Abstract
BACKGROUND.
Chronic systemic inflammation has been positively associated with structural and functional brain changes representing early markers of Alzheimer’s Disease (AD) and cognitive decline. The current study examined associations between systemic inflammation and cognitive performance among African Americans and Whites urban adults.
METHODS.
Participants were selected from the Health Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study (2004-2013, baseline age: 30-64y, mean±SD follow-up time of 4.64±0.93y, N=189-222, k=1.5-1.7 observations/participant). Cytokines known to be positively linked to AD incidence among others were tested against cross-sectional and longitudinal cognitive function, stratifying by age group (≤50y vs. >50y), sex and race. A series of mixed-effects regression models were conducted, adjusting for key confounders.
RESULTS.
Among key findings, IL1β was positively associated with a faster rate of decline on a test of executive functioning, among older adults (age>50y, γ11=+2.49±0.89, p=0.005), while in the total population, IL-6 was linked to a faster decline on a test of verbal memory (γ11=−0.011±0.004, p=0.009). Among younger participants, IL-18 was linked to a poorer performance on a test of attention at baseline (age≤50y, γ01=−0.007±0.0025, p=0.004) though a slower rate of decline with higher IL-18 was detected for a test of psychomotor speed in older adults (age>50y, γ11=+0.0010±0.0004, p=0.008). Finally, among Whites, unlike among African-Americans, IL-6 was associated with a better baseline performance on two tests of verbal and working memory.
CONCLUSIONS.
Cytokines were shown to be associated with age-related cognitive decline among middle-aged and older urban adults in an age group and race-specific manner. Further longitudinal studies are needed to replicate our findings and mediation through relevant biological and psychosocial factors need to be studied as well.
Keywords: Cytokines, inflammation, cognitive decline, aging, urban adults
INTRODUCTION
Chronic systemic inflammation has been positively associated with structural and functional brain changes representing early markers of Alzheimer’s Disease (AD) and cognitive decline during normal aging (Arfanakis et al., 2013; Bettcher et al., 2012; Corlier et al., 2018; Gu et al., 2018; Hoshi et al., 2010; Jefferson et al., 2007; Satizabal et al., 2012; Taki et al., 2013; Wada et al., 2011; Walker et al., 2017; Warren et al., 2018; Wersching et al., 2010). Recent meta-analyses have implicated inflammatory biomarkers in the onset and progression of neurodegenerative processes (Bradburn et al., 2017; Darweesh et al., 2018a; Gorelick, 2010; Lai et al., 2017). To date, studies examining the link between inflammation and cognitive health have focused primarily on C-reactive protein (CRP). This may be due, in part, to CRP’s documented utility as a non-specific proxy for inflammatory processes (Black et al., 2004). However, the expression of CRP is largely determined by interleukin 6 (IL-6) and other proinflammatory cytokines (Black et al., 2004). Relatively few studies have examined the relationship between the interleukins (IL) and cognitive health. Those that have were limited by their measures of cognitive health (Palta et al., 2015; Tegeler et al., 2016); they did not differentiate between proinflammatory and antiinflammatory markers (Jordanova et al., 2007; Metti et al., 2014); and they had for the most part a cross-sectional design (Trollor et al., 2012; Trollor et al., 2010). Further, several studies have produced mixed results which may be due, in part, to differential sample selection and population heterogeneity (Jefferson et al., 2007; Satizabal et al., 2012). Thus, despite the promise of using the interleukins diagnostically as early markers of Alzheimer’s Disease and related dementias (ADRD) (Hull et al., 1996), it remains unclear whether and to what extent these markers are associated with cognitive function and decline during normal aging, which domains of cognition are affected the most, and whether those relationships can vary by age group, sex and race. In fact, with possibly one exception,(Simpson et al., 2013) none of the studies previously showing an association between cytokines such as IL-6 and cognitive performance or change in specific domains of cognition have systematically stratified their sample by age group, race and/or sex (Elderkin-Thompson et al., 2012; Frydecka et al., 2015; Heringa et al., 2014; Marsland et al., 2006; Mooijaart et al., 2013; Simpson et al., 2013). Recent meta-analyses suggests that among the various cytokines which were available in our present study, IL-1β, IL-6 and IL-18 (and not IL-10 or IL-12) were shown to be adversely related to cognitive function, while increasing the risk of all-cause and AD dementia (Bradburn et al., 2017; Darweesh et al., 2018a; Gorelick, 2010; Lai et al., 2017). Whereas the vast majority of the primary studies included in these reviews did not compare findings across key socio-demographic groups, to our knowledge, only a handful of studies reported differential associations between cytokines and cognitive performance across sex, race and/or age (Canon and Crimmins, 2011; Goldstein et al., 2015; Simpson et al., 2013; Yaffe et al., 2003). Three of these studies were cross-sectional,(Canon and Crimmins, 2011; Goldstein et al., 2015; Simpson et al., 2013) while the only prospective cohort study among them utilized a single global measure (3MS) for cognitive assessment (Yaffe et al., 2003).
Furthermore, it is important to assess those stratum-specific associations given that various inflammatory markers have been shown to vary by age, sex and race groups (Allison et al., 2006; Aulock et al., 2006; Chapman et al., 2009; Ferrucci et al., 2005; Lu et al., 2017; McCarrey et al., 2016b; Mehta and Yeo, 2017; Miles et al., 2001; O'Connor et al., 2007; Ranjit et al., 2007; Slopen et al., 2010; Stepanikova et al., 2017; Weuve et al., 2018; Yaffe et al., 2013) Specifically, one study has shown that men had more elevated levels of IL-6 and IL-1β compared to women in response to an immune challenge,(Aulock et al., 2006), while IL-6 was generally shown to be higher among women in other studies(Chapman et al., 2009; O'Connor et al., 2007). Nevertheless, it was shown that cytokines including IL-6 and IL-18 tended to increase with age among both men and women (Ferrucci et al., 2005). It was more consistently shown that African-Americans had higher levels of systemic inflammation compared to Whites, including a higher level of IL-6 (Allison et al., 2006; Ranjit et al., 2007; Slopen et al., 2010; Stepanikova et al., 2017). In addition, various cognitive aging measures, including prevalent and incident dementia, were shown to differ across those same socio-demographic factors (McCarrey et al., 2016b; Mehta and Yeo, 2017; Miles et al., 2001; Weuve et al., 2018; Yaffe et al., 2013). For instance, men were shown to have accelerated decline on measures of mental status, perceptuomotor speed and integration, and visuospatial ability compared to women (McCarrey et al., 2016a). More consistently, it was shown that African-Americans had worse cognitive outcomes than Whites that were for the most part explained by lower socio-economic status (Mehta and Yeo, 2017; Miles et al., 2001; Weuve et al., 2018; Yaffe et al., 2013).
The current study examined associations between systemic inflammation and cognitive performance among African Americans and Whites urban adults participating in the Health Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Cytokines known to be positively linked to AD incidence among others (IL-1β, IL-6 and IL-18) were tested against cross-sectional and longitudinal cognitive function, stratifying by age group, sex and race.
MATERIALS AND METHODS
Database
HANDLS is a prospective cohort study that was initiated in 2004, with primary focus on cardiovascular disease and cognitive aging in the context of health disparities, by recruiting an ethnically and socio-economically diverse urban adult population. An area probability sampling strategy was adopted to recruit African American and White urban adults (baseline age: 30-64y) both above and below poverty who resided within thirteen Baltimore city, MD neighborhoods (Evans et al., 2010). Our present study extracted data from baseline (visit 1, 2004-2009) and the first follow-up examination (visit 2; 2009-2013), with a follow-up period ranging between <ly and ~8y, mean±SD of 4.64±0.93y. Data collected included an extensive battery of cognitive tests measured both at visits 1 and 2 and markers of inflammation measured at visit 1. As part of the study protocol, written informed consent was obtained from all participants who were also provided with a booklet and a video explaining key study procedures. The National Institute on Environmental Health Sciences Institutional Review Board of the National Institutes of Health approved the study protocol.
