Abstract
Patient: Male, 75
Final Diagnosis: Coronary artery disease
Symptoms: Chest pain
Medication: —
Clinical Procedure: Open abdominal aortic aneurysm repair
Specialty: Anesthesiology
Objective:
Challenging differential diagnosis
Background:
Global longitudinal strain (GLS) detected by echocardiography has been shown to have a prognostic role in the evaluation of myocardial ischemia in several clinical settings. A case is presented where GLS was used to detect intraoperative myocardial ischemia in a high-risk patient undergoing open abdominal aortic aneurysm repair.
Case Report:
A 75-year-old Caucasian man with non-insulin dependent diabetes mellitus and a 60 pack-year smoking history presented with a one-week history of exertional chest pain. Two-dimensional (2D) speckle-tracking echocardiography was used to calculate myocardial velocities and deformation parameters, including GLS. A reduced baseline GLS of −18.2% was found with dysfunction of the basal anterior, inferior, and mid anterolateral wall of the left ventricle. During aortic cross-clamping, his basal segments became mildly hypokinetic, although his ejection fraction (EF) remained unchanged at 50–55%. Despite normal left ventricular systolic function on visual assessment, his GLS decreased to −14.2% during aortic cross-clamping with similar segmental changes noted in the baseline GLS analysis. After the release of the aortic cross-clamp, his basal segments returned to normal and his left ventricular systolic function improved with an EF of 60–65% and the GLS recovered to −18.4% with improvement in the basal segmental function.
Conclusions:
This case report showed that detection of GLS by echocardiography was a sensitive indicator of myocardial dysfunction that was superior to regional ventricular wall assessment. Detection of early changes in myocardial function by evaluating GLS may assist in guiding anesthetic management in high-risk patients with ischemic heart disease.
MeSH Keywords: Aortic Aneurysm, Echocardiography, Myocardial Ischemia
Background
Global longitudinal strain (GLS) has been extensively studied in recent years. Its prognostic utility has been demonstrated in a multitude of clinical settings, such as acute myocardial infarction [1], aortic stenosis [2,3], and in the management of heart failure [4]. Some authors make a strong case for the use of GLS in routine clinical decision-making and routine incorporation into echocardiographic studies [5].
Perioperative assessment of GLS has also gained recent attention and quantitation of left ventricular (LV) myocardial strain can be undertaken using speckle-tracking echocardiography [6]. Preoperative strain imaging prior to cardiac surgery helps to predict outcomes, including survival, as well as assisting in risk stratification of surgical patients [7] and serves as an adjunct to expert visual assessment [8]. The feasibility of assessing right ventricular strain using transesophageal echo-cardiography (TEE) in ventilated patients has been assessed [9]. Kroijer et al. successfully used the detection of GLS in a clinical setting in non-cardiac surgery [10].
This report describes a case of myocardial ischemia detected by GLS using intraoperative speckle-tracking echocardiography in a high-risk patient undergoing abdominal aortic aneurysm repair.
Case Report
A 75-year-old Caucasian man with non-insulin dependent diabetes and a 60 pack-year smoking history presented with a one-week history of exertional chest pain. A stress test demonstrated inducible ischemia of the lateral wall. Coronary catheterization showed complete occlusion of the mid circumflex coronary artery, a 90% lesion in a long trifurcating ramus intermedius branch, a 50% segmental non-occlusive lesion in the mid left anterior descending coronary artery, and a 50–60% occlusive lesion in the mid-portion of a small right coronary artery. Due to difficulties in advancing a femoral wire, no coronary artery stents were placed, and the patient underwent an abdominal computed tomography (CT) angiogram that showed a 9.5 cm infrarenal abdominal aortic aneurysm. After multi-disciplinary consultation with interventional cardiology, vascular surgery, and cardiac anesthesiology, it was determined that the coronary artery lesion responsible for the symptoms of angina was the ramus intermedius branch. The decision was made that intervention would improve his angina but was unlikely to change his perioperative or long-term outcome. Given the urgency of his surgery for an abdominal aortic aneurysm, the clinical team decided to proceed with surgery the following day, without coronary intervention.
