Abstract
The brain is adapted to learn from interactions with the environment that predict or enable the procurement of rewards (Schultz, 2010). For infants, the main caregiver (often the mother) is most associated with primary biological rewards such as food and warmth, as well as the most likely provider of emotional and social rewards such as comfort and responsiveness. In this study we capitalize on the reward value of mother to examine reward learning mechanisms in infancy using multiple eye-tracking measures. Converging lines of research have demonstrated links between reward-related striatal dopamine activity and measurable changes in spontaneous eye-blink rate (EBR) and pupil dilation (Eckstein et al., 2017). We presented 7-month-old infants with video stimuli that parametrically increased in social-emotional value (male stranger, female stranger, mother) or in visual attention value (static image, slowed silent cartoon, dynamic cartoon). After establishing infants’ baseline responses to these stimuli, we paired the videos with arbitrary shape cues in an associative learning task. Infants showed superior learning from their own mother’s video and a heightened anticipatory arousal response to the mother-associated cue following learning. Both learning measures were predicted by infants’ baseline EBR to their mother’s video, providing the first evidence of reward learning and transfer in human infants.
Keywords: Infant learning, Reward learning, Eye-blink rate, Pupil diameter
1. Introduction
Learning from reward and in order to maximize the procurement of reward (e.g., nutrients) is adaptive for an organism’s survival. For human infants, the primary caregiver (often the mother) is the main source of primary biological rewards such as food and warmth, as well as the most likely provider of emotional and social rewards such as comfort and responsiveness. There is mounting evidence that infants treat their mother as a special stimulus, as measured by attentional preference, social and emotional responses, and distinctive neural activity (Bushneil et al., 1989; DeCasper and Fifer, 1980; De Haan and Nelson, 1997; Nakato et al., 2011). The mother’s presence and responsive caregiving have also been found to be formative in the development, connectivity, and reactivity of key neural circuits for emotion and attention regulation (Gee et al., 2014; Hostinar et al., 2014; Moriceau and Sullivan, 2006; Tottenham, 2012; Tottenham et al., 2012). Furthermore, the quality of mother-infant interaction is positively associated with children’s cognitive, emotional, and social development (Bornstein and Tamis-LeMonda, 1989, 1997; Landry et al., 1997, 2006; Lewis and Coates, 1980; Maccoby, 1992; Olson et al., 1984; Pettit et al., 1997; Tamis-LeMonda et al., 2001) with the best outcomes linked to the long-term stability of highly supportive mothering practices, such as engaging in frequent joint attention (Fuligni et al., 2013). The mother or primary caregiver may be especially salient in scaffolding infant learning by 1) providing routine and structured experiences with predictable, learnable sequences of events, 2) responding to the infant’s behavior, enabling the infant to discover the affordances of her own actions [e.g., Lewis and Goldberg, 1969], and 3) instilling feelings of safety and security that encourage the infant to explore and engage with her surroundings [e.g., Bowlby, 1988; Ainsworth, 1978; Bell and Ainsworth, 1972]. It follows that reward learning mechanisms in infancy may be triggered in the context of rewarding caregivers and may be observable in the transfer of value from the mother to mother-associated cues. Here we leverage the reward value of mother to examine mechanisms of reward learning in human infants, using behavioral and physiological eye tracking measures.
1.1. Mechanisms of reward learning
Rewards power learning, or change in behavior, by positively reinforcing actions that lead to rewarding outcomes and increasing engagement with reward-related stimuli, while discouraging actions that result in no reward or in aversive outcomes (Pavlov, 1927; Skinner, 1938). Reward learning engages firing of dopamine neurons in the striatum of the basal ganglia. Dopamine is a neurotransmitter related to pleasure that is also involved in many aspects of learning, memory and goal-directed behavior (Westbrook and Braver, 2016). In the first studies linking dopamine and reward in rats, Olds & Milner (Olds and Milner, 1954) administered low-voltage electrical stimulation in regions of the midbrain containing a high density of dopamine neurons; the rats learned to press levers, run mazes, and were even willing to forgo food and sex to receive this stimulation. In the monkey, Schultz and colleagues found that midbrain dopamine neurons fired in response to primary rewards such as food and liquid, but also to conditioned rewards, such as a light, picture, or sound that was predictive of a primary reward (Schultz, 1986; Schultz et al., 1993, 1997). Following learning, if an expected reward was withheld, then the dopaminergic response was suppressed compared to when the expected reward was delivered. In contrast, if an unexpected reward was delivered, then the dopamine cells fired more strongly. In this way, dopaminergic activity is a learning signal in that it codes for prediction error and signals to the organism that new actions must be learned to continue to maximize reward outcomes (Schultz, 2010).
Critically, when a reward is repeatedly paired with a predictive cue, the dopamine response gradually decreases following the reward itself, and increases in response to the reward-predicting cue (Schultz et al., 1997). Thus, the predictive cue acquires value in eliciting a dopaminergic response and the associated power to engage learning mechanisms. For example, when macaque monkeys were trained to select between cues correlated or uncorrelated with the size of an upcoming juice reward, the activity of striatal dopamine neurons was modulated by the predictive, not by the random, cues (Bromberg-Martin and Hikosaka, 2009). By responding to abstract cue stimuli that contain predictive information, the dopaminergic reward system enables the brain to form expectations in situations of varying uncertainty, to anticipate the outcomes of behavioral decisions, and to update those expectations in light of new or surprising evidence.
Reward learning mechanisms (and reward-related dopaminergic activity) have been implicated in shaping attention and cognitive control processes (Westbrook and Braver, 2016; Puig et al., 2014). In adults, reward has been shown to enhance attention to task-relevant stimuli and influence attentional performance (Anderson et al., 2011; Della Libera and Chelazzi, 2006, 2009). For example, Anderson and colleagues demonstrated that an arbitrary stimulus could capture attention automatically if it acquired value through reward learning (Anderson et al., 2011). Indeed, attending to stimuli that offer predictive information about potential reward is an effective strategy for preparing actions that maximize the chance of obtaining reward.
Reward learning mechanisms have been illustrated in monkeys, mice, human adults, and in children [e.g., Delgado et al., 2000; Elliott et al., 2000; Galvan et al., 2005; May et al., 2004; O’Doherty et al., 2003; Schultz, 2006]; it would follow that these mechanisms are also available to human infants. However, a primary challenge that researchers confront in studying reward learning in early development is a lack of access to the subcortical regions that comprise the dopaminergic reward system. Here we address this challenge using eye-tracking indices of neurotransmitter response. Converging lines of research with non-human animals, patient populations, and adult neuroimaging have established links between reward-related striatal dopamine activity and observable changes in spontaneous eye-blink rate (EBR) and pupil diameter, both measurable in infants (see (Eckstein et al., 2017) for a comprehensive review).
1.2. Measurements of reward learning
Spontaneous eye blinks, in the absence of provocation by an external stimulus such as an object approaching the eye, are believed to reflect activity of the central dopamine system (Bacher and Smotherman, 2004; Karson, 1983). The precise neural pathways controlling eye-blink rate (EBR) are still under investigation, but a number of studies point to a strong link between dopamine activity and EBR. Direct evidence for this relationship comes from the administration of dopamine agonists and antagonists, which increase and decrease EBR in monkeys and human adults (Karson, 1983; Blin et al., 1990; Elsworth et al., 1991; Kleven and Koek, 1996; Taylor et al., 1999). Further evidence comes from clinical populations in which dopamine levels and EBR are affected (e.g., reduced in Parkinson’s disease (Dauer and Przedborski, 2003; Deuschel and Goddemeier, 1998), and elevated in schizophrenia (Karson et al., 1990; Kegeles et al., 2010; Mohr et al., 2005)). Studies have also demonstrated superior performance on frontostriatal cognitive flexibility and attention-shifting tasks that implicate dopaminergic pathways in individuals with higher baseline EBR (Aartes et al., 2012; Dreisbach et al., 2005; Lackner et al., 2010). Barkley-Levinson and Galvan (Barkley-Levenson and Galvan, 2016) established that EBR is a predictor of dopaminergic activity and reward maximization during risky decision-making in adolescence.
EBR increases from infancy to childhood (from <3 to >6 blinks per minute) and reaches a plateau at adult levels by late adolescence (10–20 blinks per minute) (Bacher and Allen, 2009; Zametkin et al., 1979). In infants, rate of blinking increases during feeding and following the introduction of new stimuli (Bacher and Smotherman, 2004); both novelty and feeding are pleasurable to human infants and are modulated by a dopamine antagonist in rats (Pitts and Horvitz, 2000). Recently, EBR was found to increase in infants during a frontostriatal reinforcement learning task when learned pairings switched (Werchan et al., 2015, 2016), indicating that EBR may index a dopaminergic response to prediction error in infants and not only to the hedonic value of stimuli.
