Abstract
Patient: Female, 69
Final Diagnosis: Mid ventricular type of Takotsubo cardiomyopathy
Symptoms: Chest discomfort
Medication: —
Clinical Procedure: Angiography • 2D echocardiogram
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
Takotsubo cardiomyopathy (TC) is characterized as acute left ventricular dysfunction precipitated by intense emotional or physiological stress. The mid-ventricular variant of TC usually has akinesis, with or without ballooning of the mid-ventricular segment, and a hyperdynamic base and apex. Recurrence of the typical and atypical (reversed and mid-ventricular type) forms has been reported in only a very small number of cases. We report a forme fruste presentation of mid-ventricular variant of TC.
Case Report:
A 69-year-old woman with a prior history of stress-induced cardiomyopathy presented with complaint of moderate intensity, persistent, sub-sternal chest discomfort. She reported that her symptoms were similar to those she had during a previous hospitalization in 2015, and this time cited the death of her mother as an inciting stressor. No significant obstructive flow-limiting coronary artery disease was found on cardiac catheterization. However, the left ventriculogram was suggestive of mid-ventricular pattern of TC. Her first symptomatic episode of apparent TC did not reveal completion of the mid-ventricular pattern of the TC variant. The subsequent episode, during this hospitalization, manifested as a completed version of her initial apparent forme fruste of mid-ventricular variant of TC.
Conclusions:
TC may present in a myriad of clinical forms that must be considered in the evaluation of patients with suspected acute coronary syndromes or cardiomyopathy. Treatment is mainly supportive, and recurrence rates range from 7.7% to 11.4%. To the best of our knowledge, this forme fruste presentation has not been previously reported in recurrent variants of TC.
MeSH Keywords: Acute Coronary Syndrome, Echocardiography, Takotsubo Cardiomyopathy
Background
Takotsubo cardiomyopathy (TC) is characterized as acute left ventricular dysfunction precipitated by intense emotional or physiological stress [1–4]. It was first recognized in Japan in 1990. The word Takotsubo means an octopus trap in Japanese [5,6]; the narrow neck and round bottom of the octopus trap resembles the shape of the heart in TC [7]. It generally mimics acute coronary syndrome (ACS) and presents in approximately 1–2% of patients with suspected ACS, described more often in postmenopausal women [6,8,9]. Although most patients recover in a few weeks, the mortality rate in the index hospitalization may be as high as 4% to 5% [1,10], which is comparable to the mortality from ST elevation myocardial infarction (STEMI) in the current era [1]. The exact pathophysiology of TC has not been fully elucidated. It has been hypothesized that catecholamine stress, endothelial dysfunction, and microvascular coronary artery spasm may lead to an increased cardiac workload, as well as myocardial injury [1–4,11]. Another hypothesis includes oxidative/inflammatory stress-induced myocardial dysfunction [3].
Multiple clinical variants of TC have been reported. Recurrence of TC appears to be a rare phenomenon [12–17]. We herein describe a unique case of a recurrent TC variant with an index event representing an apparent forme fruste of mid-ventricular TC.
Case Report
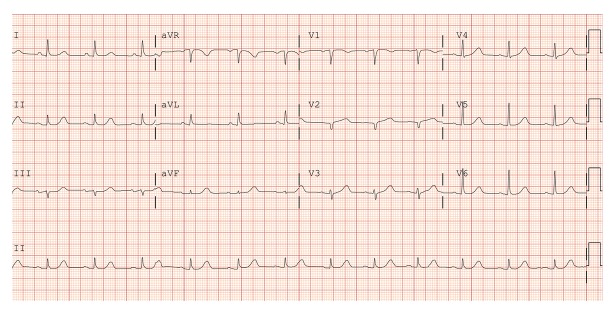
A 69-year-old woman with a prior history of stress-induced cardiomyopathy, moderate non-obstructive coronary artery disease, hypertension, and hyperlipidemia presented to our institution with a complaint of moderate intensity, persistent, sub-sternal chest discomfort. She reported that her symptoms were reminiscent to those of a presentation at another hospital in 2015, and cited the death of her mother as an inciting stressor for the current event. Upon admission, her vital signs were in normal range and physical exam was unremarkable. The EKG showed poor R wave progression in V1 and V2 (Figure 1), and troponin T levels were elevated at 0.527, 0.251, and 0.110.