The patient was treated with beta-blockers to reduce the heart rate to 50 bpm the day before surgery. Anesthetic management included general endotracheal and epidural anesthesia with an arterial line, pulmonary artery catheter, and transesophageal echocardiography (TEE). His baseline intraoperative TEE showed normal left ventricular (LV) systolic function with an ejection fraction of 50–55%, no regional wall motion abnormalities, stage II diastolic dysfunction, and mild left ventricular hypertrophy. The right ventricle was moderately to severely dilated with normal systolic function and there was biatrial dilation with no significant valvular lesions.
Speckle-tracking echocardiography showed reduced baseline global longitudinal strain (GLS) of −18.2% with dysfunction noted in the basal anterior and inferior walls as well as the mid anterolateral wall (Figure 1). During aortic cross-clamping, his basal segments became mildly hypokinetic on 2D examination, although his ejection fraction (EF) remained unchanged at 50–55% (Figure 2, Video 1). Despite generally normal appearing LV systolic function by visual assessment, his GLS decreased to −14.2% during cross-clamping with similar segmental changes noted in the baseline strain analysis (Figure 3). Care was taken to optimize coronary perfusion pressure by maintaining adequate systemic diastolic pressure with norepinephrine infusion. A suprarenal cross-clamp was in place for 26 minutes, and an infrarenal cross-clamp was placed for an additional 36 minutes. After release of the aortic cross-clamp, his basal segments returned to normal and his overall left ventricular systolic function improved to an EF of 60–65% by two-dimensional (2D) examination. The GLS recovered to −18.4% with similar improvements in the basal segmental function (Figure 4).
Figure 1.
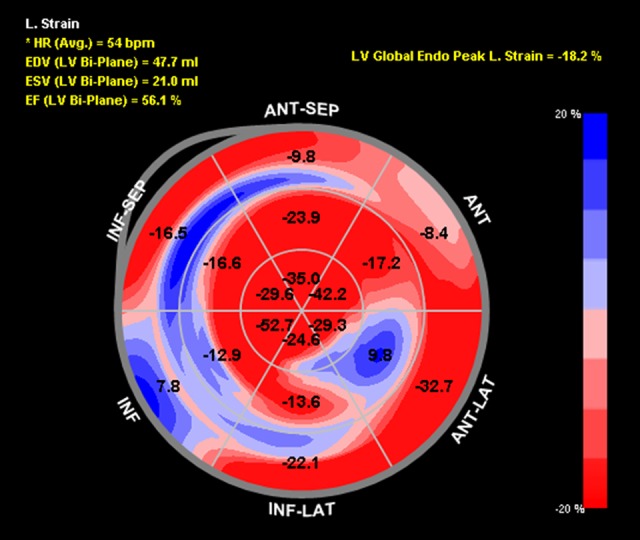
Baseline global longitudinal strain bull’s-eye plot of the 16 established myocardial segments. The numbers represent strain. The less negative the number, the worse the strain. The regions are labeled as follows: ANT-SEP (anteroseptal), ANT (anterior), ANT-LAT (anterolateral), INF-LAT (inferolateral), INF (inferior), INF-SEP (inferoseptal). Calculated left ventricular ejection fraction is labeled as EF (LV Bi-Plane). Left ventricular global peak longitudinal strain is labeled as LB Global Endo Peak L. Strain.
Figure 2.
