Abstract
BACKGROUND:
Depression is very common in the elderly population. Physical exercise is one of the non-pharmacological procedures that promise to be a solution to improve the severity of depression. Brain-Derived Neurotrophic Factor (BDNF) plays a role in maintaining the survival of neuronal cells and in the regulation of synapse plasticity, affecting serotonin production in the hippocampus and thus the depressive symptoms.
AIM:
This study aimed to assess the role of physical exercise in affecting BDNF levels in elderly with depression.
METHODS:
Thirty-five elderly women (age ≥ 50 years) with depressive episodes based on Diagnostic and Statistical Manual of Mental Disorders (DSM)-V criteria were enrolled as treatment group, and 35 elderly women without depressive episodes were enrolled as control group, and underwent physical exercise in the form of treadmill with a speed of 6 km/h for 15 minutes. Physical exercise was carried out once a day for 28 days. As much as 1 ml of blood from the study, subjects were obtained from the cubital vein before the exercise commenced. Brain-Derived Neurotrophic Factor (BDNF) serum level was assessed by Enzyme-Linked Immunosorbent Assay (ELISA). Data were presented in the form of mean ± SD. An independent T-test was used to test levels after exercise in the depression group compared to the non-depression group.
RESULTS:
Pre-exercise BDNF levels in the depression group were lower than the group of elderly without depression. Physical exercise increased BDNF production in both elderly groups with and without depression. In the depression group, the increasing percentage of BDNF level was higher compared to non-depressive elderly.
CONCLUSION:
The increasing percentage of BDNF level was found to be higher in depressive elderly performing physical exercise. Physical exercise may be beneficial in supporting the therapy of elderly with depression.
Keywords: Exercise, Brain-Derived Neurotrophic Factor, Depression
Introduction
Depression is one major health problem experienced by more than 300 million people worldwide. This disorder is characterised by the presence of typical symptoms in the form of sadness or anhedonia which are persistent and followed by somatic, cognitive, and symptomatic disorders in the form of decreased appetite, sleep disturbances, feeling unenergized, decreased concentration, guilt and suicidal thoughts or attempts [1]. Depression is very common in the elderly population. The elderly population generally has retired, which sometimes initiates a slight psychic disorder in the form of feeling no longer useful and post-power syndrome. These psychological changes generally initiate depression in the elderly population [2].
In addition to psychological problems, depressive disorders that generally occur in the elderly can be caused by physiological changes in the body. Ageing will be followed by a decrease in bodily functions, including a decrease in brain function and neurotransmitters. Neurobiological depression is caused by a decrease in the serotonin neurotransmitter in the hippocampus. The decrease in serotonin is caused by a decrease in neuronal cells. Brain-Derived Neurotrophic Factor (BDNF) is a growth factor that plays a role in maintaining the survival of neuronal cells and in the regulation of synapse plasticity. Decreasing BDNF levels will reduce the survival of neuronal cells, which will reduce serotonin production in the hippocampus, leading to the occurrence of depressive symptoms [3].
Physical exercise is one of the non-pharmacological procedures that promise to be a solution to improve the severity of symptoms in people with depression. Previous studies showed the role of physical exercise in increasing BDNF levels. Physical exercise in the form of jogging for about 30 minutes every day is believed to be able to improve cardiovascular function and improve cognitive function through regulation of BDNF production [4], [5].
This study aimed to assess the role of physical exercise in affecting BDNF levels in elderly with depression.
Material and Methods
Study Population
The study was conducted on the geriatric population at Kambang Iwaki Palembang elderly community. This study included 70 subjects, where 35 older women (age ≥ 50 years) with depressive episodes based on Diagnostic and Statistical Manual of Mental Disorders (DSM)-V criteria in the past 1 year, enrolled as the treatment group. As many as 35 older women (age ≥ 50 years) without any psychotic symptoms or depressive episodes were enrolled as a control group. Assessment of the degree of depression was done by referring to the Hamilton Rating Scale for Depression (HRSD). Subjects were categorised as depressed when HRSD score > 7. Baseline assessment of the level of physical activity was carried out by The Habitual Physical Activity (HPA) score. Subjects who were determined to experience depression were first treated with an antidepressant of Selective Serotonin Reuptake Inhibitors (SSRI) for 3 months before the study was conducted. They were subjected to routine blood tests in the form of blood glucose levels, cholesterol levels, uric acid levels and vital sign examinations to determine the overall health status of the subjects before the exercise. Also, subjects were tested for cognitive function, with the Mini-Mental State Examination (MMSE) test.
