparently healthy adults and healthy blood donors in Afar region (north-east Ethiopia) and Gondar (north-west Ethiopia) was reported as 31.2% and 51%, respectively [9].
Latent tuberculosis infection (LTBI) in immuno-compromised individuals may progress to severe active tuberculosis (TB) disease and serve as a reservoir for future transmission of TB disease [10]. The identification and successful treatment of individuals with LTBI is important for the fundamental understanding of the pathogenesis of the disease, support ongoing efforts to develop new TB vaccines, and reduce the subsequent risk for the re-activation and development of active TB [11]. Currently, LTBI screening is recommended for target populations such as patients receiving tumor necrosis factor treatment, cases co-infected with HIV and children aged less than 5 years who are at high risk of developing tuberculosis. Meanwhile, prophylaxis treatment is an alternative for tuberculosis control for high risk populations [12].
Immuno-compromised persons with latent tuberculosis infection are at increased risk for tuberculosis reactivation compared with the general population [13]. Therefore, it is important to determine the prevalence of LTBI among individuals who are at higher risk of developing active tuberculosis. However, the burden of latent tuberculosis among PLWH compared to healthy blood donors is not fully understood in Ethiopia. Therefore, the aim of this study was to determine the prevalence of LTBI and associated risk factors among HIV positive individuals and healthy blood donors.
Main text
Materials and methods
Study area, design and period
The study was conducted at the University of Gondar referral hospital located in Gondar town, one of the ancient and densely populated towns in Ethiopia. According to the 2015 population and housing census result of Ethiopia, the town had total population of 206,987.
Populations
The source population was all patients seeking health service at the University of Gondar referral hospital during the study period. The study populations were individuals living with HIV and apparently healthy blood donors who visited the University of Gondar referral hospital for health service and blood donation, respectively, from February 2016 to May 2017.
Inclusion and exclusion criteria
HIV positive individuals with CD4+ T cell count ≥ 200 cells/mm3 and apparently healthy blood donors were included in the study. HIV positive individuals who had CD4+ T cell count less than 200 cells/mm3 and individuals that had active TB infection were excluded from the study.
Study variables
The prevalence of latent tuberculosis infection was used as the dependent variable, while socio-demographic characteristics, BMI, TB contact, BCG vaccination, smoking habit, CD4+ cell count, family history of TB, previous uses of INH prophylaxis, current use of INH prophylaxis, ART status and duration of ART therapy were used as the independent variables.
Sample size determination and sampling technique
Sample size was determined by using a 50% prevalence of LTBI. We used a 20% (P1) and a 30% (P2) prevalence of LTBI among HIV+ patients and blood donors, respectively. At a 95% confidence interval and 80% power of test, n1 versus n2 1:1 ratio the initial sample size was determined 206. Considering a 10% non-response rate, the final sample size was 226 (113 HIV positive and 113 healthy controls).
Sampling techniques
Study participants were selected by using the systematic random sampling. Data of the Anti-Retroviral Treatment (ART) clinic showed that 1800 patients visited the clinic per month. Data collection period for this study was planned for 3 months and 3 × 1800 = 5400: k = N/n, 5400/113 = 48. Then, the first individual was selected randomly from patients registered 1 to 48 and included in the study. Then, every 48th individual who visited the ART clinic was selected and included in the study until the required numbers of study subjects were obtained. The required number of individuals among the apparently healthy individuals was selected by using the systematic random sampling method, too. Nearly 30 clients visited the Gondar Red Cross blood bank center every day which equals 30 × 30 × 3 = 2700, and 2700/113 = 24. Therefore, every 24th apparently healthy blood donor was recruited and included in the study, and the 1st client was selected randomly from blood donors registered 1 to 24.
Socio-demographic and clinical data collection
Socio-demographic characteristics of participants were collected using a pretested and structured questionnaire prepared in English and translated into the local language, Amharic. The prepared questionnaire was checked for completeness and validity prior to the collection of data at Gondar Poly clinic.
Sample collection and processing
Blood sample was collected by strictly following the standard operational procedures of the University of Gondar hospital laboratory. Six milliliter of venous blood was collected and 3 ml deposited into a tube containing EDTA anticoagulant and QFT-GIT test tubes. Following the standard operational procedures of the University of Gondar hospital laboratory, the blood samples were tested for quantiferon (QFT-GIT test) and CD4+ T cell count.
