Abstract
Patient: Female, 9
Final Diagnosis: Mandibular hypoplasia secondary to Shprintzen-Goldberg Syndrome
Symptoms: Difficulty swallowing
Medication: —
Clinical Procedure: Bilateral mandibular osteotomy and distraction for mandibular hypolasia
Specialty: Neurosurgery
Objective:
Rare disease
Background:
Shprintzen-Goldberg syndrome (SGS) is an extremely rare collagenopathy, most often caused by autosomal-dominant mutations in the SKI proto-oncogene, which is a component of the transforming growth factor beta (TGF-β) signaling pathway. Approximately 50–60 cases of SGS have been recorded in the literature worldwide since its discovery in 1982. This collagen disorder affects bone and vascular development throughout the body, resulting in craniosynostosis, scoliosis, chest deformities, and aortic root dilation. Patients may have problems in the central nervous system, including Chiari 1 malformation, hydrocephalus, and dilation of the lateral ventricles. Unfortunately, the symptoms of SGS closely parallel those of related collagenopathies involving mutations in the TGF-β signaling pathway, which makes accurate diagnosis difficult without genetic testing, especially in cases with complex presentation.
Case Report:
In this report we present the unique and complex disease manifestations in a 9-year-old girl with SGS. The patient had severe cervical spinal instability that resolved after surgical occipital-C4 fusion with an autograft from the rib. Midface distraction surgery was used to treat the patient’s craniosynostosis and related facial deformities. This surgery was complicated by loss of 750 mL of blood due to insufficient dura and prominent vasculature.
Conclusions:
Connective tissue symptoms associated with SGS can involve dural and vascular problems, as seen in this case report. Thus, the risk of extreme blood loss should be anticipated any time midface distraction surgery is performed on an SGS patient. Continued research is needed to define how this case relates to the SGS patient population.
MeSH Keywords: Craniosynostosis; DiGeorge Syndrome; Dura Mater; Osteogenesis, Distraction; Spinal Cord Compression
Background
Shprintzen-Goldberg syndrome (OMIM #182212) is an ultra-rare autosomal-dominant genetic collagenopathy. Common characteristics include marfanoid body habitus, characteristic craniofacial abnormalities, craniosynostosis, severe scoliosis, rib abnormalities, intellectual disability, abdominal and umbilical hernias, and aortic dilation. SGS is molecularly heterogeneous, with mutations most often found in the R-SMAD binding region of exon 1 of the SKI (Sloan-Kettering Institute) gene. Mutations in this gene result in an overactive SMAD-dependent pathway of TGF-β signaling.
The proto-oncoprotein SKI normally inhibits SMAD proteins by preventing them from entering the nucleus to transcribe the TGF-β gene. The TGF-β pathway is essential for cell growth, proliferation, and programmed cell death. Its dysregulation results in many of the cardiovascular and connective tissue deformities seen in SGS [1]. Less frequently, SGS patients have mutations in the fibrillin 1 (FBN1) gene, which also codes for a TGF-β regulatory protein. Mutations in other proteins on this pathway can also result in excess activity, leading to similar phenotypic presentations as seen in Marfan and Loeys-Dietz syndromes [2]. There is often an extensive delay preceding SGS diagnosis because it is extremely difficult to distinguish between these related collagenopathies. Delay of diagnosis in SGS can have fatal consequences, as will be discussed later in this report.
Fortunately, the differential expressions of various proteins in the TGF-β pathway lead to slight differences between related collagenopathies. For example, aortic abnormalities are usually milder in SGS than in Loeys-Dietz syndrome because the SKI gene is expressed less pervasively in the aorta than are TGF-β receptor genes[1]. Moreover, intellectual disability appears in SGS patients more often than in Loeys-Dietz patients [1]. The present patient’s unique presentation of SGS most notably involves severe cervical spinal cord compression, abnormal facial vasculature, and insufficient dura mater.
The dura mater is the outermost layer of the meninges, which provides a protective covering for the brain and spinal cord. The dura mater forms a barrier between cerebrospinal fluid and blood. Thus, cerebrospinal fluid will leak if the dura is compromised. Cerebrospinal fluid leakage is a major neurosurgical complication that can result in pneumocephalus, meningitis, improper wound healing, and infections of the graft-bone or epidural space [3]. Our patient presented with insufficient dura, resulting in CSF leakage during a combination Monobloc advancement and cranial vault remodeling surgery aimed to treat midface hypoplasia. Midface hypoplasia is common among SGS patients and can result in lagophthalmos, obstruction of the upper airway, and obstructive sleep apnea [4]. Midface hypoplasia and its resultant problems are often addressed in SGS patients with surgical treatment involving opening of the skull. However, such surgeries are extremely risky in patients with collagen disorders affecting the dura and surrounding vascularity. Thus, the prevalent connective tissue problems must be assessed prior to surgical intervention in SGS patients.