Study sample
The original sample of HANDLS consisted of 3,720 participants (Phase I, visit 1). At Phase II of visit 1 (or the Medical Research Vehicle (MRV) baseline visit), biochemical indices and cognitive performance data were obtained from a sub-set of the total Phase I sample. Specifically, sample sizes varied for the cognitive tests between 2,044 and 2,582 at baseline and between 1,728 and 2,139 for visit 2 follow-up MRV visits. However, the serum samples used for cytokine analysis were collected at the baseline MRV visit from HANDLS participants who were selected for studying DNA repair and age-related changes in microRNA. More details are provided elsewhere (Noren Hooten et al., 2012; Noren Hooten et al., 2013). This sample consisted of 244-249 participants with IL-1β, IL-6 or IL18 data available. Consequently, the final analytic sample was determined based on mutual exposure and covariate non-missingness at baseline and cognitive performance measure completeness at either visit (N=189-222, k=1.5-1.7) and is summarized in Figure SI.
Cognitive assessment
Cognitive performance was assessed with 8 tests from which 11 test scores were computed, 7 distinctive domains were identified (Global, attention, learning/memory, executive function, visuo-spatial/visuo-construction ability, psychomotor speed, language/verbal). Those were the Mini-Mental State Examination (MMSE), the California Verbal Learning Test (CVLT) immediate (List A) and Delayed Free Recall (DFR), Digit Span Forward and Backwards tests (DS-F and DS-B), the Benton Visual Retention Test (BVRT), Animal Fluency test (AF), Brief Test of Attention (BTA), Trails A and B and the Clock Drawing Test (CDT) (Supplemental method 1). Following a probe for understanding the protocol, HANDLS participants undergoing examination at the MRV were able to complete informed consent. Despite the lack of dementia diagnosis, all participants were screened using the MMSE as a global mental status test, which for the most part they completed successfully (total score ≥24). In cases where MMSE was low (~6.6% were <24 at visit 1 and 1.9% at visit 2), the cause was judged to be poor literacy rather than dementia.
Cytokines
The IL cytokines were assayed by Aushon (https://www.aushon.com/). Aushon Ciraplex® ULTRA Ultrasensitive Assays combine the power of multiplexing and ultrasensitivity with femtogram/ml (fg/ml) levels of detection. The serum samples were collected from HANDLS participants for studies of DNA repair and age-related microRNA changes (Noren Hooten et al., 2012; Noren Hooten et al., 2013). As stated earlier, cytokines selected for this analysis included IL-1β, IL-6 and IL-18 (in pg/mL) (thus excluding IL-10 and IL-12), which were shown to be adversely related to cognitive function and increase risk of all-cause and AD dementia based on several recent meta-analyses (Bradburn et al., 2017; Darweesh et al., 2018a; Gorelick, 2010; Lai et al., 2017).
Covariates
Covariates were included based on prior evidence of their association with main outcome of interest, mainly cognitive decline (Barnes and Yaffe, 2011). Socio-demographic characteristics included baseline age, sex, race (White vs. African American), marital status (married vs. not), educational attainment (<High School (HS); HS, >HS) and poverty income ratio (PIR<125% for “poor”), employment status (employed vs. not) and a continuous score reflecting literacy, the Wide Range Achievement Test (WRAT). Among lifestyle and health-related factors, analyses tested potential confounding effects of measured body mass index (BMI, kg/m2), opiate, marijuana or cocaine use (“current” vs. “never or former”), smoking status (“current” vs. “never or former”). Depressive symptoms were measured using the 20-item Center for Epidemiological Studies-Depression scale (CES-D) and the total score was considered as a potential confounder as well. (See Supplemental Method 1) The overall quality of diet was operationalized using the total score on the Healthy Eating Index (HEI-2010), averaging two 24-hr recalls that were administered at the baseline visit (v1). The computation of the total HEI-2010 score and each of its 12 components are outline in: http://appliedresearch.cancer.gov/tools/hei/tools.html and http://handls.nih.gov/06Coll-dataDoc.html. Finally, other health-related baseline covariates that were considered as potential confounders included self-reported history of type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease (stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation), inflammatory disease (multiple sclerosis, systemic lupus, gout, rheumatoid arthritis, psoriasis, thyroid disorder and Crohn’s disease) and use of NSAIDs (prescription and over-the-counter) over the past two weeks, were considered as covariates, as was done in previous studies (Bettcher et al., 2012; Gimeno et al., 2009). Moreover, a sensitivity analysis was conducted by adding alcoholic beverage consumption as an additional covariate into the models. This covariate was centered and imputed at its mean when missing to obtain the same sample size for the full and the reduced models.
Statistical analysis
Analyses were conducted using Stata release 15.0 (STATA, 2017). Design complexity was accounted for by inclusion of sampling weights for estimating population means and proportions. Linear regression models (svy:reg) were used to contrast means across key stratifying variables (e.g. age/sex or race), while distributions of categorical variables across those socio-demographic groups were compared using svy:tab and design-based F-tests. A series of mixed-effects regression models with 11 continuous cognitive test score as outcomes were conducted and each of 3 cytokines as a separate exposure. Each model included the TIME variable, reflecting years elapsed between waves of data, which was entered as a fixed and random effect (along with the intercept), interacting it with key covariates including the main exposure variable, namely the 3 cytokines of interest. Assuming that the outcome was missing at random with repeated measures of ~1.5-1.7 visits/person, the models also took into account variability in follow-up time (See Supplemental Methods 2) (Ibrahim and Molenberghs, 2009). All socio-demographic covariates were included in the models. Thus, rather than using scores already standardized by age and education, we have included age, education, literacy as well as other potentially confounding factors in the linear mixed model both to adjust the intercept (baseline performance) and the slope (annual rate of change), to be consistent with other similar analyses (Beydoun et al., 2018a; Beydoun et al., 2018c). Additional covariates were selected if they were found to be associated with each cytokine exposure at a type I error level of 0.05 based on a separate OLS regression model. Furthermore, selected significant findings from mixed-effects linear regression models were visualized using predictive margins of outcomes which were standardized as z-scores along with the exposures (i.e. cytokines) which were estimated and plotted across TIME (y), stratifying by cytokine levels (−1=mean – 1 SD, 0=mean, 1=mean + 1 SD).
Moderating effects by sex, age group or race were tested by adding 2-way and 3-way interaction terms between exposure, the effect modifier and TIME. Models were also stratified by sex, age group and race separately (Supplemental method 2), as various inflammatory markers have been shown to vary by age, sex and race groups (Lu et al., 2017).
Selection bias due to systematic differences on major characteristics between the selected sample and those excluded from the target population can occur. Accounting for this bias can be achieved in mixed-effect regression models through a 2-stage Heckman selection process. During the first sage, a binary outcome (selected=1 vs. unselected=0) is entered into a probit model and predicted by age, sex, race and poverty income ratio along with educational attainment. The predicted probability of being selected conditional on those characteristics is then used to compute an inverse mills ratio. At a second stage, this inverse mills ratio is entered into the final mixed-effects regression model as a covariate, as was done in prior studies (Beydoun et al., 2013).
Type I errors of 0.05 and 0.10 were chosen for main effects and interaction terms, respectively, (Selvin, 2004) prior to correcting for multiple testing. Correction for multiple testing was done using a familywise Bonferroni procedure by taking into account cognitive test multiplicity while assuming each exposure as a distinctive substantive hypothesis (Hochberg, 1987). Thus, a p<0.0045 (0.05/11) was considered significant for main effects, while a critical p-value was reduced to (0.10/11=0.0090) for 2-way interaction terms. Finally, for 3-way interaction terms, critical p-value were reduced to 0.05, an overall approach similar to previous studies.(Beydoun et al., 2016)
Data Availability:
Data are available upon request to researchers with valid proposals who agree to the confidentiality agreement as required by our Institutional Review Board. We publicize our policies on our website https://handls.nih.gov. Requests for data access may be sent to the PIs or the study manager, Jennifer Norbeck at norbeckje@mail.nih.gov. These data are owned by the National Institute on Aging at the National Institutes of Health. The Principal Investigators, have restricted public access to these data because (1) the study collects medical, psychological, cognitive, and psychosocial information on racial and poverty differences that could be misconstrued or willfully manipulated to promote racial discrimination; and (2) although the sample is fairly large, there are sufficient identifiers that the PIs cannot guarantee absolute confidentiality for every participant as we have stated in acquiring our confidentiality certificate.