Figure 1.
EKG on admission.
Coronary angiography demonstrated no evidence of obstructive flow-limiting coronary artery disease. Left ventriculography revealed hyperkinesis of the base and apex with akinesis of the mid-anterolateral and diaphragmatic wall segments suggestive of mid-ventricular pattern of Takotsubo cardiomyopathy (Figure 2). Transthoracic echocardiography (TTE) similarly demonstrated hyperkinesis of the basal and apical wall segments and akinesis of the mid-septal, mid-inferior, mid-inferolateral, and mid-anterior walls. Overall left ventricular systolic function was moderately reduced, with an ejection fraction (LVEF) of 35% to 40%. The wall motion abnormalities did not correspond to a single coronary artery distribution and appeared likely to represent an acute stress-induced cardiomyopathy. She remained asymptomatic following hospitalization, had an uncomplicated course, and was discharged following guideline-directed optimization of medical regimen. A post-convalescent phase TTE at 3 months demonstrated normalization of LVEF with mild hypokinesis of the mid-ventricular walls, and normal apical and basal wall motion.
Figure 2.
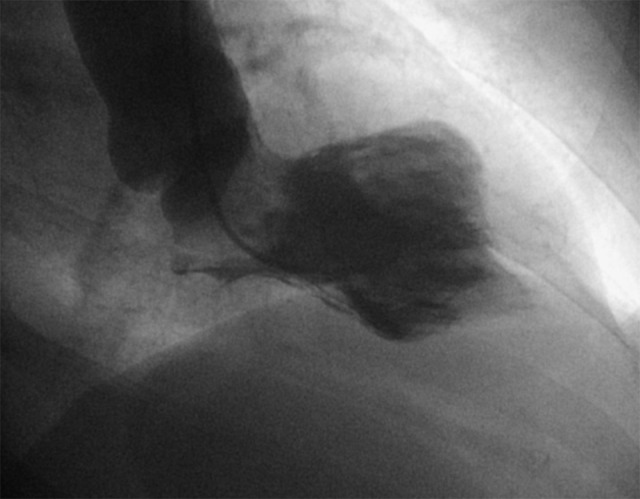
Left ventriculogram in 2018 showing complete presentation of mid-ventricular variant.
A review of her 2015 presentation to another hospital (Figure 3), also triggered by an emotional stressor, revealed angiographic demonstration of non-obstructive coronary artery disease and mid-anterior left ventricular wall akinesis – LVEF 60%. Convalescent phase TTE demonstrated an LVEF of 65% without regional wall motion abnormality.
Figure 3.
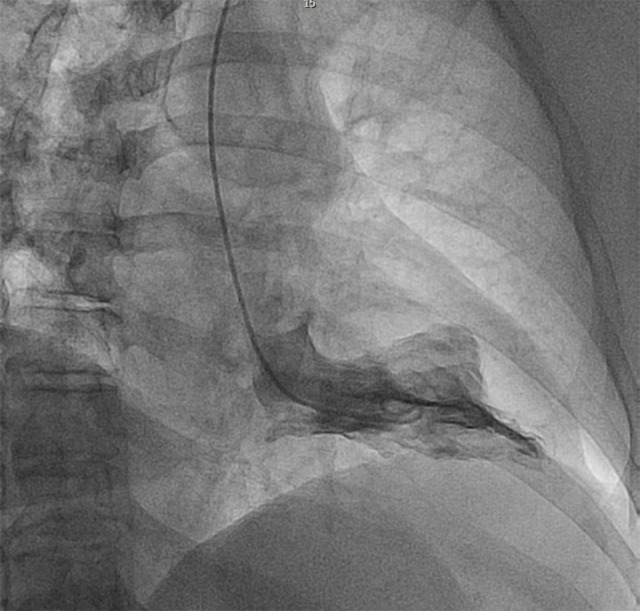
Left Ventriculogram 2015 showing incomplete presentation.