Case Report
This patient was a full-term baby, birth weight 8 pounds 11 ounces, born to a G5P5 35-year-old mother. The pregnancy was complicated by spotting at 7 weeks, difficulty picking up heartbeat at 19 weeks, and a 2-vessel umbilical cord. Although the vaginal delivery was relatively easy, the baby had a fractured clavicle at birth. The patient’s dysmorphic facial features included frontal bossing, low-set ears, hairy ear lobes, and facial features resembling trisomy 21. The patient displayed moderate hypotonia, loose hips, and significant head lag. These concerns led to immediate transfer from the birthing center to the local hospital and subsequent transfer via life-flight to the regional hospital. Karyotype was normal (46XX) and Fluorescence in situ hybridization (FISH) assays were negative for all trisomies. Upon discharge, the patient had difficulties feeding and gaining weight, resulting in 3 hospitalizations for failure to thrive during the first year. Facial deformities and hypotonia contributed to her inability to innervate muscles needed for eating and swallowing. After supplementary high-calorie formula and breastfeeding showed limited success, a gastrostomy tube (G-tube) was placed at 4 months. Adequate caloric intake and expected growth for age were attained. G-tube feedings continue to be the primary form of nutrition to date.
Many of the patient’s symptoms corresponded with collagen-related disorders. Observed bony abnormalities included cervical spinal instability, 13 pairs of ribs, recurrent left knee subluxation, coxa valga, contractures, joint hyperflexibility, and focal reversal of lordosis at T12–L1. Finger abnormalities included camptodactyly, clinodactyly, hypoplastic thumbs, and arachnodactyly. This patient exhibited an asymmetric chest deformity involving both the pectus excavatum and carinatum. Notable craniofacial abnormalities included craniosynostosis, midface hypoplasia, exophthalmos, hypertelorism, ptosis, lagophthalmos, low-set ears, retrognathia, and a high narrow palate. At 2 years, the patient was diagnosed with obstructive sleep apnea and prescribed continuous positive airway pressure (CPAP), which improved energy and progress with developmental milestones (see Table 1 for a full list of patient symptoms).
Table 1.
Complete list of symptoms seen in this patient.
Craniofacial abnormalities:
|
Orthopedic/skeletal abnormalities:
|
Cutaneous symptoms:
|
Cardiovascular symptoms:
|
Neurological abnormalities:
|
Ophthalmology features:
|
Pulmonary:
|
Gastrointestinal Symptoms:
|
Allergy related symptoms:
|
This patient was tested for Loeys-Dietz syndrome, otopalatodigital syndrome, Sticklers syndrome, Zellweger syndrome, Marshall-Smith syndrome, and Marshall syndrome prior to diagnosis. Finally, a genetic test at age 4 years revealed a c.104C>A transversion mutation in exon one of the SKI gene, which converts a proline to a glutamine. This mutation causes SGS. Through genetic consult, an official SGS diagnosis was made 2 months later.
Doctors conducted risk assessment tests upon diagnosis, including echocardiogram of heart and imaging of the cervical spinal cord. Imaging of this patient’s cervical spine revealed severe stenosis and radiographic evidence of myelomalacia at the craniocervical junction (Figure 1). This prompted surgical decompression of the foramen magnum and an occipital-cervical fusion. Postoperatively, her strength markedly improved and she became ambulatory. The echocardiogram showed mild aortic root dilation in the sinus of Valsalva and the ascending aorta, as well as mild regurgitation in the mitral valve. Losartan was prescribed at age 6 years to prevent further dilation.
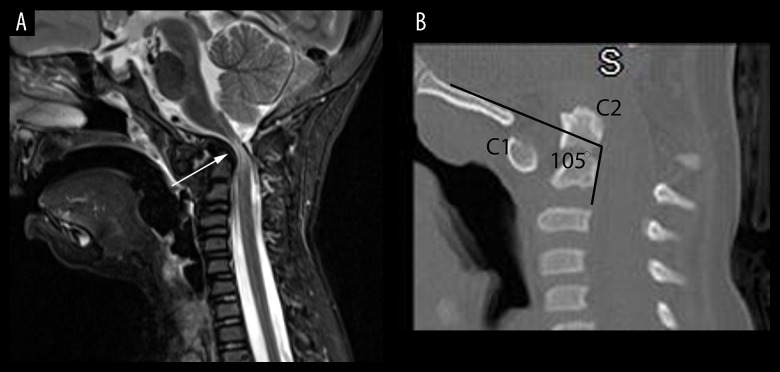
Figure 1.
(A, B) Radiological findings of craniocervical compression (A) A sagittal, T2-Stir-weighted magnetic resonance imaging (MRI) of cervical spine (A) shows severe stenosis at the craniocervical junction with evidence of spinal cord myelomalacia (arrow). (B) A sagittal computed tomography (CT) reconstruction demonstrates platybasia (PB) with a clival-cervical angle of 105°. Dynamic imaging revealed gross instability. High-resolution CT scanning showed a clival-cervical angle of 105 degrees with anterior and inferior displacement of the C1 ring. A clival-cervical angle of less than 125 degrees indicates severe platybasia and is a marker for craniocervical instability. The Pb-C2 line, a measurement of retroflexion of the odontoid as defined by Grabb and Oakes, measured 15 mm, well above the critical value of 9 mm. A value above 9 mm generally indicates severe compression of the spinal canal; her canal width measured 3 mm. The patient underwent a decompression of the foramen magnum and an occipital-cervical fusion. Postoperatively, strength markedly improved and the patient became ambulatory.
The patient’s craniosynostosis involved midface hypoplasia (Figure 2), a symptom in which the middle of the face is not fully developed. This was treated at age 5 years with a combination Monobloc osteotomy and LeFort III distraction surgery. Table 2 provides a full description of the preoperative CT scan and surgical procedure. During the surgery, the surgeons discovered dura that was “less than toilet paper thin” adjacent to the sinus bilaterally and nonexistent over multiple brain sections, including about 4 cm of the sagittal sinus. The dura was also not sticking to the skull. This was not identified on the preoperative CT scan. DuraGen was applied to partially seal the dura. In spite of this insufficiency, there was enough dura at the skull base for removal for subsequent anterior vault reconstruction. The patient experienced a surgical blood loss of approximately 750 ml requiring a total of 17 blood transfusions. Blood flow was stopped with light pressure and application of Tisseel sealant. The surgeons also noted very thin periorbital tissue with pervasive fat herniation and a thin periosteal flap manifesting in adjacent connective tissue problems.