RESULTS
Tables 1 summarizes selected baseline study characteristics, stratified by age group, sex and race. Lower education attainment was observed among older participants compared to their younger counterparts, a difference also noted for African-Americans when compared to Whites. Other important differentials by race include lower literacy (WRAT total score), income and employment rates among African-Americans, a higher likelihood of current smoking and drug use among younger participants and African-Americans, a higher mean BMI in the older group, and a higher mean HEI-2010 reflecting better overall dietary quality among Whites. Older participants were also more likely than their younger counterparts to have co-morbid conditions such as type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease. Furthermore, compared to Whites, African-Americans had a higher prevalence of hypertension and cardiovascular disease and a more elevated mean IL-6.
Table 1.
Selected baseline (Visit 1) and time-dependent study participant characteristics by age group and sex for HANDLS participants with complete and reliable baseline MMSE scores and cytokine measures (N=195)a
| All | Younger (≤50y) |
Older (>50y) |
PAgeb | Women | Men | Psexb | Whites | African- Americans |
Praceb | |
|---|---|---|---|---|---|---|---|---|---|---|
| %±SE | (N=195) | 62.0±5.0 (N=97) |
38.0±5.0 (N=98) |
65.0±5.0 (N=127) |
35.0±5.0 (N=68) |
29.0±5.0 (N=63) |
71.0±4.0 (N=132) |
|||
| Age at baseline, y | 46.90±1.00 (N=195) |
40.38±0.82 (N=97) |
57.67±0.64 (N=98) |
<0.001 | 46.7±1.31 (N=127) |
47.29±1.49 (N=68) |
0.78 | 46.14±1.81 (N=63) |
47.22±1.21 (N=132) |
0.62 |
| Sex, % male | 35.0±0.05 (N=195) |
35.0±7.0 (N=97) |
33.0±7.0 (N=98) |
0.84 | — | — | 40.8±8.0 (N=63) |
32.1±6.4 (N=132) |
0.39 | |
| Race, % African-American | 70.9±4.5 (N=195) |
72.6±6.3 (N=97) |
68.1±6.0 (N=98) |
73.6±5.5 (N=127) |
65.7±7.7 (N=68) |
0.40 | ||||
| Married, % | 30.1±5.5 (N=176) |
27.8±7.5 (N=90) |
34.4±7.3 (N=86) |
0.52 | 31.8±7.3 (N=120) |
26.4±7.5 (N=56) |
0.60 | 38.0±8.9 (N=57) |
26.9±6.8 (N=119) |
0.31 |
| Education, % | ||||||||||
| <HS | 3.8±1.6 | 0.3±0.2 | 9.7±4.2 | 0.017 | 4.7±2.4 | 2.2±1.6 | 0.59 | 1.7±1.5 | 4.7±2.3 | <0.001 |
| HS | 52.7±5.7 | 57.2±8.1 | 45.2±6.9 | 53.2±7.5 | 51.6±8.6 | 26.1±6.7 | 63.6±7.3 | |||
| >HS | 43.0±5.7 | 42.5±8.2 | 43.9±7.0 | 42.0±7.5 | 44.9±8.6 | 70.7±6.9 | 31.7±7.2 | |||
| Missing | 0.4±0.5 (N=195) |
0.0 (N=97) |
1.2±1.2 (N=98) |
0.0 (N=127) |
1.3±1.3 (N=68) |
1.5±1.6 (N=63) |
0.0 (N=132) |
|||
| Literacy (WRAT score) | 44.1±0.7 (N=193) |
44.4±0.9 (N=95) |
43.7±1.3 (N=98) |
0.65 | 43.6±0.9 (N=126) |
45.0±1.1 (N=67) |
0.31 | 49.26±0.73 (N=62) |
41.99±0.9 (N=131) |
<0.001 |
| PIR <125%, % | 23.7±36.2 (N=195) |
21.5±4.8 (N=97) |
27.2±5.2 (N=98) |
0.42 | 22.0±4.5 (N=127) |
26.8±6.0 (N=68) |
0.53 | 8.3±2.9 (N=63) |
30.0±5.3 (N=132) |
<0.001 |
| Employment, % | ||||||||||
| Yes | 46.4±5.7 | 56.0±8.1 | 30.7±6.0 | 0.038 | 47.2±7.5 | 44.4±8.8 | 0.27 | 60.0±7.6 | 40.9±7.3 | 0.011 |
| Missing | 5.7±2.1 (N=195) |
4.9±2.2 (N=97) |
7.1±4.0 (N=98) |
3.2±1.2 (N=127) |
45.0±8.4 (N=68) |
12.2±4.6 (N=63) |
3.1±2.1 (N=132) |
|||
| Current smoking status, % | ||||||||||
| Currently smoking | 43.2±5.7 | 49.0±8.2 | 33.8±6.6 | 0.28 | 42.1±4.2 | 45.2±8.7 | 0.69 | 24.4±6.3 | 51.0±7.3 | 0.019 |
| Missing | 3.3±1.6 (N=195) |
3.1±2.2 (N=97) |
3.8±2.0 (N=98) |
4.2±2.3 (N=127) |
1.7±1.3 (N=68) |
4.3±2.5 (N=63) |
2.9±1.9 (N=132) |
|||
| Current use of illicit drags, % | ||||||||||
| Used any type | 46.4±5.7 | 54.4±8.2 | 33.3±6.3 | 0.028 | 41.0±7.3 | 56.8±8.4 | 0.21 | 37.4±8.5 | 50.2±7.3 | <0.001 |
| Missing | 10.8±2.7 (N=195) |
12.9±3.9 (N=97) |
7.2±3.1 (N=98) |
10.0±3.3 (N=127) |
12.1±4.5 (N=68) |
28.8±7.0 (N=63) |
3.4±2.0 (N=132) |
|||
| Body mass index, kg.m−2 | 30.8±0.99 (N=195) |
29.25±1.36 (N=97) |
33.29±1.21 (N=98) |
0.027 | 31.79±1.36 (N=127) |
28.86±1.17 (N=68) |
0.10 | 28.7±1.04 (N=63) |
31.63±1.31 (N=132) |
0.081 |
| HEI-2010 total score | 44.36±1.37 (N=160) |
43.84±1.94 (N=82) |
45.26±1.62 (N=78) |
0.60 | 43.91±1.83 (N=105) |
45.24±1.78 (N=55) |
0.58 | 48.24±2.09 (N=50) |
42.97±1.66 (N=110) |
0.049 |
| Depressive symptoms | ||||||||||
| CES-D score | 15.08±1.42 (N=193) |
14.8±2.09 (N=97) |
15.55±1.55 (N=96) |
0.77 | 15.12±1.94 (N=125) |
15.01±1.92 (N=68) |
0.97 | 12.38±1.99 (N=61) |
16.15±1.79 (N=132) |
0.16 |
| Diabetes, % | 20.0±4.6 (N=174) |
6.6±4.3 (N=83) |
41.4±7.5 (N=91) |
<0.001 | 22.7±6.0 (N=115) |
15.4±7.1 (N=59) |
0.46 | 13.9±5.4 (N=49) |
22.2±5.7 (N=125) |
0.31 |
| Hypertension, % | 54.9±6.3 (N=169) |
43.3±9.4 (N=80) |
72.2±5.9 (N=89) |
0.010 | 59.0±8.0 (N=112) |
46.9±9.6 (N=57) |
0.33 | 35.1±9.4 (N=46) |
60.0±7.4 (N=123) |
0.043 |
| Dyslipidemia, % | 25.3±5.0 (N=169) |
11.9±5.5 (N=80) |
45.3±7.5 (N=89) |
0.002 | 24.9±6.2 (N=112) |
26.0±8.5 (N=57) |
0.92 | 26.2±8.9 (N=46) |
25.0±5.9 (N=123) |
0.91 |
| Cardiovascular diseasec, % | 21.6±4.6 (N=174) |
9.2±4.7 (N=83) |
40.8±7.4 (N=91) |
0.001 | 20.7±5.6 (N=115) |
23.3±7.9 (N=59) |
0.79 | 7.4±3.7 (N=49) |
25.9±5.9 (N=125) |
0.011 |
| Inflammatory conditionsd, % | 14.2±4.6 (N=174) |
10.8±6.5 (N=83) |
19.5±6.2 (N=91) |
0.37 | 13.2±6.0 (N=115) |
