Abstract
Dopaminergic signaling in striatum is strongly implicated in executive functions including cognitive flexibility. However, there is a paucity of multimodal research in humans defining the nature of relationships between endogenous dopamine, striatal network activity, and cognition. Here, we measured dopamine synthesis capacity in young and older adults using the PET tracer 6-[18F] fluoro-L-m-tyrosine and examined its relationship with cognitive performance and functional connectivity during an fMRI study of task switching. Aging is associated with alteration in dopamine function, including profound losses in dopamine receptors but an apparent elevation in dopamine synthesis. A compensatory benefit of upregulated dopamine synthesis in aging has not been established. Across young and older adults, we found that cognitive flexibility (low behavioral switch cost) was associated with stronger task-related functional connectivity within canonical fronto-striato-thalamic circuits connecting left inferior frontal gyrus, dorsal caudate nucleus (DCA) and ventral lateral/ventral anterior thalamic nuclei. In young adults, functional connectivity mediated the influence of DCA dopamine synthesis capacity on switch cost. For older adults, these relationships were modified such that DCA synthesis capacity and connectivity interacted to influence switch cost. Older adults with most elevated synthesis capacity maintained the pattern of connectivity-cognition relationships observed in youth, whereas these relationships were not evident for older adults with low synthesis capacity. Together, these findings suggest a role of dopamine in tuning striatal circuits to benefit executive function in young adults and clarify the functional impact of elevated dopamine synthesis capacity in aging.
INTRODUCTION
Cognitive flexibility, the ability to dynamically update behavior in accordance with shifting task goals, is a core component of executive function. Task switching reliably increases activation in the left lateral pFC (Stelzel, Fiebach, Cools, Tafazoli, & D’Esposito, 2013; Jimura & Braver, 2010; Stelzel, Basten, Montag, Reuter, & Fiebach, 2010; Braver, Reynolds, & Donaldson, 2003; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Kimberg, Aguirre, & D’Esposito, 2000) and is sensitive to striatal dopamine (see Klanker, Feenstra, & Denys, 2013, for a review). Here, we used fMRI to test the hypothesis that task-related functional connectivity within canonical frontostriatal networks underlies the updating functions integral to task switching. Furthermore, we used the PET tracer 6-[18F]fluoro-L-m- tyrosine ([18F]FMT) to investigate how striatal dopamine may influence task-related connectivity and, in turn, individual differences in cognitive flexibility in youth and aging.
Striatal dopamine function has been shown to affect cognitive flexibility. Performance on a variety of executive function tasks has been linked to striatal dopamine function measured using human PET imaging (Klostermann, Braskie, Landau, O’Neil, & Jagust, 2012; Rieckmann, Karlsson, Fischer, & Bäckman, 2011; Landau, Lal, O’Neil, Baker, & Jagust, 2009; Cools, Gibbs, Miyakawa, Jagust, & D’Esposito, 2008), including tasks specifically requiring flexible updating (Berry et al., 2016; Samanez-Larkin et al., 2013; Dang, Donde, Madison, O’Neil, & Jagust, 2012; Bäckman et al., 2011; Nyberg et al., 2009; Monchi, Ko, & Strafella, 2006). Critically, these PET studies localize dopaminergic effects on performance within striatum and join investigations in animal models that specifically implicate dorsal caudate nucleus (DCA) dopamine in modulating cognitive flexibility (reviewed in Klanker et al., 2013).
Normal aging is associated with parallel declines in many indices of striatal dopamine function (see Karrer, Josef, Mata, Morris, & Samanez-Larkin, 2017, for metaanalysis) and executive ability (see Verhaeghen & Cerella, 2002, for meta-analysis). [18F]FMT studies reveal dopamine synthesis capacity is preserved and even elevated in aging relative to youth, purportedly reflecting upregulation of synthesis (Braskie et al., 2008; DeJesus, Endres, Shelton, Nickles, & Holden, 2001). These findings are consistent with observations in early Parkinson’s disease where losses in dopaminergic function are initially met with stable or elevated synthesis rates (Nandhagopal et al., 2011; Lee et al., 2000).
Defining the functional impact of elevated dopamine synthesis in aging is an area of active research. Previously, we have found significant relationships between dopamine synthesis capacity and cognitive performance in young adults, which were not apparent in older adults (Berry et al., 2016; Braskie et al., 2008). Other findings have been mixed. Although there is a suggestion that dopamine synthesis capacity is positively correlated with working memory capacity in older adults (Landau et al., 2009), it has also been linked with weaker functional connectivity during performance of a task for which weaker connectivity was associated with worse performance (Klostermann et al., 2012).
The mechanisms by which striatal dopamine influences flexible performance in humans have received little empirical testing, even in young adults. DCA dopamine may affect cognitive flexibility by modulating activity within circuits connecting pFC and DCA. Studies in animal models have mapped striatal connectivity as segregated, parallel loops connecting pFC, striatum, and thalamus (Alexander, DeLong, & Strick, 1986). In humans, fronto-striatal functional connectivity increases during task switching and is affected by individual differences in dopamine function as determined by genetic polymorphism (Stelzel et al., 2010) and pharmacological manipulation (Stelzel et al., 2013; Nagano-Saito et al., 2008). However, because of a lack of in vivo dopamine measurement, these studies could not establish direct relationships between measured dopamine function and connectivity across individuals and were limited in their ability to specify a DCA locus of dopamine’s effects.
This study takes critical steps to advance our understanding of age-related changes in the dopaminergic mechanisms supporting cognitive flexibility beyond what has been defined to date. We previously reported findings relating dopamine synthesis capacity with univariate activation during task switching and intrinsic (resting-state) functional connectivity in young and older adults (Berry et al., 2016). These intrinsic connectivity findings support the proposal that DCA dopamine synthesis capacity shapes DCA network connectivity; an inverted-U function explained relationships between dopamine and connectivity across young and older adults. However, dopamine-connectivity relationships were weak and not significant for either young or older adults independently, limiting our interpretation of effects of age. Here, we present new psychophysiological interaction (PPI; Friston et al., 1997) analyses, which have the advantage of examining task-induced functional connectivity, which may be more closely related to DCA dopamine and directly relevant to successful task-switching performance.
The goals of this study were to (1) test the hypothesis that greater task-related DCA functional connectivity strength is associated with lower behavioral switch cost in young and older adults; (2) test the hypothesis that, in young adults, the influence of DCA dopamine synthesis capacity on switch cost is mediated by functional connectivity; and (3) explore the impact of age-related upregulation of dopamine synthesis on relationships between DCA synthesis capacity, functional connectivity, and performance in older adults. These analyses provide rare empirical testing of the fronto-striato-thalamic mechanisms supporting striatal dopamine’s influence on cognition in humans and establish the impact of age-related elevation of dopamine synthesis on the functional net-works supporting cognitive flexibility.
METHODS
Participants
Fifteen older adults (70–83 years old, M = 77.43, SD = 3.87, nine women) and 21 young adults (20–31 years old, M = 23.80, SD = 2.59, 10 women) participated in [18F]FMT PET and MRI sessions and are included in the present report (Table 1). Participants were recruited from the Berkeley Aging Cohort Study. The study was approved by the institutional review boards at the University of California, Berkeley, and Lawrence Berkeley National Laboratory. All participants provided written consent and received monetary compensation. Data from these 36 participants were reported in a previous study (Berry et al., 2016) examining the relationships between DCA dopamine synthesis capacity, task-switching performance, univariate BOLD activation, and resting-state connectivity. To avoid confusion, we largely reproduce the text describing the overall structure of the data collection and analysis procedures. Those readers familiar with our earlier article may wish to focus on the descriptions of the task-based functional connectivity analysis and tests of mediation and moderation.
Table 1.
Group Demographics, Task Performance, and Dopamine
| Effect size |
|||||
|---|---|---|---|---|---|
| Young | Older | t | Cohen’s d | ||
| Age (years) | M | 23.80 | 77.43 | ||
| SD | 2.59 | 3.87 | |||
| Task switch RT | M | 979.71 | 1,167.50 | t = 3.71 | 1.29 |
| SD | 167.09 | 120.60 | p < .001 | ||
| Task repeat RT | M | 902.37 | 1,079.07 | t = 3.54 | 1.25 |
| SD | 175.99 | 94.05 | p = .001 | ||
| Switch cost (%) | M | 9.42 | 8.25 | t< 1 | 0.15 |
| SD | 8.59 | 7.02 | p > .05 | ||
| DCA [18F]FMT Ki | M | 0.026 | 0.032 | t = 5.07 | 1.67 |
| SD | 0.0003 | 0.0003 | p < .001 | ||
Participants had normal or corrected-to-normal vision, did not take medication affecting cognition, and had no neurological or psychological conditions. Older adults were characterized as cognitively normal using an interview and test battery described previously (Berry et al., 2016; Scholl et al., 2016), which included several subtests of the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1987) and the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975). Older adults scored at least 25 on the MMSE (range: 25–30, M = 28.33, SD = 1.59) and were screened for current depression using the Geriatric Depression Scale (range: 0–8, M = 3.36, SD = 2.76; Yesavage et al., 1982). Removal of the single older adult with an MMSE score of 25 from our analyses does not change the main conclusions of the present report. Furthermore, this participant scored 29 on the MMSE during their recent annual neuropsychological testing that occurred after their participation in this study.