QuantiFERON-TB Gold In-Tube assay (QFN-GIT)
One milliliter of blood sample was transferred to each of the three QFT-GIT test blood collection tubes (a phytohemagglutinin, positive control (Mitogen) and a negative (Nil) control tube). Interferon-γ concentration was determined from the whole-blood supernatant by ELISA following the manufacturers’ description.
CD4+ lymphocyte count
CD4+ T cell count was conducted by incubating anti-coagulated whole blood with monoclonal antibodies. The specimens were analyzed on a flowcytometer to determine the proportion of cells of a particular phenotype. Tests were performed based on the manufacturer’s instructions.
Data analysis and interpretation
Data was checked for completeness, cleaned manually and entered into Epi Info Version7 and analyzed using SPSS version 20 computer software. P-values less than 0.05 were considered as statistically significant.
Results and discussion
Latent tuberculosis infection is a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens without evidence of clinically a manifested active TB [14]. HIV infection has contributed to a significant increase in the worldwide incidence of TB [15]. Because of the underlying immune deficiency, HIV-infected individuals with LTBI are at 26-fold higher risk for TB reactivation [16]. There are study reports that highlighted the importance of baseline LTBI screening for all HIV patients for minimizing the occurrence of clinical TB diseases and reducing TB incidence in the population [17].
Socio demographic characteristics
Almost half of the participants were females (53.5%). About 63% of the HIV+ cases were females but 56% of the apparently health blood donors were males. The mean ages of the HIV cases and apparently healthy blood donors were 37 and 28 years, respectively. Data also showed that 92% of the HIV positive cases and 85.8% of the apparently healthy blood donors were urban dwellers (Table 1).
Table 1.
Socio demographic characteristics and risk factors of PLWH and apparently healthy blood donors at Gondar University referral hospital, Northwest Ethiopia, 2017
| Characteristics | PLWH n (%) | Blood donor’s n (%) | Total n (%) |
|---|---|---|---|
| Mean (± SD) Age | 36.9 (± 6.6) | 27.9 (± 5.5) | 32.4 (± 7.5) |
| Age (years) | |||
| < 30 | 16 (14.2) | 73 (64.6) | 89 (39.4) |
| 30–39 | 57 (50.4) | 38 (33.6) | 95 (42.0) |
| > 39 | 40 (35.4) | 2 (1.8) | 42 (18.6) |
| Sex | |||
| Male | 42 (37.2) | 63 (55.8) | 105 (46.5) |
| Female | 71 (62.8) | 50 (44.2) | 121 (53.5) |
| Educational status | |||
| Unable to read and write | 20 (17.7) | 5 (4.4) | 25 (11.1) |
| Primary | 57 (50.4) | 24 (21.2) | 81 (35.8) |
| Secondary | 21 (18.6) | 43 (38.1) | 64 (28.3) |
| Collage and above | 15 (13.3) | 41 (36.3) | 56 (24.8) |
| Occupation | |||
| House wife | 37 (32.7) | 8 (7.1) | 45 (19.9) |
| Farmer | 5 (4.4) | 5 (7.1) | 12 (5.3) |
| Merchant | 29 (25.7) | 19 (16.8) | 47 (20.8) |
| Employee | 29 (25.7) | 52 (46.0) | 81 (35.8) |
| Student | 2 (1.8) | 25 (22.1) | 27 (11.9) |
| Daily labor | 11 (9.7) | 4 (3.5) | 14 (6.2) |
| Religion | |||
| Orthodox | 66 (58.4) | 99 (87.6) | 165 (73) |
| Muslim | 28 (24.8) | 8 (7.1) | 36 (15.9) |
| Protestant | 19 (16.8) | 6 (5.3) | 25 (11.1) |
| Ethnicity | |||
| Amhara | 104 (92) | 103 (87.6) | 207 (92.5) |
| Oromo | 6 (5.3) | 5 (4.4) | 11 (4.4) |
| Tigrie | 3 (2.7) | 5 (4.4) | 8 (3.1) |
| Monthly income | |||
| < 800 | 43 (38.1) | 15 (13.3) | 58 (25.7) |
| 800–1500 | 29 (25.7) | 33 (29.2) | 62 (27.4) |
| 1501–3025 | 26 (23.0) | 24 (21.2) | 50 (22.1) |
| > 3025 | 15 (13.3) | 41 (36.3) | 56 (24.8) |
Co-morbidities among PLWH and blood donors
Tuberculosis contact was found on 37.2% of the HIV positive cases and 31% of the blood donors. Close contact with pulmonary tuberculosis positive cases is a risk factor for acquisition of LTBI [18]. Marks et al. reported that the prevalence of LTBI was about 36% in close contacts of persons with infectious TB as assessed by TST in a study conducted in Iran [19]. INH prophylaxis was given to 25.7% of the HIV positive cases and 64.1% of them had ART for a duration of 5–10 years; 58.4% of the HIV positive individuals had CD4 count < 500 cells/mm3. On the other hand, only 3.5% of the apparently healthy blood donors had CD4+ T cell count < 500 cells.