16.2±7.0 (N=59) |
0.74 | 10.7±4.2 (N=49) |
15.3±5.8 (N=125) |
0.52 |
| NSAIDSe, % | 32.5±5.9 (N=176) |
30.0±8.7 (N=83) |
36.3±6.8 (N=93) |
0.58 | 35.0±7.9 (N=116) |
27.8±7.8 (N=60) |
0.52 | 27.8±9.6 (N=51) |
34.0±7.1 (N=125) |
0.61 |
| IL-1β | 3.4±1.24 (N=195) |
3.96±1.96 (N=97) |
2.48±0.58 (N=98) |
0.47 | 4.4±1.88 (N=127) |
1.52±0.24 (N=68) |
0.13 | 1.55±0.31 (N=63) |
4.16±1.74 (N=132) |
0.14 |
| IL-6 | 12.21±1.37 (N=195) |
10.96±1.49 (N=97) |
14.27±2.68 (N=98) |
0.28 | 10.37±0.97 (N=127) |
15.68±3.27 (N=68) |
0.12 | 8.76±1.03 (N=63) |
13.62±1.85 (N=132) |
0.023 |
| IL-18 | 157.43±11.86 (N=195) |
145.78±15.79 (N=97) |
176.63±17.01 (N=98) |
0.18 | 141.87±12.11 (N=127) |
186.74±22.39 (N=68) |
0.078 | 149.9±12.1 (N=63) |
160.51±16.0 (N=132) |
0.59 |
Key: CES-D=Center for Epidemiologic Studies-Depression; MMSE=Mini-Mental State Examination; PIR=poverty income ratio; WRAT=Wide Range Achievement Test.
Values are weighted mean±SEM or percent±SEP. Largest sample size is N=195.
P-value was based on linear regression models when row variable is continuous (svy:reg) with sex/age group/race and design-based F-test when row variable is categorical (svy:tab).
Cardiovascular disease include self-reported stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation.
Inflammatory conditions include multiple sclerosis, systemic lupus, gout, rheumatoid arthritis, psoraiasis, thyroid disorder and Crohn’s disease.
Non-steroidal anti-inflammatory drugs (NSAIDS) include over the counter and prescription drugs in that category.
Table 2 displays results for age, sex and race differentials in cognitive performance within each wave of data, as well as between wave differentials. Most notably, marked racial disparities were observed in cognitive performance, which persisted across waves of data, reflecting poorer performance among African-Americans, for most tests. Out of the 11 selected cognitive test scores, marked decline was detected only in two within the overall population (CVLT-List A and DFR) within a marginally significant decline observed for BVRT (P=0.05), mostly detected in women and among African-Americans.
Table 2.
Cognitive performance test scores at baseline (Visit 1), follow-up (Visit 2), and change between visits, by age group, sex and race, for HANDLS participants with complete and reliable baseline and/or follow-up cognitive scores and complete data on cytokinesa
| All | Younger (≤50y) |
Older (>50y) |
Women | Men | Whites | African-Americans | |
|---|---|---|---|---|---|---|---|
| Mini-Mental State Exam, total score | |||||||
| Visit 1 | 27.733±0.221 (N=195) |
27.898±0.259 (N=97) |
27.461±0.398 (N=98) |
27.713±0.307 (N=127) |
27.771±0.269 (N=68) |
28.681±0.196d
(N=63) |
27.344±0.294 (N=132) |
| Visit 2 | 28.104±0.23 (N=138) |
27.967±0.316 (N=76) |
28.392±0.233 (N=62) |
27.958±0.323 (N=93) |
28.381±0.221 (N=45) |
29.06±0.17d
(N=44) |
27.677±0.303 (N=94) |
| P (Visit2-Visit1) | 0.25 | 0.87 | 0.045 | 0.58 | 0.081 | 0.15 | 0.43 |
| California Verbal Learning Test (CVLT), List A | |||||||
| Visit 1 | 25.248±0.758 (N=167) |
26.208±1.107 (N=84) |
23.664±0.785 (N=83) |
26.088±1.023 (N=104) |
23.919±0.957 (N=63) |
29.377±1.429d
(N=46) |
23.85±0.798 (N=121) |
| Visit 2 | 20.422±0.754 (N=140) |
21.291±1.036 (N=73) |
18.869±1.006 (N=67) |
20.232±1.03 (N=95) |
20.772±1.005 (N=45) |
24.015±1.126d
(N=46) |
18.709±0.907 (N=94) |
| P (Visit2-Visit1) | <0.001 | 0.001 | <0.001 | <0.001 | 0.025 | 0.004 | <0.001 |
| CVLT, free delayed recall | |||||||
| Visit 1 | 7.282±0.339 (N=164) |
7.87±0.456b (N=82) |
6.317±0.444 (N=82) |
7.443±0.456 (N=103) |
7.018±0.471 (N=61) |
9.114±0.597 d
(N=46) |
6.654±0.388 (N=118) |
| Visit 2 | 5.706±0.38 (N=131) |
6.104±0.568 (N=66) |
5.053±0.415 (N=65) |
5.8±0.45 (N=91) |
5.507±0.701 (N=40) |
7.536±0.611d
(N=41) |
4.953±0.422 (N=90) |
| P (Visit2-Visit1) | 0.002 | 0.016 | 0.039 | 0.011 | 0.075 | 0.067 | 0.003 |
| Benton Visual Retention Test | |||||||
| Visit 1 | 5.837±0.618 (N=192) |
5.828±0.897 (N=95) |
5.852±0.71 (N=97) |
6.065±0.907 (N=122) |
5.439±0.605 (N=70) |
4.727±0.599 (N=63) |
6.316±0.833 (N=129) |
| Visit 2 | 7.651±0.689 (N=148) |
7.075±0.933 (N=80) |
8.742±0.933 (N=68) |
8.75±0.969 (N=100) |
5.606±0.6 (N=48) |
4.744±0.71561d
(N=47) |
8.927±0.889 (N=101) |
| P (Visit2-Visit1) | 0.05 | 0.34 | 0.14 | 0.044 | 0.84 | 0.99 | 0.033 |
| Brief Test of Attention | |||||||
| Visit 1 | 6.781±0.256 (N=186) |
7.021±0.337 (N=90) |
6.398±0.371 (N=96) |
6.962±0.347 (N=120) |
6.411±0.296 (N=66) |
7.436±0.301 d
(N=61) |
6.484±0.346 (N=125) |
| Visit 2 | 6.816±0.25 (N=135) |
6.894±0.363 (N=70) |
6.679±0.28 (N=65) |
6.615±0.351 c
(N=90) |
7.144±0.328 (N=45) |
7.15±0.337 (N=45) |
6.647±0.339 (N=90) |
| P (Visit2-Visit1) | 0.92 | 0.79 | 0.55 | 0.48 | 0.099 | 0.525 | 0.737 |
| Animal Fluency | |||||||
| Visit 1 | 18.557±0.634 (N=195) |
19.333±0.954 (N=93) |
17.339±0.635 (N=102) |
17.945±0.909 (N=125) |
19.645±0.661 (N=70) |
23.173±1.057 d
(N=63) |
16.641±0.63 (N=132) |
| Visit 2 | 18.837±0.62 (N=151) |
19.409±0.834 (N=80) |
17.812±0.883 (N=71) |
18.288±0.706 (N=101) |
19.835±1.222 (N=50) |
22.669±0.997 d
(N=47) |
17.13±0.684 (N=104) |
| P (Visit2-Visit1) | 0.75 | 0.95 | 0.66 | 0.77 | 0.89 | 0.73 | 0.59 |