Older adults previously underwent Pittsburg compound B ([11C] PiB) PET imaging under a different protocol to measure amyloid-p pathology. Distribution volume ratios were low (range: 0.91–1.09, M = 1.00, SD = 0.04) with 14 of 15 PiB negative (Villeneuve et al., 2015).
Behavioral Task
Participants performed a two-condition task-switching experiment. The task was generated using PsychoPy 1.80.06 (Peirce, 2007). On each trial, a cue (square or diamond) was presented for 300 msec, after which a number between 1 and 9 was presented for 2000 msec. For square cues, participants determined whether the number was odd (right button press) or even (left button press). For diamond cues, participants determined whether the number was greater than 5 (right button press) or less than 5 (left button press). The number 5 was never presented. Participants were given 2000 msec to respond and were instructed to respond as quickly as possible without sacrificing accuracy. Fixation periods of duration 2000, 4000, or 6000 msec separated each trial. Responses were limited to two buttons, rather than four buttons, which introduced response overlap.
Participants performed six functional runs composed of 48 trials each. Four runs contained both conditions, whereas two runs contained just a single condition. The single-condition runs are not included in the present report. For the two-condition runs, stimuli were pseudorandomized to ensure the same number stimulus was not presented twice in a row, the same cue was not presented more than four times in a row, consecutive right versus left hand responses did not repeat more than three times in a row, and there were no more than three consecutive rule repeat trials or rule switch trials. Participants performed out-of-scanner practice with and without trial-to-trial feedback, until they reached 60% accuracy. In the scanner, participants performed a brief practice to ensure they could see the stimuli.
Behavioral Analysis
The primary performance measure was RT “switch cost,” the difference in RT for all task switch trials (correct responses preceded by correct responses for the different condition) versus all task repeat trials (correct responses preceded by correct responses for the same condition). In this framework, low switch cost reflects cognitive flexibility. We tested relationships between switch cost (percent change in RT for switch trials relative to repeat trials) and DCA functional connectivity using correlation and voxel-wise regression approaches described below. Shapiro-Wilk testing confirmed all variables submitted to correlation analyses were normally distributed (all Ws > .95).
PET Data Acquisition
[18F]FMT PET tracer was used to measure dopamine synthesis capacity. Similar to 6-[18F]fluorodopa, [18F]FMT is a substrate for aromatic amino acid decarboxylase, an enzyme in the dopamine synthesis cascade. Although not the rate-limiting step in synthesis, its activity provides an estimate of dopamine synthesis capacity when provided with enough substrate (Dejesus, 2003). [18F]FMT was synthesized at Lawrence Berkeley National Laboratory using methods previously described (VanBrocklin et al., 2004). Participants ingested 2.5 mg/kg of carbdopa approximately 1 hr before scanning to minimize the peripheral metabolism of [18F]FMT. Data were acquired using a Siemens Biograph Truepoint 6 PET/CT (Siemens Medical Systems). After a short CT scan, participants were injected with approximately 2.5 mCi of [18F]FMT as a bolus in an antecubital vein. Dynamic acquisition frames were obtained over 90 min in 3-D mode (25 frames total: 5 × 1 min, 3×2 min, 3×3 min, 14 × 5 min). Data were re-constructed using an ordered subset expectation maximization algorithm with weighted attenuation, corrected for scatter, and smoothed with a 4-mm FWHM kernel.
Structural MRI Data Processing
Images were acquired on a Siemens 1.5T Magnetom Avanto System (Siemens Medical Systems) with a 12-channel head coil. Each participant was scanned using a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) protocol (repetition time [TR] =2110 msec, echo time [TE] = 3.58 msec, flip angle = 15°, matrix = 256 × 256, field of view [FOV] = 256, sagittal plane, voxel size = 1 × 1×1 mm, 160 slices). MPRAGE scans were segmented using FreeSurfer version 5.1 (surfer.nmr.mgh.harvard. edu/). Cerebellar gray matter was used as the reference region in [18F]FMT PET analyses because this region shows very little tracer uptake and has an extremely low density of dopamine receptors and metabolites relative to striatum (Hall et al., 1994; Levey et al., 1993; Camps, Cortes, Gueye, Probst, & Palacios, 1989; Farde, Hall, Ehrin, & Sedvall, 1986). The most anterior ¼ of cerebellar gray was removed from the reference region to limit contamination of signal from the substantia nigra and ventral tegmental area. PET analyses focused on dopamine synthesis capacity measured in dorsal DCA ROIs, which were manually drawn on each participant’s MPRAGE as previously described (Mawlawi et al., 2001) using Mango software (ric.uthscsa. edu/mango/). Inter-rater reliability was high. For ROIs of five participants drawn by two raters, the Sorensen-Dice coefficient ranged from .80 to .86, and the intraclass correlation coefficient ranged from .95 to .99.
PET Data Analysis
[18F]FMT PET data were preprocessed using SPM8 software (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007). To correct for motion between frames, images were realigned to the middle (12th) frame. The first five images were summed before realignment to improve realignment accuracy, as these early images have relatively low signal contrast. Structural images (including posterior cerebellar reference region and DCA ROIs) were coregistered to PET images using the mean image of frames corresponding to the first 20 min of acquisition as a target. Graphical analysis for irreversible tracer binding was performed using Patlak plotting (Patlak & Blasberg, 1985) with posterior cerebellar gray matter used as the reference region. Ki images were generated from PET frames corresponding to 25–90 min (Ito et al., 2006, 2007), which represent the amount of tracer accumulated in the brain relative to the cerebellum. These images are comparable to Ki images obtained using a blood input function but are scaled to the volume of tracer distribution in the reference region.
ROI-based partial volume correction was applied (Rousset, Ma, & Evans, 1998) to generate the best approximation of [18F]FMT signal originating from DCA and to minimize differences in Ki driven by age-related changes in gray matter, white matter, and cerebrospinal fluid volume. To apply the partial volume correction in native space, we used FreeSurfer-generated ROIs for gray matter cortical and subcortical regions, white matter, and cerebrospinal fluid with hand-drawn striatal ROIs (including dorsal puta-men and ventral striatum) substituting for the automated striatal segmentation.
fMRI Data Acquisition and Preprocessing
Six functional task runs were collected in the same session as the T1 MPRAGE. Functional runs were acquired with a T2*-weighted EPI sequence (TR = 2200 msec, TE = 50 msec, flip angle = 50°, matrix 64 × 64, FOV = 220 mm, voxel size = 3.44 × 3.44 × 3.91 mm). Twenty-eight axial slices oriented to the AC-PC line were acquired in ascending order to give whole-brain coverage. The first 10 volumes acquired for each functional scan were discarded to allow for magnetization preparation and stabilization. In-plane turbo inversion recovery images (TR = 2000 msec, TE = 11 msec, matrix = 256 × 256, FOV = 220, voxel size = 0.43 × 0.43 × 3.91 mm, 28 slices) were collected as an intermediate target for registration of MPRAGE and EPI images.
fMRI data were analyzed using SPM12 software. Functional images were corrected for differences in slice timing and were realigned to the first volume to correct for head movement. To spatially normalize functional images to the Montreal Neurological Institute (MNI) template, the in-plane and MPRAGE images were used as intermediates. Following normalization, images were resliced to voxel size 2 × 2 × 2 mm and were smoothed with an 8-mm FWHM isotropic Gaussian kernel and high-pass filtered (128 sec).
fMRI General Linear Model
Data were analyzed using a multisession general linear model implemented in SPM 12 (Friston, Frith, Turner, & Frackowiak, 1995). Four predictors modeled switch and repeat trials. Specifically, two predictors modeled switch and repeat trials for which the correct response (left vs. right index finger) overlapped with the nonactive task rule. Two predictors modeled switch and repeat trials for which the correct response did not overlap with the nonactive task rule. Primary analyses focused on the contrast all switch > all repeat trials to maintain power. All omissions, error responses, and trials following errors were modeled together as a single predictor and are not included in the present analysis. Predictors were time-locked to the onset of the number stimulus and convolved with the canonical hemodynamic response function. Six motion regressors derived from individual subject realignment were included in the model. In addition, a parametric regressor was created for individual trial RT to control for possible time-on-task effects (Grinband, Wager, Lindquist, Ferrera, & Hirsch, 2008). Specifically, we created an index for each trial by subtracting the mean RT across all conditions and dividing by SD (Yarkoni, Barch, Gray, Conturo, & Braver, 2009).
