Abstract
Patient: Male, 66
Final Diagnosis: Symptomatic sinus bradycardia in the setting of partial empty sella syndrome
Symptoms: Dizziness • fatigue
Medication: —
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
Empty sella syndrome (ESS) occurs when the pituitary gland becomes flattened or shrinks, which leads to filling of the sella turcica with cerebrospinal fluid. This causes the appearance of empty sella on imaging. ESS is often diagnosed after the workup of pituitary disorders, or as an incidental finding after brain imaging. To the best of our knowledge, this is the first case report in which ESS was diagnosed after an incidental finding of bradycardia pre-operatively.
Case Report:
We present the case of a 66-year-old man who was admitted electively to undergo a transforaminal lumbar interbody fusion at the level of L5-S1. He was found to be bradycardic pre-operatively. Upon further workup, he was found to have a thyroid-stimulating hormone (TSH) level of 0.358 uIU/ML (normal range, 0.465–4.680 uIU/ML) and a free thyroxine (FT4) level of 0.8 ng/dL (normal range, 0.8–2.2ng/dL). He also had a history of hypotestosteronemia. He was diagnosed with central hypothyroidism at the time of admission. Subsequently, a magnetic resonance imaging (MRI) scan of the brain was performed, and partial empty sella syndrome was diagnosed.
Conclusions:
Bradycardia in the setting of empty sella syndrome is a rare condition. It is of utmost importance for clinicians to keep in mind a wide differential that includes other non-cardiac causes for common cardiac symptoms such as bradycardia.
MeSH Keywords: Bradycardia, Empty Sella Syndrome, Hypothyroidism, Preoperative Period
Background
Empty sella syndrome (ESS) is a disease that occurs when the pituitary gland becomes flattened or shrinks, which leads to the filling of the sella turcica with cerebrospinal fluid. This causes the appearance of an empty sella on imaging. ESS is often diagnosed after the workup of pituitary disorders, or as an incidental finding after brain imaging. It is defined as partial empty sella if the cerebrospinal fluid (CSF) fills less than 50% of the sellar space, and it is defined as complete empty sella if more than 50% of the sellar space is filled with CSF. There are 2 types of ESS: primary and secondary. Primary occurs when a small anatomical defect in the diaphragmatic sella above the gland allows unobstructed pulsatile movement and leakage of CSF into the sella, which increases the pressure and causes the gland to flatten.
The exact cause for primary empty sella (PES) is still unclear, but congenital abnormalities such as incomplete formation of the sellar diaphragm or variance in pituitary volume can be possible mechanisms. Associated risk factors for PES include female sex, sleep apnea, multiple pregnancies, arterial hypertension, benign intracranial hypertension, and obesity.
Patients with PES can be completely asymptomatic. The most common symptoms are headaches (in 80% of cases), visual disturbances (in 20% of cases), and rhinorrhea. Less commonly, patients will have decreased libido due to secondary hypogonadism, increased lactation and menstrual abnormalities due to hyperprolactinemia, polyuria and polydipsia due to diabetes insipidus, and/or symptoms of central hypothyroidism (e.g., fatigue, dry skin, bradycardia, cool extremities, alopecia, and weight gain) in the setting of panhypopituitarism
Secondary empty sella syndrome is more common and is related to different pathological processes involving the cellar region, including destruction of the pituitary gland after surgery, radiation, or presence of a tumor. Other associated risk factors include medical therapy for pituitary adenomas, pituitary apoplexy, traumatic brain injury, congenital hypopituitarism, and Sheehan’s syndrome. Symptoms are similar to PES and depend on how much of the pituitary gland is involved
Herein, we report a rare and an unusual presentation of a 66-year-old man who was diagnosed with partial empty sella syndrome after he was worked-up pre-operatively for symptomatic bradycardia.
Case Report
We present the case of a 66-year-old white man with severe neural foraminal stenosis who presented to the hospital for a transforaminal lumbar interbody fusion at the level of L5–S1 by neurosurgery. Pre-operatively, the Internal Medicine team was consulted for bradycardia. The patient reported occasional dizziness, lightheadedness, cognitive slowing, and fatigue for the past 3 years, but was asymptomatic during his hospital stay. He also reported a history of decreased libido that was treated successfully with testosterone injections. He denied angina, symptoms of heart failure, syncope, headaches, visual disturbances, rhinorrhea, or convulsions.