| Digits Span, Forward | |||||||
| Visit 1 | 7.767±0.283 (N=194) |
7.94±0.408 (N=96) |
7.48±0.332 (N=98) |
7.68±0.401 (N=122) |
7.914±0.339 (N=72) |
8.842±0.363 d
(N=64) |
7.293±0.365 (N=130) |
| Visit 2 | 7.376±0.203 (N=147) |
7.245±0.25 (N=77) |
7.602±0.335 (N=70) |
7.211±0.266 (N=99) |
7.658±0.3 (N=48) |
8.551±0.289d
(N=48) |
6.787±0.235 (N=99) |
| P (Visit2-Visit1) | 0.26 | 0.15 | 0.79 | 0.33 | 0.57 | 0.53 | 0.24 |
| Digits Span, Backward | |||||||
| Visit 1 | 6.035±0.244 (N=193) |
6.099±0.315 (N=95) |
5.934±0.384 (N=98) |
6.227±0.35 (N=121) |
5.726±0.276 (N=72) |
6.936±0.375 d
(N=65) |
5.613±0.31 (N=128) |
| Visit 2 | 5.574±0.215 (N=147) |
5.66±0.271 (N=77) |
5.425±0.358 (N=70) |
5.424±0.305 (N=99) |
5.83±0.253 (N=48) |
6.548±0.311 d
(N=48) |
5.086±0.277 (N=99) |
| P (Visit2-Visit1) | 0.16 | 0.29 | 0.33 | 0.084 | 0.78 | 0.43 | 0.20 |
| Clock, command | |||||||
| Visit 1 | 8.811±0.14 (N=194) |
8.975±0.167 (N=95) |
8.538±0.241 (N=99) |
8.638±0.194 c
(N=126) |
9.135±0.152 (N=68) |
9.208±0.162 d
(N=65) |
8.639±0.188 (N=129) |
| Visit 2 | 8.88±0.165 (N=148) |
9.03±0.161 (N=80) |
8.596±0.355 (N=68) |
8.853±0.236 (N=100) |
8.929±0.184 (N=48) |
9.367±0.119 d
(N=48) |
8.658±0.233 (N=100) |
| P (Visit2-Visit1) | 0.75 | 0.81 | 0.89 | 0.48 | 0.39 | 0.43 | 0.95 |
| Trailmaking test, Part A | |||||||
| Visit 1 | 35.935±2.035 (N=185) |
32.493±2.007 b
(N=92) |
42.02±4.198 (N=93) |
37.382±2.958 (N=120) |
33.256±1.666 (N=65) |
27.881±1.724 d
(N=61) |
39.422±2.725 (N=124) |
| Visit 2 | 47.296±10.695 (N=142) |
35.881±5.925 (N=77) |
69.62±28.291 (N=65) |
50.433±15.294 (N=96) |
41.44±11.156 (N=46) |
27.904±1.669 (N=48) |
56.394±15.705 (N=94) |
| P (Visit2-Visit1) | 0.29 | 0.59 | 0.33 | 0.4 | 0.47 | 0.99 | 0.29 |
| Trailmaking test, Part B | |||||||
| Visit 1 | 144.572±19.57 (N=185) |
135.24±27.558 (N=92) |
161.073±24.538 (N=93) |
134.035±16.447 (N=120) |
164.079±45.361 (N=65) |
67.823±5.893 d
(N=61) |
177.801±26.704 (N=124) |
| Visit 2 | 149.918±24.57 (N=138) |
139.106±33.301 (N=76) |
171.669±33.268 (N=62) |
145.891±19.754 (N=91) |
157.105±58.258 (N=47) |
73.436±8.229 d
(N=46) |
186.16±34.571 (N=92) |
| P (Visit2-Visit1) | 0.87 | 0.93 | 0.79 | 0.64 | 0.93 | 0.58 | 0.85 |
Key: CES-D=Center for Epidemiologic Studies-Depression; MMSE=Mini-Mental State Examination; PIR=poverty income ratio; WRAT=Wide Range Achievement Test.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
p<0.05 for null hypothesis of no difference in means of cognitive test scores by Age group (referent category: Younger) within each visit. Wald test from svy:reg command.
p<0.05 for null hypothesis of no difference in means of cognitive test scores by sex (referent category: Women) within each visit. Wald test from svy:reg command.
p<0.05 for null hypothesis of no difference in means of cognitive test scores by race within each visit (referent category: Whites). Wald test from svy:reg command.
To test our key hypotheses, a series of mixed-effects linear regression models (Table 3-5) were conducted. Adjusting for multiple testing for 11 test scores, IL1β was positively associated with a faster rate of decline on a test of executive functioning, Trails B only among older participants aged >50y [γ11=+2.49±0.89, p=0.005, Figure 1A (standardized effect size (ES))]. Moreover, in the total population, IL-6 was linked to a faster decline on a test of verbal memory, the CVLT-DFR [γ11=−0.011±0.004, p=0.009, Figure 1B (ES)]. Finally, among younger participants, IL-18 was linked to a poorer performance on a test of attention (BTA) at baseline (γ01=−0.007±0.0025, p=0.004), but slower rate of decline on a test of psychomotor speed (Digits Span, Forward), among older individuals [γ11=+0.0010±0.0004, p=0.008, Figure 1C (ES)]. Additionally, among Whites, unlike among African-Americans, IL-6 was associated with a better baseline performance on CVLT-DFR and DS-B tests, reflecting verbal and working memory, respectively. Further in-depth analysis of the findings indicated that Whites had lower levels of IL-6 than AA at baseline, with a smaller variability (SD=12.6 vs. 18.7), coupled with better performance on the CVLT-List A and the DS-B tests that had a larger variability than among AA (SD=3.4 vs. 2.8 for CVLT-List A, SD=2.3 vs. 1.99). Thus, a positive association between IL-6 and cognitive performance on CVLT-List A and DS-B should be interpreted as an association between moderate level of this cytokine with variations in verbal and working memory, rather than the highly elevated level found among AAs. None of the sex-specific mixed-effects linear regression models suggested a significant association between the cytokines of interest and cognitive performance or change over time after adjustment for multiple testing. In addition, when formally testing heterogeneity by age group, sex and race (using 2-way and 3-way interaction terms), none of the key associations were shown to differ significantly by those socio-demographic factors at a type I error level of 0.05. Additional control for alcoholic beverage consumption in a sensitivity analysis did not alter the key findings for all 3 cytokines.
Table 3.