Task-based Functional Connectivity Analysis
PPI Analysis during Task Switching
We hypothesized that increased functional connectivity of DCA within defined fronto-striato-thalamic loops during task switching underlies successful performance. To test this, we performed both exploratory voxelwise analyses and targeted analyses assessing DCA’s functional connectivity with pFC and thalamus (Haber & Calzavara, 2009; Haber, Lynd-Balta, & Spooren, 1994; Alexander & Crutcher, 1990; Alexander et al., 1986). Targeted analyses assessed functional connectivity between DCA and anatomically defined ROIs in bilateral pFC and thalamus and a functionally defined ROI in pFC based on our previous univariate fMRI analysis (Berry et al., 2016; see details below). These complementary exploratory and ROI-based approaches allowed for comprehensive testing of DCA functional connectivity’s relationship with behavior in young and older adults.
First, we generated whole-brain PPI (Friston et al., 1997) maps implemented in SPM 12 using a seed region drawn in DCA. Seed region masks are publicly available through NeuroVault (Gorgolewski et al., 2015) and can be found at https://neurovault.org/collections/3474/. Bilateral DCA nucleus was drawn manually on the ch2better. nii structural template in MNI space (Mawlawi et al., 2001). To generate individual participant PPI maps, a first-level model contained separate regressors for seed region time series, all switch > all repeat contrast, interaction (the multiplication of the deconvolved BOLD time series from the seed and contrast regressor), and six motion regressors. For each participant, voxelwise PPI effects were estimated, and statistical parametric maps were generated for the interaction term.
For whole-brain analyses, we generated a voxelwise multiple regression model to (1) identify regions whose functional connectivity with DCA increased for switch relative to repeat trials and (2) identify regions whose functional connectivity with DCA was correlated with performance. Individual participant PPI maps (DCA seed described above) were submitted to the model with switch cost entered as the regressor. To mitigate potential concerns that individual differences in head motion contributed to relationships between PPI connectivity and switch cost, we included AFNI’s Euclidian norm of motion parameters (∥d∥L2) as a covariate. Group analyses combined young and older adults. Secondary analyses examined differences in functional connectivity strength between young and older adults. For voxelwise significance testing, an initial cluster forming threshold of p < .001 was applied, which is more conservative than the commonly used p < .01 (Eklund, Nichols, & Knutsson, 2016; Woo, Krishnan, & Wager, 2014). An additional minimum cluster extent threshold was applied using 3DClustSim in AFNI (https://afni.nimh.nih.gov/; cluster-level threshold p < .05). AFNI’s 3dFWHMx was applied to group-level beta maps to estimate the spatial auto-correlation using a mixed-model function (rather than Gaussian-shaped function). This principled extent thresh-olding method accounts for spatial correlations of voxels and does not make assumptions about data distribution and smoothness. A cluster extent threshold of 139 voxels was required for whole-brain analyses. A cluster extent threshold of 32 voxels was required for analyses restricted to bilateral frontal cortex and thalamus (anatomically defined mask; Maldjian, Laurienti, Kraft, & Burdette, 2003). To preview our results, we found no significant clusters for whole-brain voxelwise contrasts between switch and repeat trials. Regression results did, however, identify a 40-voxel cluster in thalamus whose functional connectivity was negatively correlated with switch cost. This cluster survived cluster-level significance thresholding for targeted analyses restricted to pFC and thalamus. There were no clusters in pFC whose functional connectivity was negatively correlated with switch cost and survived significance thresholding (whole-brain or within an anatomically defined pFC and thalamus mask).
To further test a role of pFC-DCA connectivity in taskswitching performance, we performed targeted analyses using a functionally defined pFC ROI. Although pFC was not identified in multiple regression analyses, left lateral pFC is highly implicated in task switching (Armbruster-Geng, Ueltzhöffer, & Fiebach, 2016; Jimura & Braver, 2010; Stelzel et al., 2010, 2013; Braver et al., 2003; Dove et al., 2000; Kimberg et al., 2000), and its functional connectivity with DCA has been linked to task performance (Stelzel et al., 2010, 2013). We tested the hypothesis that the left pFC-DCA connectivity would be correlated with switch cost using an ROI in the left inferior frontal gyrus (IFG) approximating BA 44/BA 9 defined based on previously reported univariate results for the contrast switch > repeat (Berry et al., 2016). Specifically, we measured PPI connectivity between DCA and a spherical IFG ROI with an 8-mm radius centered on MNI −54, 14, 18 and tested the relationship between IFG-DCA functional connectivity and switch cost. Previous univariate analyses also identified a region in the right IFG (MNI 54, 6,10) that showed increased BOLD response for the contrast switch > repeat contrast (Berry et al., 2016). Analyses here focus on the left pFC connectivity due to precedent in the literature (Stelzel et al., 2010, 2013), but we include parallel analyses for the right pFC-DCA connectivity in Supplementary Materials and Figure S1.1 Partial correlations were used to control for possible effects of head motion (∥d∥L2) as has been done previously (Berry, Sarter, & Lustig, 2017).
To ensure that relationships between IFG-DCA functional connectivity and performance were specific (i.e., that DCA’s connectivity throughout the brain was not uniformly related to performance), we performed parallel analyses in a control region in visual cortex (8-mm sphere surrounding MNI −28, −96, −6; Anderson, Ferguson, Lopez-Larson, & Yurgelun-Todd, 2011; Kiviniemi et al., 2009). Though visual cortex is critical for successful task performance, we had no hypothesis that differences in its connectivity with DCA during switch trials would be related to performance.
Tests of Mediation and Moderation
To examine the role of DCA dopamine function in producing individual differences in switch cost, we conducted tests of mediation. Mediation analysis tested the hypothesis that DCA [18F]FMT Ki (X) influences switch cost (Y) by altering functional connectivity (M). To examine differences between young and older adults, age group was included as a categorical moderating variable (W). Moderated mediation analyses were conducted in SPSS v. 24 (IBM Corp.) using the PROCESS module v2.16.3 (Hayes, 2013) Model 8 (Figure 1). To preview our results, this analysis revealed dopamine’s influence on switch cost was mediated by functional connectivity for young but not older adults.
Figure 1.
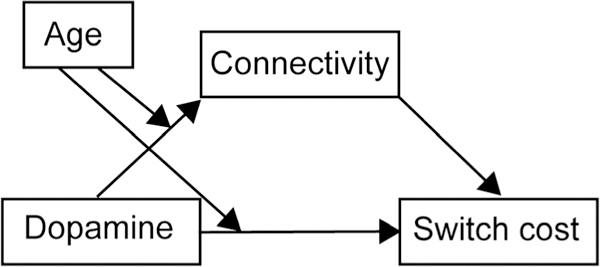
Model of moderated mediation. Model 8 from the PROCESS module v2.16.3 (Hayes, 2013) tested relationships between dopamine ([18F]FMT), functional connectivity (PPI), switch cost, and age.
To further probe the nature of relationships between dopamine, functional connectivity, and switch cost in older adults, we conducted post hoc moderation analyses to test whether striatal dopamine function acts as a moderator, affecting the strength of relationships between functional connectivity and switch cost for older adults rather than directly predicting flexible performance. Primary moderation analyses were performed on older adults alone (PROCESS Model 1). However, for completeness we also include analyses on young adults alone. Moderation analyses used hierarchical regression to first test the relationships between the predictor (functional connectivity) and the outcome (switch cost), and the moderator (DCA [18F]FMT Ki) and the outcome (conditional effects) and then test the interaction between the predictor and the moderator (i.e., how the effect of the predictor on the outcome changes as the moderator changes). We report where in the distribution of striatal dopamine (moderator) functional connectivity (predictor) is related to switch cost (outcome; i.e., Johnson-Neyman technique). Given the small sample size (n = 15 older adults, n = 21 young adults), moderation analyses must be interpreted with caution. Statistical values were considered significant at a final alpha level of .05 to prevent Type I error inflation. Head motion (∥d∥L2) was included as a covariate for mediation and moderation analyses.