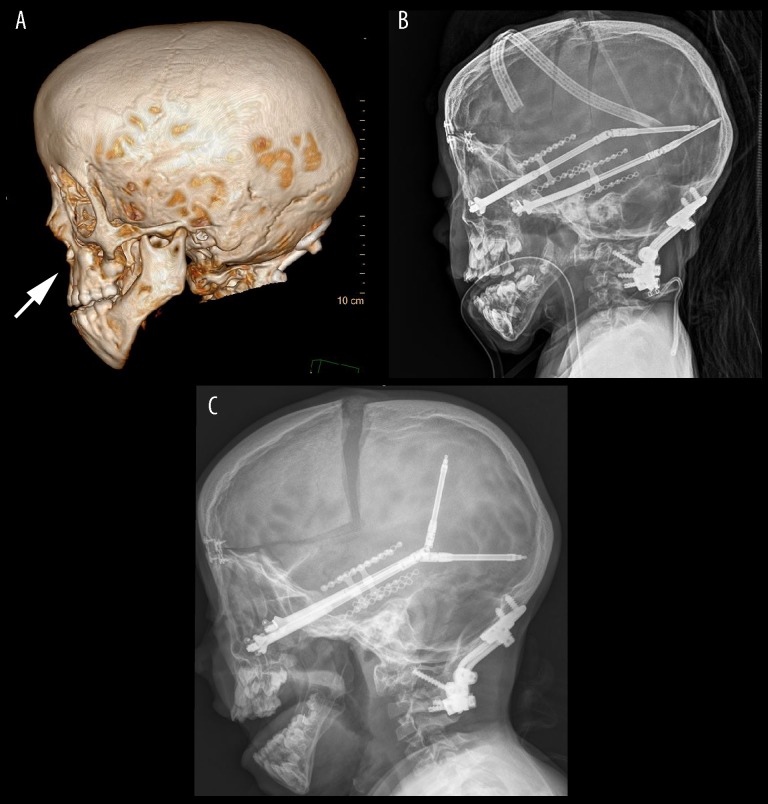
Figure 2.
(A–C) Radiological Visualization of Midface Distraction. (A) 3D CT reconstruction shows pronounced midface hypoplasia (arrow). (B). Lateral x-ray shows the immediate postoperative anatomy after a midface distraction procedure. (C) Lateral x-ray after distraction demonstrates advancement of midface and frontal calvarium.
Table 2.
Description of preoperative CT scan and midface distraction surgery as found in the medical record.
Preoperative CT scan:
|
Reconstructive surgery:
|
Neurosurgery:
|
Blood transfusions
|
|
Postoperative observations: Resulted in more normal midface/jaw relationship, improved exophthalmia, slightly improved sleep apnea, improved hypertelorism, open bite from midface advancement, velopharyngeal insufficiency(VPI) that limited speech, mild prominence of bilateral orbital rims that was shaved down upon distraction removal. |
The patient was placed in a medically induced sedation for 72 hours postoperatively to aid in recovery from the blood loss and difficult surgery. The patient was discharged after 7 days, without complications. Distractors were advanced 1 mm per day for 3 weeks and removed after 9 weeks (Figure 2B, 2C). This surgery normalized the midface-to-jaw relationship, improved exophthalmos and hypertelorism, and slightly improved sleep apnea.
Discussion
This case relates to many other SGS cases presented in the literature, and the present report improves understanding of this disease. A comprehensive summary of SGS cases is shown in Tables 3–5. Notably, this patient’s scoliosis was mild relative to other patients in the literature. This patient’s focal reversal of lordosis at T12–L1 does not currently require surgery. A previous report presented 4 SGS patients requiring surgical scoliosis repair [5]. These surgeries involved numerous bone density-related complications during the operation and in the years following the procedure. Our patient’s cardiac symptoms were also milder than some of those seen in the literature. In contrast, SGS patients may display severe aortic dilation resulting in aneurysm [6,7]. Moreover, 1 patient required mitral valve replacement to treat severe regurgitation. Our patient presented with mild aortic root dilation and mitral valve regurgitation, suggesting that the cardiac symptoms were relatively mild. Our patient presented with a c.104C>A in exon 1 of the SKI gene, which is considered the genetic hotspot for SGS mutations.
Table 3.