Cognitive performance test scores by IL-1β, stratified by age group, sex and race, for HANDLS participants with complete and reliable baseline and/or follow-up cognitive scores: mixed-effects regression modelsa
| All | Younger (≤50y) |
Older (>50y) |
Women | Men | Whites | African- Americans |
|
|---|---|---|---|---|---|---|---|
| Mini-Mental State Exam, total score | |||||||
| IL-1β | −0.004±0.014 | −0.019±0.016 | +0.012±0.037 | −0.011±0.016 | +0.080±0.073 | +0.039±0.036 | −0.015±0.019 |
| IL-1β ×TIME | +0.005±0.003 (N=216; k=1.6) |
+0.006±0.004 (N=103; k=1.7) |
+0.003±0.008 (N=113; k=1.6) |
+0.004±0.004 (N=143; k=1.7) |
−0.004±0.022 (N=73; k=1.6) |
−0.013±0.009 (N=70; k=1.7) |
+0.009±0.004b
(N=146; k=1.6) |
| California Verbal Learning Test (CVLT), List A | |||||||
| IL-1β | +0.060±0.050 | +0.111±0.063 | +0.130±0.100 | +0.091±0.053 | +0.182±0.231 | +0.086±0.289 | +0.135±0.057b |
| IL-1β ×TIME | −0.007±0.010 (N=207; k=1.6) |
−0.017±0.012 (N=100; k=1.6) |
−0.003±0.023 (N=107; k=1.5) |
−0.005±0.010 (N=135; k=1.6) |
−0.126±0.098 (N=72; k=1.5) |
−0.051±0.069 (N=68; k=1.5) |
−0.012±0.011 (N=139; k=1.6) |
| CVLT, Free Delayed Recall | |||||||
| IL-1β | +0.044±0.023 | +0.055±0.030 | +0.044±0.045 | +0.046±0.024 | +0.142±0.099 | +0.111±0.120 | +0.063±0.027b |
| IL-1β ×TIME | −0.009±0.005 (N=206; k=1.5) |
−0.012±0.006 (N=100; k=1.5) |
−0.020±0.011 (N=106; k=1.5) |
−0.008±0.005 (N=136; k=1.6) |
−0.07±0.043 (N=70; k=1.5) |
−0.045±0.028 (N=66; k=1.5) |
−0.009±0.006 (N=140; k=1.6) |
| Benton Visual Retention Test | |||||||
| IL-1β | +0.036±0.038 | +0.022±0.042 | +0.028±0.090 | +0.030±0.042 | −0.15±0.182 | +0.221±0.208 | −0.055±0.155 |
| IL-1β ×TIME | −0.001±0.009 (N=217; k=1.7) |
−0.001±0.008 (N=103; k=1.8) |
+0.044±0.033 (N=114; k=1.6) |
+0.001±0.010 (N=141; k=1.7) |
+0.011±0.059 (N=76; k=1.6) |
−0.236±0.149 (N=44; k=1.7) |
+0.053±0.050 (N=111; k=1.7) |
| Brief Test of Attention | |||||||
| IL-1β | −0.011±0.018 | −0.034±0.022 | +0.086±0.061 | −0.019±0.020 | +0.112±0.076 | +0.111±0.088 | −0.039±0.024 |
| IL-1β ×TIME | +0.003±0.004 (N=212; k=1.6) |
+0.009±0.005 (N=101; k=1.7) |
−0.026±0.017 (N=111; k=1.6) |
+0.005±0.004 (N=141; k=1.6) |
−0.040±0.039 (N=71; k=1.6) |
−0.028±0.021 (N=71; k=1.7) |
+0.008±0.005 (N=141; k=1.6) |
| Animal Fluency | |||||||
| IL-1β | −0.032±0.036 | +0.02±0.046 | +0.003±0.080 | −0.023±0.037 | +0.184±0.183 | +0.037±0.108 | +0.020±0.043 |
| IL-1β ×TIME | +0.004±0.008 (N=222; k=1.7) |
+0.001±0.01 (N=104; k=1.7) |
+0.006±0.017 (N=118; k=1.6) |
+0.003±0.007 (N=145; k=1.7) |
+0.059±0.071 (N=77; k=1.6) |
−0.043±0.030 (N=72; k=1.7) |
+0.002±0.009 (N=150; k=1.6) |
| Digits Span, Forward | |||||||
| IL-1β | +0.015±0.016 | +0.025±0.020 | −0.106±0.057 | +0.020±0.017 | −0.115±0.078 | −0.046±0.046 | +0.020±0.021 |
| IL-1β ×TIME | +0.000±0.003 (N=219; k=1.7) |
−0.003±0.004 (N=103; k=1.7) |
+0.031±0.016 (N=116; k=1.6) |
−0.001±0.004 (N=144; k=1.7) |
−0.038±0.028 (N=75; k=1.6) |
+0.025±0.010b
(N=72; k=1.7) |
−0.003±0.004 (N=147; k=1.6) |
| Digits Span,Backward | |||||||
| IL-1β | +0.008±0.015 | −0.003±0.017 | +0.071±0.057 | +0.011±0.016 | −0.068±0.064 | +0.034±0.044 | −0.011±0.018 |
| IL-1β ×TIME | −0.003±0.003 (N=219; k=1.7) |
+0.000±0.003 (N=103; k=1.7) |
−0.029±0.016 (N=116; k=1.6) |
−0.004±0.004 (N=144; k=1.7) |
+0.011±0.026 (N=75; k=1.6) |
+0.007±0.011 (N=72; k=1.7) |
−0.001±0.004 (N=147; k=1.6) |
| Clock, Command | |||||||
| IL-1β | +0.009±0.010 | −0.001±0.013 | −0.024±0.036 | +0.008±0.011 | +0.016±0.045 | +0.000±0.025 | −0.008±0.014 |
| IL-1β ×TIME | −0.002±0.002 (N=219; k=1.7) |
+0.001±0.003 (N=104; k=1.8) |
+0.009±0.010 (N=115; k=1.6) |
−0.002±0.002 (N=145; k=1.7) |
+0.031±0.018 (N=74; k=1.6) |
+0.003±0.007 (N=73; k=1.7) |
+0.001±0.003 (N=146; k=1.7) |
| Trailmaking Test, Part A | |||||||
| IL-1β | −0.174±0.367 | −0.116±0.538 | −0.176±0.822 | −0.067±0.335 | −1.061±2.629 | −0.278±0.251 | −0.254±0.530 |
| IL-1β ×TIME | +0.043±0.111 (N’=352) |
+0.010±0.151 (N’=177) |
+0.105±0.313 (N’=175) |
+0.016±0.100 (N’=236) |
−0.866±1.251 (N’=116) |
−0.001±0.084 (N’=122) |
+0.045±0.163 (N’=230) |
| Trailmaking Test, Part B | |||||||
| IL-1β | −1.099±1.147 | +0.220±1.276 | −1.214±2.788 | −0.295±1.354 | −2.333±6.138 | −1.221±1.265 | −0.769±1.657 |
| IL-1β ×TIME | +0.387±0.243 (N=207; k=1.7) |
+0.026±0.201 (N=99; k=1.8) |
+2.492±0.893d
(N=108; k=1.6) |
+0.387±0.404 (N=230) |
+0.604±2.877 (N=117) |
−0.159±0.257 (N=69; k=1.7) |
+0.709±0.375 (N=138; k=1.6) |
Key: CES-D=Center for Epidemiologic Studies-Depression; IL=Interleukin; k=number of observations/participant; MMSE=Mini-Mental State Examination; NSAIDs=Non-steroidal anti-inflammatory drugs; PIR=poverty income ratio; N=number of participants; N’=Number of observations (when ordinary least square linear regression is used instead of mixed-effects regression models); WRAT=Wide Range Achievement Test.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds). Models were controlled for socio-demographic factors, namely age (centered at 50y), sex, race, poverty status, education, marital status, literacy, and employment status and the inverse mills ratio. Additional covariates were considered for inclusion namely current smoking status, current drug use, body mass index (BMI, centered at 30), CES-D total score (centered at 15), HEI-2010 (centered at 40), self-reported diabetes, hypertension, high cholesterol, cardiovascular disease, inflammatory conditions, NSAIDs. Only those that were associated with IL-1β in a separate bivariate OLS regression model were included (current smoking). All covariates were interacted with TIME. All inverse mills ratios were centered at zero.
P<0.05 for null hypothesis that γ=0;
P<0.004 for null hypothesis that γ=0 for main effect IL-1β;
P<0.009 for null hypothesis that γ=0 for interaction between IL-1β and TIME.
p<0.05 for null hypothesis of no difference by Age group, sex or race, based on 2-way and 3-way interaction terms with IL-1β and TIME.
Table 5.