RESULTS
Behavior
Behavioral results for switch cost were previously reported (Berry et al., 2016) but are included here for completeness. Briefly, participants were slower to respond to switch trials (M ± SD all: 1057.95 ± 174.90) than repeat trials (all: 976.00 ± 170.42), and there was no difference in the magnitude of switch cost between young and older adults (see Table 1 for M ± SD). This was confirmed by an ANOVA demonstrating main effects of Trial type, F(1, 34) = 40.13, p < .001, = 0.05, and Age, F(1, 34) = 14.06, p = .001, = 0.28, but no Age × Trial type interaction, F(1,34) < 1. For both groups combined, the percent increase in RT for switch trials was 8.93 ± 7.89. Absence of age differences in measures of local switch cost replicates patterns in the literature (see Wasylyshyn, Verhaeghen, & Sliwinski, 2011; Verhaeghen & Cerella, 2002, for meta-analyses, but see Discussion for possible limitations in interpreting age differences in neural effects given matched performance).
Task-based Functional Connectivity Analysis
Greater DCA-thalamus Connectivity is Associated with Lower Switch Cost
PPI analyses were conducted using bilateral DCA as the seed region (Figure 2A). Voxelwise analysis of whole-brain DCA connectivity for the all switch > all repeat contrast showed no significant clusters and no clusters demonstrating significant age group differences (contrast maps are publicly available at https://neurovault.org/collections/3474/). Similarly, voxelwise analysis of whole-brain DCA connectivity for the all repeat > all switch contrast showed no significant clusters and no clusters demonstrating significant age group differences.
Figure 2.
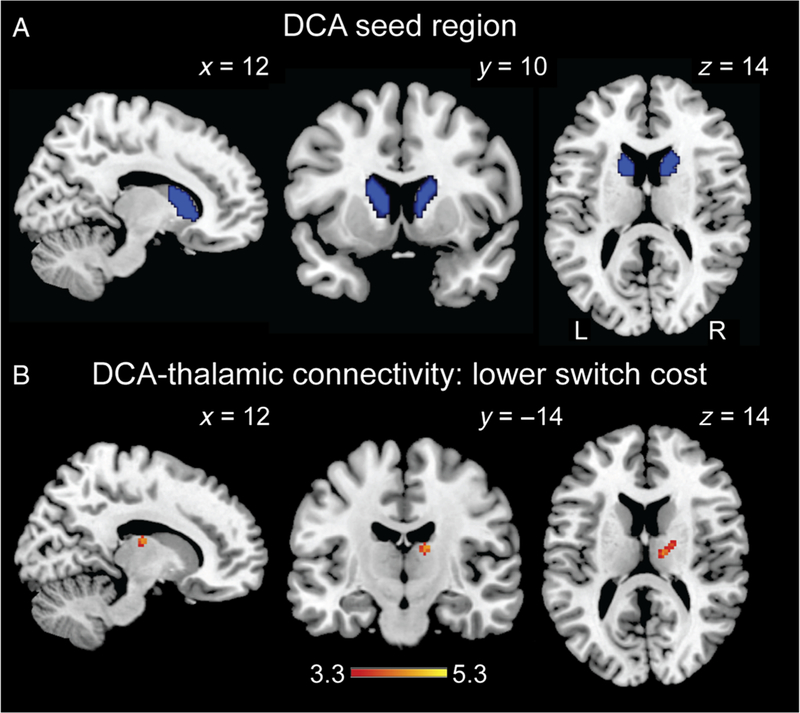
Striato-thalamic functional connectivity associated with task-switching performance. (A) The bilateral DCA seed region used in PPI analyses is displayed in blue. (B) Regression analysis indicated bilateral DCA functional connectivity with thalamus (right ventral lateral/ventral anterior; MNI 14, −14, 16) was associated with reduced switch cost. This voxelwise contrast is available at https://neurovault.org/collections/3474/. Scale bar reflects t value. t Maps are displayed on ch2better template. L = left, R = right.
Voxelwise multiple regression analysis identified regions whose connectivity with DCA was associated with smaller switch cost. This analysis thus identified pathways that did not necessarily show increased DCA connectivity when averaged across all participants, but where individual differences in increased connectivity were associated with individual differences in switch cost. There were no clusters that reached significance for a whole-brain search. Targeted analyses within the anatomically defined mask, including bilateral frontal cortex and thalamus, identified a 40-voxel cluster in the right thalamus (MNI 14, −14, 16), which spanned the ventral lateral nucleus and ventral anterior nucleus, with greater connectivity associated with lower switch cost (Figure 2B; https://neurovault.org/collections/3474/). There was no difference in DCA-thalamus functional connectivity between young and older adults, t(34) < 1. Our regression analyses did not identify any significant clusters for which increased connectivity was associated with higher switch cost.
Greater IFG-DCA Connectivity Is Associated with Lower Switch Cost
We examined individual differences in functional connectivity between bilateral DCA and the left IFG (8-mm sphere centered on MNI −54, 14, 18; Figure 3A) for switch relative to repeat trials. There was no significant difference in functional connectivity between age group, t(34) < 1. There was a significant correlation between switch cost and IFG-DCA connectivity controlling for head motion such that greater connectivity was associated with smaller switch cost for analyses including both young and older adults (r = −.46, p = .005). Considering groups independently, the relationship was marginal for older adults (r = −.48, p = .09) and was significant for young adults (r = −.56, p = .01; Figure 3B), though correlation coefficients were not significantly different between groups ( p = .77).
Figure 3.
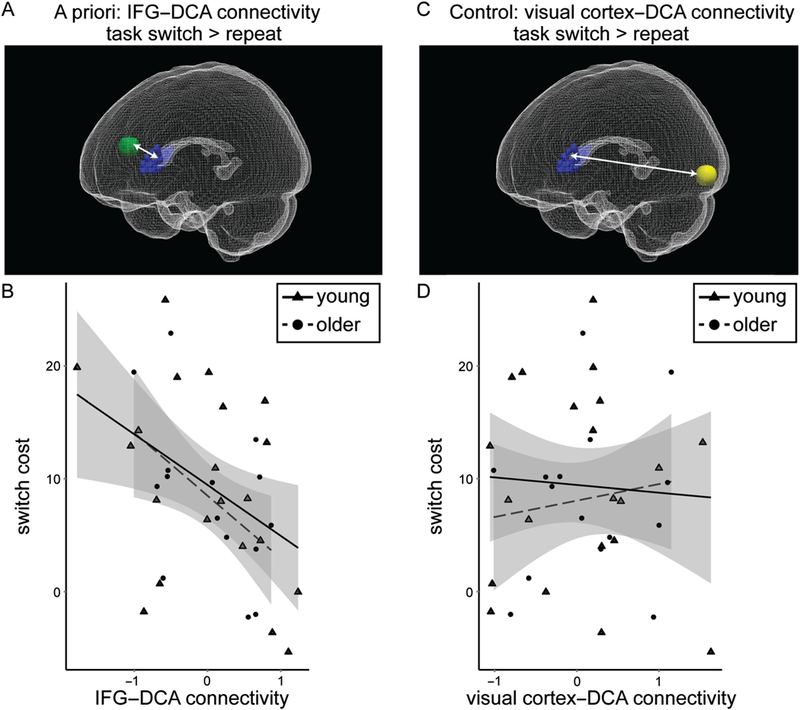
Frontostriatal functional connectivity associated with task-switching performance. (A) PPI functional connectivity was measured between bilateral DCA (blue) and left IFG (8-mm sphere surrounding MNI −54, 14, 18; green) for task switch > repeat trials. This image was created using ITK-Snap (Yushkevich et al., 2006) and ParaView (Ahrens, Geveci, & Law, 2005). (B) IFG-DCA functional connectivity was correlated with switch cost (r = −.46, p = .005) for young and older adults combined. Young adults are displayed as triangles with a solid line indicating the correlation for young adults alone, and older adults are displayed as circles with a dashed line indicating the correlation for older adults alone. 90% Confidence interval is displayed in gray. (C) Functional connectivity was measured between bilateral DCA and left visual cortex (8-mm sphere surrounding MNI −28, −96, −6; yellow) for task switch > repeat trials. (D) Visual cortex-DCA functional connectivity was not correlated with switch cost (r = −.01, p = .97) for young and older adults combined.
We performed a parallel control analysis examining functional connectivity between bilateral DCA and left visual cortex (8 mm sphere centered on MNI − 28, −96, −6; Figure 3C). There was no significant difference in functional connectivity between age group, t(34) < 1, and there was no correlation between visual cortex-DCA functional connectivity and switch cost for both young and older adults (r = −.01,p = .97; Figure 3D). Considering groups independently, the relationship did not approach significance for young (r = −.21, p = .38) or older adults (r = .25, p = .39).