While EBR may be reflective of dopaminergic firing, some stimuli may also elicit sustained wide-eyed visual attention. Data have shown that spontaneous blinks are reduced in many visual tasks, particularly when sustained visual attention or object tracking is required (Bentivoglio et al., 1997; De Jong and Merckelbach, 1990; Shultz et al., 2011). Bacher (Bacher (2014)) found that 4-month-olds suppressed blinking compared to baseline when visually inspecting moving toy stimuli but not when viewing a social interaction. The eye-blink (EB) startle response, a related dopaminergic biomarker, has been successfully used to index the reward value of stimuli (Skolnick and Davidson, 2002). However, Guera and colleagues (Guerra et al., 2012) showed a reduction in this EB startle in response to loved ones’ faces (accompanied by changes in heart rate and skin conductance indicative of a positive emotional response), presumably as a function of sustained visual attention when presented with this stimulus. Studies have also found significant relationships between individual differences in EBR and reward-related cognitive performance [e.g., 58], and some have suggested that variation across subjects in dopaminergic activity and/or receptor expression may affect tonic EBR more strongly than the slight phasic changes elicited by task conditions (Eckstein et al., 2017).
Pupil dilation, under constant illumination, may be a useful indicator of arousal and the intensity of cognitive processing. Pupil dilations are modulated by the activity of the noradrenergic system’s locus coeruleus, which supplies noradrenaline (NA) to the cortex, cerebellum, and hippocampus (Wilhelm et al., 1999). Converging evidence from electrophysiology (Rajkowski et al., 1994), pharmacology (Phillips et al., 2000), anatomy (Samuels and Szabadi, 2008), and human imaging (Sterpenich et al., 2006); but see (Astafiev et al., 2010)) points to a tight link between pupil dilation and NA activity. The noradrenergic system is hypothesized to play a role in the functional integration of the brain’s attentional system (Coull et al., 1999; Sara, 2009) and particularly the alerting network (Posner and Fan, 2008), maintaining appropriate levels of arousal for cognitive performance. Thus changes in pupil diameter are thought to reflect changes in alertness, focus, and mental effort (Just and Carpenter, 1993; Kahneman, 1973). In adults, pupillary responses have been documented to emotional, painful, sexually attractive, and preferred stimuli (e.g., (Hess and Polt, 1960); see (Sirois and Brisson, 2014) for a review), as well as to increasing cognitive load such as greater numbers of items to be remembered (Beatty and Kahneman, 1966) or increased difficulty of mental calculations (Hess and Polt, 1964).
During reward learning, a predictive stimulus may also come to evoke an anticipatory arousal response that can be measured in dilation of the pupils (Anderson and Yantis, 2012; O’Doherty et al., 2006). Further, data have shown that pupil dilation during decision-making signals surprise or uncertainty (Preuschoff et al., 2011). A few recent studies have found that infants’ pupils dilate in response to violations of expectations or to physically impossible events (Jackson and Sirois, 2009; Gredeback and Melinder, 2010). In essence, pupil dilation (NA activity) seems to code for increased attention or arousal during passive viewing, and increased uncertainty during conditions of reinforcement learning, where heightened alertness may be adaptive for responding to unexpected outcomes.
In addition to EBR and pupil dilation, the present study incorporates looking time and smiling, perhaps the most accessible and widely used indices of infant attention and interest. The duration of infants’ looking, whether measured as individual fixations or the accumulation of many looks, reflects the time needed for the infant to fully process and encode a visual stimulus, and beyond that, a measure of their interest or subjective preference for it (Fantz, 1964; Oakes, 2010). Smiling is an overt behavioral response to positive, pleasurable stimuli and a demonstration of positive affect (see (Messinger et al., 2008) for a review). These measures offer a more holistic picture of infants’ response to rewards and help disambiguate them from responses reflecting general attention or interest.
1.3. The present study
The aim of the present study is to identify mechanisms of reward learning in infants’ visual behavior. Using eye-tracking, we measured individual differences in 7-month-old infants’ responses to passively viewed video stimuli that parametrically increased in social-emotional value (an unfamiliar male foreign-speaker, an unfamiliar female native-speaker, and the infant’s own mother). The video of the infant’s own mother we hypothesized to have the greatest social-emotional value to the infant and strongest association with primary biological rewards. To disentangle this from general attentional interest, we also measured infants’ responses to videos that parametrically varied in visual attention value but were not associated with primary rewards (a static grey-scale cartoon image, a slowed silent cartoon, and a colorful dynamic cartoon with soundtrack). We expected infants’ smiling, pupil dilation, and EBR to be modulated by the face stimuli, indicating differences in their reward value, whereas infants’ looking times would be modulated by the cartoon stimuli, indicating differences in visual attention but not reward.
Having measured baseline responses to the videos, we then examined the impact of varying rewards on learning in a cue-target associative learning task. Specifically, we tested whether four simple arbitrary shapes would acquire distinct values through consistent pairing with four of the videos as rewards. By presenting cue-target pairs in fixed spatial locations, we were able to measure changes in infants’ saccadic latencies as an index of spatio-temporal associative learning [e.g., Amso and Johnson, 2006; Kirkham et al., 2007; Tummeltshammer and Kirkham, 2013]. Further, we tested whether infants’ responses to the shape cues changed following their learning of the associated rewards. While infants as young as 6 months can detect the relationship between an arbitrary visual cue and subsequent reward, showing a preference for a reward-predictive stimulus compared to a distracter stimulus (Tummeltshammer et al., 2014; Wang et al., 2012), no studies have examined how infants learn from differently valued rewards and whether this value transfers to a predictive cue through reward learning. Recognizing that the rewardingness of mother and the dopaminergic response to rewards is likely to vary across mother-infant dyads, we also explored how individual differences in infants’ responses affected our learning measures.
2. Method
2.1. Participants
Fifty-one healthy full-term infants (23 females, 28 males; Mage = 7 months, 7.6 days, SD = 13.1 days) participated in a single testing session. Four additional infants were excluded due to fussiness, inattention, or poor calibration. Participants were recruited from the community via advertisements and birth records. Informed consent was received from all caregivers, and families were compensated for their time and travel. Based on parental report, 36 participants were Non-Hispanic White, 8 were Hispanic White, 5 were Black, 5 were Asian, and 1 did not report a race/ethnicity. Parents completed a questionnaire indicating maternal education, occupation, income and family size, as well as a survey about caregiving arrangements and screen-viewing habits (see Appendix for survey data).
2.2. Eye tracking apparatus
Eye movements were recorded using a remote eye tracker (SensoMotoric Instruments RED system) with a 22″ monitor. Stimuli were presented using the SMI Experiment Center software at a resolution of 1920 × 1080 pixels, and sounds were played through external stereo speakers. A digital video camera with infrared night vision (Canon ZR960) was placed above the monitor to observe and record infants’ head movements. At the beginning of the testing session, each infant’s point-of-gaze was calibrated using a 5-point calibration sequence (the four corners and center of the screen) provided by the SMI software. The looming calibration stimulus was then presented again in the four corners to validate the accuracy of calibration. If fewer than four points were accurately calibrated, the sequence was repeated. Average deviation was 1.9° (SD = 1.3°), suitable for assessing eye movements within the specified areas of interest.
2.3. Stimuli
Infants viewed six different parametrically varying visual stimuli: three faces and three cartoons. The faces included the infant’s own mother, a female stranger, and a foreign-language speaking male stranger. The faces were filmed against a blank background under the same ambient lighting conditions. Models were instructed to maintain direct eye contact, smile, and speak in infant-directed speech; however, they did not adhere to a particular script, encouraging mothers to speak as they naturally would to their infants. The cartoons were actually identical clips of a popular children’s song (“Five Little Monkeys”) with audio-visual features manipulated at 3 levels: colorful and dynamic with an accompanying soundtrack, slowed with no sound, and gray-scale static with no sound. Comparing samples of 100 frames randomly selected from the face and cartoon videos confirmed that average luminance did not differ within condition (Faces: F(2,97) = 0.950, p = 0.391, ηp2 = 0.022; Cartoons: F(2,97) = 0.01, p = 0.974, ηp2 = 0.001). However, between conditions, the faces were slightly more luminous than the cartoons, on average (Faces M = 68.61, Cartoons M = 59.01; F(1,198) = 57.87, p < 0.001, ηp2 = 0.226). Since pupillary and eye-blink responses are sensitive to such low-level differences in luminance, we processed them in separate analyses for the faces and cartoons.
