Abstract
Background
Lupus nephritis is one of the most serious complications of systemic lupus erythematosus (SLE) and is associated with patient mortality. This study aimed to investigate the proteomic profiles of the glomerulus in the NZB/W F1 hybrid mouse model of mild and severe lupus nephritis using two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) combined with matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS).
Material/Methods
Female NZB/WF1 mice (n=60) at 28 weeks of age were divided into the mild proteinuria group (+1), the moderate proteinuria group (+2), and the severe proteinuria group (+3) using paper strip urine testing, and then later divided into a mild (≤1+) and severe (≥3+) proteinuria group to allow comparison of upregulation and down-regulation of proteins between the two groups. Renal glomeruli were isolated following renal perfusion with magnetic beads. Protein expression was determined by Western blot, immunohistochemistry, 2D-DIGE, and MALDI-TOF-MS.
Results
A total of 56 differentially expressed proteins were identified from 133 protein spots, of which 18 were upregulated and 23 were down-regulated between groups 1 and 2. Expression of the proteins Ras-related GTP-binding protein B (RRAGB), serine/threonine-protein kinase 1 (SMG1), angiopoietin 2 (ANGP2), methylmalonate semialdehyde (MMSA), and ATP beta chain (ATPB) were identified by Western blot and SMG1, ANGP2, and MMSA were identified by immunohistochemistry.
Conclusions
In a mouse model of lupus nephritis, expression of SMG1, MMSA, and ATPB were down-regulated, and RRAGB and ANGP2 were upregulated.
MeSH Keywords: Lupus Nephritis, Proteomics, Two-Dimensional Difference Gel Electrophoresis
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease that is associated with the production of autoantibodies that result in immune damage to several organs, and commonly affects the kidneys [1–3]. The etiology and pathogenesis of SLE remain unclear, but genetic, epigenetic, and environmental factors have been described [4–6]. Between 40–70% of patients with SLE have clinical lupus nephritis [7]. Lupus nephritis is an immune complex-mediated glomerulonephritis, and the severity of renal damage determines the prognosis of lupus nephritis [8]. Up to 10% of patients with lupus nephritis develop end-stage renal disease, which is a leading cause of patient morbidity and mortality due to SLE [9].
Proteins are key players in cellular processes, and human diseases can be caused by abnormal protein expression at multiple levels. Therefore, understanding disease in proteomic terms can help to explain the molecular basis of disease, with the aim of disease prevention, diagnosis, and treatment. Mass spectrometry (MS)-based proteomics has made a significant contribution to understanding these basic biological processes and is able to measure hundreds of thousands of proteins in any biological system test platform [10]. During the past decade, with the application of MS-based proteomic technologies, discoveries have been made that have provided insight into the pathogenesis of some important diseases. Also, the development of proteomics databases has provided information about peptide and protein amino acid sequence and quantitative information for research studies [11,12]. SLE and lupus nephritis have also been studied with the use of proteomics techniques with urine being most commonly used in proteomics studies [13], followed by peripheral blood mononuclear cells [14], plasma [15], and renal cortical tissue [16]. However, the changes in protein expression at the level of the glomerulus in lupus nephritis remain unknown.
A new method has been developed that uses magnetic beads to isolate renal glomeruli from mice, which provides the opportunity for the proteomic profiling of the glomerulus [17]. However, this method can be expensive to use, and so a new and simpler technique that uses fewer magnetic beads but can obtain the same amount of protein has provided new opportunities for proteomic studies of the glomerulus [18].
Therefore, this study aimed to investigate the proteomic profiles of the glomerulus in the NZB/W F1 hybrid mouse model of mild and severe lupus nephritis using two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) combined with matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS).
Material and Methods
Animals
Specific pathogen-free (SPF) 12-week-old female NZB/W F1 hybrid mice (n=60) were purchased from the Jackson Laboratory (Bar Harbour, ME, USA) and SPF 12-week-old female C57BL/6 mice (n=60) were purchased from Beijing Vitonghua Laboratory Animal Technology Co., Ltd. NZB/W F1 hybrid mice were chosen because they spontaneously developed lupus nephritis. The mice were bred in a Chinese Medical University SPF animal room, at 23°C (±3°C), with a humidity of 50% (±20%), a 12-hourly light and dark cycle, and free access to food and drink, in accordance with the guidelines of the Chinese National Standard (GB 14925-2001). The study complied with the protocols approved by the Institutional Animal Care and Use Committee at China Medical University (Approval Date: Dec14, 2016) (IACUC Issue No. 2016101). At the end of the study, all mice were euthanized with sodium pentobarbital anesthesia.
Animal groups
The onset of renal disease was monitored by weekly testing of fresh urine specimens with protein test strips. Mice were grouped according to the degree of proteinuria as +1 (10 mg/dl), +2 (100 mg/dl), +3 (300 mg/dl), and +4 (>1,000 mg/dl). Mice with proteinuria of 300 mg/dl or more in repeated tests were regarded as having severe proteinuria [19]. At the age of 28 weeks, the mice were divided into two groups based upon their degree of renal disease: light lupus nephritis (proteinuria ± – +) and severe lupus nephritis (proteinuria +3 – +4).
