Abstract
Microsatellite instability was searched for at six different loci on chromosome arms 5q, 18q, 15q, 17p, 19q, and 11p in 22 patients (12 men and 10 women; average age of 31.8 years, range of 20–55 years) with giant cell tumor of bone (GCT). These loci were chosen because of their use in microsatellite instability studies in other tumors such as colorectal cancer (e.g., 5q, 18q, 17p) or because of the presence of chromosomal abnormalities such as telomeric associations commonly occurring at 19q and 11p termini (thus the reason for including the 19q and 11p termini microsatellites in our study of GCT). No microsatellite instability or loss of heterozygosity were detected when comparing normal and tumor cells from any of the GCT patients. Unlike several other tumors, our study indicates that microsatellite instability does not appear to play a role in the tumorigenesis of GCT although other abnormal cytogenetic, biochemical, and molecular genetics data do exist for this musculoskeletal tumor.
INTRODUCTION
Giant cell tumor of bone (GCT) is a benign locally aggressive neoplasm that has a predilection for the epiphysis of long bones. Most individuals affected are in their third or fourth decade of life. Although GCT is a benign tumor, it has a high local recurrence rate and manifests its biologic aggressiveness with a rate of benign pulmonary metastasis in about 2% of patients [1]. After surgical excision, rates of recurrence varies from 0% to 47% [2].
Approximately 60% of GCT patients share cytogenetic abnormalities, particularly telomeric associations, in the majority of cells [3, 4]. Telomeric integrity studies generally show a reduction in DNA telomere size, isolated from tumor in GCT patients, compared to age-matched control tissue [5]. In addition, the presence of telomerase in tumor cells from GCT patients supports DNA instability for GCT oncogenesis [6]. These data support genetic instability of GCT, which led us to look for microsatellite instability, which has been reported in other malignancies [7, 8, 9, 10].
Microsatellites are interspersed, highly polymorphic [11], tandem repeats of nucleotide base pairs scattered throughout the human genome [12]. They are very short DNA sequence repeats designated (CA)n, with the range of n being 15–30. Thibodeau and others [9] have shown that distinct genetic alterations occur in microsatellites of patients with colon cancer. These alterations may be either amplification or deletion of base pairs within the microsatellite regions. This alteration of DNA has been termed microsatellite instability, and has been reported in non–small-cell lung carcinomas [7], hereditary nonpolyposis colon cancer [8], colorectal cancer [9], and endometrial carcinoma [10]. Although the significance of microsatellite instability remains unknown, it appears to be an indirect marker of global alterations in the maintenance/house-keeping of DNA in patients with cancer.
Herein, we report our experience with six microsatellites from different chromosomes in 22 patients with histologically proven GCT.
MATERIALS AND METHODS
Twenty-two patients (12 men and 10 women; average age, 31.8 years, range of 20—55 years) with histologically confirmed giant cell tumor of bone treated at a single institution were analyzed in this study. Many of these patients had previously reported cytogenetic findings [4], chromosome telomeric integrity studies [5], and telomerase assays [6]. The clinical, biochemical, and genetic data for the patients are shown in Table 1. Genomic DNA was isolated routinely from tumor samples and peripheral blood specimens from each of the 22 patients with GCT. Tumor DNA was isolated from an estimated 250-mg sample cut from the center of the tumor specimen and frozen in liquid nitrogen before DNA isolation.
Table 1.