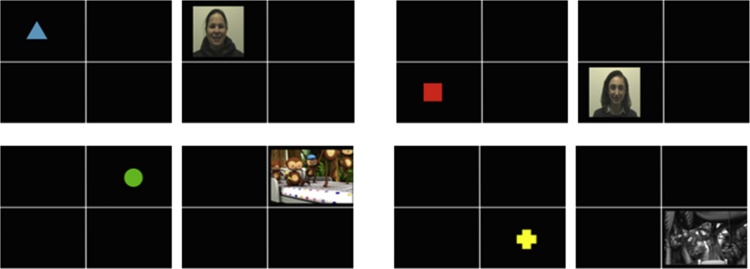
Infants also viewed four different colored shapes (a red square, a blue triangle, a green circle, and a yellow cross). In the associative learning task, the four shapes acted as peripheral cues; each was presented in one of the four quadrants, equidistant from the center of the screen, and followed by a unique reward video in the same quadrant (see Fig. 1). The reward videos were the infant’s own mother, the female stranger, the dynamic cartoon with soundtrack, and the gray-scale static cartoon with no sound. Cue-target pairings and their quadrant locations were counter-balanced across infants. The stimuli were filmed, edited, and animated using Adobe Flash and Premiere Pro software packages.
Fig. 1.
Schematic of the associative learning task. On each trial, a peripheral cue appeared in one of the four quadrants, followed by its paired reward video in the same quadrant.
2.4. Procedure
All infants were tested individually in a quiet room, seated at a distance of 60 cm from the eye-tracking monitor on their caregiver’s lap1 . To ensure equal luminance during all recordings, the testing room was windowless and the artificial lighting was controlled at an identical level for all participants. Following successful calibration, a colorful attention-grabbing stimulus was presented to draw infants’ fixation to the center of the screen. After ensuring fixation, the experimenter manually initiated the first trial.
The experiment consisted of four parts: the video baseline, the cue pre-test, the associative learning task, and the cue post-test. First, the six videos were presented centrally for 8 s each. Each video was presented twice (for a total of 12 randomized trials) separated by the colorful attention-grabbing stimulus to ensure that infants maintained fixation on the screen. Next the four shape cues were presented one at a time in counter-balanced order in the center of a blank screen. Each shape was presented once and remained onscreen for 4 s, separated by a blank screen for 500 ms and a ringing sound to prompt the infant to fixate the screen. Next came the associative learning task, in which the four shapes acted as peripheral cues and predicted the unique spatial location of four paired reward videos (the infant’s own mother, the female stranger, the dynamic cartoon, and the static cartoon). On each trial, a fixation stimulus attracted infants’ gaze to the center; then a shape cue appeared in one of the four quadrants of the screen, followed closely by the paired reward video. Cues were presented for 1 s and rewards were presented for 3 s with a 500-millisecond gap in between. Each cue-target pair occurred 6 times, for a total of 24 randomized trials, and all cues were 100% predictive. The locations of the four cue-target pairs were fixed across trials within a single participant and counter-balanced across participants. Finally, the four shape cues were presented again in a post-test, identical to the pre-test. A break, in the form of an unrelated 15-second video clip, was inserted before and after the associative learning task. The entire experiment lasted approximately 5.5 min.
2.5. Data analysis
Unfiltered eye movement data were analyzed in SMI’s BeGaze analysis software. Trials were excluded if missing more than 50% of samples due to tracking error or inattention, or if the infant failed to orient to the cued quadrant during associative learning (8.7% of baseline videos, 14.5% of pre/post-test trials, 19.8% of associative learning trials). The following dependent variables (DVs) were coded from available eye-tracking and video data: looking time, smiling, pupil dilation, and eye-blink rate. See Appendix for a principle components analysis (PCA) and brief discussion of how these DVs correlate.
Looking time. Looking times for the video baseline and the cue pre- and post-tests were computed as total dwell time, which is the summed duration of all samples falling within the stimulus area of interest (AOI). Total dwell time is arguably a less biased measure of looking time, as it does not require the application of a fixation filter.
Smiling. Digital video recordings of the infants’ faces were coded for instances of smiling by a trained research assistant, blind to the order of stimulus presentation. On each video baseline trial, the presence or absence of a smile was scored categorically to avoid any ambiguity with regard to the smile’s onset or offset. These data were double-coded in 10% of participants by a second trained research assistant, and the intraclass correlation coefficient between coders was 0.910 (p < 0.001).
Pupillary data. The SMI eye-tracker, recording at 60 HZ, takes a sample of the infant’s pupil diameter every 16.67 s. Whenever data was available for both eyes, the mean pupil diameter was computed; otherwise, the value for the available eye was used. Gaps in the data tended to be due to flicker or tracking error (<100 ms duration), eye blinks (100–400 ms in duration), or looks away from the screen (> 400 ms duration). Because of these disparate sources of data loss and their variable durations, the gaps were not interpolated. Rather, the mean pupil size was computed over 100-ms intervals (approximately 6 samples) to generate time series for each trial for each infant.
For the video baseline, face and cartoon videos differed slightly in average luminance and each video was presented on two distinct trials; therefore, pupil size values required adjustment by subtracting the mean pupil diameter in the first 500 ms of the trial from the mean over the remaining intervals. This adjustment has been shown in infants to best accommodate variation in the pupil as a result of differing initial light-reflex responses (i.e., the initial dilation and contraction that occurs when any new stimulus is presented as the eye adjusts to the light (Nyström et al., 2015)). Pupil dilation was computed as the change in mean pupil diameter from the first 500 ms to the second half of the trial (between 4 and 8 s), and these dilations were compared across video stimuli. For the cue pre- and post-tests, the cue stimuli were identical in luminance, had the same preceding blank screen, and were presented only once; therefore, the pupil size values did not require adjustment. Rather, mean pupil diameter across the 4-second presentation of each cue was computed and pre- to post-test changes, resulting from reward learning, were compared.
Blink data. Blinks were identified by an automatic algorithm as events of missing data (i.e., when neither eye position nor pupil diameter could be sampled) that were 100–400 ms in duration. These data were double-coded in 10% of participants by a trained research assistant, blind to the order of stimulus presentation. The coder viewed digital video recordings of the infant’s face, marked the eye closure and reopening, and noted looks off-screen. The intraclass correlation coefficient between the automatic and manual coding was 0.889 (p < 0.001); with satisfactory inter-coder reliability, the eye-tracker values were used in analysis. Eye-blink rate (EBR) was calculated for each trial as blinks per visible second, the total number of blinks divided by the infant’s total looking time.
Associative learning task. Three of 51 infants were excluded from the latency analysis due to missing or unusable data on more than half of trials (i.e., >12 trials) or for supplying no data on one or more stimulus conditions. Latency was calculated as the time difference between the onset of the cue and the arrival of the first eye movement into the cued quadrant.
3. Results
3.1. Baseline responses to face and cartoon videos
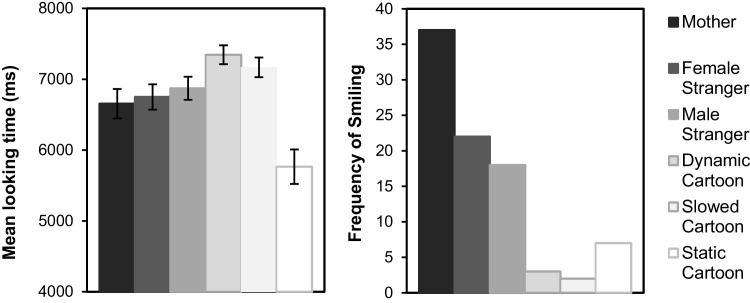
Looking time (LT) and smiling. Mean LTs did not differ between face and cartoon videos, F(1,50) = 0.000, p = 0.996, ηp2<0.001, but did differ by Parametric Value, F(2,100) = 16.03, p < 0.001, ηp2 = 0.243, with a significant Stimulus x Value interaction, F(2,100) = 21.57, p < 0.001, ηp2 = 0.301. Infants looked equally long at the face videos (p = 0.546), but looked less at the static cartoon relative to the two animated cartoons (both p < 0.001; Fig. 2). Frequency of smiling did vary by Stimulus Type, Friedman X2(5) = 52.27, p < 0.001, as infants smiled more when presented with faces than cartoons, and most for their own mother’s face (Fig. 2). These results confirm the validity of our parametric manipulations: The dynamic cartoons induced longer LTs, reflecting greater visual interest and attention value, whereas the infant’s mother induced more smiles, reflecting greater social-emotional value (i.e., positive affect, recognition and/or pleasure).