Cognitive performance test scores by IL-18, stratified by age group, sex and race, for HANDLS participants with complete and reliable baseline and/or follow -up cognitive scores: mixed -effects regression modelsa
| All | Younger (≤50y) |
Older (>50y) |
Women | Men | Whites | African - Americans |
|
|---|---|---|---|---|---|---|---|
| Mini -Mental State Exam, total score | |||||||
| IL -18 | −0.0009±0.0008 | +0.0009±0.0020 | −0.0007±0.0010 | −0.0013±0.0010 | +0.0006±0.0017 | −0.0008±0.0024 | −0.0008±0.0010 |
| IL -18 ×TIME | +0.0002±0.0003 (N=198; k=1.6) |
+0.0001±0.0006 (N=86; k=1.7) |
+0.0002±0.0004 (N=112; k=1.6) |
+0.0003±0.0005 (N=132; k=27) |
−0.0003±0.0003 (N=66; k=1.6) |
+0.0015±0.0010b
(N=57; k=1.6) |
+0.0000±0.0004 (N=141; k=1.6) |
| California Verbal Learning Test (CVLT), List A | |||||||
| IL -18 | −0.0026±0.0028 | +0.0094±0.0071 | −0.0035±0.0028 | −0.0019±0.0033 | +0.0007±0.0051 | +0.0075±0.0116 | −0.0036±0.0028 |
| IL -18 ×TIME | +0.0010±0.0010 (N=190; k=1.6) |
−0.0012±0.0019 (N=84; k=1.6) |
+0.0017±0.0011 (N=106; k=1.5) |
+0.0015±0.0014 (N=125; k=1.6) |
+0.0014±0.0015 (N=65; k=1.5) |
+0.0050±0.0020b
(N=55; k=1.5) |
+0.0014±0.0010 (N=135; k=1.6) |
| CVLT, Free Delayed Recall | |||||||
| IL -18 | +0.0001±0.0012 | +0.0038±0.0032 | +0.0002±0.0012 | −0.0001±0.0015 | +0.0011±0.0021 | −0.0008±0.0049 | +0.0001±0.0013 |
| IL -18 ×TIME | +0.0006±0.0005 (N=189; k=1.5) |
−0.0004±0.0009 (N=84; k=1.5) |
+0.0011±0.0005b
(N=105; k=1.5) |
+0.0005±0.0007 (N=126; k=1.6) |
+0.0001±0.0005b (N=63; k=1.4) |
+0.0014±0.0010 (N=53; k=1.5) |
+0.0007±0.0005 (N=136; k=1.6) |
| Benton Visual Retention Test | |||||||
| IL -18 | −0.0046±0.0023b | +0.001±0.0054 | −0.0054±0.0025b | −0.0036±0.0028 | −0.0062±0.0041 | +0.0141±0.0056b | −0.0048±0.0026 |
| IL -18 ×TIME | +0.001±0.0009 (N=199; k=1.7) |
−0.001±0.0011 (N=86; k=1.8) |
+0.0014±0.0013 (N=113; k=1.6) |
+0.0004±0.0013 (N=130; k=1.7) |
+.0002±0.0009b
(N=69; k=1.6) |
+0.0006±0.0026 (N=45; k=1.7) |
−0.0001±0.0013 (N=120; k=1.7) |
| Brief Test of Attention | |||||||
| IL -18 | −0.0015±0.0011 | −0.007±0.0025c | −0.0010±0.0011 | −0.0019±0.0012 | −0.0013±0.0019 | −0.0042±0.0031 | −0.0008±0.0011 |
| IL -18 ×TIME | −0.0001±0.0005 (N’=314) |
+0.001±0.0007 (N=86; k=1.7) |
−0.0001±0.0004 (N=109; k=1.6) |
+0.0005±0.0005 (N=131; k=1.6) |
−0.0002±0.0007 (N=64; k=1.6) |
+0.0003±0.0008 (N=57; k=1.7) |
+0.000±0.0004 (N=138; k=1.6) |
| Animal Fluency | |||||||
| IL -18 | −0.0021±0.0021 | +0.0031±0.0055 | −0.0029±0.0021 | −0.0022±0.0024 | −0.0013±0.0039 | +0.0030±0.0069 | −0.0031±0.0022 |
| IL -18 ×TIME | +0.0001±0.0008 (N=201; k=1.7) |
−0.0009±0.0014 (N=87; k=1.7) |
+0.0012±0.0008 (N=114; k=1.6) |
+0.0000±0.0011 (N=132; k=1.7) |
+0.0000±0.0011 (N=69; k=1.6) |
+0.0027±0.0019 (N=58; k=1.7) |
−0.0003±0.0008 (N=143; k=1.7) |
| Digits Span, Forward | |||||||
| IL -18 | +0.0005±0.0009 | +0.0023±0.0026 | +0.0003±0.0010 | +0.0006±0.0011 | −0.0005±0.0018 | −0.0028±0.0033 | +0.0008±0.0010 |
| IL -18 ×TIME | +0.0004±0.0003 (N=201; k=1.7) |
−0.0007±0.0006 (N=86; k=1.7) |
+0.0010±0.0004d
(N=115; k=1.6) |
+0.0004±0.0005 (N=132; k=1.7) |
+0.0006±0.0005 (N=69; k=1.6) |
+0.0007±0.0007 (N=58; k=1.7) |
+0.0004±0.0004 (N=143; k=1.6) |
| Digits Span, Backward | |||||||
| IL -18 | −0.0005±0.0008 | −0.0001±0.0020 | −0.0005±0.0009 | −0.0008±0.0010 | +0.0009±0.0014 | −0.0015±0.0030 | −0.0002±0.0008 |
| IL -18 ×TIME | +0.0004±0.0003 (N=201; k=1.6) |
+0.0002±0.0005 (N=86; k=1.7) |
+0.0006±0.0004 (N=115; k=1.6) |
+0.0012±0.0005 (N=132; k=1.7) |
−0.0002±0.0004 (N=69; k=1.6) |
+0.0011±0.0009 (N=58; k=1.7) |
+0.0003±0.0004 (N=143; k=1.6) |
| Clock, Command | |||||||
| IL -18 | −0.0004±0.0006 | −0.0009±0.0015 | −0.0005±0.0006 | −0.0002±0.0007 | −0.0023±0.0010 | −0.0033±0.0014 | −0.0002±0.0006 |
| IL -18 ×TIME | −0.0003±0.0002 (N=198; k=1.7) |
−0.0005±0.0004 (N=87; k=1.7) |
+0.0000±0.0002 (N=111; k=1.6) |
+0.0000±0.0003 (N=132; k=1.7) |
−0.0002±0.0003 (N=66; k=1.6) |
+0.0011±0.0004 (N=59; k=1.7) |
−0.0004±0.0002 (N=139; k=1.7) |
| Trailmaking Test, Part A | |||||||
| IL -18 | −0.0076±0.0238 | −0.0258±0.0775 | −0.0108±0.0239 | −0.0019±0.0234 | −0.0187±0.0741 | −0.0281±0.0178 | −0.0066±0.0302 |
| IL -18 ×TIME | +0.0094±0.0105 (N’=324) |
−0.0072±0.0240 (N’=150) |
+0.0286±0.0122 (N’=174) |
+0.0272±0.0120 (N’=220) |
−0.0149±0.0248 (N’=104) |
+0.0061±0.0067 (N’=98) |
+0.0103±0.0138 (N’=226) |
| Trailmaking Test, Part B | |||||||
| IL-18 | −0.0807±0.0718 | +0.0789±0.1719 | −0.1324±0.0833 | −0.1443±0.0838 | +0.0156±0.1444 | +0.0386±0.1143 | −0.1043±0.0828 |
| IL -18 ×TIME | +0.0477±0.0249 (N=193; k=1.7) |
+0.0185±0.0287 (N=85; k=1.8) |
+0.0621±0.037 (N= 108; k=1.6) |
+0.0756±0.0421 (N=129; k=1.7) |
−0.0189±0.0082 (N=64; k=1.6) |
+0.0081±0.0434 (N’=96) |
+0.0481±0.0314 (N’=223) |
Key: CES-D=Center for Epidemiologic Studies-Depression; IL=Interleukin; k=number of observations/participant; MMSE=Mini-Mental State Examination; NSAIDs=Non-steroidal anti-inflammatory drugs; PIR=poverty income ratio; N=number of participants; N’=Number of observations (when ordinary least square linear regression is used instead of mixed-effects regression models); WRAT=Wide Range Achievement Test.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds). Models were controlled for socio -demographic factors, namely age (centered at 50y), sex, race, poverty status, education, marital status, literacy, and employment status and the inverse mills ratio. Additional covariates were considered for inclusion namely current smoking status, current drug use, body mass index (BMI, centered at 30), CES -D total score (centered at 15), HEI -2010 (centered at 40), self -reported diabetes, hypertension, high cholesterol, cardiovascular disease, inflammatory conditions, NSAIDs. Only those that were associated with IL -18 in a separate bivariate OLS regression model were included (current smoking, inflammatory conditions).