The subjects had received informed consent regarding the study. This study was by the Declaration of Helsinki and had received ethical approval from the ethics committee for medical and health research, Bioethics and Humanities Unit, Faculty of Medicine, Sriwijaya University (No.153/kptfkunsri-rsmh/2018).
Physical Exercise
Physical exercise was carried out in a measurable manner, where each study subject conducted a treadmill with a speed of 6 km/h for 15 minutes. Physical exercise was carried out once a day for 28 days.
Blood Collection
Before the commencement of the physical exercise, as much as 1 ml of blood from the study, subjects were obtained from the cubital vein. Furthermore, the blood of the research subjects was stored in the centrifuge tube. The samples that had been stored in the centrifuge tube were then centrifuged at a speed of 5000 rpm for 10 minutes at 25°C. Then, the supernatant was separated from the pellet and put into a 1.5 ml centrifuge tube. Samples were stored at -20°C.
Measurement of BDNF
BDNF serum level was assessed by Enzyme-Linked Immunosorbent Assay (ELISA) (detection limit of 62.5 pg/ml). ELISA kit (Cloud-Clone Corp, Texas, USA) consisted of 96-well microplate pre-coated with antibodies for BDNF. A total of 10 ul samples were inserted into the microplate, then incubated for 30 minutes, at 37°C. Next, horseradish peroxidase (HRP)-conjugate (Sigma-Aldrich, St. Louis, Missouri, USA) was added as much as 50 ul on each microplate, then pre-incubated at 37°C for 30 minutes. As much as 50 ul of chromogen A and B were added to each microplate. The stop solution was added and read at a wavelength of 450 nm so that the optical density value was obtained.
Data Analysis
Data were presented in the form of mean ± SD (standard deviation). Variables of age, HRSD, MMSE, (Body Mass Index) BMI, HPA, systolic and diastolic blood pressure, respiratory rate, hemoglobin, blood glucose, cholesterol level, uric acid level were analyzed by independent T-test to determine the differences in overall health status of the subjects and to put aside the possibility of chronic diseases involvement in physical exercise results. BDNF serum was analysed by dependent T-test to assess the levels before and after exercise. An independent T-test was used to test levels after exercise in the depression group compared to the non-depression group. Significance value was set at p < 0.05. The analysis was carried out with SPSS 24.0 (SPSS Inc., Chicago, USA).
Results
As seen in Table 1, the differences in baseline characteristics between the depression and non-depression groups were not statistically significant. The difference between the depression and non-depression groups was observed only in the HRSD score, which exhibited that the depression group was indeed depressed, while the control was less likely to experience depression. Statistics of independent T-test on age, MMSE, BMI, HPA, systolic and diastolic blood pressure, respiratory rate, haemoglobin, blood glucose, cholesterol level, uric acid level showed no difference between depression and non-depression groups. These results, of course, exhibited that both groups were comparable in overall health status and only differed in depressive psychological status.
Table 1.