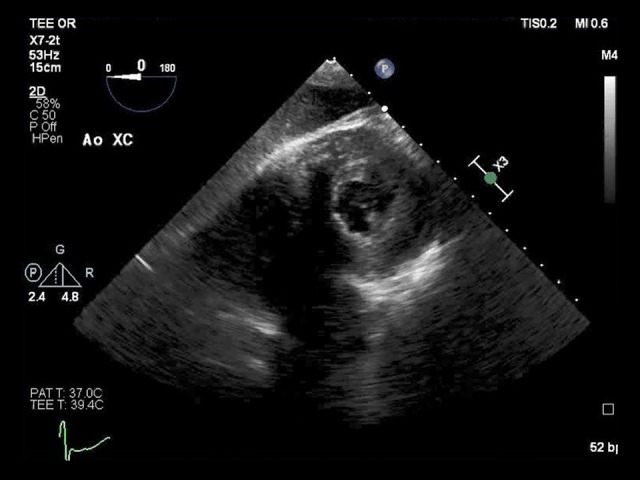
Transgastric mid-papillary short-axis view during aortic cross-clamping shows a normal left ventricular ejection fraction. This image corresponds to Video 1. The left ventricle is seen at the top right-hand side of the video and the right ventricle, while not clear, is to the left of the left ventricle, slightly toward the bottom of the image.
Video 1.
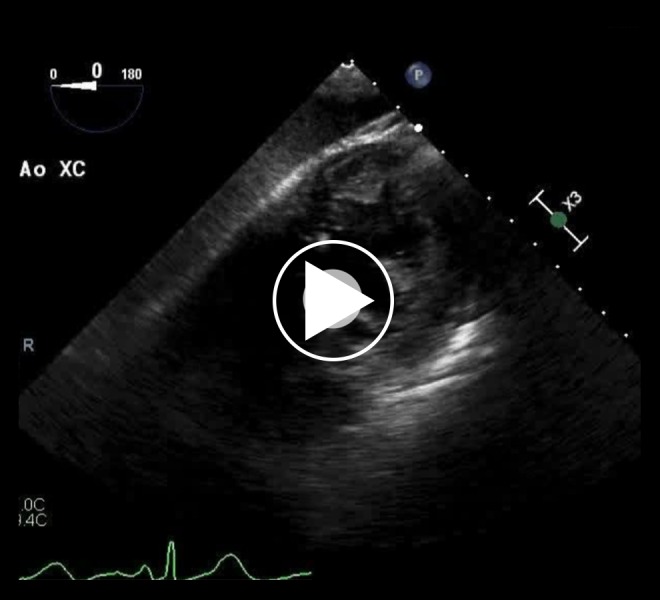
Transgastric mid-papillary short-axis view during aortic cross-clamping showing normal left ventricular ejection fraction. The left ventricle is seen at the top right-hand side of the video. The right ventricle, while not clear, is to the left of the left ventricle, slightly toward the bottom of the image.
Figure 3.
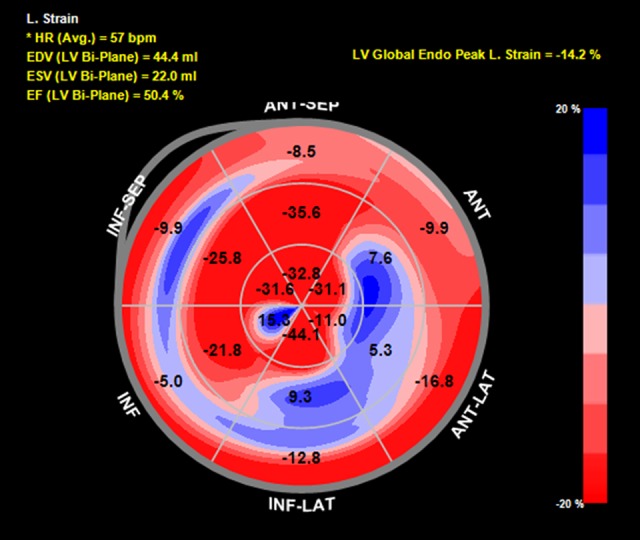
Global longitudinal strain bull’s-eye plot of the 16 established myocardial segments during cross-clamp. The numbers represent strain. The less negative the number, the worse the strain. The regions are labeled as follows: ANT-SEP (anteroseptal), ANT (anterior), ANTLAT (anterolateral), INF-LAT (inferolateral), INF (inferior), INF-SEP (inferoseptal). Calculated left ventricular ejection is labeled as EF (LV Bi-Plane); left ventricular global peak longitudinal strain is labeled as LB Global Endo Peak L. Strain.