The use of magnetic beads to separate the glomeruli
Isolation of the mouse renal glomeruli through the thoracic aorta was performed according to the method previously described, but with some modifications [17]. Briefly, ligation of the distal abdominal aorta and inferior vena cava was performed, followed by ligation of the superior mesenteric artery and the peritoneal artery. Thoracotomy was performed to insert an intravenous indwelling needle. A 0.3 cm opening was made close to the ligation of the inferior vena cava, and phosphate-buffered saline (PBS) at 4°C was used to perfuse the kidney.
Magnetic beads (Dynal Company, Union City, CA, USA) were used to perfuse the kidney, at a rate of 7.2 ml/min/gm of kidney tissue and using 4×107 beads/ml. The renal tissue was removed and placed on ice and the renal cortex was sectioned into 1 mm3 slices. The remaining renal tissue was stored at −70°C. Collagenase A was used to digest the protein at 37°C, with the lysate passed twice through a mesh with a 100 μm pore size. The washed glomeruli were isolated using a magnet, with the entire procedure was performed on ice. The glomerular proteins were isolated using a 2-D Clean-Up Kit (GE Healthcare Life Sciences, Logan, UT, USA). The protein content was determined using electrophoresis with an Ettan TM 2-D Quant Kit (GE Healthcare Life Sciences, Logan, UT, USA), according to the manufacturer’s instructions.
Two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) of glomerular proteins
Ettan IPGphor III (GE Healthcare Life Sciences, Logan, UT, USA) was used to perform the isoelectric focusing (IEF) program, referred to as the first electrophoresis. For each specimen that had been sampled from mice in the proteinuria ≤1+ group and proteinuria in the ≥3+ group, 50 μg of purified glomerular protein was labeled with 1 μl (400 pmol) of CyDye (GE Healthcare Life Sciences, Logan, UT, USA), and an internal standard (the equivalent mixture of the above two glomeruli protein samples) (50 μg), which was labeled with the same content of Cy2 on the same 2D gel. The fluorescent dye was incubated in the dark for 30 minutes. To each centrifuge tube was added with 1 μL of 10 mM of lysine to stop the reaction, followed by mixing for 10 s. The labeling reaction was quenched by centrifugation at 10,000×g and 4°C for 1 min and then stored on ice for 10 min in a dark environment.
The proteins were labeled with Cy3, Cy5, and Cy2 for mass spectrometry (600 μg), that required preparing volumes of 2*DTT, IPG pyrolysis solution, 1*DTT, IPG hydration solution and the final volume of 450 μL, which were added to the labeled samples. After IEF electrophoresis, the Ettan DALT six vertical electrophoresis system (GE Healthcare Life Sciences, Logan, UT, USA) was used to run the second dimension of the 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel overnight using a low voltage. When the bromophenol blue staining was 1 cm from the bottom of the glass, electrophoresis was stopped. The dry gel was removed, washed with deionized water, and placed in the Typhoon TRIO scanner (Bruker, Billerica, MA, USA) for image acquisition. SYPRO® Ruby protein gel staining (Thermofisher Scientific, Waltham, MA, USA) was used, in the dark and on a shaking platform overnight. The gel was washed and placed in the Typhoon TRIO scanner (Bruker, Billerica, MA, USA), and the images were collected after adding 100 ml of fresh dye and refrigerating at 4°C.
Matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS)
Gel images were analyzed using DeCyder 2-D differential analysis software version 7.2. Trypsin (Promega, Madison, WI, USA) was added at 37°C overnight, and 0.4 mg of α-cyano-hydroxy-cinnamic acid (HCCA) (Bruker, Billerica, MA, USA) was dissolved in 300 μl of 1% of trifluoroacetic acid (TFA) and 700 μl of acetonitrile to form the substrate. Peptide labeling was diluted 240 times as standard. MALDI-TOF mass spectrometry (Bruker, Billerica, MA, USA) was used to obtain the ionic peaks of the protein spots. Flexi Analysis™ version 3.0 software was used to obtain the peptide list and fingerprint of protein spots. Mascot software from Matrix Science was used to retrieve the information for the protein spots in the SWISS-PROT curated protein sequence database to obtain the full protein sequence and the protein score of mass spectrometry identification coverage sequence. A score of >50 and fold change >1.5 times indicated a significant match and accurate identification of the proteins.