Fig. 2.
Mean looking time (left) with error bars indicating ±1 SE, and frequency of smiling (right).
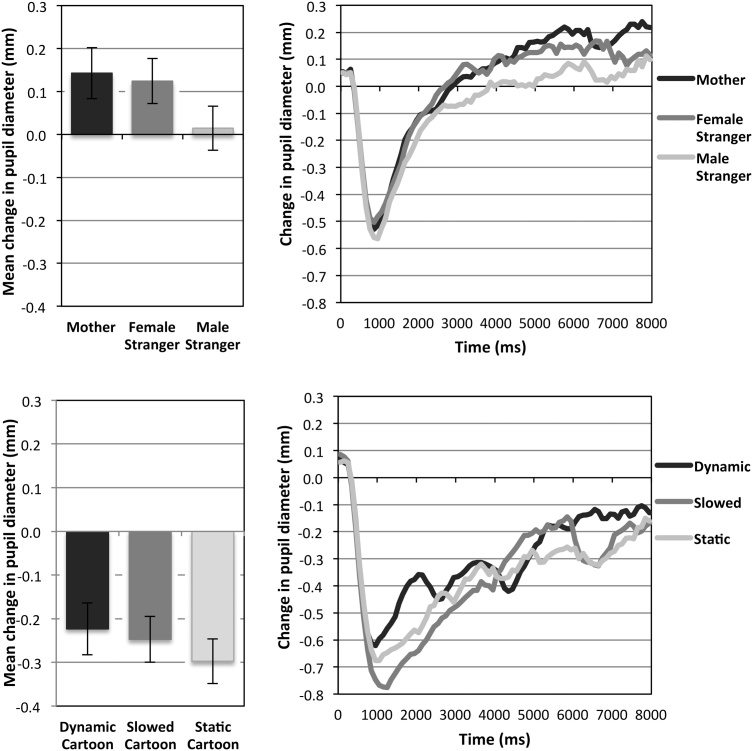
Pupil dilation. Mean pupil dilations (Fig. 3) varied significantly across face videos (main effect of Parametric Value, F(2,96) = 3.83, p = 0.025, ηp2 = 0.074), with larger dilations observed for the Mother and Female Stranger compared to the Male Stranger (t(49) = 2.40, p = 0.020, and t(49) = 2.44, p = 0.018 respectively). Pupil dilations did not vary across cartoon videos (no effect of Parametric Value, F(2,96) = 0.62, p = 0.540, ηp2 = 0.013). These data indicate that the pupillary response, reflecting increased arousal, was sensitive to changes in the social-emotional value of the faces, but not the visual attention value of the cartoons. This result mirrors the pattern of infant smiling, which also increased with the social-emotional value of the faces, but not with the visual attention value of the cartoons.
Fig. 3.
Mean pupil dilation (i.e., change in pupil diameter from the first 500 ms to the second half of the trial) for faces (top) and cartoons (bottom), plotted in (A) as a function of stimulus. Error bars indicate ±1 SE. Raw change in pupil diameter plotted in (B) as a function of time. NB: Face videos elicited larger dilations despite the fact that they were slightly more luminous, on average, than cartoon videos (pupil constriction would be expected if the pupillary changes were simply due to a light-reflex response).
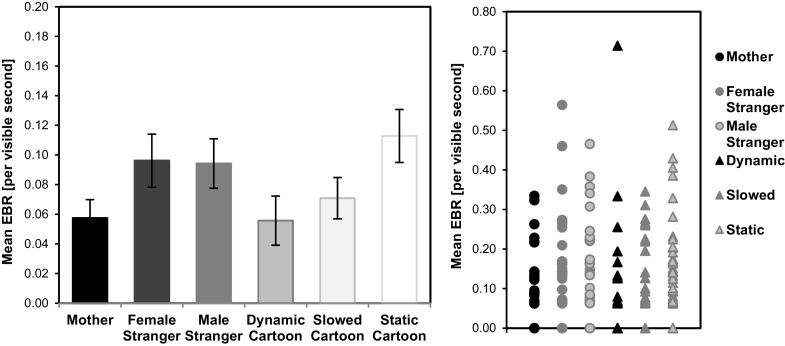
Eye-blink rate (EBR). Mean EBR (Fig. 4) differed significantly across face videos (main effect of Parametric Value, F(2,98) = 3.02, p = 0.053, ηp2 = 0.058), due to less blinking for the Mother relative to the Female and Male Stranger’s faces (t(49) = 2.23, p = 0.030, and t(50) = 2.06, p = 0.045 respectively). Mean EBR also differed significantly across cartoon videos (main effect of Parametric Value, F(2,100) = 3.72, p = 0.028, ηp2 = 0.069), due to less blinking for Dynamic compared to Static cartoons (t(50) = 2.16, p = 0.035), and marginally less for Slowed compared to Static cartoons (t(50) = 1.85, p = 0.070). Although one might expect a higher EBR for higher valued rewards, indicating reward-related dopaminergic firing, here we found average EBR was significantly reduced when viewing both the mother’s face and the dynamic cartoon videos, perhaps due to their sustained attention demands [e.g., 67,69]
Fig. 4.
Mean eye-blink rate (left) and individual differences in eye-blink rate (right) plotted as a function of stimulus. Error bars indicate ±1 SE.
Despite these group-level reductions in EBR for highly valued stimuli, it is nonetheless possible that individual differences in EBR may index a reward-related dopaminergic response that would be elicited selectively by social-emotionally valuable stimuli. A few studies have indicated that variation across subjects in dopaminergic activity and/or receptor expression may affect EBR and reward-related cognitive performance more strongly than the slight phasic changes elicited by task conditions (Eckstein et al., 2017; Barkley-Levenson and Galvan, 2016). We examined infants’ individual differences in EBR with respect to the parental survey measures (see Appendix) of Time Spent with Mother and Total Time Exposed to Screen-based Media, considering whether these experiential factors may correlate with EBR to highly valued stimuli. For Dynamic cartoons, no significant correlations with EBR emerged (Time w/ Mother: r(45) = 0.151, p = .321; Screen Time: r(42)=-0.137, p = 0.387). For Mother’s face, a marginally positive correlation was present between EBR and Time w/ Mother, r(45) = 0.251, p = 0.096, such that the more time infants spent with their mother, the higher their EBR to their mother’s video.
Summary. Taken together, the video baseline results suggest that the mother’s video elicited the greatest reward-related response from infants. Specifically, the mother’s video prompted larger pupil dilations than the male stranger or cartoon videos, and more smiling than any other stimulus, reflecting a greater emotional arousal response. The mother’s video also evoked meaningful individual differences in EBR that positively correlated with the amount of time mothers reported spending with their infants. This pattern of response was distinct from the pattern elicited by the highest value cartoon, which prompted longer looking time and reduced blinking, but no increase in smiling or pupil dilation. Next we examined whether the relative value of the mother reward would induce measurable changes in infants’ behavior (i.e., learning), and further, whether the value of mother and the pattern of responses she elicited would transfer onto the mother-predictive cue.
3.2. Evidence of reward learning
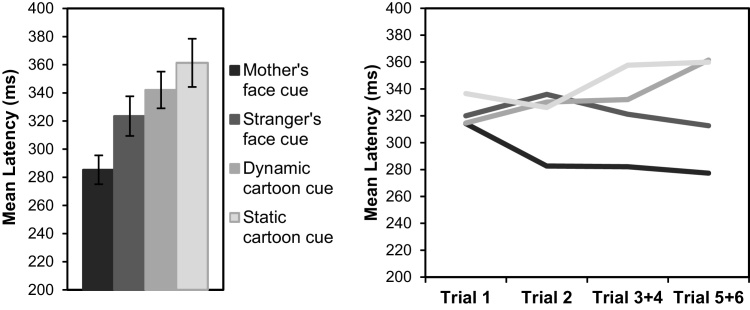
Latency. If infants’ learning were modulated by the relative value of the four rewards (the infants’ own mother, a female stranger, a dynamic cartoon, and a static grey-scale cartoon), then we would expect to see a relative decrease in latency across the 6 trials for cues that predicted higher value rewards. Results of a repeated measures ANOVA, displayed in Fig. 5, showed that mean saccadic latencies were faster for cues paired with the Mother relative to the Stranger or the cartoons (main effect of Stimulus, F(3,141) = 7.07, p < 0.001, ηp2 = 0.131; all post-hoc comparisons, p < 0.03). Comparing latencies on Trial 1 confirmed that this difference was not present initially or due to arbitrary bias (F(3,78) = 1.11, p = 0.352, ηp2 = 0.041), but rather emerged rapidly during the associative learning task.