Figure 4.
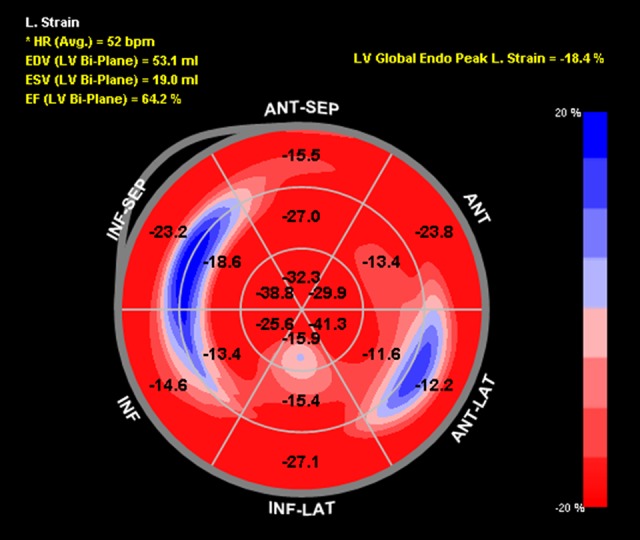
Global longitudinal strain bull’s-eye plot following cross-clamp of the 16 established myocardial segments. The numbers represent strain. The less negative the number, the worse the strain. The regions are labeled as follows: ANT-SEP (anteroseptal), ANT (anterior), ANT-LAT (anterolateral), INF-LAT (inferolateral), INF (inferior), INF-SEP (inferoseptal). The calculated left ventricular ejection is labeled as EF (LV Bi-Plane); left ventricular global peak longitudinal strain is labeled as LB Global Endo Peak L. Strain.
After a total of 62 minutes of aortic cross-clamping and five hours of total operative time, the patient was extubated and transported to the intensive care unit (ICU). The mean arterial pressures were maintained between 70–90 mmHg with a moderate-dose norepinephrine infusion that was weaned within 12 hours of extubation. The postoperative electrocardiogram (ECG) showed normal sinus rhythm with nonspecific ST changes. The patient had negative troponins for the first 24 hours. On postoperative day 2, his cardiac troponin levels slowly began to rise and peaked at 0.098 ng/ml on postoperative day 4. The patient remained stable and was discharged home on postoperative day 5.
Discussion
Assessment of global longitudinal strain (GLS) by speckle-tracking echocardiography is a sensitive tool for the evaluation of global and regional myocardial function. The displacement of two speckles in each myocardial segment is tracked through systole and diastole. The percentage change in length from diastole to systole is referred to as the longitudinal strain, which can be segmentally mapped (Figures 1, 3, 4) [6]. The average of each of the standard 17 myocardial segments is referred to as the GLS, which has been shown to correlate well with long-term outcomes in several patient populations [1,11–15]. Longitudinal strain is a semi-objective quantitative means of assessing myocardial function and has been shown to detect subclinical dysfunction prior to overt changes in regional or global function [6]. Strain has also been shown to be a better predictor of outcomes than the ejection fraction (EF) [16,17]. This patient had a known history of obstructive coronary artery disease, which increased his risk for perioperative myocardial ischemia [18]. Therefore, the addition of intraoperative GLS analysis to routine transesophageal echocardiography (TEE) monitoring detected intraoperative myocardial dysfunction prior to the appearance of regional wall motion abnormalities.
Abnormal strain patterns are well documented in the setting of coronary artery disease [6, 19]. Therefore, the areas of reduced segmental strain in this patient were not unexpected at baseline. Sub-endocardial ischemia causes an early reduction in longitudinal strain [20]. However, normal values for strain can differ between patients and within individual patients [21,22]. Yang et al. have suggested a longitudinal peak strain cutoff of −14.08% for detecting myocardial ischemia and −6.65% for detecting myocardial infarction [23]. According to these values, this patient had myocardial segments at risk for infarction during the period of aortic cross-clamping.