Western blot
The extraction method of glomeruli protein was the same as described above. The BCA method was used to determine the concentration of glomerular protein. SDS-PAGE protein electrophoresis was performed (the loading quantity of the sample was 35 μg) with a voltage of 15V for 2 h on the polyvinylidene fluoride membrane (PVDF), incubated in 5% dried skimmed milk powder (prepared with TBST) and mixed for 1 h. After 1 h, the membranes were washed three times with TBST for 10 min each, followed by incubation in the primary antibody, diluted 1: 200, overnight at 4°C. The membranes were then incubated with horse-radish peroxidase (HRP)-conjugated IgG secondary antibodies to Ras-related GTP-binding protein B (RRAGB), serine/threonine-protein kinase 1 (SMG1), angiopoietin 2 (ANGP2), methylmalonate semialdehyde (MMSA), and ATP beta chain (ATPB), and Annexin A5 (ANXA5) at a dilution of 1: 1000. The PVDF membrane was washed with TBST for 10 min and then incubated for 2 h in the secondary antibody. The images were captured using Beyo ECL Plus reagent (Beyotime, Shanghai, China). Western blot was performed in triplicate, and representative photomicrograph images were obtained. Chemiluminescent gel imaging system MicroChemi (DNR Co., Israel) was used to quantify the relative expression levels of the candidate proteins.
Immunohistochemistry
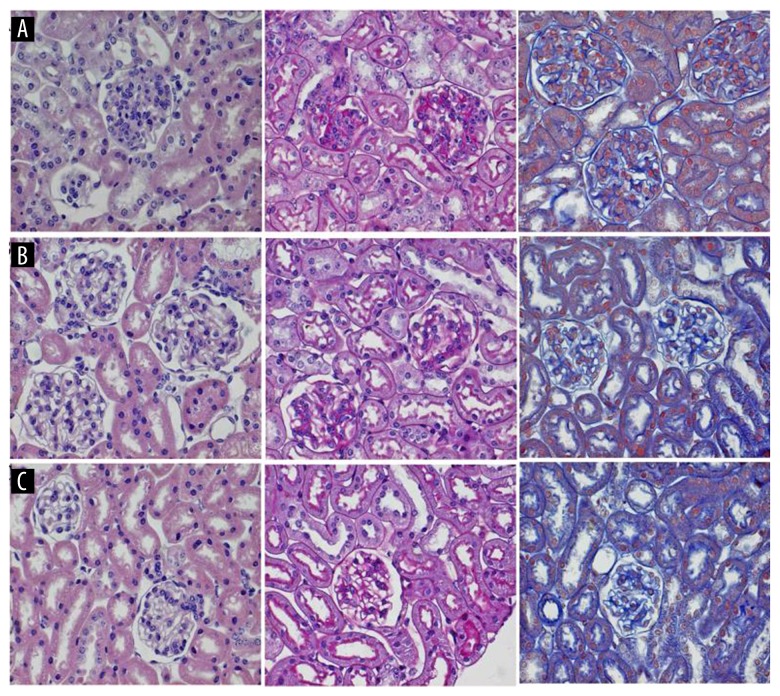
Paraffin sections of mouse kidney were deparaffinized with xylene and rehydrated in a graded series of alcohols. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 10 min. The sections were incubated with primary antibodies to ANGPT2, SMG1, and ANXA5 overnight at 4°C. HRP-conjugated and biotinylated secondary antibodies were added for 20 min at room temperature, incubated at 37°C for 30 min, and washed three times for 5 min each in PBS. Then, 100 μl of the brown chromogen, 3,3′-diaminobenzidine (DAB) (Solarbio Science & Technology Co. Ltd., Beijing, China) was added and incubated on the tissue section until a brown reaction developed. Tissue sections were counterstained, washed, dehydrated, and mounted for viewing by light microscopy. Histochemical stains used to study the histology of the mouse model and control kidney tissue included hematoxylin and eosin (H&E), Periodic acid Schiff (PAS), and Masson’s trichrome (Figure 1).
Figure 1.
Photomicrographs of the histology of the kidney in the NZB/W F1 hybrid mouse model of mild and severe lupus nephritis shows the renal glomeruli. (A) NZB/W F1 hybrid mouse model. Severe proteinuria (the proteinuria ≥3+ group). (B) NZB/W F1 hybrid mouse model. Mild proteinuria (the proteinuria ≤+ group). (C) C57BL/6 age-matched mouse control group. Images from left to right, hematoxylin and eosin (H&E), magnification ×400; Periodic acid Schiff (PAS), magnification ×400; Masson’s trichrome, magnification ×400. Severe lupus nephritis in the NZB/W F1 hybrid mouse model is shown by glomerular mesangial cell proliferation, sclerosis, nuclear fragmentation and shrinkage (pyknosis), and neutrophil infiltration.
Statistical analysis
Protein expression data were expressed as the mean ± standard deviation (SD) of triplicate gel images. Statistical analysis was performed using SPSS version 16.0 software (IBM, Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
Results
Histology of the kidney tissue of the NZB/W F1 hybrid mouse model of lupus nephritis
The main histological changes of lupus nephritis of the NZB/W F1 hybrid mice were glomeruli mesangial cell proliferation and mesangial matrix hyperplasia. Compared with the mild proteinuria group, the severe lupus nephritis F1 mice showed glomerular mesangial cell proliferation, and glomerular sclerosis, cell nuclear fragmentation, and nuclear shrinkage (pyknosis), with neutrophil infiltrates. The glomerular basement membrane was thickened, and some tubules were necrotic (Figure 1).