Mediation and Moderation Analyses
Moderated mediation analyses tested the hypothesis that striatal dopamine influences cognitive flexibility via modulation of functional connectivity and examined possible age-related differences in these relationships. DCA [18F] FMT K (see Table 1 for M ± SD; Figure 4A) was used as the independent variable, switch cost was the dependent variable, IFG-DCA-thalamus PPI connectivity was used as the mediating variable, and age group was used as the dichotomous moderating variable. IFG-DCA-thalamus PPI connectivity was calculated for each participant by averaging IFG-DCA connectivity calculated from the ROI analysis (8-mm sphere surrounding MNI −54, 14, 18) and DCA-thalamus connectivity identified by the multiple regression analysis described above (8-mm sphere surrounding MNI 14, −14, 16; Figure 4B). We designed this analysis to incorporate connectivity with both frontal and thalamic elements of the DCA circuit because we hypothesized DCA dopamine influences connectivity with both regions. We report mediation and moderation results for IFG-DCA and DCA-thalamus functional connectivity separately (not averaged) in the Supplementary Materials and Figures S2 and S3. Results largely mirror those reported below. In instances for which results differ, we report those statistics here in the main text in addition to Supplementary Materials and discuss possible implications in the Discussion.
Figure 4.
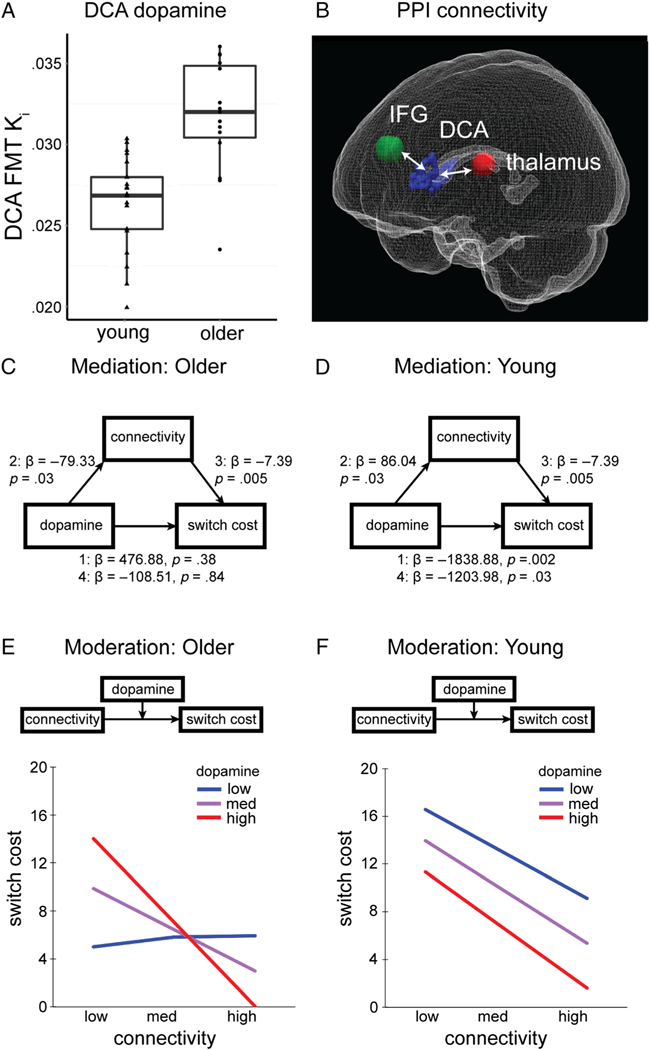
Mediation and moderation analyses. (A) Box and whisker plots display group differences in DCA [18F]FMT Ki. Data for individual participants is displayed for young (triangles) and older adults (circles). (B) A 3-D view of IFG (green) and thalamus (red) ROIs whose PPI functional connectivity with DCA (blue) was averaged and submitted to mediation and moderation analyses. (C) For older adults, there was no significant mediation. The bootstrapped unstandardized indirect effect of DCA [18F]FMT Ki on switch cost was not significantly different from zero. (D) For young adults, PPI connectivity significantly mediated the influence of DCA [18F]FMT Ki on switch cost. The bootstrapped unstandardized indirect effect of DCA [18F]FMT Ki on switch cost was −635.53 and significantly differed from zero, as revealed by a 95% bootstrap confidence interval that was entirely below zero (confidence interval [−1510.68, −15.53]). (E) For older adults, DCA [18F]FMT Ki and PPI connectivity interacted to influence switch cost (b = −2052.38,p = .01). The conditional effect of PPI connectivity on switch cost was present for high (red) DCA [18F]FMT Ki (1 SD above the mean), t(10) = 3.57, p = .005, was at trend level for moderate (purple) levels DCA [18F]FMT Ki, t(10) = 1.89, p = .09, but was not significant for low (blue) DCA [18F]FMT Ki (−1 SD), t(10) = .05,p = .97. (F) For young adults, both DCA [18F]FMT Ki and PPI connectivity were significant predictors of switch cost (b = −1119.98,p = .03; b = −7.80, p = .01, respectively) and did not interact (b = −361.09, p = .43). We observed similar relationships between PPI connectivity and switch cost for low, medium, and high levels of DCA [18F]FMT Ki.
It is important to note that, because we identified DCA-thalamus connectivity based on its relationship with switch cost (i.e., using voxelwise regression), we expect a negative relationship between PPI connectivity and switch cost. However, three questions remain. First, does PPI connectivity in the IFG-DCA-thalamus pathway correlate with individual differences in DCA dopamine? Second, does the relationship between IFG-DCA-thalamus PPI connectivity and switch cost remain after accounting for DCA dopamine? Third, can this relationship explain DCA dopamine’s effect on switch cost?
The Influence of Striatal Dopamine on Flexible Performance Is Mediated by Functional Connectivity in Young Adults
The moderated mediation was significant and indicated that, overall, the effect of dopamine on switch cost was mediated by functional connectivity, but that this relationship differed between young and older adults. Specifying the overall mediation as follows: Step 1: The direct effect of dopamine on switch cost (independent of functional connectivity) was significant (β = −1838.88, p = .002). Step 2: The effect of dopamine on functional connectivity was significant (β = 86.04, p = .03). Step 3: The effect of functional connectivity on switch cost (accounting for dopamine) was significant (β = −7.39, p = .005). Step 4: The effect of dopamine on switch cost was reduced when accounting for functional connectivity (β = −1203.98, p = .03).
However, age group significantly moderated these relationships. Age group interacted with dopamine to significantly moderate dopamine’s direct relationship with switch cost (Dopamine × Age interaction: β = 2315.77, p = .005) as well as dopamine’s relationship with functional connectivity (Dopamine × Age interaction: β = 165.38, p = .003; see Figure 1 for model). The index of moderated mediation was 1221.48, and bootstrapped confidence interval was entirely above zero [171.18, 2517.20], indicating the indirect effect of dopamine on switch cost via functional connectivity significantly differed across groups. Indeed, the indirect effect of dopamine on switch cost was not significant for older adults (estimated indirect effect = 585.96, bootstrapped CI [−81.60, 1351.13]), though it was significant for young adults (−635.53 [−1510.68, −15.53]). These results indicate the mediation was not significant for older adults. Figure 4 (C, D) displays results for age-conditional effects for young and older adults generated from the moderated mediation model. Age was not included as a moderator for the path relating functional connectivity and switch cost, so those values are held constant across groups.
For analyses considering functional connectivity between IFG-DCA only, the mediation was not significant for young or older adults. Similar to Step 3 reported above, the regression of IFG-DCA connectivity, controlling for DCA [18F]FMT Ki, on switch cost was significant (Step 3: β = −3.59, p = .05). However, in Step 2, the regression of DCA [18F]FMT Ki on connectivity did not reach statistical significance for young (β = 97.46, p = .09) or older adults (β = −77.72,p = .16; full age-conditional results are reported in Figure S2). Analyses for DCA-thalamus connectivity alone replicate primary findings described above (Figure S3).