Fig. 5.
Mean latency of first eye movement to each of the four cued quadrants (left). Error bars indicate ±1 SE. Change in latency across the six trials for each of the four cues (right).
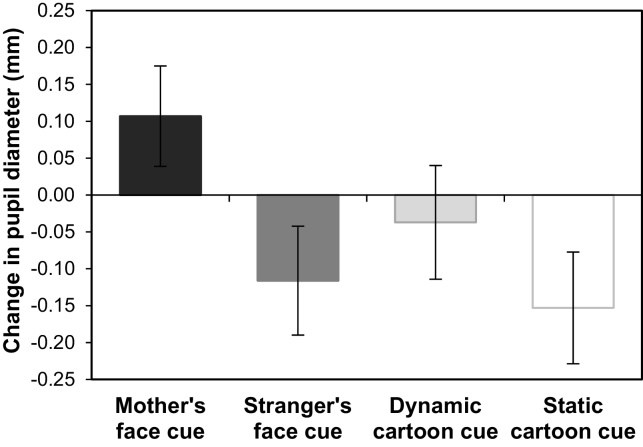
Transfer of value to cues. Comparing pre- and post-test responses to the isolated cues allowed us to examine whether associating arbitrary cues with differently valued rewards resulted in a transfer of value from the reward onto the reward-predictive cue. We compared mean changes in infants’ responses (i.e., mean differences in looking time, pupil diameter, and EBR to the four cues from pre-test to post-test) in separate repeated measures ANOVAs. The ANOVAs showed no significant differences across cues in infants’ mean changes in looking time or EBR (both p > 0.646); however, the pupil dilation analysis did show a significant effect of Cue, F(3,90) = 2.54, p = 0.061, ηp2 = 0.078. Post-hoc tests showed that pupil diameter for the mother-associated cue increased relative to the stranger-associated cue (t(34) = 2.58, p = 0.015) and relative to the static cartoon-associated cue (t(33) = 2.54, p = 0.016) (Fig. 6).
Fig. 6.
Mean change in pupil diameter to the four shapes from pre-test to post-test. Error bars indicate ±1 SE.
Predicting learning and transfer from baseline responses. Next we considered whether infants’ enhanced learning of the mother-associated cue (i.e., latency and increased pupillary response) could be predicted from their baseline responses to their mother’s video (i.e., smiling, pupil dilation, and EBR). For each DV, we collapsed the infants’ responses into a single difference score: for example, the Latency Difference Score was calculated as the difference between mean latency to the mother-associated cue and mean latency to the remaining three cues. Use of a difference score eliminates variability that may be shared across all stimulus conditions (e.g., some infants may have generally faster eye movements) and provides a measure of the infant’s response to mother isolated from the other videos.
The first multiple regression model was constructed to predict infants’ Latency Difference Score from the following predictors: Age, Time Spent with Mother, Smiling Difference Score, Pupil Difference Score, and EBR Difference Score. This regression model was significant, F(535) = 3.09, p = 0.022, with an overall fit of R2 = 0.326. Results indicate that infants’ Age (β=-0.484, p = 0.005) and EBR Difference Score (β=-0.438, p = 0.008) were significant negative predictors of their Latency Difference Score; Time Spent with Mother, Smiling and Pupil were not significant predictors (all p > 0.105). Older infants were more likely to have a smaller Latency Difference Score, which we interpret as a byproduct of generally faster processing and eye movement speeds with age. Importantly, larger EBR Difference Scores, reflecting higher EBR when viewing the Mother compared to all other videos, were predictive of faster latencies to the mother-associated cue. Thus infants who found their mother’s video more rewarding (as measured by a higher reward-related EBR response) showed better learning from their mother’s video in the associative learning task.
The second multiple regression model was constructed to predict infants’ Pupil Change Score (i.e., the difference in mean pupil diameter to the mother-associated cue from pre-test to post-test) from the following predictors: Age, Time Spent with Mother, Pupil Difference Score, EBR Difference Score, and Latency Difference Score. The regression model was significant, F(5,28) = 3.09, p = 0.024, with an overall fit of R2 = 0.356. Results indicate that infants’ EBR Difference Score (β = 0.492, p = 0.006) and Time Spent with Mother (β = 0.349, p = 0.049) were significant positive predictors of their Pupil Change Score; Age, Pupil Difference Score, and Latency Difference Score were not significant predictors (all p > 0.145). More time with their mothers, as well as larger EBR Difference Scores reflecting higher EBR when viewing their mother’s video, were predictive of larger increases in pupil dilation to the mother-associated cue. Thus infants who found their mother’s video more rewarding (as measured by a higher reward-related EBR response) showed greater transfer of the pupillary response from their mother’s video onto the mother-associated cue through reward learning.
4. Discussion
Our results provide evidence that the same reward learning mechanisms observed in human adults and non-human animals are also operational in human infants; specifically, when a highly valued reward is repeatedly paired with a predictive cue, we found evidence that infants’ visual responses to the reward itself indeed transfer onto the reward predictive cue (Schultz et al., 1997). Prior to learning, a video of the infant’s mother elicited a pattern of behavioral and physiological response indicative of greater social-emotional value (i.e., larger pupil dilations than the male stranger or cartoon videos and more infant smiling than any other stimulus, as well as meaningful changes in EBR). The mother’s video motivated the strongest spatiotemporal learning (i.e., faster latency to the cue paired with mother) and the extent of learning was predicted by individual differences in infants’ baseline EBR for the mother’s video. Further, pupil dilations increased to the mother-predictive cue following learning, indicating that the cue had acquired value in eliciting a pupillary response. This increase in pupil dilation was also predicted by individual differences in infants’ baseline EBR for the mother’s video.
The present study capitalized on the accessibility of pupil and eye-blink responses in human infants and offers new insights into their use as indirect measures of reward-related neurotransmitter activity. Pupil dilations, modulated by the activity of the noradrenergic system, are thought to reflect changes in arousal and attentional focus (Coull et al., 1999; Sara, 2009). Infants showed differences in pupil dilation across face stimuli that varied in social-emotional value, but not across cartoon stimuli that varied in visual attention/interest value. Larger pupil dilations were observed for their own mother, who also elicited the most smiles from infants, as well as for the female stranger, who was arguably most similar to the mother and may have evoked a sense of familiarity. These results are consistent with a large body of research that has documented pupillary responses to emotionally relevant stimuli (Sirois and Brisson, 2014). Further they indicate that pupillary responses are not simply indexing differences in attentional demand (as reflected in looking times), but rather the intrinsic value of the stimulus in eliciting an emotional arousal response. In the context of reward learning, pupil dilations may reflect an anticipatory arousal response that follows a predictive cue in expectation of receiving a reward (Anderson and Yantis, 2012; O’Doherty et al., 2006). After seeing repeated cue-target pairings, infants showed increased pupil dilation to the cue that predicted their mother’s video. This anticipatory arousal was not apparent for the other three cues. Since the mother video had elicited the largest dilations prior to learning, this increased pupillary response to the mother-predictive cue signifies a transfer of value from the reward onto the cue as a result of reward learning.
Spontaneous eye-blink rate, modulated by the activity of the dopaminergic system, has been found to predict performance on frontostriatal cognitive control, reward maximization, and reinforcement learning tasks (Aartes et al., 2012; Dreisbach et al., 2005; Lackner et al., 2010; Barkley-Levenson and Galvan, 2016; Werchan et al., 2015, 2016). Infants’ EBR differed across both face and cartoon videos, indicating that it may be sensitive to variations in both social-emotional and visual attention/interest value. In general, one might expect a higher EBR, indicating greater reward-related dopaminergic firing, for the mother’s face and/or dynamic cartoon videos, which we manipulated to be of highest value. However, here group-level EBR was significantly reduced when viewing the both the mother’s face and dynamic cartoon videos. This reduction is consistent with previous work that observed blink suppression during sustained attention while tracking moving objects (Bacher, 2014) or looking at videos of loved ones (Guerra et al., 2012). Our smiling, looking time, and pupillary data suggest that separate processes may be responsible for the same reduction in EBR due to wide-eyed sustained attention to these highest value stimuli (namely, recognition and a stronger social-emotional response to the mother’s face, and effortful audio-visual processing and particularly motion tracking of the dynamic cartoon).