Clinical, biochemical, and genetic data from patients with giant cell tumor of bone
| Patient | Age (years) | Sex | Location | Treatment | Microsatellite Instability | Telomere Associations | Telomere Size | Telomerase Activity |
|---|---|---|---|---|---|---|---|---|
| GCT-1a | 20 | M | Femur | Excision | None | Yes | Reduced | Not tested |
| GCT-2 | 23 | M | Femur | Excision | None | No | Not tested | Not tested |
| GCT-3 | 23 | M | Femur | Excision | None | Yes | Not tested | Not tested |
| GCT-4 | 24 | M | Ulna | Excision | None | No | Not tested | Yes |
| GCT-5 | 32 | M | Radius | Excision | None | Not tested | Not tested | Not tested |
| GCT-6b | 32 | M | Tibia | Excision | None | No | Reduced | Yes |
| GCT-7 | 34 | M | Femur | Excision | None | No | Not tested | Not tested |
| GCT-8a | 39 | M | Tibia | Excision | None | No | No difference | Not tested |
| GCT-9 | 39 | M | Tibia | Excision | None | Not tested | Not tested | Not tested |
| GCT-10 | 44 | M | Femur | Excision | None | No | Not tested | Not tested |
| GCT-11a | 47 | M | Femur | Excision | None | Yes | Reduced | Not tested |
| GCT-12 | 55 | M | Tibia | Amputation | None | Not tested | Not tested | Not tested |
| GCT-13b | 20 | F | Sacrum | Excision | None | Not tested | Reduced | Yes |
| GCT-14b | 23 | F | Tibia | Excision | None | Yes | Elongated | Yes |
| GCT-15 | 23 | F | Tibia | Excision | None | Not tested | Not tested | Not tested |
| GCT-16a | 25 | F | Scapula | Excision | None | Not tested | Reduced | Not tested |
| GCT-17a | 26 | F | Tibia | Excision | None | No | Reduced | Not tested |
| GCT-18 | 33 | F | Radius | Excision | None | No | Not tested | Not tested |
| GCT-19b | 33 | F | Femur | Excision | None | Yes | Reduced | Yes |
| GCT-20 | 34 | F | Femur | Excision | None | Yes | Not tested | Not tested |
| GCT-21a | 42 | F | Tibia | Excision | None | Yes | Reduced | Not tested |
| GCT-22b | 43 | F | Tibia | Excision | None | Yes | No difference | Yes |
Standard polymerase chain reaction (PCR) was performed on genomic DNA from both GCT and leukocytes. Tumor and leukocyte DNA were amplified at six separate microsatellites localized to chromosome arms 5q(D5S107), 18q(D18S34), 15q(D15S165), 17p(D17S786), 19q(D19S254), and 11p(D11S861). The heterozygosity indices for these loci are: 82%, 81%, 80%, 77%, 78%, and 70%, respectively.
Polymerase chain reactions were performed on 40 ng of tumor and leukocyte DNA following established protocols [13] and ran at 25–27 cycles, as described below. The forward primer was end-labeled with gamma 32P-dATP (Amersham Co., Arlington Heights, IL) for each of the six microsatellites used in this study.
Conditions for the PCR varied depending on which primer was studied. Primers D5S107 and D18S34 underwent 27 step cycles with 30 seconds at 94°C, 75 seconds at 55°C, 15 seconds at 72°C, and 6 minutes at 72°C. Primers D11S861 and D19S254 were cycled at 25 step cycles with 60 seconds at 94°C, 2 minutes at 55°C, 2.5 minutes at 72°C, and 10 minutes at 72°C. Primer D15S165 underwent 25 step cycles with 4 minutes at 95°C, 1 minute at 94°C, 2 minutes at 57°C, and 7 minutes at 72°C. Primer D17S786 underwent 27 step cycles with 1 minute at 94°C, 2 minutes at 55°C, and 2 minutes at 72°C, followed by 10 minutes at 72°C. The samples were then denatured for 5 minutes at 94°C. Following denaturing, the samples were immediately placed on ice and 1.5 mL of the sample was added to a 6% acrylamide gel. The gels were run at 90 watts for 1.5 hours (for D5S107, D18S34, D17S786, D11S861, and D19S254) or 2.5 horns (for D15S165) and after cooling were transferred to gel blot paper. The blot paper was then placed in an autoradiography cassette (Fisher Scientific, Pittsburgh, PA) and exposed to radiographic film for 1–5 days.
RESULTS
Six microsatellites were examined for genetic instability and loss of heterozygosity. Sequences located on chromosomes 5,17, and 18 were chosen as sites because of previously reported genetic instability in cancer, specifically colon [9]. In addition, chromosome 19q and 11q termini were selected because they are common sites for telomeric associations in GCT [4]. Microsatellites were chosen at these two chromosome regions (D19S254 and D11S861). An additional microsatellite of chromosome number 15 was also analyzed.
Table 1 displays the characteristics of the 22 patients selected for study including cytogenetic, telomere length and telomerase activity, and microsatellite data. There were no identifiable genetic alterations or loss of heterozygosity between tumor and leukocytes at the sites chosen. Figures 1 and 2 show representative microsatellite data generated by PCR amplification of genomic DNA from tumor and control tissue from selected GCT patients. The heterozygosity of the microsatellites observed in our patients was similar to the reported data (e.g., the reported heterozygosity for D5S107 is 82% while 91% heterozygosity was observed in our 22 GCT patients). No loss of heterozygosity was seen in our GCT patients for any of the six microsatellites. Conversely, microsatellite instability was observed with D5S107 from the DNA of tumor and control tissue from two colorectal cancer patients with known instability, courtesy of Dr. S. Thibodeau using the methodology described in our study.