Striatal Dopamine and Functional Connectivity Interact to Influence Cognitive Flexibility in Aging
Given the lack of mediation observed in older adults, we conducted post hoc moderation analyses. In older adults, moderation analysis indicated striatal dopamine and functional connectivity interact to produce individual differences in cognitive flexibility (Figure 4E). The two predictors (DCA [18F]FMT Ki and PPI connectivity) were first entered into the regression analysis to determine each predictor’s effect on switch cost and the interaction term. The overall model explained a significant amount of variance (R2 = .63, F(4,10) = 4.27,p = .03). For older adults, results indicated that DCA [18F]FMT Ki was not a significant predictor of switch cost (b = 249.56, t(10) = 0.49, p = .64), but connectivity was a significant predictor (b = 58.22, t(10) = 2.37, p = .04) of switch cost. The interaction between DCA [18F]FMT Ki and connectivity was significant (b = −2052.38, t(10) = 2.78, p = .01), and explained a significant increase in variance in switch cost (change R2 = .29, F(1, 10) = 7.73, p = .02). Thus, the influence of PPI connectivity on switch cost depends on dopamine synthesis capacity.
The unstandardized simple slopes were tested for low (−1 SD), moderate and high levels (+1 SD) of dopamine synthesis in older adults. Different patterns in the slope of the regression line with varying levels of dopamine synthesis show that the relationship between PPI connectivity and switch cost is unique and depends on the level of dopamine. Results suggest that the conditional effect of connectivity on switch cost was present for high dopamine synthesis capacity (1 SD above the mean), t(10) = 3.57, p = .005, was at trend level for moderate levels of dopamine synthesis capacity, t(10)= 1.89, p = .09, but was not significant for low dopamine (−1 SD), t(10) = 0.05, p = .97. To further characterize the nature of this interaction, we used the johnson-Neyman technique to identify points in the range of the moderator variable where the effect of the predictor on the outcome transitions from being statistically not significant to significant (Hayes, 2013). We found that when DCA [18F]FMT Ki is greater than or equal to .0323, the confidence bands were entirely below zero. DCA [18F]FMT Ki values for approximately 38% (6/15) of older adults fell in this range, but no young adults (0/21; Figure 4A). Indeed, a DCA [18F] FMT Ki value of .0323 is over 2 SD greater than the mean Ki value measured in young adults. Together, our results indicate that, for older adults, individual differences in dopamine moderate the impact of functional connectivity on switch cost. This conditional relationship was generally present in those with dopamine synthesis capacity outside the normal range of young adults.
For young adults, moderation analysis revealed no interaction between striatal dopamine and functional connectivity in producing individual differences in cognitive flexibility (Figure 4D). The overall model explained a significant amount of variance (R2 = .70, F(4, 16) = 9.24, p < .001). Both DCA [18F]FMT Ki and connectivity were significant predictors of switch cost (FMT: b = −1119.98, t(16) = 2.40, p = .03; PPI: b = −7.80, t(16) = 2.93, p = .01), but there was no interaction (b = −361.09, t(16) = 0.43, p = .43). Thus, the influence of PPI connectivity on switch cost did not interact with synthesis capacity.
DISCUSSION
The presumption that individual differences in neurochemistry produce variability in cognition is widely held, yet there is surprisingly little research in humans linking in vivo measures of neuromodulator function, neural activity, and behavior. This multimodal study supports a model by which DCA dopamine affects striato-thalamo-cortical functional connectivity to produce individual differences in flexible performance. These findings strengthen and specify the role of DCA in task switching while revealing substantial age differences in the nature of the relationships between dopamine, functional connectivity, and cognitive flexibility. Below we discuss the major contributions of this study in the context of previous data and outline important avenues for future experimentation.
Our functional connectivity results build on a rich body of research in describing the anatomical connections between pFC, basal ganglia structures, and thalamus underlying distinct cognitive operations (van Schouwenburg et al., 2013, 2014; Middleton & Strick, 2000; Alexander & Crutcher, 1990; Alexander et al., 1986). Relevant to flexible performance, DCA is posited to control inputs to pFC from thalamus, “opening the gate” to allow the updating of pFC representations (Frank, Loughry, & O’Reilly, 2001; Braver & Cohen, 1999). Importantly, our connectivity results trace the canonical “executive function” loop connecting dorsolateral pFC, DCA, and thalamus (medial dorsal/ventral anterior nuclei; Alexander et al., 1986) in humans and directly connect its successful engagement with performance. Of the thalamic nuclei implicated in executive function, our fMRI study confirmed a role of the ventral anterior nucleus though failed to detect regions in the dorsomedial nucleus whose connectivity with DCA predicted performance. Interestingly, connectivity with ventral lateral nucleus, a component of the motor loop, was also associated with enhanced performance. These findings may not be surprising as lateral aspects of DCA are associated with motor processes (Zhuravin, Brozek, & Bures, 1994) but also highlight the mutual contribution of motor and executive processes to successful task switching.
Notably, we found no evidence of age differences in functional connectivity strength between pFC-DCA and DCA-thalamus. Although it is difficult to interpret null results, they complement our behavioral findings indicating no group difference in switch cost (also discussed below). The conjoint maintenance of connectivity strength and performance in older adults and correlation between these factors across participants (though only marginal in older adults alone) works against the notion that there is a fundamental reorganization of the circuits supporting task switching in older adults. Instead, fronto-striato-thalamic networks appear to be engaged across adulthood with critical alterations in the nature of dopamine’s modulation of these networks.
Our findings converge with evidence from animal studies to suggest striatal dopamine tunes fronto-striato-thalamo-cortical circuits during cognitive performance (Parikh, Cole, Patel, Poole, & Gould, 2016; Agnoli, Mainolfi, Invernizzi, & Carli, 2013; Berger et al., 2005; Walters, Ruskin, Allers, & Bergstrom, 2000; Ruskin, Bergstrom, Mastropietro, Twery, & Walters, 1999). Specifically, dopamine signaling in the striatum may affect activity of striatal circuits by modulating glutamatergic signaling at both frontostriatal and thalamo-striatal synapses (Moss & Bolam, 2008; Bamford et al., 2004). In our study, DCA synthesis capacity was associated with PPI connectivity in both young and older adults (mediation analysis Path 2). However, it is important to note that correlations between DCA synthesis capacity and PPI connectivity for IFG-DCA alone were not statistically significant for young or older adults (p = .09–.16). This is a potentially interesting null effect, as it suggests that the relationship between individual differences in striatal dopamine and striatal functional connectivity is more consistent for DCA’s connection with thalamus rather than pFC. The weak relationship could reflect the unidirectional nature of pFC projections from IFG to DCA. DCA does not directly project to IFG; thus, the influence of DCA dopamine function on IFG-DCA functional connectivity may be muted relative to its influence on DCA-thalamus connectivity. Further research with larger sample sizes is needed to test this possibility. To date, there have been relatively few studies linking in vivo dopamine measures with task-related functional connectivity, though notably, two studies have revealed correlations between caudate dopamine PET measures and functional connectivity during working memory performance within frontoparietal cortex (Rieckmann et al., 2011) and between pFC and DCA (Klostermann et al., 2012).
There is growing interest in dissociating the contributions of dopaminergic modulation of cognitive function via pFC versus striatal mechanisms, as well as dissociating the contributions of varying temporal components of its neurotransmission (Arbuthnott & Wickens, 2007), which have been described as both tonic and phasic (Grace, Floresco, Goto, & Lodge, 2007; Grace, 1991). Emerging models of cognitive control posit that successful performance depends both on cognitive stability (e.g., maintenance of working memory representations, resistance to distractions), as well as flexibility (e.g., ability to update goals, respond to changing environmental demands; Armbruster, Ueltzhöffer, Basten, & Fiebach, 2012; Banich, 2009; Miyake et al., 2000). These complementary though counteractive cognitive states may be differentially augmented by tonic pFC and phasic striatal dopaminergic mechanisms, respectively (Westbrook & Braver, 2016; Cools & D’Esposito, 2011; Braver & Barch, 2002; Cohen, Braver, & Brown, 2002). Here, it was not possible to measure dopamine synthesis capacity in cortex due to low signal-to-noise ratio, though future pharmacological studies pairing PET and fMRI may address these questions (see Cameron, Wallace, Al-Zughoul, Kayser, & D’Esposito, 2018, for behavioral example).
Although DCA dopamine synthesis capacity was related to functional connectivity for both young and older adults, we observed age differences in the direction of these relationships and in their influence on switch cost. Specifically, significant regressions in Mediation Path 2 determined a positive relationship between DCA synthesis capacity and functional connectivity in young adults and a negative relationship in older adults. Similar dissociations in the direction of dopaminergic modulation of fMRI activity in aging have been reported previously for reward processing (Dreher, Meyer-Lindenberg, Kohn, & Berman, 2008) and were evident in univariate analyses in this same task-switching data set (Berry et al., 2016). These findings may reflect a transformation of the mechanisms by which dopamine influences behavior over the lifespan, which we probed further in path analyses.