Two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) imaging
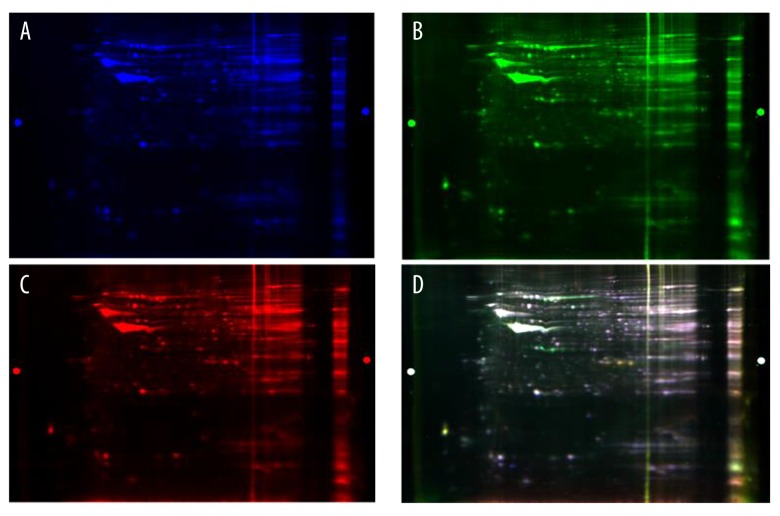
The gels were analyzed using a Typhoon Trio scanner (GE Healthcare Life Sciences, Logan, UT, USA) for two-dimensional differential gel electrophoresis (2D-DIGE) imaging. The dyed blue map by Cy2 was an internal standard, and the dyed green map from Cy3 was from the severe proteinuria group, the dyed red map from Cy5 was from the mild proteinuria group. The final image was created by the superposition of the three fluorescent channels (Figure 1). If the color was yellow, the protein expression levels were close to each other; if it was red, the protein (Cy3) was upregulated; and if the color was green, the protein was down-regulated (Figure 2).
Figure 2.
(A–D) Images of the two-dimensional fluorescence difference gel electrophoresis (2D-DIGE). The gels were analyzed using a Typhoon TRIO scanner, which acquired the two-dimensional differential gel electrophoresis image (2D-DIGE). The dyed blue map using Cy2 is the internal standard group, the dyed green map using Cy3 is the severe proteinuria group, the dyed red map using Cy5 is the mild proteinuria group. The last image is the superposition of three fluorescence channels.
A total of 1630 protein points were matched, and a total of 56 differentially expressed proteins were obtained from 133 protein spots identified by mass spectrometry. After removing the damaged colloidal particles using an Ettan spot picker and comparing the two groups, there were 13 upregulated proteins and 15 down-regulated protein expression. Details of the proteins identified are shown in Tables 1 and 2.
Table 1.
Comparison of upregulated protein expression in the renal glomeruli of the NZB/W F1 hybrid mouse model of lupus nephritis between the mild proteinuria group (group 1) (≤1+) and the severe proteinuria group (group 2) (≥3+).
| Spot ID | MW (Da) | Protein name | SWISS-PROT accession | Ratio | Protein score |
|---|---|---|---|---|---|
| 1499 | 43620 | Ras-related GTP-binding protein B | RRAGB | 3.07 | 59 |
| 1476 | 40669 | cATP-dependent protein kinase catalytic subunit PRKX | PRKX | 2.47 | 53 |
| 1369 | 8930 | Protein FAM229B | F229B | 2.07 | 70 |
| 1327 | 96750 | A-kinase anchor protein 3 | AKAP3 | 1.93 | 50 |
| 1472 | 38937 | Annexin A2 | ANXA2 | 1.92 | 124 |
| 541 | 175669 | NACHT domain and WD repeat-containing protein 1 | NWD1 | 1.88 | 50 |
| 406 | 56996 | Angiopoietin-2 | ANGP2 | 1.78 | 59 |
| 538 | 67839 | Moesin | MOES | 1.66 | 57 |
| 806 | 135640 | Wings apart-like protein homolog | WAPL | 1.6 | 50 |
| 795 | 129164 | Coiled-coil domain-containing protein 150 | CC150 | 1.6 | 50 |
| 1529 | 35787 | Annexin A5 | ANXA5 | 1.54 | 56 |
| 2072 | 19824 | Myosin regulatory light chain12B | ML12B | 1.5 | 53 |
| 1588 | 36178 | Annexin A4 | ANXA4 | 1.51 | 101 |
Table 2.