Discussion
Variants of TC are classified according to various wall motion abnormality patterns [6,7]. Classical or typical variant of TC is more common than the atypical forms, and is characterized by apical hypokinesis and basal hyperkinesis [8,9]. The mid-ventricular variant demonstrates akinesis, with or without ballooning, of the mid-ventricular segment and a hyperdynamic base and apex [7,18–23]. Reverse or inverted TC presents an akinetic base and hyperdynamic apex [19]. Recurrence of both the typical and atypical (reverse and mid-ventricular type) forms have been reported [12–15,21]. Biventricular and isolated right ventricular forms also have been described [20,29]. It has been proposed that a heterogeneous density of adrenoceptors in apical, middle, and basal regions of the left ventricle may explain these clinical variants [24]. The apical variant has been reported to occur most often in postmenopausal women, as they generally have more adrenoceptors in the apex than in the base [25]. Differential autonomic innervation of the anterior wall compared to the apex and inferior wall and differences in local catecholamine release are also postulated as mechanisms for TC [26]. Different variants in the same patient have also been previously reported, and postulated reasons offered include variability in stress exposure, catecholamine levels, and adrenergic receptor sensitivity [22,27]. Estrogen deprivation mediated subsequent to endothelial dysfunction may also play a role, and this might explain the relative overall preponderance of TC among postmenopausal women [1,2].
Despite the various mechanisms proposed, there is no clearly established consensus on the underlying pathophysiology [2]. Many patients with TC present with elevations in cardiac biomarkers and EKG changes mimicking ACS. Takotsubo syndrome accounts for about 2% of all suspected STEMI presentations [28]. Patients with TC usually, although not exclusively, demonstrate an unremarkable coronary angiogram without epicardial coronary obstruction [4,20,26]. In most cases, the akinetic or hypokinetic wall segments do not correlate to a single epicardial coronary artery distribution pattern [1,16]. In addition, there is often discordance between the severity of LV systolic dysfunction and the degree of elevation in cardiac biomarkers [16]. Furthermore, systolic dysfunction from TC usually normalizes over the course of a few weeks. Obstructive CAD and acute coronary syndromes have been described in conjunction with TC, and may serve as a potential stressor for the subsequent development of TC. Apical ballooning is a distinct entity and is usually not observed in acute coronary syndrome [16,26]. Coronary artery spasm and left ventricular outflow obstruction have been observed in a few cases, but reports are inconsistent [30].
TC is often precipitated by either emotional or physical triggers; the usual ones are fear or panic after death of a relative, natural catastrophe, or a sense of self-danger. In addition to ACS, other medical conditions like hypoglycemia, GI bleed, acute asthma, and pneumothorax triggering TC have also been reported [13]. Sympathomimetic medications like beta2 agonist nebulization, and alpha agonist medications like midodrine, have also reportedly precipitated recurrent TC events in a few patients [13,31].
The clinical presentation varies, with most cases following a benign course; although congestive heart failure, and cardiogenic shock have been reported. Complete recovery is observed in over 95% of patients, and the incidence of cardiac arrest is reportedly 5% [3].
Treatment is mainly supportive, and recurrence rates range from 7.7% to 11.4% [13]. Recurrent TC has also been reported with a few cases of pheochromocytoma [15,32].
In our patient, the first symptomatic episode of apparent TC did not reveal completion of the mid-ventricular pattern of the TC variant. The subsequent episode, during this hospitalization, manifested as a completed version of her initial apparent forme fruste of the mid-ventricular variant TC. To the best of our knowledge, this apparent forme fruste presentation has not been previously reported in recurrent TC variants.
Conclusions
Takotsubo cardiomyopathy may present in a myriad of clinical forms that must be considered in the evaluation of patients with suspected acute coronary syndromes or cardiomyopathy.
Video 1.

Left ventriculography during her subsequent presentation in 2018, showing evidence of hyperkinesis of the base and apex with akinesis of the midanterolateral and diaphragmatic wall segments suggestive of mid-ventricular pattern of Takotsubo cardiomyopathy (complete presentation of mid-ventricular pattern).
Video 2.