Aortic cross-clamping results in a multitude of physiologic effects, including hemodynamic changes, acid-base disturbances, activation of the renin-angiotensin system, increase in serum catecholamine levels, and production of reactive oxygen species (ROS) [24]. Hemodynamic changes are affected by the level of the clamp, but an increase in both systemic vascular resistance and preload consistently occur [24]. These changes increase myocardial oxygen demand and reduce oxygen supply to the myocardium [25]. In healthy individuals, the Anrep effect, or the autoregulation of myocardial contractility that increases with increased afterload, assists with physiologic compensation. However, in patients with coronary artery disease, left ventricular filling pressures remain elevated with a decreased cardiac index due to an inability to adapt to ischemia by increasing coronary flow and cardiac inotropy [24]. Therefore, it is possible that a significant acute episode of demand ischemia may have gone unnoticed with only mild hypokinesis of the basal segments. In this patient, intraoperative anesthetic management focused on maintaining coronary artery perfusion pressure during surgery and his segmental strain and GLS improved following the period of aortic cross-clamping. These findings indicate that the brief period of ischemia recovered completely, which was confirmed by the mild elevation in postoperative troponin levels.
An acute change GLS is a sensitive indicator of myocardial dysfunction and has been shown to be superior to regional wall assessment, even in the setting of poor acoustic windows on echocardiography [26]. Other abnormal strain patterns associated with coronary ischemia are post-systolic shortening, pre-stretch, and early diastolic decreases in strain rate [19,27,28]. These findings, in addition to standard echocardiographic findings of regional and global LV function, can increase the ability of the intraoperative echocardiographer to detect early changes in myocardial function and may assist in guiding anesthetic management [6].
Conclusions
Transesophageal echocardiography (TEE) is a useful tool in detecting and managing intraoperative ischemia. Evaluations of global longitudinal strain (GLS) is a sensitive indicator of myocardial ischemia and may be able to predict morbidity and mortality. The addition of strain analysis to an intraoperative TEE examination in a high-risk patient may allow for earlier detection of myocardial ischemia, help guide clinical care, and may be useful in perioperative decision-making. Therefore, strain analysis by speckle-tracking echocardiography can increase the utility of intraoperative TEE in detecting myocardial ischemia and should be considered in patients at high risk of ischemic heart disease.
Footnotes
Conflict of interest
None.
References:
- 1.Choi SW, Park JH, Sun BJ, et al. Impaired two-dimensional global longitudinal strain of left ventricle predicts adverse long-term clinical outcomes in patients with acute myocardial infarction. Int J Cardiol. 2015;196:165–67. doi: 10.1016/j.ijcard.2015.05.186. [DOI] [PubMed] [Google Scholar]
- 2.Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012;13(10):827–33. doi: 10.1093/ehjci/jes115. [DOI] [PubMed] [Google Scholar]
- 3.Kusunose K, Goodman A, Parikh R, et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7(6):938–45. doi: 10.1161/CIRCIMAGING.114.002041. [DOI] [PubMed] [Google Scholar]
- 4.Saito M, Negishi K, Eskandari M, et al. Association of left ventricular strain with 30-day mortality and readmission in patients with heart failure. J Am Soc Echocardiogr. 2015;28(6):652–66. doi: 10.1016/j.echo.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2):260–74. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Duncan AE, Alfirevic A, Sessler DI, et al. Perioperative assessment of myocardial deformation. Anesth Analg. 2014;118(3):525–44. doi: 10.1213/ANE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard-Quijano K, Salem A, Barkulis C, et al. Preoperative three-dimensional strain imaging identifies reduction in left ventricular function and predicts outcomes after cardiac surgery. Anesth Analg. 2017;124(2):419–28. doi: 10.1213/ANE.0000000000001440. [DOI] [PubMed] [Google Scholar]