Prevalence of latent tuberculosis infection
The overall prevalence of LTBI was 46.0%, comparable with the prevalence of LTBI reported previously in Uganda (49%) and Zambia (40%) [15, 20]. The prevalence of LTBI among HIV positive cases was 44.2%, while that of the apparently healthy blood donors was 47.8%. The prevalence of LTBI among PLWH found in the current study was by far higher than those of studies conducted in Saudi Arabia (5.0%) [21], Atlanta (8.0%) [22], Norway and the UK (10%) [23]. A 51% prevalence of LTBI was reported among blood donors in the same study area previously [24].
Among the HIV positive cases, 93 were on anti-retroviral therapy; the other 20 were treatment naïve. The prevalence of LTBI among HIV positive cases taking ART and ART naïve groups was 39.8% and 65%, respectively (Fig. 1). Moreover, ART naïve HIV positive patients were 3 times more likely to have LTBI than patients under ART treatment [P = 0.04; COR (95% CI) 2.8 (1.02–7.7)]. It has previously been shown that antiretroviral therapy decreases the incidence of tuberculosis among HIV-infected persons [25–27]. The reduction in TB disease with antiretroviral therapy may be due to an improved ability to control LTBI. Antiretroviral therapy has also been associated with lower TB recurrence rates and the decrease of mortality [28].
Fig. 1.
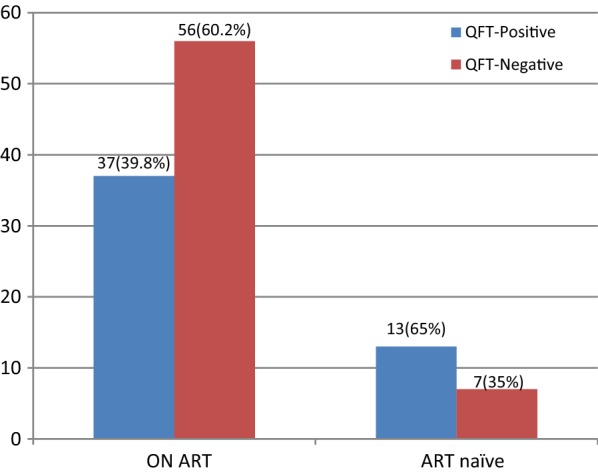
Prevalence of LTBI among HIV positive cases who are taking ART compared with ART naïve patients at the University of Gondar referral hospital, Northwest Ethiopia, 2017
Association of risk factors with LTBI among PLWH and blood donors
Among PLWH, the majority (52.5%) of the LTBI individuals were found within 30–39 years of age, but 62.5% of the blood donors that had LTBI were under 30 years of age. Previously, Lee et al. reported that LTBI prevalence increased with age. The risk of infection from household and community sources increased from birth until 20 years of age [29]. Among PLWH slightly over half (50.7%) of the LTBI positive individuals were females. In fact, a higher prevalence of LTBI (43% versus 33%) was reported among males compared to females [30]. In both cases, the majority had BMI greater than 17 kg/m2. On the other hand, PLWH and apparently healthy blood donors who had close contacts with TB patient were about 5 and 4 times more likely to develop LTBI as compared to individuals who had no close contacts with TB patients [P = 0.003, AOR = 5.2 (95% CI 1.7–15.8) and P = 0.003, AOR = 4.5 (95% CI 1.7–11.9)], respectively. Similarly, PLWH and apparently healthy blood donors who had family history of TB infection had about 4 times higher association to LTBI compared to those who did not have such history [P = 0.023, AOR = 3.8 (95% CI 1.2–12 and P = 0.025, AOR = 3.9 (95% CI 1.2–13), respectively] (Table 2). Cheng et al. previously reported that close contact with tuberculosis was a risk factor for LTBI [31].