In young adults, higher DCA dopamine was related to greater cognitive flexibility (lower switch costs) through the mediation of higher functional connectivity. These relationships were not obtained in older adults, in whom DCA synthesis capacity and functional connectivity interacted to produce individual differences in switch cost. In older adults, the expected negative relationships between switch cost and functional connectivity were observed only for participants with high DCA synthesis capacity and were marginal for midrange levels of DCA synthesis capacity. Even though older adults with high dopamine synthesis capacity generally had weaker functional connectivity, this connectivity was reliably correlated with performance, thus mimicking the relationships observed in youth. Although overall, older adults with low synthesis capacity showed higher functional connectivity, this connectivity strength was dissociated from performance. Future functional connectivity studies may explore factors contributing to higher connectivity in older adults with low dopamine synthesis capacity, including differential engagement of circuits implicated in attentional effort and motivation (Lustig & Sarter, 2016; Botvinick & Braver, 2015).
These findings refine our understanding of the role of upregulated dopamine synthesis in aging—suggesting that higher levels of synthesis may preserve (or reinstate) some of the relationships between neurochemistry, brain activity, and performance observed in youth. Notably, we found that DCA Ki of .0323 was the threshold at which the conditional effect of PPI on switch cost was significant in aging; this level of measured synthesis capacity is outside the normal range (M ±2 SD) found in young adults. To be clear, we do not provide evidence that levels of dopamine synthesis outside the normal range in youth benefit cognitive function. Instead, our results leave open the possibility that elevated synthesis, which we observed in approximately 38% of older adults, reestablishes consistent relationships between connectivity and cognition. Elevated synthesis capacity may restore balance between pre- and postsynaptic dopaminergic function. Progressive loss of D2/3 postsynaptic receptors in striatum has been estimated to be ~8% per decade and may begin by 20 years (Karrer et al., 2017). These changes likely precede alteration in synthesis capacity (Dejesus et al., 2001; Lee et al., 2000). Therefore, the dissociation in relationships between connectivity and switch cost for older adults with low synthesis capacity may reflect this early phase in age-related alteration of dopaminergic function. Further studies are needed to resolve these mechanisms, which may include longitudinal testing of dopamine synthesis and cognition changes as well as testing relationships between D2/3 receptor density and dopamine synthesis capacity within participants (see Berry et al., 2018; Ito et al., 2011, for examples in young adults).
This study has a number of limitations, which should be considered. First, the number of participants is small. This is particularly relevant for interpreting results of the moderation analyses examining interactions between dopamine synthesis capacity and functional connectivity for which the small group of older adults (n = 15) is parsed into subgroups based on dopamine synthesis capacity. Although it is encouraging that the moderation effects in older adults were consistent for analyses considering IFG-DCA and DCA-thalamus connectivity separately, these results must be interpreted with caution while pending replication. However, we believe that these analyses offer valuable targets for future hypothesis testing and are thus reported here. Second, the task-switching procedure did not yield age differences in local switch cost. Although this effect may be due in part to attributes of the older adults enrolled in the Berkeley Aging Cohort Study, absence of age differences in switch cost are consistent with previous meta-analyses (Wasylyshyn et al., 2011; Verhaeghen & Cerella, 2002). It is possible that matched performance underlies our finding that functional connectivity strength was similar between groups. As such, we are careful not to draw conclusions that age-related changes in dopamine function cause changes in cognitive flexibility or functional connectivity strength. Instead, our findings suggest that dopaminergic changes alter the relationships between functional connectivity and performance observed in youth. Finally, although our mediation analyses posit directionality of dopamine’s effects on functional connectivity and performance, complementary pharmacological studies are needed to infer causality.
Together, our findings implicate well-described striatal circuits in high-level cognition and suggest a role of striatal dopamine function in tuning the strength of these net-works in young and older adults. Importantly, these results are in agreement with evolving models describing the mechanisms of striatal influences on complex cognitive function (van Schouwenburg, den Ouden, & Cools, 2015; Badre, Doll, Long, & Frank, 2012; Hazy, Frank, & O’Reilly, 2007), as well as cognitive theories suggesting a spatially selective mapping of dopamine’s influence on distinct aspects of executive function (Westbrook & Braver, 2016; Cools & D’Esposito, 2011). Here, we describe a neurochemical basis by which fronto-striato- thalamic connectivity affects cognition and provide preliminarily evidence for compensatory upregulation of dopamine synthesis in aging.
Supplementary Material
Acknowledgments
The authors declare no competing financial interest. This work was supported by NIH grants AG044292 and AG047686. We thank Stephanie Leal for helpful discussions.
Footnotes
Note
Supplementary Materials for this paper can be retrieved from https://doi.org/10.6078/D1W973.
REFERENCES
- Agnoli L, Mainolfi P, Invernizzi RW, & Carli M (2013). Dopamine D1-like and D2-like receptors in the dorsal striatum control different aspects of attentional performance in the five-choice serial reaction time task under a condition of increased activity of corticostriatal inputs. Neuropsychopharmacology, 38, 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens J, Geveci B, & Law C (2005). Paraview: An end-user tool for large data visualization. Burlington, MA: Elsevier. [Google Scholar]
- Alexander GE, & Crutcher MD (1990). Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neurosciences, 13, 266–271. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, & Yurgelun- Todd D (2011). Reproducibility of single-subject functional connectivity measurements. American Journal of Neuroradiology, 32, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott GW, & Wickens J (2007). Space, time and dopamine. Trends in Neurosciences, 30, 62–69. [DOI] [PubMed] [Google Scholar]
- Armbruster DJN, Ueltzhöffer K, Basten U, & Fiebach CJ Prefrontal cortical mechanisms underlying individual differences in cognitive flexibility and stability. Journal of Cognitive Neuroscience, 24, 2385–2399. [DOI] [PubMed] [Google Scholar]
- Armbruster-Genç DJN, Ueltzhöffer K, & Fiebach CJ (2016). Brain signal variability differentially affects cognitive flexibility and cognitive stability. Journal of Neuroscience 36, 3978–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, et al. (2011). Effects of working-memory training on striatal dopamine release. Science, 333, 718. [DOI] [PubMed] [Google Scholar]
- Badre D, Doll BB, Long NM, & Frank MJ (2012). Rostrolateral prefrontal cortex and individual differences in uncertainty-driven exploration. Neuron, 73, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, et al. (2004). Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron, 42, 653–663. [DOI] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18, 89–94. [Google Scholar]
- Berger A, Sadeh M, Tzur G, Shuper A, Kornreich L, Inbar D, et al. (2005). Task switching after cerebellar damage. Neuropsychology 19, 362–370. [DOI] [PubMed] [Google Scholar]
- Berry AS, Sarter M, & Lustig C (2017). Distinct frontoparietal networks underlying attentional effort and cognitive control. Journal of Cognitive Neuroscience, 29, 1212–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Baker SL, Vogel JW, O’Neil JP, Janabi M, et al. (2016). Aging affects dopaminergic neural mechanisms of cognitive flexibility. Journal of Neuroscience 36, 12559–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Furman DJ, White RL, Baker SL, O’Neil JP, et al. (2018). Dopamine synthesis capacity is associated with D2/3 receptor binding but not dopamine release. Neuropsychopharmacology, 43, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, & Braver T (2015). Motivation and cognitive control: From behavior to neural mechanism. Annual Review of Psychology, 66, 83–113. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Wilcox CE, Landau SM, O’Neil JP, Baker SL, Madison CM, et al. (2008). Relationship of striatal dopamine synthesis capacity to age and cognition. Journal of Neuroscience 28, 14320–14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, & Barch DM (2002). A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience & Biobehavioral Reviews 26, 809–817. [DOI] [PubMed] [Google Scholar]
- Braver TS, & Cohen JD (1999). Dopamine, cognitive control, and schizophrenia: The gating model. Progress in Brain Research 121, 327–349. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, & Donaldson DI (2003). Neural mechanisms of transient and sustained cognitive control during task switching. Neuron, 39, 713–726. [DOI] [PubMed] [Google Scholar]
- Cameron IGM, Wallace DL, Al-Zughoul A, Kayser AS, & D’Esposito M (2018). Effects of tolcapone and bromocriptine on cognitive stability and flexibility. Psychopharmacology, 235, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Cortes R, Gueye B, Probst A, & Palacios JM (1989). Dopamine receptors in human brain: Autoradiographic distribution of D2 sites. Neuroscience 28, 275–290. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, & Brown JW (2002). Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology 12, 223–229. [DOI] [PubMed] [Google Scholar]
- Cools R, & D’Esposito M (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69, e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, & D’Esposito M (2008). Working memory capacity predicts dopamine synthesis capacity in the human striatum. Journal of Neuroscience, 28, 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LC, Donde A, Madison C, O’Neil JP, & Jagust WJ (2012). Striatal dopamine influences the default mode network to affect shifting between object features. Journal of Cognitive Neuroscience, 24, 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus OT (2003). Positron-labeled DOPA analogs to image dopamine terminals. Drug Development Research 59, 249–260. [Google Scholar]