Comparison of down-regulated protein expression in the renal glomeruli of the NZB/W F1 hybrid mouse model of lupus nephritis between the mild proteinuria group (group 1) (≤1+) and the severe proteinuria group (group 2) (≥3+).
| Spot ID | MW (Da) | Protein name | SWISS-PROT accession | Ratio | Protein score |
|---|---|---|---|---|---|
| 1971 | 413559 | Serine/threonine-protein kinase SMG1 | SMG1 | 2.2 | 64 |
| 1377 | 89167 | Myotubularin-related protein 10 | MTMRA | 1.96 | 60 |
| 1335 | 36792 | Alcohol dehydrogenase [NADP(+)] | AK1A1 | 1.74 | 51 |
| 807 | 58335 | Methylmalonate-semialdehyde dehydrogenase | MMSA | 1.72 | 80 |
| 800 | 15119 | Profilin-1 | PROF1 | 1.66 | 60 |
| 2494 | 12035 | Pterin-4-aipha-carbinolamine dehydratase | PHS | 1.64 | 60 |
| 2628 | 149978 | Lysine-specific demethylase 3A | KDM3A | 1.64 | 52 |
| 141 | 130344 | Pyruvate carboxylase, mitochondrial | PYC | 1.61 | 66 |
| 844 | 59337 | Alpha-aminoadipic semialdehyde dehydrogenase | AL7A1 | 1.57 | 51 |
| 1331 | 39938 | Fructose-bisphate aldolase B | ALDOB | 1.56 | 64 |
| 439 | 22461 | Coiled-coil domain-containing protein 85B | CC85B | 1.54 | 54 |
| 443 | 12625 | NADH dehydrogenase [ubiquinon] a-subunit 7 | NDUA7 | 1.53 | 50 |
| 766 | 54751 | Dihydrolipoyl dehydrogenase | DLDH | 1.52 | 74 |
| 819 | 56265 | ATP synthase subunit beta, mitochondrial | ATPB | 1.51 | 99 |
| 2625 | 61088 | 60 KDa heat shock protein, mitochondrial | CH60 | 1.51 | 84 |
Western blot analysis of representative candidate proteins
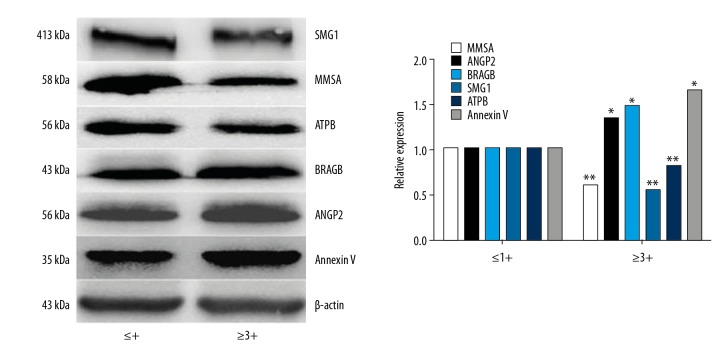
According to the 2-D and MS results, the following were selected, as the candidate proteins, Ras-related GTP-binding protein B (RRAGB), serine/threonine-protein kinase 1 (SMG1), angiopoietin 2 (ANGP2), methylmalonate semialdehyde (MMSA), and ATP beta chain (ATPB). Annexin A5 (ANXA5) was a positive control. Western blot was used to confirm these proteins (Figure 3).
Figure 3.
Western blot analysis of protein expression in the kidney glomeruli from the NZB/W F1 hybrid mouse model group 1 (mild proteinuria) and group 2 (severe proteinuria). The Western blot images on the left are the mild proteinuria ≤1+ group, and the images on the right are the severe proteinuria ≥3+ group. The expression of Ras-related GTP-binding protein B (RRAGB) and angiopoietin 2 (ANGP2) are upregulated and the expression of serine/threonine-protein kinase 1 (SMG1), methylmalonate semialdehyde (MMSA), and ATP beta chain (ATPB) are down-regulated. The gray value histogram of the Western blot validation results shows that the gray value of the ratio of each control group and the internal control are all 1 on the left side. On the right side, the same pattern is found. The gray values of the corresponding proteins were first compared with internal control and then compared with the base value. * Proteinuria ≥3+ group compared with proteinuria ≤1+ group, P<0.05. ** Proteinuria ≤1+ group compared with proteinuria ≥3+ group, P<0.05.
Immunohistochemistry
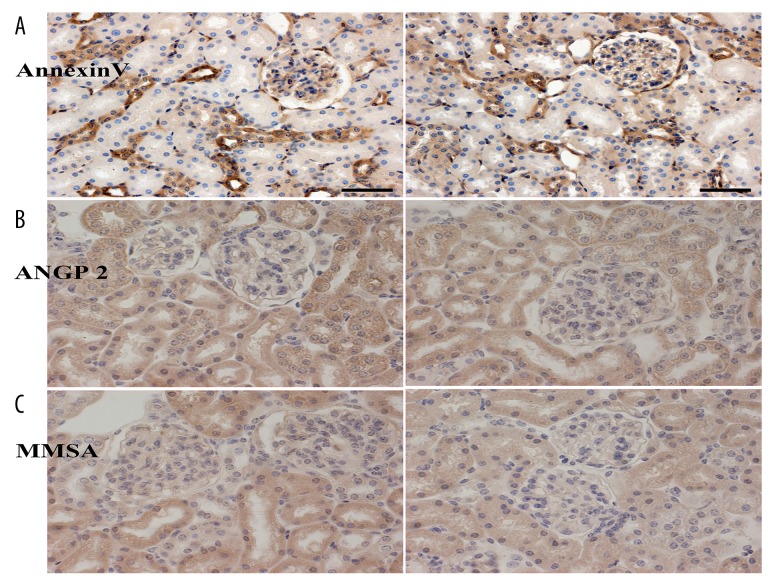
Using positive staining for ANXA5, in mice in group 2 the protein expression was greater than for group 1. There were differences in expression levels of MMSA and ANGP2 between the two groups (Figure 4).