Shprintzen-Goldberg syndrome symptoms, a literature review 2010–2019.
| 2010–2019 | [44] O’Dougherty | [43] Zhang 2019 | [7] Kimura 2018 | [42] Saito 2017 | [40] Minocha 2016 | [6] Poninska 2016 | [39] Ingle 2016 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [37] Schepers 2015 | [35] Shah 2014 | [31] Zhu, 2013 | 29] Shanske 2012 | [29] Shanske 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [1] Doyle 2012 | [28] Watanabe 2011 | [28] Watanabe 2009 | [28] Watanabe 2010 | [28] Watanabe 2011 | [27] Gupta 2010 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 12 | 10 | 7 | 3 | 26 | 12 | 50 | 44 | 5 | 4 | 10 | 10 | 12 | 16 | 9 | 13 | 22 | 5 | 2 | 9 | 5 | 43 | 6 | 16 | 12 | 22 | 21 | 2 | 6 | 5 | 4 | 8 | 4 | 11 | 12 | 5 | |
| Sex | F | F | M | F | M | M | F | M | F | M | F | F | M | M | M | F | F | M | M | F | F | M | M | M | F | M | F | M | M | F | F | M | F | M | F | ||
| Craniofacial | |||||||||||||||||||||||||||||||||||||
| Lack of dura mater | ++ | − | ** | ||||||||||||||||||||||||||||||||||
| Craniosynostosis | ++ | − | + | + | + | − | − | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| Dolico-/scaphocephaly | − | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + | + | + | |||||||||||||
| Brachiocephaly | + | ||||||||||||||||||||||||||||||||||||
| Hypertelorism | + | − | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| Downslanting palpebral fissures | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Proptosis | + | − | + | + | + | + | + | + | + | + | + | − | − | + | + | + | + | − | + | + | + | − | + | + | + | + | + | + | + | + | + | ||||||
| Low set ears | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| High/narrow palate | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | +* | + | + | + | + | + | + | + | + | + | ||||||
| Chiari malformation | + | − | + | + | + | ||||||||||||||||||||||||||||||||
| Micro/retrognathia | ++ | − | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Skeletal | |||||||||||||||||||||||||||||||||||||
| Cervical spine abnormalities | ++ | ++ | + | + | − | − | + | + | + | + | |||||||||||||||||||||||||||
| Arachnodactlyly | + | + | ++ | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| Camptodactyly | + | − | + | + | + | − | + | + | − | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | |||||||||||||
| Scoliosis/kyphosis | + | + | + | + | + | ++ | + | + | − | − | + | − | − | + | + | + | + | + | + | + | + | − | − | + | + | + | ++ | ++ | + | ||||||||
| Pectus deformiity | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + | − | + | + | + | |||||||||
| Joint hypermobility | + | − | + | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | |||||||||
| Foot malposition | + | − | + | + | + | + | − | − | + | − | − | + | + | − | − | + | + | + | + | ||||||||||||||||||
| Joint contracture | − | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | |||||||||||||||||||
| Neurological | |||||||||||||||||||||||||||||||||||||
| Developmental delay | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| Intellectual disability | + | − | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Cardiovascular | |||||||||||||||||||||||||||||||||||||
| Mitral valve prolapse | − | − | + | − | − | − | − | − | + | − | − | − | − | + | + | + | − | − | − | + | + | + | |||||||||||||||
| Aortic dilatation | + | − | ++ | − | + | − | − | − | − | − | − | + | + | − | − | + | + | + | + | + | + | + | − | − | + | + | |||||||||||
| Obstructive apnea | + | − | + | + | + | ||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||||
| Hearing loss | + | − | + | ||||||||||||||||||||||||||||||||||
| Inguinal hernia | + | − | + | + | + | ||||||||||||||||||||||||||||||||
| Umbilical hernia | + | − | + | + | + | + | |||||||||||||||||||||||||||||||
| Hypotonia | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Dural ectasia | + | + | |||||||||||||||||||||||||||||||||||
| Malrotation of intestines | + |
Prominent venous structure in posterior Fossa. ‘+’ − present; ‘++’ − severe; ‘−‘ − not present; blank − no information.
Table 4.
Shprintzen-Goldberg Syndrome Symptoms, a literature review 1981–2008.
| 1981–2008 | [26] Stheneur 2008 | [25] Kosaki 2006 | [25] Kosaki 2007 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [24] Robinson 2005 | [23] Greally 1998 | [23] Greally 1998 | [23] Greally 1998 | [23] Greally 1998 | [23] Greally 1998 | [21] Shprinzten and Goldberg 1991 | [21] Shprinzten and Goldberg 1991 | [20] Sugarman, Vogel 1981 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 17 | 17 | 21 | 6 | 4 | 2 | 17 | 10 | 6 | 2 | 7 | 25 | 16 | 17 | 16 | 12 | 12 | 12 | 5 | ∼6 | 17 | ||||
| Sex | M | M | M | M | M | M | M | F | M | M | M | M | M | M | M | M | M | F | M | M | M | F | M | M | M |
| Craniofacial | |||||||||||||||||||||||||
| Lack of dura mater | |||||||||||||||||||||||||
| Craniosynostosis | + | + | + | + | − | − | + | − | + | + | + | − | + | + | + | + | + | ||||||||
| Dolicocephaly | + | + | + | + | + | + | + | − | + | + | + | + | + | − | − | + | + | + | + | ||||||
| Scaphocephaly | |||||||||||||||||||||||||
| Hypertelorism | + | + | + | + | + | − | + | + | + | + | − | + | + | − | − | − | + | + | + | + | |||||
| Downslanting palpebral fissures | + | + | − | − | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| Proptosis | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | |||||||
| Low set ears | + | + | + | + | + | + | + | ||||||||||||||||||
| High/narrow palate | + | + | +* | + | +* | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| Chiari malformation | |||||||||||||||||||||||||
| Micro/retrognathia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | |
| Skeletal | |||||||||||||||||||||||||
| Cervical spine abnormalities | + | + | |||||||||||||||||||||||
| Arachnodactlyly | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| Camptodactyly | − | − | + | − | − | + | + | + | − | + | − | + | − | + | + | + | + | + | |||||||
| Scoliosis/kyphosis | + | + | + | + | + | + | + | − | + | + | − | + | + | + | + | + | + | + | − | + | + | ||||
| Pectus deformiity | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| Joint hypermobility | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| Foot malposition | + | + | + | + | + | + | |||||||||||||||||||
| Joint contracture | + | + | − | + | + | + | |||||||||||||||||||
| Neurological | |||||||||||||||||||||||||
| Developmental delay | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | |
| Intellectual disability | + | + | + | − | + | ++ | + | + | + | − | + | + | + | ||||||||||||
| Cardiovascular | |||||||||||||||||||||||||
| Mitral valve prolapse | − | + | + | − | + | − | + | − | − | − | − | − | + | + | |||||||||||
| Aortic dilatation | + | + | − | − | + | + | + | − | + | − | − | − | − | − | |||||||||||
| Obstructive apnea | − | − | − | − | + | + | + | − | + | ||||||||||||||||
| Other | + | ||||||||||||||||||||||||
| Hearing loss | + | + | + | ||||||||||||||||||||||
| Inguinal hernia | + | + | + | − | + | − | + | + | − | − | + | + | + | + | + | + | + | ||||||||
| Umbilical hernia | + | − | − | + | − | − | + | − | − | + | + | + | |||||||||||||
| Hypotonia | ++ | − | + | + | + | ++ | + | + | + | + | + | + | + | + | |||||||||||
| Dural ectasia |
‘+’ − present; ‘++’ − severe; ‘−‘ − absent; blank − no information; ‘*’ − cleft lip.