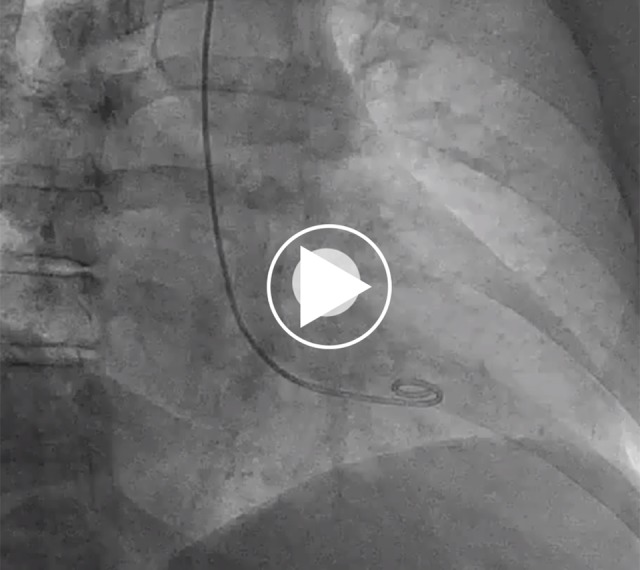
Left ventriculography during her initial presentation in 2015, showing evidence of mid-anterior left ventricular wall akinesia with preserved ejection fraction (60%) (incomplete presentation of mid-ventricular pattern).
Footnotes
Department and Institution where work was done
Department of Internal Medicine and Division of Cardiology, Saint Vincent Hospital, Worcester, MA, U.S.A.
Conflict of interest
None.
References:
- 1.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135(24):2426–41. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 2.Akashi YJ, Nef HM, Lyon AR. Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev Cardiol. 2015;12(7):387–97. doi: 10.1038/nrcardio.2015.39. [DOI] [PubMed] [Google Scholar]
- 3.Komamura K, Fukui M, Iwasaku T, et al. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602–9. doi: 10.4330/wjc.v6.i7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Dilsizian V. Exploring the pathophysiology of Takotsubo cardiomyopathy. Curr Cardiol Rep. 2017;19(6):53. doi: 10.1007/s11886-017-0865-7. [DOI] [PubMed] [Google Scholar]
- 5.Sato H, T H, Uchida T. [Takotsubo-type cardiomyopathy due to multivessel spasm.] In: Kodama K, Haze K, Hon M, editors. [Clinical aspect of myocardial injury: From ischemia to heart failure] Tokyo: Kagakuhyouronsya; 1990. pp. 56–64. [in Japanese] [Google Scholar]
- 6.Takotsubo cardiomyopathy (broken-heart syndrome). It’s named after an octopus trap – and that’s not all that’s unusual about this reversible heart condition. It occurs almost exclusively in women. Harv Womens Health Watch. 2010;18(3):6–7. [PubMed] [Google Scholar]
- 7.Velankar P, Buergler J. A mid-ventricular variant of Takotsubo cardiomyopathy. Methodist Debakey Cardiovasc J. 2012;8(3):37–39. doi: 10.14797/mdcj-8-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YP, Poh KK, Lee CH, et al. Diverse clinical spectrum of stress-induced cardiomyopathy. Int J Cardiol. 2009;133(2):272–75. doi: 10.1016/j.ijcard.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Zeb M, Sambu N, Scott P, Curzen N. Takotsubo cardiomyopathy: A diagnostic challenge. Postgrad Med J. 2011;87(1023):51–59. doi: 10.1136/pgmj.2010.102475. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with Takotsubo cardiomyopathy: A study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215–21. doi: 10.1016/j.ahj.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Gopalakrishnan P, Zaidi R, Sardar MR. Takotsubo cardiomyopathy: Pathophysiology and role of cardiac biomarkers in differential diagnosis. World J Cardiol. 2017;9(9):723–30. doi: 10.4330/wjc.v9.i9.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maroules CD, Linz NA, Boswell GE. Recurrent Takotsubo cardiomyopathy. J Cardiovasc Comput Tomogr. 2009;3(3):187–89. doi: 10.1016/j.jcct.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Mendoza I, Novaro GM. Repeat recurrence of Takotsubo cardiomyopathy related to inhaled beta-2-adrenoceptor agonists. World J Cardiol. 2012;4(6):211–13. doi: 10.4330/wjc.v4.i6.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novo G, Carita P, Ajello L, Assennato P. Chronic stress and early recurrence of Takotsubo cardiomyopathy: A clinical case. J Cardiovasc Echogr. 2014;24(3):83–85. doi: 10.4103/2211-4122.143976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi AP, Bing-You RG, Thomas LR. Recurrent Takotsubo cardiomyopathy associated with pheochromocytoma. Endocr Pract. 2009;15(6):560–62. doi: 10.4158/EP09005.CRR1. [DOI] [PubMed] [Google Scholar]