- 8.Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: Definition of normal range. JACC Cardiovasc Imaging. 2009;2(1):80–84. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Tousignant C, Desmet M, Bowry R, et al. Speckle tracking for the intraoperative assessment of right ventricular function: A feasibility study. J Cardiothorac Vasc Anesth. 2010;24(2):275–79. doi: 10.1053/j.jvca.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Kroijer R, Eldrup N, Paaske WP, et al. Left ventricular longitudinal strain for perioperative cardiac monitoring in aortic aneurysm surgery using transthoracic 2-dimensional echocardiography: A feasibility and repeatability study. J Cardiothorac Vasc Anesth. 2010;24(1):37–42. doi: 10.1053/j.jvca.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Park JH, Lee HS, et al. Impaired RV global longitudinal strain is associated with poor long-term clinical outcomes in patients with acute inferior STEMI. JACC Cardiovasc Imaging. 2015;8(2):161–69. doi: 10.1016/j.jcmg.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Alashi A, Mentias A, Abdallah A, et al. Incremental prognostic utility of left ventricular global longitudinal strain in asymptomatic patients with significant chronic aortic regurgitation and preserved left ventricular ejection fraction. JACC Cardiovasc Imaging. 2018;11(5):673–82. doi: 10.1016/j.jcmg.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–80. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 14.Jha AK, Malik V, Gharde P, et al. Echocardiographic predictors of immediate postoperative outcomes in patients with severe left ventricular systolic dysfunction undergoing on-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2017;31(1):184–90. doi: 10.1053/j.jvca.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Biering-Sorensen T, Biering-Sorensen SR, Olsen FJ, et al. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging. 2017;10(3) doi: 10.1161/CIRCIMAGING.116.005521. pii: e005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnasamy R, Isbel NM, Hawley CM, et al. Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS One. 2015;10(5):e0127044. doi: 10.1371/journal.pone.0127044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 18.Patel AY, Eagle KA, Vaishnava P. Cardiac risk of noncardiac surgery. J Am Coll Cardiol. 2015;66(19):2140–48. doi: 10.1016/j.jacc.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260–74. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Reant P, Labrousse L, Lafitte S, et al. Experimental validation of circumferential, longitudinal, and radial 2-dimensional strain during dobutamine stress echocardiography in ischemic conditions. J Am Coll Cardiol. 2008;51(2):149–57. doi: 10.1016/j.jacc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 21.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: A meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–91. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Belghitia H, Brette S, Lafitte S, et al. Automated function imaging: A new operator-independent strain method for assessing left ventricular function. Arch Cardiovasc Dis. 2008;101(3):163–69. doi: 10.1016/s1875-2136(08)71798-4. [DOI] [PubMed] [Google Scholar]
- 23.Yang ZR, Zhou QC, Lee L, et al. Quantitative assessment of left ventricular systolic function in patients with coronary heart disease by velocity vector imaging. Echocardiography. 2012;29(3):340–45. doi: 10.1111/j.1540-8175.2011.01585.x. [DOI] [PubMed] [Google Scholar]
- 24.Zammert M, Gelman S. The pathophysiology of aortic cross-clamping. Best Pract Res Clin Anaesthesiol. 2016;30(3):257–69. doi: 10.1016/j.bpa.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82(4):1026–60. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Macron L, Lairez O, Nahum J, et al. Impact of acoustic window on accuracy of longitudinal global strain: A comparison study to cardiac magnetic resonance. Eur J Echocardiogr. 2011;12(5):394–99. doi: 10.1093/ejechocard/jer029. [DOI] [PubMed] [Google Scholar]
- 27.Voigt JU, Exner B, Schmiedehausen K, et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107(16):2120–26. doi: 10.1161/01.CIR.0000065249.69988.AA. [DOI] [PubMed] [Google Scholar]
- 28.Liang HY, Cauduro S, Pellikka P, et al. Usefulness of two-dimensional speckle strain for evaluation of left ventricular diastolic deformation in patients with coronary artery disease. Am J Cardiol. 2006;98(12):1581–86. doi: 10.1016/j.amjcard.2006.07.038. [DOI] [PubMed] [Google Scholar]