His past medical history is significant for hypotestosteronemia, depression, hypertension and chronic back pain. He has no prior history of head trauma or any autoimmune diseases.
His past surgical history was significant for left L5–S1 hemilaminectomy and microdiscectomy 2 years prior to admission, appendectomy, and cholecystectomy. He denied any brain surgery or radiation therapy. His social history was significant for occasional alcohol use. He was a former smoker who quit 22 years ago. He denied any history of illicit drug abuse.
His family history was significant for cardiovascular disease in his father and mother, who died at the ages of 72 and 75 years, respectively. He was married and had 3 children.
Home medications included oral aspirin (81 mg once daily) for primary cardiovascular disease prevention, oral clonazepam (1 mg twice a day as needed) for anxiety, oral gabapentin (100 mg 3 times a day) for chronic back pain, oral morphine (15 mg twice a day) for chronic back pain, oral oxycodone/acetaminophen (10/325 mg twice a day) for chronic back pain, oral tizanidine (4 mg 3 times a day as needed) for back spasms, topical lidocaine (5% twice a day as needed) for back pain, oral losartan (100 mg once daily) for hypertension, oral hydrochlorothiazide (12.5 mg once daily) for hypertension, oral omeprazole (10 mg once daily) for reflux, oral paroxetine (30 mg once daily) for depression, and subcutaneous testosterone (30 mg once weekly) for hypotestosteronemia. Other than the above history, his review of systems was not contributory.
His vital signs revealed a blood pressure of 174/73 mmHg and a heart rate of 40 bpm. His body temperature was 98.1ºF (36.7°C), respiratory rate was 18/min, and a blood oxygen saturation of 97% on room air. His body mass index (BMI) was 29.3 kg/m2. He was not in any distress. His head exam showed a normocephalic, atraumatic head with no palpable or visible masses. His cardiovascular exam was significant for a slow heart rate, with normal S1 and S2. No murmurs or thrills were appreciated. His neck exam showed no lymphadenopathy, jugular venous distention, or carotid bruits. His abdomen was soft, nondistended, and non-tender, with normal bowel sounds and no organomegaly. His breath sounds were clear and symmetric bilaterally, without any crackles, wheezes, or rhonchi.
A skin exam showed good turgor, with no rashes. His extremities showed no rashes, lesions, or lower-extremity edema. Radial, posterior tibial, and dorsalis pedis pulses of all bilateral extremities were 3+ throughout. On neurological exam, the patient was awake, alert, and oriented to time, place, and person. His cranial nerves 2–12 were grossly intact. The sensation was 3 out of 5 in the left lower extremity. Otherwise, sensory and motor exam were 5 out of 5 throughout. Gait was not assessed due to back pain pre-operatively.
An electrocardiogram showed sinus bradycardia with a rate of 40 beats per minute (bpm), an upright p wave in I, II, and AVL, and an inverted p wave in AVR. PR interval was 194 milliseconds (ms), and QRS interval was 112 ms (Figure 1). Based on our electronic medical record, 3 years prior to presentation, the patient was found to have a heart rate of 40–55 bpm. An electrocardiogram 3 years prior showed sinus bradycardia with a rate of 49 bpm, an upright p wave in I, II, and AVL, and an inverted p wave in AVR.
Figure 1.

Sinus bradycardia with a rate of 40 bpm, an upright p wave in I, II, and AVL, and an inverted p wave in AVR. PR interval was 194 ms, and QRS interval was 112 ms.
Laboratory workup revealed a white blood cell count of 4300/mm3, hemoglobin of 14 g/dL, and platelet count of 250 000/mm3. A chemistry panel showed a sodium of 141 mmol/L, potassium of 3.4 mmol/L, carbon dioxide of 29 mmol/L, blood urea nitrogen (BUN) of 21 mg/dL, creatinine of 0.8 mg/dL, calcium of 9.9 mg/dl, thyroid-stimulating hormone (TSH) of 0.358 uIU/ML (normal range, 0.465–4.680 uIU/ML), and a free thyroxine (FT4) of 0.8 ng/dL (normal range, 0.8–2.2 ng/dL).