Table 5.
Imaging results and treatment interventions for Shprintzen-Goldberg syndrome patients, a literature review 1981–2019.
| Test Performed | Results | [44] O’Dougherty 2019 | [43] Zhang 2019 | [7] Kimura 2018 | [42] Saito 2017 | [40] Minocha 2016 | [6] Poninska 2016 | [39] Ingle 2016 | [31] Zhu 2013 | [29] Shanske 2012 | [29] Shanske 2012 | [1] Doyle 2012 case 1 of 10 | [1] Doyle 2012 8 of 10 | [28] Watanabe 2011 | [28] Watanabe 2011 | [28] Watanabe 2011 | [28] Watanabe 2011 | [27] Gupta 2012 | [26] Stheneur | [25] Kosaki 2006 | [25] Kosaki 2006 | [24] Robinson 2005(1) | [24] Robinson 2005(3) | [24] Robinson 2005(4) | [24] Robinson 2005(7) | [24] Robinson 2005(8) | [24] Robinson 2005(9) | [24] Robinson 2005(11) | [24] Robinson 2005(12) | [24] Robinson 2005(14) | [23] Greally Case 3 of 5 | [23] Greally 5 of 5 | [21] Shprintzen 1982 | [21] Shprintzen 1982 | [20] Sugarman 1981 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * Spinal MRI | Cervical spine instability | + | + | + | + | + | |||||||||||||||||||||||||||||
| *Dural ectasia | +TL | +L | |||||||||||||||||||||||||||||||||
| Severe spondylolysis | + | ||||||||||||||||||||||||||||||||||
| *Spinal cord impingement | +C | +MO | +C | ||||||||||||||||||||||||||||||||
| Brain MRI | Small pituitary gland | + | |||||||||||||||||||||||||||||||||
| Enlarged ventricles | + | + | + | + | + | + | |||||||||||||||||||||||||||||
| EEG | Seizure activity | + | + | − | |||||||||||||||||||||||||||||||
| Spine Xray | Scoliosis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||
| Spina bifuda occulta | + | + | |||||||||||||||||||||||||||||||||
| Skull Xray | Midface hypoplasia | + | + | + | + | + | + | ||||||||||||||||||||||||||||
| Craniosynostosis | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||
| Echocardiogram | Atrial septal defect | + | − | + | + | ||||||||||||||||||||||||||||||
| Mitral valce prolapse | + | + | − | + | |||||||||||||||||||||||||||||||
| Aortic root dilatation | + | − | + | − | + | + | − | + | + | + | + | + | + | + | |||||||||||||||||||||
| CT chest | Recurrent pneumonia | + | + | ||||||||||||||||||||||||||||||||
| *** Aneurysm | ThAAA | TAA | SAA | SAA | |||||||||||||||||||||||||||||||
| Ultrasound | Undescended testes | + | + | ||||||||||||||||||||||||||||||||
| Surgery/treatment | Gastrostomy tube placed | + | + | + | |||||||||||||||||||||||||||||||
| Tracheostomy | + | + | |||||||||||||||||||||||||||||||||
| Tonsillo-adenoidectony | + | + | + | ||||||||||||||||||||||||||||||||
| Mandibular osteotomy and distraction | + | ||||||||||||||||||||||||||||||||||
| Cranioplasty/craniectomy | + | + | + | ||||||||||||||||||||||||||||||||
| Cervical spine surgery | + | + | |||||||||||||||||||||||||||||||||
| ***Hernia | +UI | − | +UI | +U | + | +U | +I | +U | +I | + | + I | +I | + | + | |||||||||||||||||||||
| Mitral valve prolapse repair | + | ||||||||||||||||||||||||||||||||||
| Aortic valve repair | + | ||||||||||||||||||||||||||||||||||
| ***TAA, ThAAA, SAA repair | + | SAA | SAA | ||||||||||||||||||||||||||||||||
| Atrial septal defect repair | + | ||||||||||||||||||||||||||||||||||
| Scoliosis surgery | + | + | + | + | + | + | |||||||||||||||||||||||||||||
| Metatarsus adductus surgery | + | + | |||||||||||||||||||||||||||||||||
| Knee surgery | + | ||||||||||||||||||||||||||||||||||
| Genu recurvatum repair | + | + | + | ||||||||||||||||||||||||||||||||
| Cleft palate repair | + | + | + | ||||||||||||||||||||||||||||||||
| Continuous positive airway pressure (CPAP) | + | + | + | ||||||||||||||||||||||||||||||||
| Orchidoplexy | + | + | |||||||||||||||||||||||||||||||||
| Seizure meds | + |
‘+’ – present; ‘−‘ – not present; blank – no information.