Figure 1.
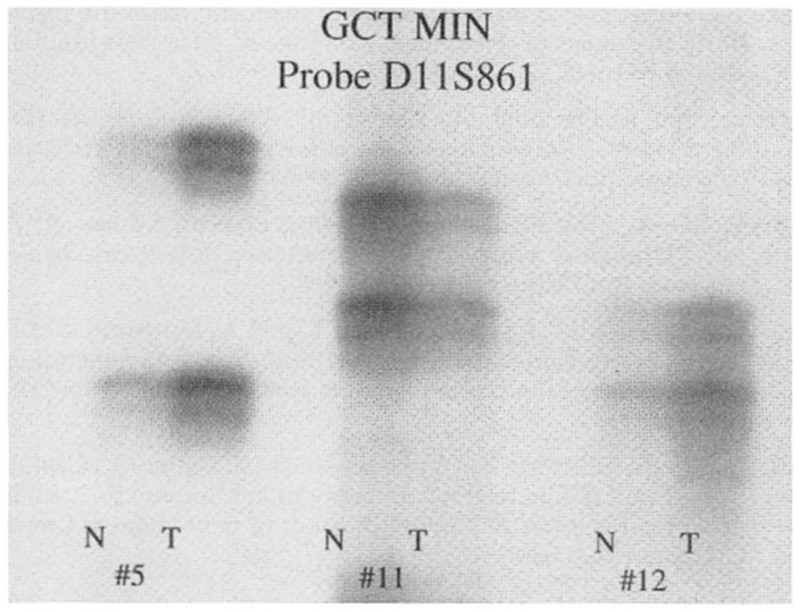
Giant cell tumor of bone microsatellite instability (GCT MIN) studies in three representative patients (GCT-5, GCT-11, and GCT-12) of locus D11S861 showing lack of instability when comparing normal (N) and tumor (T) tissue from each patient.
Figure 2.
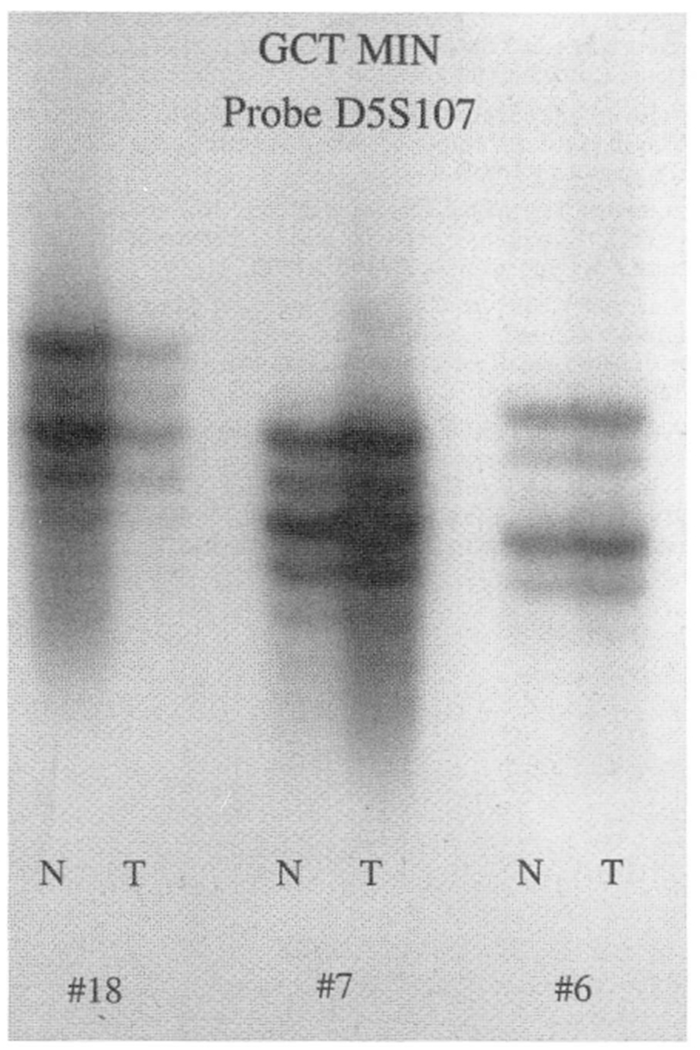
Giant cell tumor of bone microsatellite instability (GCT MIN) in three representative patients (GCT-6, GCT-7, and GCT-18) of locus D5S107 showing lack of instability when comparing normal (N) and tumor (T) tissue from each patient.