Figure 4.
(A–C) Immunohistochemical of kidney tissue sections in the NZB/W F1 mouse model group 1 (mild proteinuria) and group 2 (severe proteinuria) groups. Angiopoietin 2 (ANGP2) and Annexin A5 (ANXA5) (as the control) expression in the glomeruli are increased in the proteinuria ≥3+ group (group 2) of NZB/W F1 mice, but the expression of methylmalonate semialdehyde (MMSA) in the glomeruli is reduced. The left images show the mild proteinuria group (proteinuria ≤1+), and the right images show the severe proteinuria group (proteinuria ≥3+).
Discussion
Proteomics complements genomics. Following the completion of the human genome project, scientists realized that many problems related to proteins cannot be completely explained solely by genomics. Protein ultimately implements the function of not only coding genes but also non-coding genes, including microRNA (miRNA) and long noncoding RNA (lncRNA).
The aims of this study were to investigate the proteomic profiles of the glomerulus in an NZB/W F1 hybrid mouse model of lupus nephritis using two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) combined with matrix-assisted laser desorption time of flight mass spectrometry (MALDI-TOF-MS). In this study, differences in fluorescence using 2D-DIGE combined with MALDI-TOF-MS proteomic demonstrated the protein profile of the glomerulus in a spontaneous mouse model of lupus nephritis, the NZB/W F1 mouse. A total of 56 differentially expressed proteins were screened during the development of lupus nephritis. As representitive the expression of RRAG and ANGPT2 being up-regulated and the expression of SMG1, MMSA and ATPB being down-regulated were identified by wetern blot detection. SMG1, MMSAand and ANGP2 were identified by Immunohistochemical method. These results suggest that these proteins may be involved in the progression of lupus nephritis.
Rag GTPases (include Rag A, B, C, D) belong to the family of guanine nucleotide-binding proteins, and Rag A and B, and Rag C and D can form four dimers [20,21]. Rag GTPases played an important role in the activation of the mechanistic target of rapamycin complex 1 (mTORC1) [21]. The formation of the dimer of Rag GTPases is crucial to the activation of mTORC1, and the role of Rag A/B seems to be more important than Rag C/D in the activation of mTORC1 [20,21]. Studies have shown that in the lysosome, Rag GTPases mediate signaling of amino acids to mTORC1 [22,23]. Recently mTORC1 has been shown to affect Th17 and Treg cell differentiation, and Th17/Treg imbalance can cause renal damage in SLE [24]. A study showed that RagA/B GTPases act as important components of the lysosome and can affect the function of cardiac myocytes [25].
In the present study, the proteomic profile of NZB/W F1 mice when the group with urine protein ≤1+ and the group with urine protein ≥3+ were compared, showed that the expression of RagA increased 3.07 times with increased proteinuria. This finding may suggest that at the onset of lupus nephritis, the mTORC1 pathway may have a role. Also, mTORC1 can regulate the phosphorylation of proteins, synthesize ribosomes and lipids, and prevents autophagy. It is possible that mTORC1 has a role in SLE and lupus nephritis through Rag GTPases.
SMG-1 is a newly-described member of the phosphoinositide kinase-like kinase (PIKK) family [26]. SMG-1 can regulate the proliferation of cells and maintain the genome. The stability or maturity of PIKK family members depends in part on a molecular chaperone called tel2-tti1-tti2 (TTT) [27], which, together with HSP90, participates in the proper folding of newly synthesized PIKKs [28]. As a regulator of the DNA damage response, PIKKs promote stability and maintain the activity of mTORC1 and mTORC2 complexes, which may regulate telomere length, SMG1, and mRNA expression in response to stress [29]. SMG-1 participates in epithelial-mesenchymal transition (EMT), triggered by activation of the Wnt pathway in gastric cancer, and EMT can be associated with chronic kidney damage [30].
ATPB is a subunit of ATP that is associated with oxidative stress, which is increased in lupus nephritis [31]. Studies have shown that the expression of ATPB differs between patients with SLE with and without lupus nephritis [32]. Angiostatin, an antagonist of angiogenesis, may down-regulate endothelial cell proliferation and migration by binding to the α/β-subunits of ATP synthase on the cell surface [33]. Therefore, some factors that affect ATP synthase may also affect kidney vascular endothelial cells and may promote the development of SLE and lupus nephritis.
Angiopoietin 2 (ANGP2) can mediate endothelial cell activity and is associated with the endothelial receptor tyrosine kinase, Tie-2 [34]. Increased levels of circulating ANGP2 are associated with renal involvement in SLE, and renal expression of ANGP2 is upregulated in patients with lupus nephritis [35]. These results support the findings of the present study. Increased serum levels of ANGP2 can reflect the extent of endothelial cell activation and are correlated with renal involvement in patients with SLE [36]. The present study was the first to investigate the expression of ANGP2 in the kidney in lupus nephritis using proteomics analysis.