C – cervical; T – thoracic; L – lumbar; MO – medulla oblongata.
Thoracic, Abdominal Aortic (ThAAA); Thoracic Aortic (TAA); Splenic Aortic (SAA).
Umbilical (U) or Inguinal (I) Hernia; UI – both.
Our patient’s combination Monobloc osteotomy and LeFort III distraction surgery has not been previously recorded anywhere in the literature, as they are rarely performed concurrently. The procedural method for Monobloc osteotomy is described in Dr. Laure’s 2014 surgical case study [4,8], while the procedure for LeFort III distraction id presented in Ianetti et al’s 2012 meta-analysis on this procedure [9]. Any midface distraction surgery comes with a high risk of blood loss. However, our patient’s extreme intraoperative blood loss of 750 mL had syndromic causes. The patient had significant holes and paucity in the dura mater that were not observable on preoperative CT scans and could not be completely sealed using DuraGen. This represents an unprecedented symptom of SGS.
Our patient’s insufficient dura mater is likely syndromic. Collagen disorders, including SGS, may affect all connective tissues, including the dura mater. Furthermore, dural issues have been identified in other heritable connective tissue disorders. In a study by Dr. E. Reinstein, 9 patients with hereditary connective tissue disorders experienced CSF leakage due to dural fragility, 7 of whom had Ehlers-Danlos or Marfan syndromes. All these cases resolved with epidural blood patching [10]. Our case was more severe, with pervasively thin dura and gaps as large as 4 cm. DuraGen could not seal the gaps and epidural blood patching would not have resolved this case. Thus, the dural problems presented in this SGS case are more severe than those recorded in related connective tissue disorders.
Unfortunately, it is difficult to detect dural insufficiencies preoperatively. CSF leakage may be indicative of compromised dura because the dura mater holds in the CSF [11]. CT and MRI scans do not allow for proper examination of the dura unless it is inflamed.
Once detected, dural deficiency can be treated in several ways. In our case, patches of DuraGen, a dural-sealing adhesion barrier matrix, were placed. Additional dural supplements have been used to treat CSF leakage. A 2014 paper by Goldschmidt provided evidence that growth factors, including insulin, FGF-2, and human serum, can aid in dural closure by facilitating cell migration [12]. Epidural blood patches can be used to noninvasively treat symptoms of CSF leakage and perforated dura. This method exerts the “mass effect”, which is when injection of the patient’s own blood propels CSF into the cranium and increases intracranial pressure [13]. In a 2011 study by Burkett, patients who received dural sealants had shorter average hospital stays and time in intensive care units, decreased need for additional incisions, and decreased lumbar CSF drainage than those treated with autologous fat graft and lumbar drain replacement [14]. This indicates that application of dural sealants may be the most effective treatment for compromised dura.
Collagen is a crucial part of the cardiovascular system because it is a protein in the matrix that supports the shape of blood vessels [15]. Thus, collagenopathies are linked to aortic and peripheral aneurysms [16].
In our patient, surgery was used as treatment for midface hypoplasia, which was caused by craniosynostosis [17,18]. This symptom spurred surgical intervention because it hindered quality of life. In our patient, midface hypoplasia caused lagophthalmos and airway obstruction, which contributed to breathing difficulties and obstructive sleep apnea. The severity of these symptoms should be weighed against the risk of this type of surgery, considering the findings in the present report. Posterior distraction may be a safer alternative to the external methods used here. Dural issues and consequent blood loss may be avoided in this procedure because the dura remains attached to the “endocranial surface of the vault bone.” This surgery was successful in treating midface hypoplasia in an Antley-Bixler Syndrome patient, but further investigation is needed regarding application to SGS [19].
Conclusions
This case report presents dural insufficiency as a previously unreported symptom of SGS. We also discussed the risk of major blood loss in combination Monobloc osteotomy and LeFort III distraction surgery for treatment of symptoms stemming from craniosynostosis. Our literature review assessed how this case relates to previous findings in SGS.
Acknowledgments
Special thanks to all members of the patient’s family for assistance and support, as well as to Ms. Corianne Kellems for providing administrative and editorial help.
Abbreviations:
- SGS
Shprintzen-Goldberg Syndrome;
- LDS
Loeys-Dietz Syndrome;
- MRI
magnetic resonance imaging;
- CT scan
computerized axial tomography scan;
- CSF
cerebrospinal fluid
Footnotes
Conflict of interest
None.