- DeJesus OT, Endres CJ, Shelton SE, Nickles RJ, & Holden JE (2001). Noninvasive assessment of aromatic L-amino acid decarboxylase activity in aging rhesus monkey brain in vivo. Synapse, 39, 58–63. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, & von Cramon DY (2000). Prefrontal cortex activation in task switching: An event-related fMRI study. Cognitive Brain Research 9, 103–109. [DOI] [PubMed] [Google Scholar]
- Dreher J-C, Meyer-Lindenberg A, Kohn P, & Berman KF (2008). Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences, U.S.A, 105, 15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, U.S.A, 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Hall H, Ehrin E, & Sedvall G (1986). Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science, 231, 258–261. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, & O’Reilly RC (2001). Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive Affective & Behavioral Neuroscience, 1, 137–160. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, & Penny WD (2007). Statistical parametric mapping: The analysis of functional brain images. Amsterdam: Academic. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, & Dolan RJ (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, & Frackowiak RS (1995). Characterizing evoked hemodynamics with fMRI. Neuroimage, 2, 157–165. [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, et al. (2015). NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA (1991). Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience, 41, 1–24. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, & Lodge DJ (2007). Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences, 30, 220–227. [DOI] [PubMed] [Google Scholar]
- Grinband J, Wager TD, Lindquist M, Ferrera VP, & Hirsch J (2008). Detection of time-varying signals in event-related fMRI designs. Neuroimage, 43, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Calzavara R (2009). The cortico-basal ganglia integrative network: The role of the thalamus. Brain Research Bulletin, 78, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Lynd-Balta E, & Spooren WPJM (1994). Integrative aspects of basal ganglia circuitry In Percheron G, McKenzie JS, & Feger J (Eds.), The basal ganglia IV (pp. 71–80). New York: Plenum Press. [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, & Farde L (1994). Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 11, 245–256. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press. [Google Scholar]
- Hazy TE, Frank MJ, & O’Reilly RC (2007). Towards an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 362, 1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, et al. (2011). Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: Implication of dopaminergic tone. Journal of Neuroscience, 31, 7886–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Ota M, Ikoma Y, Seki C, Yasuno F, Takano A, et al. (2006). Quantitative analysis of dopamine synthesis in human brain using positron emission tomography with L-[β−11C] DOPA. Nuclear Medicine Communications, 27, 723–731. [DOI] [PubMed] [Google Scholar]
- Ito H, Shidahara M, Takano H, Takahashi H, Nozaki S, & Suhara T (2007). Mapping of central dopamine synthesis in man, using positron emission tomography with L-[β−11C] DOPA. Annals of Nuclear Medicine, 21, 355–360. [DOI] [PubMed] [Google Scholar]
- Jimura K, & Braver TS (2010). Age-related shifts in brain activity dynamics during task switching. Cerebral Cortex, 20, 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer TM, Josef AK, Mata R, Morris ED, & Samanez- Larkin GR (2017). Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: A meta-analysis. Neurobiology of Aging, 57, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, & D’Esposito M (2000). Modulation of task-related neural activity in task-switching: An fMRI study. Cognitive Brain Research, 10, 189–196. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, et al. (2009). Functional segmentation of the brain cortex using high model order group PICA. Human Brain Mapping 30, 3865–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, & Denys D (2013). Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in Neuroscience 7, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann EC, Braskie MN, Landau SM, O’Neil JP, & Jagust WJ (2012). Dopamine and frontostriatal networks in cognitive aging. Neurobiology of Aging 33, 623.e615–623.e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, Baker S, & Jagust WJ(2009). Striatal dopamine and working memory. Cerebral Cortex, 19, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. (2000). In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Annals of Neurology 47, 493–503. [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, et al. (1993). Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proceedings of the National Academy of Sciences, U.S.A, 90, 8861–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, & Sarter M (2016). Attention and the cholinergic system: Relevance to schizophrenia. Current Topics in Behavioral Neurosciences 28, 327–362. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I Accuracy and precision of D2 receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism 21, 1034–1057. [DOI] [PubMed] [Google Scholar]
- Middleton FA, & Strick PL (2000). Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain and Cognition, 42, 183–200. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, & Strafella AP (2006). Striatal dopamine release during performance of executive functions: A [11C] raclopride PET study. Neuroimage 33, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, & Bolam JP (2008). A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. Journal of Neuroscience, 28, 11221–11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, & Dagher A (2008). Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. Journal of Neuroscience 28, 3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J, et al. (2011). Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain 134, 3290–3298. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Andersson M, Forsgren L, Jakobsson-Mo S, Larsson A, Marklund P, et al. (2009). Striatal dopamine D2 binding is related to frontal BOLD response during updating of long-term memory representations. Neuroimage 46, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Parikh V, Cole RD, Patel PJ, Poole RL, & Gould TJ (2016). Cognitive control deficits during mecamylamine-precipitated withdrawal in mice: Possible links to frontostriatal BDNF imbalance. Neurobiology of Learning and Memory, 128, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, & Blasberg RG (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. Journal of Cerebral Blood Flow and Metabolism 5, 584–590. [DOI] [PubMed] [Google Scholar]
- Peirce JW (2007). PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods, 162, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Karlsson S, Fischer H, & Backman L (2011). Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. Journal of Neuroscience 31, 14284–14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, & Evans AC (1998). Correction for partial volume effects in PET: Principle and validation. Journal of Nuclear Medicine, 39, 904–911. [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Mastropietro CW, Twery MJ, & Walters JR (1999). Dopamine agonist-mediated rotation in rats with unilateral nigrostriatal lesions is not dependent on net inhibitions of rate in basal ganglia output nuclei. Neuroscience, 91, 935–946. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. (2013). A thalamocorticostriatal dopamine network for psychostimulant-enhanced human cognitive flexibility. Biological Psychiatry, 74, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. (2016). PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, & Fiebach CJ(2010). Frontostriatal involvement in task switching depends on genetic differences in D2 receptor density. Journal of Neuroscience, 30, 14205–14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Fiebach CJ, Cools R, Tafazoli S, & D’Esposito M(2013). Dissociable fronto-striatal effects of dopamine D2 receptor stimulation on cognitive versus motor flexibility. Cortex, 49, 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HEM, & Cools R (2015). Selective attentional enhancement and inhibition of fronto-posterior connectivity by the basal ganglia during attention switching. Cerebral Cortex, 25, 1527–1534. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, Onnink AMH, ter Huurne N, Kan CC, Zwiers MP, Hoogman M, et al. (2014). Cognitive flexibility depends on white matter microstructure of the basal ganglia. Neuropsychologia, 53, 171–177. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, Zwiers MP, van der Schaaf ME, Geurts DEM, Schellekens AFA, Buitelaar JK, et al. (2013). Anatomical connection strength predicts dopaminergic drug effects on fronto-striatal function. Psychopharmacology, 227, 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBrocklin HF, Blagoev M, Hoepping A, O’Neil JP, Klose M, Schubiger PA, et al. (2004). A new precursor for the preparation of 6-[18F]Fluoro-L-m-tyrosine ([18F]FMT): Efficient synthesis and comparison of radiolabeling. Applied Radiation and Isotopes, 61, 1289–1294. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, & Cerella J (2002). Aging, executive control, and attention: A review of meta-analyses. Neuroscience & Biobehavioral Reviews 26, 849–857. [DOI] [PubMed] [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. (2015). Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain 138, 2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Ruskin DN, Allers KA, & Bergstrom DA (2000). Pre- and postsynaptic aspects of dopamine-mediated transmission. Trends in Neurosciences, 23, S41–S47. [DOI] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, & Sliwinski MJ (2011). Aging and task switching: A meta-analysis. Psychology and Aging 26, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Wechsler Memory Scale—Revised, manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Westbrook A, & Braver TS (2016). Dopamine does double duty in motivating cognitive effort. Neuron 89, 695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage, 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, & Braver TS (2009). BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS One 4, e4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research 17, 37–49. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. (2006). User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage, 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
- Zhuravin IA, Brozek G, & Bures J (1994). Differential contribution of motor cortex and caudate nucleus to instrumental tongue-forelimb synchronization in rats: A functional ablation study. Neuroscience 58, 193–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