When saccadic latencies and the transfer of pupillary responses indicated superior reward learning from the mother video, predictive models revealed EBR to be the key predictor of both learning measures. Interestingly, as a group infants had the lowest rates of blinking for their mother’s video, but it was higher EBR that was predictive of better learning and transfer. We interpret this result as reflecting the competing influences of attention and reward value on EBR, as both are consistent with previous findings. The lower EBR observed in the group mean is likely a consequence of increased attentional focus, as it was similarly observed for the mother’s video and the dynamic cartoons, and has been documented in other visual tasks where sustained visual attention is required (Bentivoglio et al., 1997; De Jong and Merckelbach, 1990; Shultz et al., 2011; Bacher, 2014; Guerra et al., 2012). The higher EBR that was predictive of superior reward learning is more likely to relate to dopaminergic firing, possibly reflecting individual differences in dopamine receptor expression, and is consistent with positive predictive relationships between EBR and performance on other reward and reinforcement tasks (Aartes et al., 2012; Lackner et al., 2010; Barkley-Levenson and Galvan, 2016). Thus while infants may have suppressed blinking to their mother’s video to preserve visual access and continuity of processing, infants whose mother elicited a larger reward-related EBR within that tight range showed better subsequent learning from their mother’s video.
In addition to providing primary rewards (e.g., food, warmth, comfort), our findings suggest that the infant’s mother can play a critical role in driving early learning. A number of studies have found predictive relationships between the quality of mother-infant interaction and/or maternal responsiveness and infants’ cognitive development (Bornstein and Tamis-LeMonda, 1989, 1997; Landry et al., 1997, 2006; Lewis and Coates, 1980; Maccoby, 1992; Olson et al., 1984; Pettit et al., 1997; Tamis-LeMonda et al., 2001). These studies indicate that the mother is likely to play a significant foundational role in her infant’s learning. Our results are consistent with the idea that this role may be, in part, to motivate early learning by engaging infants’ reward learning pathways as a cognitive reinforcer. The mother’s video proved to be a highly valued stimulus that produced a unique pattern of behavioral and physiological responses: specifically, infants exhibited the most smiles, largest pupil dilations, and lowest blink rates when viewing their mother’s video. During the associative learning task, the mother’s video indeed functioned as a reward (motivating faster saccades) and recruited reward-learning mechanisms (as seen in the transfer of pupillary responses onto the mother-predictive cue). The use of difference scores in our regression models provides further evidence that these effects were specific to the mother reward; larger EBR Difference Scores indicating higher EBR to mother above and beyond the other videos was predictive of faster latencies and larger pupillary responses to the mother-predictive cue above and beyond the other cues. In other words, the prospect of seeing their own mother’s face motivated infants to learn, possibly engaging dopaminergic reward-related pathways in a way that the stranger’s face and the cartoon stimuli did not.
This result suggests that attention, learning and memory for new information may be enhanced in the context of mother. If the mother indeed motivates faster orienting and better learning, then it may be the case that infants will show better processing and encoding of mother-associated information. This relationship is likely to vary across infant-mother dyads: the extent of transfer of infants’ pupillary response to the mother-predictive cue following learning was also predicted by the amount of time that mothers reported spending with their infants, indicating a strong effect of infants’ day-to-day experience with their mother on this relationship. Thus, individual differences in attachment style, maternal sensitivity, and the quality of mother-child interaction may have a pervasive effect not only on infants’ early social-emotional bonding, but also on infants’ early learning. Future models may likely find that more sensitive measures of mother-child interaction quality (e.g., maternal responsiveness) are stronger moderators of reward learning than our gross measure of quantity, as a number of studies have found interaction quality to be an important predictor of cognitive outcomes (Bornstein and Tamis-LeMonda, 1989, 1997; Landry et al., 1997, 2006; Lewis and Coates, 1980; Maccoby, 1992; Olson et al., 1984; Pettit et al., 1997; Tamis-LeMonda et al., 2001).
In addition, our results indicate that arbitrary stimuli can acquire value through their association with mother, so it follows that mother could also serve as a value-assigning cue, guiding her infant toward positive and pleasurable interactions with the environment and away from negative ones. Indeed, by 10–12 months of age, infants begin to reference their mothers for positive or negative feedback prior to engaging with a new object or individual (social referencing (Baldwin and Moses (1996); Campos and Stenberg, 1981; Feinman et al., 1992; Sorce et al., 1985)) and rely on mother’s cues when making decisions about whether to undertake risky or uncertain actions (Tamis-LeMonda et al., 2008). Moreover, infants tend to show measurable distress when mothers are unresponsive or fail to provide appropriate, contingent social cues (e.g., the Still-Face paradigm (Mesman et al., 2009; Moore et al., 2009; Tronick et al., 1978)). Feedback from the mother may be more potent in influencing infants’ behavior precisely because it engages powerful reward learning mechanisms. In this capacity, the mother has the potential to serve as both a reward and reward-maximizing cue.
While infants demonstrated learning and transfer effects that were specific to their mother’s video and mother-predictive cues, the reward mechanisms implicated in that learning should not be limited to the mother per se. Theoretically any stimulus so strongly associated with social-emotional value and the fulfillment of primary biological needs should recruit the same mechanisms (e.g., an alternate caregiver or a personally significant object such as the infant’s milk bottle). A limitation of this work is that we have restricted means of accessing what is rewarding to infants; we cannot ask them, we cannot easily put them to work for reward, and we are not able to measure dopaminergic activity directly. However, by using indirect measures such as smiling and EBR to quantify whether and which stimuli elicit a relative response, and then assessing the effect of these responses on subsequent learning, we can contribute to a fuller picture of the pathways these mechanisms take to motivate behavior change early in life.
In conclusion, the present study has provided evidence that reward mechanisms indeed power learning in infancy and can be measured by changes in neurotransmitter-related visual responses (i.e., pupil dilation and EBR). Reward learning was observed most strongly in the context of the infant’s mother, indicating that primary caregivers have a significant role to play in early cognitive development by assigning value to novel stimuli and motivating opportunities for learning. Furthermore, this study demonstrates how visual responses can provide a window into learning-linked processes in human infants, making reward and reinforcement circuitry more accessible for future investigations.
Conflict of interest
None.
Acknowledgements
We thank Heidi Baumgartner and Kelley Gunther for help with testing a recruitment, and Bella Ehrlich for help with behavioral coding. This work was funded in part by a James S. McDonnell Scholar Award for Understanding Human Cognition to DA, an R01 research grant (MH099078) to DA from the National Institutes of Health, and an NRSA fellowship (1-F32-MH108278-01) to KT from the National Institutes of Health.