References:
- 1.Doyle AJ, Doyle JJ, Bessling SL, et al. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44:1249–54. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler JB, Ikonomidis JS, Jones JA. Connective tissue disorders and cardiovascular complications: The indomitable role of transforming growth factor-beta signaling. Adv Exp Med Biol. 2015;802:107–27. doi: 10.1007/978-94-007-7893-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinaci A, Algra A, Heuts S, et al. Effectiveness of dural sealants in prevention of CSF leakage after craniotomy: A systematic review. World Neurosurg. 2018;118:368–76. doi: 10.1016/j.wneu.2018.06.196. [DOI] [PubMed] [Google Scholar]
- 4.Laure B, Moret A, Joly A, et al. Orbitofrontal monobloc advancement for Crouzon syndrome. J Cranio Maxill Surg. 2014;42(6):335–38. doi: 10.1016/j.jcms.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrhop SK, McElroy MJ, Dietz HC, et al. High prevalence of cervical deformity and instability requires surveillance in Loeys-Dietz syndrome. J Bone Jt Surg. 2015;95(5):411–19. doi: 10.2106/JBJS.N.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poninska JK, Bilinska ZT, Franaszczyk M, et al. Next-generation sequencing for diagnosis of thoracic aortic aneurysms and dissections: Diagnostic yield, novel mutations and genotype phenotype correlations. J Transl Med. 2016;14(1):115. doi: 10.1186/s12967-016-0870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura N, Inaba Y, Kameyama K, Shimizu H. Thoraco-abdominal aortic aneurysm rupture in a patient with Shprintzen-Goldberg syndrome. Interact Cardiovasc Thorac Surg. 2018;26(6):1039–40. doi: 10.1093/icvts/ivy003. [DOI] [PubMed] [Google Scholar]
- 8.Al-Namnam NMN, Hariri F, Rahman ZAA. Distraction osteogenesis in the surgical management of syndromic craniosynostosis: A comprehensive review of published papers. Br J Oral Maxillofac Surg. 2017;56(5):353–66. doi: 10.1016/j.bjoms.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Iannetti GT, Ramieri V, Pagnoni M, et al. Le Fort III external midface distraction: Surgical outcomes and skeletal stability. J Craniofac Surg. 2012;23(3):896–900. doi: 10.1097/SCS.0b013e31824e2549. [DOI] [PubMed] [Google Scholar]
- 10.Reinstein E, Pariani M, Bannykh S, et al. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: A prospective study. Eur J Hum Genet. 2013;21(4):386–90. doi: 10.1038/ejhg.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schievink WI, Dodick DW, Mokri B, et al. Diagnostic criteria for headache due to spontaneous intracranial hypotension: A perspective. Headache. 2011;51(9):1442–44. doi: 10.1111/j.1526-4610.2011.01911.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt E, Ielpi M, Loresi M, et al. Assessing the role of selected growth factors and cytostatic agents in an in vitro model of human dura mater healing. J Neurol Res. 2014;36(12):1040–46. doi: 10.1179/1743132814Y.0000000429. [DOI] [PubMed] [Google Scholar]
- 13.Agerson A, Scavone B. Prophylactic epidural blood patch after unintentional dural puncture for the prevention of postdural puncture headache in parturients. Anesth Analg. 2012;115(1):133–36. doi: 10.1213/ANE.0b013e31825642c7. [DOI] [PubMed] [Google Scholar]
- 14.Burkett CJ, Patel S, Tabor MH, et al. Polyethylene glycol (PEG) hydrogel dural sealant and collagen dural graft matrix in transsphenoidal pituitary surgery for prevention of postoperative cerebrospinal fluid leaks. J Clin Neurosci. 2011;18(11):1513–17. doi: 10.1016/j.jocn.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta. 2014;1842(11):2106–19. doi: 10.1016/j.bbadis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaulieu RJ, Lue J, Ehlert BA. Surgical management of peripheral vascular manifestations of Loeys-Dietz syndrome. Ann Vasc Surg. 2017;38:10–16. doi: 10.1016/j.avsg.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Carmignac V, Thevenon J, Ades L, et al. In-frame mutations in Exon 1 of SKI cause dominant Shprintzen-Goldberg syndrome. Am J Hum Genet. 2012;91(5):950–57. doi: 10.1016/j.ajhg.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzen JT, McCarthy JG. Syndromes involving craniosynostosis and midface hypoplasia. Otolaryngol Clin North Am. 2000;33(6):1257–84. doi: 10.1016/s0030-6665(05)70280-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang, Jessica S, Lin K. Posterior distraction osteogenesis in syndromic craniosynostosis: A case series. American Medical Student Research Journal. 2015;2(1):21–27. [Google Scholar]
- 20.Sugarman G, Vogel MW. Case report 76: Craniofacial and musculoskeletal abnormalities – A questionable connective tissue disease. Synd Ident. 1981;7:16–17. [Google Scholar]
- 21.Shprintzen RJ, Goldberg RB. A recurrent pattern syndrome of craniosynostosis associated with arachnodactyly and abdominal hernias. J Craniofac Genet Dev Biol. 1982;2:65–74. [PubMed] [Google Scholar]