Table 2.
Association between the prevalence of LTBI and risk factors among PLWH at University of Gondar referral hospital, Northwest Ethiopia, 2017
| Characteristics | QuantiFERON test result | |||||
|---|---|---|---|---|---|---|
| Positive n (%) |
Negative N (%) |
P-value | COR (95% C.I.) | P-value | AOR (95% C.I.) | |
| Contact with TB case | ||||||
| Yes | 31 (73.8) | 11 (26.2) | 0.0 | 7.71 (3.2–18.3) | 0.003* | 5.2 (1.7–15.8)* |
| No | 19 (26.8) | 52 (73.2) | – | 1.0 | – | 1.0 |
| Family history with TB | ||||||
| Yes | 26 (74.3) | 9 (25.7) | 0.0 | 6.5 (2.6–16) | 0.023* | 3.8 (1.2–12)* |
| No | 24 (30.8) | 54 (69.2) | – | 1.0 | – | 3.79 (1.0) |
| INH prophylaxis | ||||||
| Yes | 4 (13.8) | 25 (86.2) | 0.0 | 0.1 (0.1–0.4) | 0.005* | 0.1 (0.03–0.6)* |
| No | 46 (54.8) | 38 (45.2) | – | 1.0 | – | 1.0 |
| ART status | ||||||
| Yes | 37 (39.8) | 56 (60.0) | – | 1.00 | – | – |
| No | 13 (65.0) | 7 (35.0) | 0.04 | 2.8 (1–7.7) | 0.138 | 2.6 (0.7–9.1) |
ART antiretroviral therapy, HIV human immunodeficiency virus, INH isonicotinylhydrazide (Isoniazid)
* P-value = < 0.05
Conclusion
The overall prevalence of LTBI was 46%, and the prevalence of LTBI among PLWH was slightly lower than that of the blood donors. Close contacts with known TB cases and family history of TB were found risk factors for LTBI. Therefore, screening all HIV positive cases for LTBI could be valuable to initiate tuberculosis treatment and to prevent its progress to active tuberculosis infection.
Limitations
In this study, population groups less than 18 years of age were not included. Because the study design was cross-sectional, the exact relationship to develop LTBI among HIV patients which could have been assessed in a cohort study was not determined, and that was the limitation of the attempt.
Acknowledgements
We would like to thank the Department of Medical Microbiology, College of Medicine and Health Sciences, and the University of Gondar for all the support given for us to conduct this study.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- ART
anti retroviral therapy
- CD4
cluster of differentiation 4
- ELISA
Enzyme Linked Immuno Sorbent Assay
- ESAT-6
early secreted antigenic target 6
- HIV
human immunodeficiency virus
- IFN
interferon
- IGRA
interferon gamma release assay
- INH
isonicotinylhydrazide
- IPT
isoniazid preventive therapy
- LTBI
latent tuberculosis infection
- MTB
Mycobacterium tuberculosis
- PLWH
people living with HIV
- PPD
purified protein derivative
- QFT-GIT
QuantiFERON-TB Gold In-Tube method
- TB
tuberculosis
- TST
tuberculin skin test
- WHO
World Health Organization
Authors’ contributions
MT: Designed the study, collected and analyzed the data; AS: Participated in its design, coordination, follow up of data collection and drafted the manuscript; AK:, Participated in data collection and laboratory works and also involved during the interpretation of results; GB; involved in laboratory investigation of samples, interpretation of results and edited the manuscript; EA: Participated in conception and design of the study; BG: Participated in data analysis, interpretation of results and preparation of the draft and final write up of the manuscript. All authors read and approved the final manuscript.
Funding
Funding is not applicable because we have not received any fund for this study.
Availability of data and materials
Data were registered on Microsoft excel spread sheet and the datasets are available from the correspondence author but will not be shared to ensure patient confidentiality.