Footnotes
Most often, infants came to the lab with their mothers, and thus it was the mother’s lap that they sat on during the experiment. We coded the number of times that infants turned to look at their mothers during testing and found that it was negligible and did not differ across stimulus conditions (i.e., infants were not likely to turn to their mothers when they saw their video onscreen). All caregivers were instructed not to speak or interact with their infants during testing.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.12.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aartes H., Bijleveld E., Custers R., Dogge M., Deelder M., Schutter D., van Haren N.E.M. Positive priming and intentional binding: eye-blink rate predicts reward information effects on the sense of agency. Soc. Neurosci. 2012;7:105–112. doi: 10.1080/17470919.2011.590602. [DOI] [PubMed] [Google Scholar]
- Ainsworth M.D.S. Lawrence Erlbaum Assoc.; Hillsdale, N.J: 1978. Patterns of Attachment: A Psychological Study of the Strange Situation. [Google Scholar]
- Amso D., Johnson S.P. Learning by selection: visual search and object perception in young infants. Dev. Psychol. 2006;42(6):1236. doi: 10.1037/0012-1649.42.6.1236. [DOI] [PubMed] [Google Scholar]
- Anderson B.A., Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Atten. Percept. Psychophys. 2012;74(8):1644–1653. doi: 10.3758/s13414-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.A., Laurent P.A., Yantis S. Value-driven attentional capture. Proc. Natl. Acad. Sci. U. S. A. 2011;108(25):10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev S.V., Snyder A.Z., Shulman G.L., Corbetta M. Comment on “Modafinil shifts human locus coeruleus to low-tonic, high phasic activity during functional MRI” and “homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area”. Science. 2010;328:309. doi: 10.1126/science.1177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher L.F. Development and manipulation of spontaneous eye blinking in the first year: relationships to context and positive affect. Dev. Psychobiol. 2014;56(4):783–796. doi: 10.1002/dev.21148. [DOI] [PubMed] [Google Scholar]
- Bacher L.F., Allen K.J. Sensitivity of the rate of spontaneous eye blinking to type of stimuli in young infants. Dev. Psychobiol. 2009;51(2):186–197. doi: 10.1002/dev.20357. [DOI] [PubMed] [Google Scholar]
- Bacher L.F., Smotherman W.P. Spontaneous eye blinking in human infants: a review. Dev. Psychobiol. 2004;44(2):95–102. doi: 10.1002/dev.10162. [DOI] [PubMed] [Google Scholar]
- Baldwin D.A., Moses L.J. The ontogeny of social information gathering. Child Dev. 1996;67(5):1915–1939. [Google Scholar]
- Barkley-Levenson E., Galvan A. Eye blink rate predicts reward decisions in adolescents. Dev. Sci. 2016;20(3) doi: 10.1111/desc.12412. [DOI] [PubMed] [Google Scholar]
- Beatty J., Kahneman D. Pupillary changes in two memory tasks. Psychonomic. Sci. 1966;5(10):371–372. [Google Scholar]
- Bell S.M., Ainsworth M.D.S. Infant crying and maternal responsiveness. Child Dev. 1972;43(4):1171–1190. [PubMed] [Google Scholar]
- Bentivoglio A.R., Bressman S.B., Cassetta E., Carretta D., Tonali P., Albanese A. Analysis of blink rate patterns in normal subjects. Mov. Disord. 1997;12(6):1028–1034. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- Blin O., Masson G., Azulay J.P., Fondarai J., Serratrice G. Apomorphine-induced blinking and yawning in healthy volunteers. Br. J. Clin. Pharmacol. 1990;30(5):769–773. doi: 10.1111/j.1365-2125.1990.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein M.H., Tamis-LeMonda C.S. Maternal responsiveness and cognitive development in children. New Dir. Child Adolesc. Dev. 1989;1989(43):49–61. doi: 10.1002/cd.23219894306. [DOI] [PubMed] [Google Scholar]
- Bornstein M.H., Tamis-LeMonda C.S. Maternal responsiveness and infant mental abilities: specific predictive relations. Inf. Behav. Dev. 1997;20(3):283–296. [Google Scholar]
- Bowlby J. Basic Books, Inc.; New York, NY: 1988. A Secure Base: Parent-Child Attachment and Healthy Human Development. [Google Scholar]
- Bromberg-Martin E.S., Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63(1):119–126. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushneil I.W.R., Sai F., Mullin J.T. Neonatal recognition of the mother’s face. Br. J. Dev. Psychol. 1989;7(1):3–15. [Google Scholar]
- Campos J., Stenberg C.R. Perception, appraisal and emotion: the onset of social referencing. In: Lamb M.E., Sherrod L.R., editors. Infant Social Cognition. Erlbaum; Hillsdale NJ): 1981. pp. 273–314. [Google Scholar]
- Coull J.T., Buchel C., Friston K.J., Frith C.D. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage. 1999;10(6):705–715. doi: 10.1006/nimg.1999.0513. [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- De Haan M., Nelson C.A. Recognition of the mother’s face by six-month-old infants: a neurobehavioral study. Child Dev. 1997;68(2):187–210. [PubMed] [Google Scholar]
- De Jong P.J., Merckelbach H. Eyeblink frequency, rehearsal activity, and sympathetic arousal. Int. J. Neurosci. 1990;51(1-2):89–94. doi: 10.3109/00207459009000513. [DOI] [PubMed] [Google Scholar]
- DeCasper A.J., Fifer W.P. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Della Libera C., Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychol. Sci. 2006;17(3):222–227. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Della Libera C., Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychol. Sci. 2009;20:778–784. doi: 10.1111/j.1467-9280.2009.02360.x. [DOI] [PubMed] [Google Scholar]
- Deuschel G., Goddemeier C. Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease and in normal subjects. J. Neurol. Neurosurg. Psychiatry. 1998;64:320–324. doi: 10.1136/jnnp.64.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach G., Muller J., Goschke T., Strobel A., Schulze K., Lesch K., Brocke B. Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine polymorphisms on perseveration and distractibility. Behav. Neurosci. 2005;119:483–490. doi: 10.1037/0735-7044.119.2.483. [DOI] [PubMed] [Google Scholar]
- Eckstein M.K., Guerra-Carrillo B., Miller Singley A.T., Bunge S.A. Beyond eye-gaze: what else can eyetracking reveal about cognition and cognitive development? Dev. Cogn. Neurosci. 2017;25:69–91. doi: 10.1016/j.dcn.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Friston K.J., Dolan R.J. Dissociable neural responses in human reward systems. J. Neurosci. 2000;20(16):6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth J.D., Lawrence M.S., Roth R.H., Taylor J.R., Mailman R.B., Nichols D.E., Lewis M.H., Redmond D.E. D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J. Pharmacol. Exp. Ther. 1991;259:595–600. [PubMed] [Google Scholar]
- Fantz R.L. Visual experience in infants: decreased attention to familiar patterns relative to novel ones. Science. 1964;145(3644):668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Feinman S., Roberts D., Hsieh K.F., Sawyer D., Swanson D. A critical review of social referencing in infancy. In: Feinman S., editor. Social Referencing and the Social Construction of Reality in Infancy. Springer; Boston, MA): 1992. pp. 15–54. [Google Scholar]
- Fuligni A.S., Brady-Smith C., Tamis-LeMonda C.S., Bradley R.H., Chazan-Cohen R., Boyce L., Brooks-Gunn J. Patterns of supportive mothering with 1-, 2-, and 3-year-olds by ethnicity in early head start. Parenting Sci. Pract. 2013;13:44–57. [Google Scholar]
- Galvan A., Hare T.A., Davidson M., Spicer J., Glover G., Casey B.J. The role of ventral frontostriatal circuitry in reward-based learning in humans. J. Neurosci. 2005;25(38):8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., Humphreys K.L., Goff B., Shapiro M., Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredeback G., Melinder A. Infants’ understanding of everyday social interactions: a dual process account. Cognition. 2010;114(2):197–206. doi: 10.1016/j.cognition.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Guerra P., Sanchez-Adam A., Anllo-Vento L., Ramirez I., Vila J. Viewing loved faces inhibits defense reactions: a health-promotion mechanism? PloS One. 2012;7(7) doi: 10.1371/journal.pone.0041631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess E.H., Polt J.M. Pupil size as related to interest value of visual stimuli. Science. 1960;132(3423):349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Hess E.H., Polt J.M. Pupil size in relation to mental activity during simple problem-solving. Science. 1964;143(3611):1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Hostinar C., Sullivan R.M., Gunnar M.R. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol. Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I., Sirois S. Infant cognition: going full factorial with pupil dilation. Dev. Sci. 2009;12(4):670–679. doi: 10.1111/j.1467-7687.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Just M.A., Carpenter P.A. The intensity dimension of thought: pupillometric indices of sentence processing. Can. J. Exp. Psychol. 1993;47(2):310. doi: 10.1037/h0078820. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Prentice-Hall; Englewood Cliffs, NJ: 1973. Attention and Effort. [Google Scholar]
- Karson C. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Karson C.N., Dykman R.A., Paige S.R. Blink rates in schizophrenia. Schizophr. Bull. 1990;16(2):345. doi: 10.1093/schbul/16.2.345. [DOI] [PubMed] [Google Scholar]
- Kegeles L.S., Abi-Dargham A., Frankle W.G., Gil R., Cooper T.B., Slifstein M., Laruelle M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kirkham N.Z., Slemmer J.A., Richardson D.C., Johnson S.P. Location, location, location: development of spatiotemporal sequence learning in infancy. Child Dev. 2007;78(5):1559–1571. doi: 10.1111/j.1467-8624.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- Kleven M.S., Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J. Pharmacol. Exp. Ther. 1996;279:1211–1219. [PubMed] [Google Scholar]