A multiplanar multisequence magnetic resonance imaging (MRI) scan of the brain was performed before and after administration of intravenous contrast on a 1.5 T MRI system. There was no evidence of infarct. There was a partially empty sella, and no pituitary lesion or mass effect on the optic chiasm were identified (Figure 2).
Figure 2.
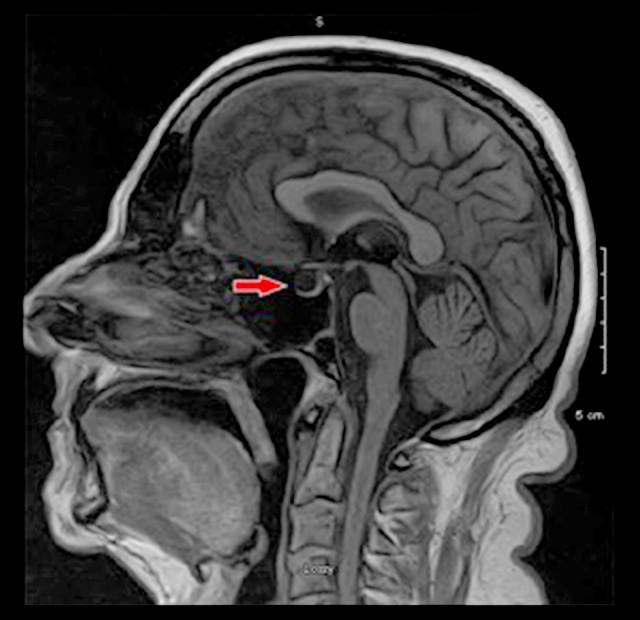
1.5 T MRI of the brain. There was no evidence of infarct. There was a partially empty sella (red arrow). No pituitary lesion or mass effect on the optic chiasm was identified.
He then underwent surgery without any complications and was sent to follow up with Endocrinology as an out-patient.
Discussion
Empty sella syndrome (ESS) was first described by Busch in 1951. It is reported to occur in 5.5–23.5% of the population, of which 25–50% will have symptoms of panhypopituitarism, diabetes insipidus, or gonadotropin deficiency, while the others will be completely asymptomatic. Common symptoms of PES include headaches (in 80% of the cases), visual disturbances (in 20% of the cases), and papilledema that are more commonly seen in patients with intracranial hypertension. Rhinorrhea is a less common symptom, which increases the risk of meningitis.
Neurological disturbances were also reported in 40% of cases, including syncope, dizziness, convulsions, and cranial nerve disorders
The reason for the consult in our case was chronic symptomatic bradycardia. Symptoms included occasional dizziness, lightheadedness, cognitive slowing, and fatigue for the past 3 years, but he was asymptomatic during the hospital stay.
Primary workup for bradycardia included an electrocardiogram (Figure 1), review of his medications, and a TSH level. The patient was found to have sinus bradycardia without any conduction disease, based on his electrocardiogram. Upon exertion, his heart rate increased to the range of 65–70 bpm, without signs of any conduction abnormalities.
His daily lidocaine gel 5%, morphine, and hydrocodone may be the possible culprits or aggravating factors for his bradycardia. Moreover, he was found to have low TSH 0.358 uIU/ML (normal range, 0.465–4.680 uIU/ML), which prompted us to measure his free T4, which also was found to be low, at a level of 0.8 ng/dL (normal range, 0.8–2.2 ng/dL). Due to the treatment with testosterone, gonadotropins were not tested, as it affects sensitivity of the results.
Based on our laboratory workup, we diagnosed the patient with central hypothyroidism. Given the history of hypotestosteronemia, we ordered an MRI scan of the brain, which showed partial empty sella (Figure 2).
The main reason for his bradycardia was likely central hypothyroidism due to hypopituitarism in the setting of ESS. His medications were a possible culprit for bradycardia, but we thought that this was less likely as his bradycardia symptoms started 2 years after initiating his pain medications. Nonthyroid illness is a possible differential diagnosis, but it is less likely since the patient has been having symptomatic bradycardia with a documented proof by ECG for the past 3 years, which is usually a long duration for sick euthyroid syndrome.