Baseline Characteristics of Depression and Non-Depression Group
| Variables | Depression N = 35 (Mean ± SD) |
Non-Depression N = 35 (Mean ± SD) |
P* |
|---|---|---|---|
| Age (years) | 58.2 ± 5.78 | 58.9 ± 6.13 | 0.567 |
| HRSD | 9.7 ± 2.1 | 2.7 ± 1.1 | 0.002 |
| MMSE | 29.3 ± 2.6 | 29.7 ± 2.3 | 0.756 |
| BMI (kg/m2) | 25.7 ± 3.4 | 24.8 ± 3.9 | 0,713 |
| HPA | 8.7 ± 2.2 | 8.4 ± 2.5 | 0.811 |
| Systolic Blood Pressure (mmHg) | 129.4 ± 10.2 | 128.7 ± 11.1 | 0.542 |
| Diastolic Blood Pressure (mmHg) | 82.7 ± 6.6 | 81.1 ± 7.3 | 0.643 |
| Respiration Rate (x/minute) | 19.7 ± 1.1 | 20.2 ± 1.8 | 0.482 |
| Hemoglobin (g/dl) | 12.9 ± 9.6 | 12.4 ± 8.4 | 0.463 |
| Blood Glucose (mg/dl) | 100.7 ± 11.3 | 97.7 ± 8.9 | 0.354 |
| Cholesterol Level (mg/dl) | 169.7 ± 9.8 | 167.9 ± 10.1 | 0.451 |
| Uric Acid Level (mg/dl) | 3.7 ± 1.1 | 3.4 ± 1.1 | 0.431 |
Independent T-test, p = 0.05; SD: Standard Deviation; HRSD: Hamilton Rating Scale for Depression; MMSE: Mini-Mental State Examination; BMI: Body Mass Index; HPA: Habitual Physical Activity.
As shown in Table 2, pre-exercise BDNF levels in the depression group were lower than non-depression This showed that patients with depression possessed lower BDNF levels than non-depression. Physical exercise increased BDNF levels in both depression and non-depression group.
Table 2.
BDNF Levels Before and After Exercise in Depression and Non-Depression Group
| Variable | Depression N = 35 |
p | Non-Depression N = 35 |
p | ||
|---|---|---|---|---|---|---|
| Pre-Exercise (Mean ± SD) |
Post-Exercise (Mean ± SD) |
Pre-Exercise (Mean ± SD) |
Post Exercise (Mean ± SD) |
|||
| BDNF (pg/mL) | 231.7 ± 18.5 | 313.5 ± 28.7 | 0.001* | 412.4 ± 22.3 | 478.9 ± 33.2 | 0.001* |
Dependent T-test, p = 0.05; SD: Standard Deviation; BDNF: Brain-Derived Neurotrophic Factor.
T-test showed that there were significant differences in BDNF levels before and after exercise. In the depression group, BDNF level increased about 35.3% from the initial value (from 231.7±18.5 to 313.5±28.7). In the non-depression group, BDNF level increased about 16.3% from the initial value (from 412.4±22.3 to 478.9±33.2). For the BDNF levels after exercise, the differences between depression group and non-depression were significant, as shown in Table 3.
Table 3.
BDNF Levels After Exercise in Depression and Non-Depression Group
| Variable | Depression N = 35 (Mean ± SD) |
Non-Depression N = 35 (Mean ± SD) |
p |
|---|---|---|---|
| BDNF (pg/mL) | 313.5 ± 28.7 | 478.9 ± 33.2 | 0.001* |
Independent T-test, p = 0.05; SD: Standard Deviation; BDNF: Brain-Derived Neurotrophic Factor.
As shown in Figure 1, depression and non-depression group yielded an increase in post-exercise BDNF level. Depression group showed lower initial BDNF level compared to non-depression that eventually elevated after physical exercise.
Figure 1.
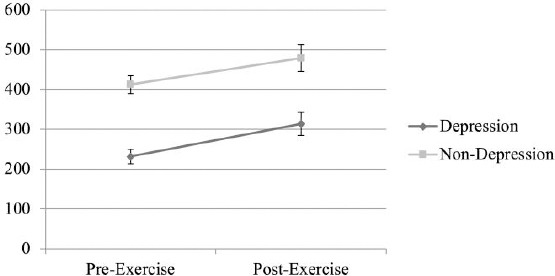
BDNF Levels in Depression and Non-Depression Group
Discussion
In this study, baseline characteristics of the subjects were similar between both study groups and only differed in depressive psychological status. The measures were taken into consideration since overall health status would affect physical activity related to the physical exercise and thus, the outcome of BDNF levels. Overall health status as may be compromised by chronic diseases that may be detected from BMI, blood pressures, respiration, as well as chemistry panel to put aside anaemic, hyper or hypoglycemia, hypercholesterolemia, or hyperuricemia conditions, that may confound results of physical exercise [6], [7]. The MMSE scores that almost reached the highest value (30) also indicated that all subjects were in normal cognitive function [8]. The cognitive evaluation was performed since depressive state, and cognitive function may be intertwined and degree of cognitive impairment is related to many factors in depression such as numbers of previous episodes, duration, and onset as well as treatment factors [9]. Evaluation of these factors and characteristics was aimed to include homogenous patient groups and make a comparison of the main result more precise.