MMSA expression has not been previously reported in SLE but has been described in other diseases, including methylmalonic aciduria [37]. Recently, MMSA was shown to promote Th1 cell-mediated immune responses and to induce dendritic cell activation, indicating that it may have a role in the immune response, including SLE and lupus nephritis [38].
Currently, MS-based proteomics analysis has identified 241 protein markers of SLE, including Annexin A5 (ANXA5) [16,39], which is a marker for stage IV lupus nephritis. In the present study, both 2D and MALDI-TOF-MS proteomics analysis methods were used, and ANXA5 was also identified. However, it is likely that more proteins remain to be identified in lupus nephritis.
Conclusions
In this study, the proteomic profiles of the glomerulus in an NZB/W F1 hybrid mouse model of lupus nephritis were analyzed using two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) combined with matrix-assisted laser desorption time of flight mass spectrometry (MALDI-TOF-MS). A total of 56 differentially expressed proteins were identified during the development of lupus nephritis in the mouse model. The expression of Ras-related GTP-binding protein B (RRAGB) and angiopoietin 2 (ANGP2) were upregulated and the expression of serine/threonine-protein kinase 1 (SMG1), methylmalonate semialdehyde (MMSA), and ATP beta chain (ATPB) were down-regulated. The findings suggest that the expression of these proteins may be involved in the progression of lupus nephritis. Further studies are required to identify the range of proteins expressed and their role in lupus nephritis.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81273297), the National Science and Technology Support Program during the 12th five-year plant period (No. 2011BAI10B04), the Science and Technology Plan of Liaoning Provincial Technology Department (No. 2012225021), and the Natural Science Foundation of Liaoning Province (No. 201202254)
Conflict of interest
None.
References
- 1.Constantin MM, Nita IE, Olteanu R, et al. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis. Exp Ther Med. 2019;17(2):1085–90. doi: 10.3892/etm.2018.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M, Yao C, Cai J, et al. LRRK2 is involved in the pathogenesis of system lupus erythematosus through promoting pathogenic antibody production. J Transl Med. 2019;17(1):37. doi: 10.1186/s12967-019-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue Z, Cui C, Xia S, et al. Identification of lncRNA Linc00513 containing lupus-associated genetic variants as a novel regulator of interferon signaling pathway. Front Immunol. 2018;9:2967. doi: 10.3389/fimmu.2018.02967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghodke-Puranik Y, Imgruet M, Dorschner JM, et al. Novel genetic associations with interferon in systemic lupus erythematosus identified by replication and fine-mapping of trait-stratified genome-wide screen. Cytokine. :2019. doi: 10.1016/j.cyto.2018.12.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H, Mi W, Luo H, et al. Whole-genome transcription and DNA methylation analysis of peripheral blood mononuclear cells identified aberrant gene regulation pathways in systemic lupus erythematosus. Arthritis Res Ther. 2016;18:162. doi: 10.1186/s13075-016-1050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adtani P, Malathi N. Epstein-Barr virus and its association with rheumatoid arthritis and oral lichen planus. J Oral Maxillofac Pathol. 2015;19(3):282–85. doi: 10.4103/0973-029X.174643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin J, Zheng Z, Li X, et al. Urinary albumin levels are independently associated with renal lesion severity in patients with lupus nephritis and little or no proteinuria. Med Sci Monit. 2017;23:631–39. doi: 10.12659/MSM.899973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin H, Wei JC, Tan CY, et al. Survival analysis of late-onset systemic lupus erythematosus: A cohort study in China. Clin Rheumatol. 2012;31(12):1683–89. doi: 10.1007/s10067-012-2073-6. [DOI] [PubMed] [Google Scholar]
- 9.Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci. 2013;346:319–23. doi: 10.1097/MAJ.0b013e31827f4ee3. [DOI] [PubMed] [Google Scholar]
- 10.Williams EG, Wu Y, Jha P, et al. Systems proteomics of liver mitochondria function. Science. 2016;352(6291) doi: 10.1126/science.aad0189. aad0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthiesen R, Trelle MB, Hojrup P, et al. VEMS 3.0: Algorithms and computational tools for tandem mass spectrometry-based identification of post-translational modifications in proteins. J Proteome Res. 2005;4(6):2338–47. doi: 10.1021/pr050264q. [DOI] [PubMed] [Google Scholar]
- 12.Tabb DL, Fernando CG, Chambers MC. MyriMatch: Highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res. 2007;6:654–61. doi: 10.1021/pr0604054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somparn P, Hirankarn N, Leelaha-vanichkul A, et al. Urinary proteomics revealed prostaglandin H(2)D-isomerase, not Zn-alpha2-glycoprotein, as a biomarker for active lupus nephritis. J Proteomics. 2012;75:3240–47. doi: 10.1016/j.jprot.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Kazemipour N, Qazizadeh H, Sepehrimanesh M, et al. Biomarkers identified from serum proteomic analysis for the differential diagnosis of systemic lupus erythematosus. Lupus. 2015;24:582–87. doi: 10.1177/0961203314558860. [DOI] [PubMed] [Google Scholar]