- 22.Sood S, Eldadah ZA, Krause WL, et al. Mutation in fibrillin-1 and the Marfanoid-craniosynostosis (Shprintzen-Goldberg) syndrome. Nat Genet. 1996;12(2):209–11. doi: 10.1038/ng0296-209. [DOI] [PubMed] [Google Scholar]
- 23.Greally MT, Carey JC, Milewicz DM, et al. Sphrintzen-Goldberg syndrome: A clinical analysis. Am J Med Genet. 1998;76(3):202–12. [PubMed] [Google Scholar]
- 24.Robinson P, Neumann L, Demuth S, et al. Shprintzen-Goldberg syndrome: Fourteen new patients and a clinical analysis. Am J Med Genet A. 2005;135(3):251–62. doi: 10.1002/ajmg.a.30431. [DOI] [PubMed] [Google Scholar]
- 25.Kosaki K, Takahashi D, Udaka T, et al. Molecular pathology of Shprintzen-Goldberg syndrome. Am J Med Genet Part A. 2006;140(1):104–8. doi: 10.1002/ajmg.a.31006. [DOI] [PubMed] [Google Scholar]
- 26.Stheneur C, Collod-Béroud G, Faivre L, et al. Identification of 23 TGFBR2 and 6 TGFBR1 gene mutations and genotype-phenotype investigations in 457 patients with Marfan syndrome type I and II, Loeys-Dietz syndrome and related disorders. Hum Mutat. 2008;29(11):E284–95. doi: 10.1002/humu.20871. [DOI] [PubMed] [Google Scholar]
- 27.Gupta AK, Divekar DS, Shah B, Dhulkhed VK. Anesthetic management of a rare case of Shprintzen-Goldberg craniosynostosis syndrome. Pediatr Anesth. 2010;20(8):771–73. doi: 10.1111/j.1460-9592.2010.03340.x. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Okada E, Kosaki K, et al. Surgical treatment for scoliosis in patients with Shprintzen-Goldberg syndrome. J Pediatr Orthop. 2011;31(2):186–93. doi: 10.1097/BPO.0b013e3182093da5. [DOI] [PubMed] [Google Scholar]
- 29.Shanske A, Goodrich J, Ala-Kokko L, et al. Germline mosaicism in Shprintzen-Goldberg syndrome. Am J Med Genet Part A. 2012;158(7):1574–78. doi: 10.1002/ajmg.a.35388. [DOI] [PubMed] [Google Scholar]
- 30.Kanda T, Kasai H, Sanefuji Y. Fiberoptic tracheal intubation through the supraglottic airway device air-Q in a patient with Shprintzen-Goldberg syndrome. Masui. 2013;62(8):942–45. [PubMed] [Google Scholar]
- 31.Zhu X, Zhang Y, Wang J, et al. 576 kb deletion in 1p36.33–p36.32 containing SKI is associated with limb malformation, congenital heart disease and epilepsy. Gene. 2013;528(2):352–55. doi: 10.1016/j.gene.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Elmistekawy E, Hudson C, Williams A, Mesana T. Double-valve surgery in Shprintzen-Goldberg syndrome. Asian Cardiovasc Thorac Ann. 2014;22(7):842–45. doi: 10.1177/0218492313485070. [DOI] [PubMed] [Google Scholar]
- 33.Billie Au PY, Racher HE, Graham JM, et al. De novo exon 1 missense mutations of SKI and Shprintzen-Goldberg syndrome: Two new cases and clinical review. Am J Med Genet. 2013;164:676–84. doi: 10.1002/ajmg.a.36340. [DOI] [PubMed] [Google Scholar]
- 34.Jeong HJ, Lee JJ, Lee BD, et al. Case of psychotic patient with suspected Shprintzen-Goldberg syndrome. Psychiatry Clin Neurosci. 2014;68(5):388–89. doi: 10.1111/pcn.12141. [DOI] [PubMed] [Google Scholar]
- 35.Shah B, Sahu S, Kalakoti P, et al. Shprintzen-Goldberg syndrome presenting as umbilical hernia in an Indian child. Australas Med J. 2014;7(2):51–57. doi: 10.4066/AMJ.2014.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannaerts E, va de Beek G, Verstraeten A, et al. TGF-B signalopathies as a paradigm for translational medicine. Eur J Med Genet. 2015;58(12):695–703. doi: 10.1016/j.ejmg.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Schepers D, Doyle AJ, Oswald G, et al. The SMAD-binding domain of SKI: A hotspot for de novo mutations causing Shprintzen-Goldberg syndrome. Eur J Hum Genet. 2015;23(2):224–28. doi: 10.1038/ejhg.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav S, Rawal G. Shprintzen-Goldberg syndrome: A rare disorder. Pan Afr Med J. 2016;23:227. doi: 10.11604/pamj.2016.23.227.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingle SY, Shah P, Karande P. A rare case of Shprintzen-Goldberg syndrome. J Evolution Med Dent Sci. 2016;5(18):914–16. [Google Scholar]
- 40.Minocha P, Sitaraman S, Goyal M. Shprintzen-Goldberg Syndrome presenting as generalized epilepsy in a child: A rare presentation. International Journal of Biomedical Research. 2016;7(6):399–401. [Google Scholar]
- 41.Zanetti-Yabur A, Butler T, Rocca JP, Graham JA. Pancreas transplantation is feasible in donors with Shprintzen-Goldberg syndrome. Transplant Proc. 2017;49(8):1883–84. doi: 10.1016/j.transproceed.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Saito T, Nakane T, Yagasaki H, et al. Shprintzen-Goldberg syndrome associated with first cervical vertebra defects. Pediatr Int. 2017;59(10):1098–100. doi: 10.1111/ped.13354. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Xu X, Sun K, et al. A de novo mutation in DHD domain of SKI causing spina bifida with no craniofacial malformation or intellectual disability. Am J Med Genet A. 2019;179(6):936–39. doi: 10.1002/ajmg.a.61088. [DOI] [PubMed] [Google Scholar]