This study showed that older adults with depression possessed BDNF levels far lower than those of the elderly without depression. The results of this study were in line with various previous studies, which stated that BDNF levels tended to be lower in the elderly with depression. Decreasing BDNF levels will cause a decrease in the ability of neuronal cells to survive, thus affecting the neuroplasticity of neuronal cells [2], [10], [11]. Neuronal cells are producers of neurotransmitters that play a role in communication between neuronal cells. One of the main neurotransmitters produced by neurons is serotonin. Serotonin produced by neuronal cells in the hippocampus holds a major role in mood regulation. Decreased serotonin production will cause an alteration in mood, and patients will tend to undergo anhedonia, or to be sad and feeling guilty. Decreased survival of neuronal cells will reduce their ability to produce serotonin [4], [5], [12].
Physical exercise increased BDNF production in both elderly groups with and without depression. In the depression group, the increasing percentage of BDNF level was higher compared to the non-depression group. Physical exercise will reduce blood glucose levels. This causes the production of ketone bodies from the liver to increase as compensation for decreasing glucose levels in the brain, to maintain the stability of energy sources for the brain [13], [14]. Ketone bodies can increase BDNF expression in neuronal cells by inducing activation of monocarboxylate transporter-2 (Mct2) in the hippocampus [15], [16]. Increased activity of Mct2 transporters will increase the expression of BDNF and tropomyosin receptor kinase B (TrkB) levels, which leads to increased survival of neuronal cells. In addition to the role of ketone bodies, the presence of intermediate compounds such as ketoglutarate is believed to also play a role in increasing BDNF expression. Ketoglutarate will initiate histone demethylation. Histone demethylation is an epigenetic factor that possesses a role in increasing transcription and translation of the BDNF gene in the hippocampus [15], [16], [17], [18], [19].
In conclusion, physical exercise increased BDNF production in both elderly groups with and without depression. The increasing percentage of BDNF level was found to be higher in depressive elderly performing physical exercise. Physical exercise may be beneficial in supporting the therapy of elderly with depression. Further studies may explore physical exercise in improving depressive symptoms as well as BDNF level as performed in an extended period of study.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;11(8):985. doi: 10.3389/fnhum.2014.00985. https://doi.org/10.3389/fnhum.2014.00985 PMid:25566019 PMCid:PMC4263078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–93. doi: 10.1038/nn1971. https://doi.org/10.1038/nn1971 PMid:17726474. [DOI] [PubMed] [Google Scholar]
- 3.Phillips C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity:Making the Neuroplastic Connection. Neural Plast. 2017;2017:7260130. doi: 10.1155/2017/7260130. https://doi.org/10.1155/2017/7260130 PMid:28928987 PMCid:PMC5591905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira DS, de Queiroz BZ, Miranda AS, Rocha NP, Felício DC, Mateo EC, et al. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women--a randomized clinical trial. Arch Phys Med Rehabil. 2013;94(8):1443–50. doi: 10.1016/j.apmr.2013.03.029. https://doi.org/10.1016/j.apmr.2013.03.029 PMid:23602881. [DOI] [PubMed] [Google Scholar]
- 5.Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol. 2010;13(5):595–602. doi: 10.1017/S1461145709991234. https://doi.org/10.1017/S1461145709991234 PMid:20067661. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi Y, Shephard RJ. Habitual physical activity and health in the elderly:the Nakanojo Study. Geriatr Gerontol Int. 2010;10(Suppl 1):S236–43. doi: 10.1111/j.1447-0594.2010.00589.x. https://doi.org/10.1111/j.1447-0594.2010.00589.x PMid:20590838. [DOI] [PubMed] [Google Scholar]