Since arterial hypertension is a common risk factor of PES, it is highly likely it was the cause of ESS in our case.
Treatment depends on the type of ESS. Weight loss is beneficial in obese patients with sleep apnea. A neurosurgical approach is indicated in patients with rhinorrhea. Hyperprolactinemia is treated with dopamine agonists, while panhypopituitarism is treated with hormone replacement. Asymptomatic patients do not require treatment.
Our patient was referred to Endocrinology to follow up in the out-patient setting. Part of the primary workup will include cortisol levels, estradiol, IGF-1, prolactin, and testosterone. It is important to be aware that testosterone and cortisol levels might be falsely elevated while the patient is on testosterone and/or prednisone treatment (for back pain).
Conclusions
Bradycardia in the setting of ESS is a rare condition. To the best of our knowledge, this is the first case report in which partial ESS was diagnosed after an incidental finding of bradycardia pre-operatively. It is of utmost importance for clinicians to keep in mind other non-cardiac causes for common cardiac symptoms such as bradycardia.
Footnotes
Conflicts of interest
None.
Department and Institution where work was done
Department of Cardiology, United Health Services Hospitals, Wilson Medical Center, Johnson City, NY, U.S.A.
References:
- 1.Melmed S, Kleinberg D, Ho K. Pituitary physiology and diagnostic evaluation. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Chapter 8 Williams Textbook of Endocrinology. 12th ed. Philadelphia: Elsevier; 2011. [Google Scholar]
- 2.Aruna P, Sowjanya B, Reddy PA, et al. Partial empty sella syndrome: A case report and review. Indian J Clin Biochem. 2014;29(2):253–56. doi: 10.1007/s12291-013-0369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiloiro S, Giampietro A, Bianchi A, et al. Primary empty sella: A comprehensive review. Eur J Endocrinol. 2017;177(6):R275–85. doi: 10.1530/EJE-17-0505. [DOI] [PubMed] [Google Scholar]
- 4.Miljic D, Pekic S, Popovic V. In: Empty Sella. Feingold KR, Anawalt B, Boyce A, et al., editors. South Dartmouth (MA): MDTextcom, Inc; 2000. 2018 Oct 1. [Google Scholar]
- 5.Auer MK, Stieg MR, Crispin A, et al. Primary empty sella syndrome and the prevalence of hormonal dysregulation. Dtsch Arztebl Int. 2018;115(7):99–105. doi: 10.3238/arztebl.2018.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bensing S, Rorsman F, Crock P, et al. No evidence for autoimmunity as a major cause of the empty sella syndrome. Exp Clin Endocrinol Diabetes. 2004;112:231–35. doi: 10.1055/s-2004-817968. [DOI] [PubMed] [Google Scholar]
- 7.Brismar K, Efendić S. Pituitary function in the empty sella syndrome. Neuroendocrinology. 1981;32(2):70–77. doi: 10.1159/000123133. [DOI] [PubMed] [Google Scholar]
- 8.Guitelman M, Garcia Basavilbaso N, Vitale M, et al. Primary empty sella (PES): A review of 175 cases. Pituitary. 2013;16:270–74. doi: 10.1007/s11102-012-0416-6. [DOI] [PubMed] [Google Scholar]
- 9.Javorsky BR, Aron DC, Findling JW, Tyrrell J. Hypothalamus and pituitary gland. In: Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology. 10th ed. New York, NY: McGraw-Hill; [Google Scholar]
- 10.Busch W. [Morphology of sella turcica and its relation to the pituitary gland]. Virchows Arch Pathol Anat Physiol Klin Med. 1951;320(5):437–58. doi: 10.1007/BF00957474. [in German] [DOI] [PubMed] [Google Scholar]
- 11.Chiloiro S, Giampietro A, Bianchi A, et al. Diagnosis of endocrine disease: Primary empty sella: A comprehensive review. Eur J Endocrinol. 2017;177:R275–85. doi: 10.1530/EJE-17-0505. [DOI] [PubMed] [Google Scholar]
- 12.Maira G, Anile C, Mangiola A. Primary empty sella syndrome in a series of 142 patients. J Neurosurg. 2005;103:831–36. doi: 10.3171/jns.2005.103.5.0831. [DOI] [PubMed] [Google Scholar]