- Lackner C.L., Bowman L.C., Sabbagh M.A. Dopaminergic functioning and preschoolers’ theory of mind. Neuropsychologia. 2010;48:1767–1774. doi: 10.1016/j.neuropsychologia.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Landry S.H., Smith K.E., Miller-Loncar C.L., Swank P.R. Predicting cognitive-language and social growth curves from early maternal behaviors in children at varying degrees of biological risk. Dev. Psychol. 1997;33(6):1040–1053. doi: 10.1037//0012-1649.33.6.1040. [DOI] [PubMed] [Google Scholar]
- Landry S.H., Smith K.E., Swank P.R. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Dev. Psychol. 2006;42(4):627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- Lewis M., Coates D.L. Mother-infant interaction and cognitive development in twelve-week-old infants. Inf. Behav. Dev. 1980;3:95–105. [Google Scholar]
- Lewis M., Goldberg S. Perceptual-cognitive development in infancy: a generalized expectancy model as a function of the mother-infant interaction. Merrill-Palmer Q Behav. Dev. 1969;15(1):81–100. [Google Scholar]
- Maccoby E.E. The role of parents in the socialization of children: an historical overview. Dev. Psychol. 1992;28(6):1006–1017. [Google Scholar]
- May J.C., Delgado M.R., Dahl R.E., Stenger V.A., Ryan N.D., Fiez J.A., Carter C.S. Event-related functional magnetic resonance imaging or reward-related brain circuitry in children and adolescents. Biol. Psychiatry. 2004;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mesman J., van IJzendoorn M.H., Bakermans-Kranenburg M.J. The many faces of the still-face paradigm: a review and meta-analysis. Dev. Rev. 2009;29(2):120–162. [Google Scholar]
- Messinger D.S., Cassel T.D., Acosta S.I., Ambadar Z., Cohn J.F. Infant smiling dynamics and perceived positive emotion. J. Nonverbal Behav. 2008;32(3):133. doi: 10.1007/s10919-008-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C., Sandor P.S., Landis T., Fathi M., Brugger P. Blinking and schizotypal thinking. J. Psychopharmacol. 2005;19(5):513–520. doi: 10.1177/0269881105056538. [DOI] [PubMed] [Google Scholar]
- Moore G.A., Hill-Soderlund A.L., Propper C.B., Calkins S.D., Mills-Koonce W.R., Cox M.J. Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Dev. 2009;80(1):209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakato E., Otsuka Y., Kanazawa S., Yamaguchi M.K., Honda Y., Kakigi R. I know this face: neural activity during mother’s face perception in 7- to 8-month old infants as investigated by near-infrared spectroscopy. Early Hum. Dev. 2011;87(1):1–7. doi: 10.1016/j.earlhumdev.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Nyström P., Gredebäck G., Bölte S., Falck-Ytter T. Hypersensitive pupillary light reflex in infants at risk for autism. Mol. Autism. 2015;6(1):10. doi: 10.1186/s13229-015-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J.P., Dayan P., Friston K., Critchley H., Dolan R.J. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P., Buchanan T.W., Seymour B., Dolan R.J. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Oakes L.M. Using habituation of looking time to assess mental processes in infancy. J. Cogn. Dev. 2010;11(3):255–268. doi: 10.1080/15248371003699977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J., Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954;47(6):419. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olson S.L., Bates J.E., Bayles K. Mother-infant interaction and the development of individual differences in children’s cognitive competence. Dev. Psychol. 1984;20(1):166–179. [Google Scholar]
- Pavlov I.P. Oxford University Press; Oxford: 1927. Conditioned Reflexes: an Investigation of the Physiological Activity of the Cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit G.S., Bates J.E., Dodge K.A. Supportive parenting, ecological context, and children’s adjustment: a seven-year longitudinal study. Child Dev. 1997;68(5):908–923. doi: 10.1111/j.1467-8624.1997.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Phillips M.A., Szabadi E., Bradshaw C.M. Comparison of the effects of clonidine and yohimbine on spontaneous pupillary fluctuations in healthy human volunteers. Psychopharmacol. 2000;150(1) doi: 10.1007/s002130000398. [DOI] [PubMed] [Google Scholar]
- Pitts S.M., Horvitz J.C. Similar effects of D1/D2 receptor blockade on feeding and locomotor behavior. Pharmacol. Biochem. Behav. 2000;65(3):433–438. doi: 10.1016/s0091-3057(99)00249-x. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Fan J. Attention as an organ system. In: Pomerantz J., editor. Topics in Integrative Neuroscience: from Cells to Cognition. Cambridge University Press; London): 2008. pp. 31–61. [Google Scholar]
- Preuschoff K., Marius’t Hart B., Einhauser W. Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Front. Neurosci. 2011:5. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig M.V., Rose J., Schmidt R., Freund N. Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents and birds. Front. Neural Circ. 2014:8. doi: 10.3389/fncir.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J., Kubiak P., Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res. Bull. 1994;35(5):607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Samuels E.R., Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic functions part I: principles of functional organization. Curr. Neuropharmacol. 2008;6(3):235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10(3):211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J. Neurophysiol. 1986;56:1439–1462. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct. 2010;6(1):24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Apicella P., Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Dayan P., Montague P.R. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shultz S., Klin A., Jones W. Inhibition of eye blinking reveals subjective perceptions of stimulus salience. Proc. Nat. Acad. Sci U. S. A. 2011;108(52):21270–21275. doi: 10.1073/pnas.1109304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois S., Brisson J. Pupillometry. Wiley Interdiscip. Rev. Cogn. Sci. 2014;5(6):679–692. doi: 10.1002/wcs.1323. [DOI] [PubMed] [Google Scholar]
- Skinner B.F. D. Appleton-Century Company; New York, London: 1938. The Behavior of Organisms: an Experimental Analysis. [Google Scholar]
- Skolnick A.J., Davidson R.J. Affective modulation of eyeblink startle with reward and threat. Psychophysiology. 2002;39(6):835–850. doi: 10.1111/1469-8986.3960835. [DOI] [PubMed] [Google Scholar]
- Sorce J.F., Emde R.N., Campos J.J., Klinnert M.D. Maternal emotional signaling: its effect on the visual cliff behavior of 1-year-olds. Dev. Psychol. 1985;21(1):195–200. [Google Scholar]
- Sterpenich V., D’Argembeau A., Desseilles M., Balteau E., Albouy G., Vandewalle G., Maquet P. The locus coeruleus is involved in the successful retrieval of emotional memories in humans. J. Neurosci. 2006;26(28):7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda C.S., Bornstein M.H., Baumwell L. Maternal responsiveness and children’s achievement of language milestones. Child Dev. 2001;72(3):748–767. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda C.S., Adolph K.E., Lobo S.A., Karasik L.B., Ishak S., Dimitropoulou K.A. When infants take mothers’ advice: 18-month-olds integrate perceptual and social information to guide motor action. Dev. Psychol. 2008;44(3):734–746. doi: 10.1037/0012-1649.44.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.R., Elsworth J.D., Lawrence M.S., Sladek J.R., Roth R.H., Redmond D.E. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp. Neurol. 1999;158:214–220. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev. Psychobiol. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Shapiro M., Telzer E.H., Humphreys K.L. Amygdala response to mother. Dev. Sci. 2012;15(3):307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E., Als H., Adamson L., Wise S., Brazelton T.B. The infant’s response to entrapment between contradictory messages in face-to-face interaction. Pediatrics. 1978;62(3):403. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Tummeltshammer K.S., Kirkham N.Z. Learning to look: probabilistic variation and noise guide infants’ eye movements. Dev. Sci. 2013;16(5):760–771. doi: 10.1111/desc.12064. [DOI] [PubMed] [Google Scholar]
- Tummeltshammer K.S., Mareschal D., Kirkham N.Z. Infants’ selective attention to reliable visual cues in the presence of salient distractors. Child Dev. 2014;85(5):1981–1994. doi: 10.1111/cdev.12239. [DOI] [PubMed] [Google Scholar]
- Wang Q., Bolhuis J., Rothkopf C.A., Kolling T., Knopf M., Triesch J. Infants in control: rapid anticipation of action outcomes in a gaze-contingent paradigm. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0030884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werchan D.M., Collins A.G., Frank M.J., Amso D. 8-month-old infants spontaneously learn and generalize hierarchical rules. Psychol. Sci. 2015;26(6):805–815. doi: 10.1177/0956797615571442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werchan D.M., Collins A.G., Frank M.J., Amso D. Role of prefrontal cortex in learning and generalizing hierarchical rules in 8-month-old infants. J. Neurosci. 2016;36(40):10314–10322. doi: 10.1523/JNEUROSCI.1351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A., Braver T.S. Dopamine does double duty in motivating cognitive effort. Neuron. 2016;89:695–710. doi: 10.1016/j.neuron.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm B., Wilhelm H., Ludkte H. Pupillography: principles and applications in basic and clinical research. In: Kuhlmann J., Bottcher M., editors. Pupillography: Principles, Methods and Applications. Zuckschwerdt Verlag; Munchen: 1999. pp. 1–11. [Google Scholar]
- Zametkin A.J., Stevens J.R., Pittman R. Ontogeny of spontaneous blinking and of habituation of the blink reflex. Ann. Neurol. 1979;5(5):453–457. doi: 10.1002/ana.410050509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.