Ethics approval and consent to participate
Ethical clearance was obtained from the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, the University of Gondar, Ethical Review Committee. A written informed consent was obtained from each study participant. The laboratory results were communicated to their attending physicians for appropriate treatment.
Consent for publication
In this study, no individual details, images or videos were used.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mekdes Tilahun, Email: mekddy78@gmail.com.
Agumas Shibabaw, Email: agumas2000@gmail.com.
Amare Kiflie, Email: amexkif@gmail.com.
Gezahegn Bewket, Email: gezahegnb123@gmail.com.
Ebba Abate, Email: ebbaabate@yahoo.com.
Baye Gelaw, Phone: +251918703723, Email: tedybayegelaw@gmail.com.
References
- 1.Horsburgh J. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350(20):2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 2.Lawn S, Bekker L, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS. 2005;19(11):1113–1124. doi: 10.1097/01.aids.0000176211.08581.5a. [DOI] [PubMed] [Google Scholar]
- 3.Jones B, Young S, Antoniskis D, Davidson P, Kramer F, Barnes P. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148(5):1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 4.Diaz A, Diez M, Bleda M, Aldamiz M, Camafort M, Camino X, Cepeda C, Costa A, Ferrero O, Geijo P. Eligibility for and outcome of treatment of latent tuberculosis infection in a cohort of HIV-infected people in Spain. BMC Infect Dis. 2010;10:267. doi: 10.1186/1471-2334-10-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanai H, Uthaivoravit W, Mastro T, Limpakarnjanarat K, Sawanpanyalert P, Morrow J, Nieburg P. Utility of tuberculin and anergy skin testing in predicting tuberculosis infection in human immunodeficiency virus-infected persons in Thailand. Int J Tuberc Lung Dis. 1997;1(5):427–434. [PubMed] [Google Scholar]
- 6.Richeldi L, Losi M, D’Amico R, Luppi M, Ferrari A, Mussini C, Codeluppi M, Cocchi S, Prati F, Paci V. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest J. 2009;136(1):198–204. doi: 10.1378/chest.08-2575. [DOI] [PubMed] [Google Scholar]
- 7.Rangaka M, Wilkinson K, Seldon R, Van Cutsem G, Meintjes G, Morroni C, Mouton P, Diwakar L, Connell T, Maartens G. Effect of HIV-1 infection on T-cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175(5):514–520. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 8.Jensen A, Jensen L, Faurholt-Jepsen D, Aabye M, Praygod G, Kidola J, Faurholt-Jepsen M, Changalucha J, Range N, Krarup H. The prevalence of latent Mycobacterium tuberculosis infection based on an interferon-γ release assay: a cross-sectional survey among urban adults in Mwanza, Tanzania. PLoS ONE. 2013;8(5):e64008. doi: 10.1371/journal.pone.0064008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legesse M, Ameni G, Mamo G, Medhin G, Bjune G, Abebe F. Community-based cross-sectional survey of latent tuberculosis infection in Afar pastoralists, Ethiopia, using QuantiFERON-TB Gold In-Tube and tuberculin skin test. BMC Infect Dis. 2011;11(1):89. doi: 10.1186/1471-2334-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Zhu T, Wang Z, Peng H, Kong W, Zhou Y, Shao Y, Zhu L, Lu W. High latent TB infection rate and associated risk factors in the Eastern China of low TB incidence. PLoS ONE. 2015;10(10):e0141511. doi: 10.1371/journal.pone.0141511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai M, Behr M. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.tbtb2-0023-2016. [DOI] [PubMed] [Google Scholar]
- 12.Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, Den Boon S, Gutierrez SMB, Bruchfeld J, Burhan E. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46(6):1563–1576. doi: 10.1183/13993003.01245-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redelman-Sidi Gil, Sepkowitz Kent A. IFN-γ release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med. 2013;188(4):422–431. doi: 10.1164/rccm.201209-1621CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . Guide to monitoring and evaluation for collaborative TB/HIV activities–2015 update. Geneva: World Health Organization; 2015. [Google Scholar]