DISCUSSION
The precise mechanism by which a normal cell becomes a tumor cell is unclear. Microsatellites apparently become unstable in several types of carcinomas studied to date. We questioned whether this instability also occurred in GCT, a benign but aggressive tumor. Our results did not show microsatellite instability at the DNA sequences studied. Although cytogenetic abnormalities, specifically telomeric associations, are frequently seen in GCT patients, there was no evidence of microsatellite instability. Additional studies are needed to further characterize the biologic aggressiveness and the status of microsatellite instability in malignancies. Telomeric reduction has also been reported in the majority of GCT patients as well as in vivo aging [5]. In addition, telomerase activity has been reported in several GCT patients [6]. By accumulating cytogenetic, biochemical, and molecular genetic data from GCT patients and correlating these with clinical findings, a better understanding of this tumor may lead to better treatment modalities.
Acknowledgments
This research was partially supported by OREF grant number 93-005 (H.S.S.). We thank Janie Falkenberg for expert preparation of the manuscript.
REFERENCES
- 1.Rock MG, Pritchard DJ, Unni KK (1984): Metastases from histologically benign giant cell tumor of bone. J Bone Joint Surg 66A:269–274. [PubMed] [Google Scholar]
- 2.O’Donnell R, Springfield D, Motwani H, Ready J, Gebhardt M, Mankin H (1994): Recurrence of giant cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg 76-A:1827–1833. [DOI] [PubMed] [Google Scholar]
- 3.Bridge JA, Neff JR, Mouron BS (1992): Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. Cancer Genet Cytogenet 58:2–13. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz HS, Butler MG, Jenkins RB, Miller DA, Moses HL (1991): Telomeric associations and consistent growth factor overexpression detected in giant cell tumor of bone. Cancer Genet Cytogenet 56:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz HS, Dahir GA, Butler MG (1993): Telomere reduction in giant cell tumor of hone and with aging. Cancer Genet Cytogenet 71:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz HS, Juliao SF, Sciadini MR, Miller LK, Butler MG (1995): Telomerase activity and oncogenesis in giant cell tumor of bone. Cancer 75:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shridhar V, Siegfried J, Hunt J, del Mar Alonso M, Smith DI (1994): Genetic instability of microsatellite sequences in many non-small cell lung carcinomas. Cancer Res 54:2084–2087. [PubMed] [Google Scholar]
- 8.Aaltonen LA, Peltomak P, Leach FS, Sistonen P, Pylkkanen L, Meckline JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, Peterson GM, Kinzler KW, Vogelstein B, de la Chapelle A (1993): Clues to the pathogenesis of familial colorectal cancer. Science (Washington DC) 260:812–816. [DOI] [PubMed] [Google Scholar]
- 9.Thibodeau SN, Bren G, Schaid P (1993): Microsatellite instability in cancer of the proximal colon. Science (Washington DC) 260:816–819. [DOI] [PubMed] [Google Scholar]
- 10.Risingu JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J (1993): Genetic instability of microsatellites in endometrial carcinoma. Cancer Res 53:5100–5103. [PubMed] [Google Scholar]
- 11.Weber JL, May PE (1989): Abundant class of human DNA polymorphisms which can be typed using polymerase chain reaction. Am J Hum Gen 44:388–396. [PMC free article] [PubMed] [Google Scholar]
- 12.Weissenbach J, Giyapay G, Dib C, Vignal A, Morisette J, Millaseau P, Vayssein G, Lathrop M (1992): A second generation linkage map of the human genome. Nature (Lond.) 359:794–801. [DOI] [PubMed] [Google Scholar]
- 13.Dahir GA, Schwartz HS, Vnencak-Jones CJ, Butler MG (1993): Dosage and allelic restriction fragment studies and PCR analysis of H-ras locus in giant cell tumor of bone. Cancer Genet Cytogenet 74:95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]