- 16.Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: First series in white patients. Heart. 2003;89(9):1027–31. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50(5):448–52. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Cacciotti L, Camastra GS, Beni S, et al. A new variant of Takotsubo cardiomyopathy: Transient mid-ventricular ballooning. J Cardiovasc Med (Hagerstown) 2007;8(12):1052–54. doi: 10.2459/JCM.0b013e32803cab4a. [DOI] [PubMed] [Google Scholar]
- 19.Cacciotti L, Camastra GS, Musaro S, et al. Stress cardiomyopathy: Transient basal ballooning. J Cardiovasc Med (Hagerstown) 2010;11(10):764–67. doi: 10.2459/JCM.0b013e328334466c. [DOI] [PubMed] [Google Scholar]
- 20.Eitel I, Schuler G, Gutberlet M, Thiele H. Biventricular stress-induced (Takotsubo) cardiomyopathy with left mid-ventricular and right apical ballooning. Int J Cardiol. 2011;151(2):e63–64. doi: 10.1016/j.ijcard.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda E, Hisamatsu K, Kijima Y, et al. Morphologically unique feature of recurrent ampulla (Takotsubo) cardiomyopathy. Circ J. 2009;73(2):371–75. doi: 10.1253/circj.cj-07-0976. [DOI] [PubMed] [Google Scholar]
- 22.Mansencal N, El Mahmoud R, Pilliere R, Dubourg O. Relationship between pattern of Takotsubo cardiomyopathy and age: From mid-ventricular to apical ballooning syndrome. Int J Cardiol. 2010;138(1):e18–20. doi: 10.1016/j.ijcard.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Daly MJ, Harbinson MT, Dixon LJ, Spence MS. An unusual case of mid-ventricular Takotsubo cardiomyopathy. QJM. 2010;103(9):695–96. doi: 10.1093/qjmed/hcq008. [DOI] [PubMed] [Google Scholar]
- 24.Balkin DM, Cohen LS. Takotsubo syndrome. Coron Artery Dis. 2011;22(3):206–14. doi: 10.1097/MCA.0b013e328342532c. [DOI] [PubMed] [Google Scholar]
- 25.Ramaraj R, Movahed MR. Reverse or inverted Takotsubo cardiomyopathy (reverse left ventricular apical ballooning syndrome) presents at a younger age compared with the mid or apical variant and is always associated with triggering stress. Congest Heart Fail. 2010;16(6):284–86. doi: 10.1111/j.1751-7133.2010.00188.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh NK, Rumman S, Mikell FL, et al. Stress cardiomyopathy: Clinical and ventriculographic characteristics in 107 North American subjects. Int J Cardiol. 2010;141(3):297–303. doi: 10.1016/j.ijcard.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Nishizawa T, Yajima K, et al. A rare case of Takotsubo cardiomyopathy with variable forms of left ventricular dysfunction: A new entity. Int J Cardiol. 2009;134(2):e73–75. doi: 10.1016/j.ijcard.2007.12.092. [DOI] [PubMed] [Google Scholar]
- 28.Sosnowska-Pasiarska B, Bakowski D, Woronowicz-Chrosciel A, Wozakowska-Kaplon B. Sudden cardiac arrest in Takotsubo cardiomyopathy – a case study. Postepy Kardiol Interwencyjnej. 2014;10(2):110–13. doi: 10.5114/pwki.2014.43517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagiyama N, Okura H, Kume T, et al. Isolated right ventricular Takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16(3):285. doi: 10.1093/ehjci/jeu207. [DOI] [PubMed] [Google Scholar]
- 30.Fang CC, Jao YT, Yi C, et al. Transient left ventricular apical ballooning syndrome: The first series in Taiwanese patients. Angiology. 2008;59(2):185–92. doi: 10.1177/0003319707305463. [DOI] [PubMed] [Google Scholar]
- 31.Matevosyan A, Duchene B, Patel S, et al. Mid-ventricular variant Takotsubo cardiomyopathy in the setting of midodrine use. Chest. 2017;152(4):A85. [Google Scholar]
- 32.Y-Hassan S. Recurrent Takotsubo syndrome triggered by undiagnosed pheochromocytoma. Int J Cardiol. 2015;187:369–71. doi: 10.1016/j.ijcard.2015.03.220. [DOI] [PubMed] [Google Scholar]