- 7.Landi F, Calvani R, Picca A, Tosato M, Martone AM, D'Angelo E, et al. Impact of habitual physical activity and type of exercise on physical performance across ages in community-living people. PLoS ONE. 2018;13(1):e0191820. doi: 10.1371/journal.pone.0191820. https://doi.org/10.1371/journal.pone.0191820 PMid:29370306 PMCid:PMC5784987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monroe T, Carter M. Using the Folstein Mini Mental State Exam (MMSE) to explore methodological issues in cognitive aging research. Eur J Ageing. 2012;9(3):265–74. doi: 10.1007/s10433-012-0234-8. https://doi.org/10.1007/s10433-012-0234-8 PMid:28804426 PMCid:PMC5547414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClintock SM, Cullum CM, Husain MM, Rush J, Knapp RG, Mueller M, et al. Evaluation of the Effects of Severe Depression on Global Cognitive Function and Memory. CNS Spectr. 2010;15(5):304–13. doi: 10.1017/s109285290002753x. https://doi.org/10.1017/S109285290002753X PMid:20448521 PMCid:PMC5718337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Håkansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, et al. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness:Associations with Working Memory Function. J Alzheimers Dis. 2017;55(2):645–657. doi: 10.3233/JAD-160593. https://doi.org/10.3233/JAD-160593 PMid:27716670 PMCid:PMC6135088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:e15092. doi: 10.7554/eLife.15092. https://doi.org/10.7554/eLife.15092 PMid:27253067 PMCid:PMC4915811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihara K, Yoshida H, Jones PB, Hashizume M, Suzuki Y, Ishijima H, et al. Serum BDNF levels before and after the development of mood disorders:a case-control study in a population cohort. Transl Psychiatry. 2016;6(4):e782. doi: 10.1038/tp.2016.47. https://doi.org/10.1038/tp.2016.47 PMid:27070410 PMCid:PMC4872405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh SH, Lin LW, Chuang YK, Liu CL, Tsai LJ, Tsuei FS, et al. Effects of Music Aerobic Exercise on Depression and Brain-Derived Neurotrophic Factor Levels in Community Dwelling Women. BioMed Res Int. 2015;2015(135893):1–10. doi: 10.1155/2015/135893. https://doi.org/10.1155/2015/135893 PMid:26075212 PMCid:PMC4446469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–58. doi: 10.1124/pr.111.005108. https://doi.org/10.1124/pr.111.005108 PMid:22407616 PMCid:PMC3310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, et al. Irisin - a myth rather than an exercise-inducible myokine. Scientific Reports. 2015;5:8889. doi: 10.1038/srep08889. https://doi.org/10.1038/srep08889 PMid:25749243 PMCid:PMC4352853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman JC, Verdin E. β-hydroxybutyrate:much more than a metabolite. Diabetes Res Clin Pract. 2014;106(2):173–81. doi: 10.1016/j.diabres.2014.08.009. https://doi.org/10.1016/j.diabres.2014.08.009 PMid:25193333 PMCid:PMC4414487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Ma XL, Geng Z, Huang SH, Zhai LK, Guo YY, et al. Up-regulation of c-Jun NH2-terminal kinase-interacting protein 3 (JIP3) contributes to BDNF-enhanced neurotransmitter release. J Neurochem. 2015;135(3):453–65. doi: 10.1111/jnc.13226. https://doi.org/10.1111/jnc.13226 PMid:26303065. [DOI] [PubMed] [Google Scholar]
- 18.Park DC, Bischof GN. The aging mind:neuroplasticity in response to cognitive training. Dialogues Clin Neurosci. 2013;15(1):109–19. doi: 10.31887/DCNS.2013.15.1/dpark. https://doi.org/10.1016/B978-0-12-380882-0.00007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–4. doi: 10.1126/science.1227166. https://doi.org/10.1126/science.1227166 PMid:23223453 PMCid:PMC3735349. [DOI] [PMC free article] [PubMed] [Google Scholar]