- 15.Coimbra I, Maruza M, Albuquerque MdFPM, Batista JDAL, Braga MC, Moura LV, Miranda-Filho DB, Montarroyos UR, Lacerda HR, Rodrigues LC. Validating a scoring system for the diagnosis of smear-negative pulmonary tuberculosis in HIV-infected adults. PLoS ONE. 2014;9(4):e95828. doi: 10.1371/journal.pone.0095828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Global tuberculosis report. Geneva: World Health Organization; 2015. [Google Scholar]
- 17.Wong NS, Leung CC, Chan KCW, Chan WK, Lin AWC, Lee SS. A longitudinal study on latent TB infection screening and its association with TB incidence in HIV patients. Scientific Rep. 9, Article number: 10093; 2019. [DOI] [PMC free article] [PubMed]
- 18.Mumpe-Mwanja D, Verver S, Yeka A, Etwom A, Waako J, Ssengooba W, Matovu J, Wanyenze R, Musoke P, Mayanja-Kizza H. Prevalence and risk factors of latent Tuberculosis among adolescents in rural Eastern Uganda. Afr Health Sci. 2015;15(3):851–860. doi: 10.4314/ahs.v15i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latorre I, Martínez-Lacasa X, Font R, Lacoma A, Puig J, Tural C, Lite J, Prat C, Cuchi E, Ausina V. IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection. BMC Infect Dis. 2010;10(1):348. doi: 10.1186/1471-2334-10-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanaube K, Hargreaves J, Fielding K, Schaap A, Lawrence K, Hensen B, Sismanidis C, Menezes A, Beyers N, Ayles H. Risk factors associated with positive QuantiFERON-TB Gold In-Tube and tuberculin skin tests results in Zambia and South Africa. PLoS ONE. 2011;6(4):e18206. doi: 10.1371/journal.pone.0018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed H, Alothman A, Baffoe-Bonnie H, Alageeb H. What is the rate of latent tuberculosis infection in human immunodeficiency virus (HIV) infected patients in Saudi Arabia? Am J Epidemiol Infect Dis. 2016;4(1):10–13. [Google Scholar]
- 22.Kall M, Coyne K, Garrett N, Boyd A, Ashcroft A, Reeves I, Anderson J, Bothamley G. Latent and subclinical tuberculosis in HIV infected patients: a cross-sectional study. BMC Infect Dis. 2012;12:107. doi: 10.1186/1471-2334-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talati N, Seybold U, Humphrey B, Aina A, Tapia J, Weinfurter P, Albalak R, Blumberg H. Poor concordance between interferon-γ release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis. 2009;9:15. doi: 10.1186/1471-2334-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalvani A, Nagvenkar P, Udwadia Z, Pathan A, Wilkinson K, Shastri J, Ewer K, Hill A, Mehta A, Rodrigues C. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183(3):469–477. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 25.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 26.Santoro-Lopes G, de Pinho A, Harrison L, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34(4):543–546. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 27.Jones J, Hanson D, Dworkin M, DeCock K, Adult/Adolescent Spectrum of HIV Disease Group HIV-associated tuberculosis in the era of highly active antiretroviral therapy The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4(11):1026–1031. [PubMed] [Google Scholar]
- 28.Lawn S, Harries A, Williams B, Chaisson R, Losina E, De Cock K, et al. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis. 2011;15(5):571–581. doi: 10.5588/ijtld.10.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Lin H, Tsai H, Su I, Yang C, Sun H, Hung C, Sy C, Wu K, Chen J. A clinical algorithm to identify HIV patients at high risk for incident active tuberculosis: a prospective 5-year cohort study. PLoS ONE. 2015;10(8):e0135801. doi: 10.1371/journal.pone.0135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelner J, Murray M, Becerra M, Galea J, Lecca L, Calderon R, Yataco R, Contreras C, Zhang Z, Grenfell B. Age-specific risks of tuberculosis infection from household and community exposures and opportunities for interventions in a high-burden setting. Am J Epidemiol. 2014;180(8):853–861. doi: 10.1093/aje/kwu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng C, Tao Z, Zhijian W, Hong P, Wen K, Yang Z, Yan S, Limei Z, Wei L. High latent Tb infection rate and associated risk factors in the eastern china of low TB incidence. PLoS ONE. 2015 doi: 10.1371/journal.pone.0141511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were registered on Microsoft excel spread sheet and the datasets are available from the correspondence author but will not be shared to ensure patient confidentiality.