- 15.Caster DJ, Korte EA, Merchant ML, et al. Autoantibodies targeting glomerular annexin A2 identify patients with proliferative lupus nephritis. Proteomics Clin Appl. 2015;9:1012–20. doi: 10.1002/prca.201400175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alaiya A, Assad L, Alkhafaji D, et al. Proteomic analysis of Class IV lupus nephritis. Nephrol Dial Transplant. 2015;30(1):62–70. doi: 10.1093/ndt/gfu215. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161(3):799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Fan Q, Yang G, et al. Isolating glomeruli from mice: A practical approach for beginners. Exp Ther Med. 2013;5(5):1322–26. doi: 10.3892/etm.2013.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Fujio K, Jiang Y, et al. Dissection of the role of MHC class II A and E genes in autoimmune susceptibility in murine lupus models with intragenic recombination. Proc Natl Acad Sci USA. 2004;101(38):13838–43. doi: 10.1073/pnas.0405807101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim E, Goraksha-Hicks P, Li L, et al. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–45. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon S, Dibble CC, Talbott G, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156(4):771–85. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Q, Wang B, Su H, et al. Elevated Th17 cells are accompanied by foxp3+ Treg cells decrease in patients with lupus nephritis. Rheumatol Int. 2012;32(4):949–58. doi: 10.1007/s00296-010-1771-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Park HW, Sciarretta S, et al. Rag GTPases are cardioprotective by regulating lysosomal function. Nat Commun. 2014;5:4241. doi: 10.1038/ncomms5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denning G, Jamieson L, Maquat LE, et al. Cloning of a novel phosphatidylinositol kinase-related kinase: Characterization of the human SMG-1 RNA surveillance protein. J Biol Chem. 2001;276:22709–14. doi: 10.1074/jbc.C100144200. [DOI] [PubMed] [Google Scholar]
- 27.Rao F, Cha J, Xu J, et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol Cell. 2014;54(1):119–32. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takai H, Xie Y, de Lange T, Pavletich NP. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes Dev. 2010;24:2019–30. doi: 10.1101/gad.1956410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horejsí Z, Takai H, Adelman CA, et al. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell. 2010;39:839–50. doi: 10.1016/j.molcel.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Peng Y, Huang Y, et al. SMG-1 inhibition by miR-192/-215 causes epithelial-mesenchymal transition in gastric carcinogenesis via activation of Wnt signaling. Cancer Med. 2018;7(1):146–56. doi: 10.1002/cam4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CK, Seto NL, Vivekanandan-Giri A, et al. Lupus high-density lipoprotein induces proinflammatory responses in macrophages by binding lectin-like oxidised low-density lipoprotein receptor 1 and failing to promote activating transcription factor 3 activity. Ann Rheum Dis. 2017;76:602–11. doi: 10.1136/annrheumdis-2016-209683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan PE, Sturgess AD, Hennessy A, Davies MJ. Serum protein oxidation and apolipoprotein CIII levels in people with systemic lupus erythematosus with and without nephritis. Free Radic Res. 2007;41:1301–12. doi: 10.1080/10715760701684809. [DOI] [PubMed] [Google Scholar]
- 33.Moser TL, Stack MS, Asplin I, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811–16. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salama MK, Taha FM, Safwat M, et al. The Tie2 receptor antagonist angiopoietin-2 in systemic lupus erythematosus: Its correlation with various disease activity parameters. Immunol Invest. 2012;41(8):864–75. doi: 10.3109/08820139.2012.711407. [DOI] [PubMed] [Google Scholar]
- 35.Kümpers P, David S, Haubitz M, et al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis. 2009;68(10):1638–43. doi: 10.1136/ard.2008.094664. [DOI] [PubMed] [Google Scholar]
- 36.El-Banawy HS, Gaber EW, Maharem DA, Matrawy KA. Angiopoietin-2, endothelial dysfunction and renal involvement in patients with systemic lupus erythematosus. J Nephrol. 2012;25(4):541–50. doi: 10.5301/jn.5000030. [DOI] [PubMed] [Google Scholar]
- 37.Marcadier JL, Smith AM, Pohl D, et al. Mutations in ALDH6A1 encoding methylmalonate semialdehyde dehydrogenase are associated with ds myelination and transient methylmalonic aciduria. Orphanet J Rare Dis. 2013;8:98. doi: 10.1186/1750-1172-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JS, Kim WS, Choi HH, et al. Mycobacterium tuberculosis MMSA, a novel immunostimulatory antigen, induces dendritic cell activation and promotes Th1 cell-type immune responses. Cell Immunol. 2015;298(1–2):115–25. doi: 10.1016/j.cellimm.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Luo N, Wu Q, et al. Transcellular distribution heterogeneity of Annexin A5 represents a protective response to lupus-related thrombophilia: A pilot proteomics-based study. Biochem Biophys Res Commun. 2012;420:357–63. doi: 10.1016/j.bbrc.2012.02.162. [DOI] [PubMed] [Google Scholar]