P<0.05 for null hypothesis that γ=0;
P<0.004 for null hypothesis that γ=0 for main effect IL-18;
P<0.009 for null hypothesis that γ=0 for interaction between IL -18 and TIME.
p<0.05 for null hypothesis of no difference by Age group, sex or race, based on 2-way and 3-way interaction terms with IL-18 and TIME.
FIGURE 1.A. Predictive margins for Trailmaking test, part B (standardized z-score) by IL-1β (mean-1SD, mean, mean + 1 SD): mixed-effects regression models: older adults (>50y of age at baseline).
KEY: TRAILS B=Trailmaking test, part B; IL-1β=Interleukin-1β.
Note: Among subgroup >50y with complete and reliable Trailmaking Test, part B: mean ±SD IL-1β: 2.19±5.45; mean±SD Trailmaking Test, part B total score (sec.): 155.8±151.9.
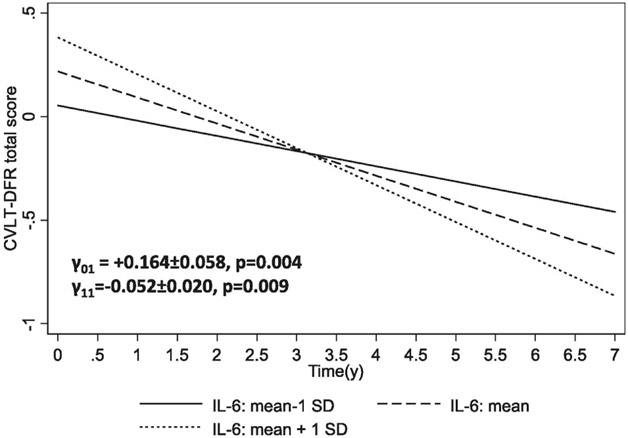
FIGURE 1.B. Predictive margins for CVLT-DFR (standardized z-score) by IL-6 (mean-1SD, mean, mean + 1 SD): mixed-effects regression models: total population.
KEY: CVLT-DFR= California Verbal Learning Test, Delayed Free Recall (List A); IL-6=Interleukin-6.
Note: Among total population with reliable CVLT-DFR: mean±SD IL-6: 12.52±15.04; mean±SD CVLT-DFR: 6.35±3.24.
FIGURE 1.C. Predictive margins for Digits Span-Forward (standardized z-score) by IL-18 (mean-1SD, mean, mean + 1 SD): mixed-effects regression models: older adults (>50y of age at baseline).
KEY: DS-F= Digits Span-Forward; IL-18=Interleukin-18.
Note: Among subgroup >50y with complete and reliable DS-F: mean ±SD IL-18: 168.3±160.1; mean±SD DS-F: 7.02±2.13.
DISCUSSION
The association between various cytokines with longitudinal trajectories of cognitive performance have been explored in our present study using a sample of bi-racial middle-aged and older urban adults, while stratifying by age, sex and race. Among key findings, IL1β was positively associated with a faster rate of decline on a test of executive functioning, among older adults (age>50y), while in the total population, IL-6 was linked to a faster decline on a test of verbal memory. Among younger participants, IL-18 was linked to a poorer performance on a test of attention (BTA) at baseline though a slower rate of decline with higher IL-18 was detected for a test of psychomotor speed in older adults. Finally, among Whites, unlike among African-Americans, IL-6 was associated with a better baseline performance on two tests of verbal and working memory.
Interleukins have been linked cross-sectionally and prospectively with various cognitive outcomes. With respect to IL-6, two recent studies—one using data from the US-based Framingham Offspring Study (Jefferson et al., 2007) and the other from the France based Three City-Dijon cohort—found evidence linking the concentration of IL-6 to markers of brain atrophy (Jefferson et al., 2007; Satizabal et al., 2012). In the Framingham Offspring Study, Jefferson and colleagues (Jefferson et al., 2007) extracted data from a sample of nearly 2,000 non-demented participants (Mean=60y, SD=9y) free of stroke and transient ischemic attacks. They found elevated IL-6 to be significantly inversely associated with total cerebral brain volume (TCBV) but not white matter hyperintensities (WMH) attained via magnetic resonance imaging (MRI). In this study, cardiovascular disease (CVD) was also associated with increased inflammatory markers which they found to be positively linked to brain atrophy. Satizabal and colleagues (Satizabal et al., 2012) used data on 1,841 men and women (Mean=72.5y, SD=4.1) from the Three City-Dijon cohort and found elevated levels of IL-6 to be cross-sectionally associated with reduced WMH integrity, gray matter, and hippocampal volume. However, these findings were not replicated using the baseline inflammatory markers and new MRI results at four years of follow-up. Based on a recent meta-analysis of 7 independent studies, adults with high circulating IL-6 were on average 1.42 times more likely to experience global cognitive decline (based on change in MMSE and other similar tests) at follow-up, over a 2–7 y period, compared to those with low IL-6 (OR=1.42, 95% CI: 1.18–1.70; P < 0.001) (Bradburn et al., 2017). Similarly, based on 4 studies mostly conducted among White Europeans, another meta-analysis concluded that higher IL-6 (top quantile vs. lowest) was linked to a HR for all-cause dementia of 1.40, with a 95% CI: 1.13-1.73, P<0.05 (Darweesh et al., 2018b). Finally, a recent meta-analysis comparing AD with health controls, found that the standardized mean difference (SMD) in the 3 cytokines of interest were: IL-1β [25 studies, SMD=0.727 (95% CI: 0.335 to 1.120) P<0.001], IL-6 [40 studies, SMD=0.522 (95% CI: 0.240 to 0.804) P<0.001] and IL-18 [9 studies, SMD= 0.945 (95% CI: 0.143 to 1.748) P=0.021] (Lai et al., 2017).
Several studies also indicate that interleukins, especially IL-6, are associated with a range of cognitive domains, including processing speed and attention (Heringa et al., 2014), executive function (Mooijaart et al., 2013), immediate and delayed recall (Elderkin-Thompson et al., 2012), visuospatial skills (Frydecka et al., 2015), and working memory in midlife (Marsland et al., 2006) and older ages (Simpson et al., 2013). Our study found that IL-6 was linked to faster decline on a test of verbal memory in the total population, though at baseline it was associated with a better performance on that test. Thus, our findings partially replicate findings by (Elderkin-Thompson et al., 2012). In contrast, among Whites, IL-6 was found to be protective against impairment in verbal and working memory at baseline, thus contradicting findings by (Elderkin-Thompson et al., 2012) and (Marsland et al., 2006) for that group. This finding should be interpreted with caution given that IL-6 cytokine levels were on average higher among AA compared to Whites, as was shown in numerous other reports (Allison et al., 2006; Ranjit et al., 2007; Slopen et al., 2010; Stepanikova et al., 2017). Thus, a protective effect among Whites is that of a moderate increase in IL-6 rather than the larger increments found among AAs. More studies are needed comparing racial groups in terms of the association between cytokines such as IL-6 and memory-related performance, both cross-sectionally and longitudinally. We did not detect any association between cytokines IL-1β, IL-6 or IL-18 and MMSE, possibly due to the younger age of our cohort compared to previous studies [e.g. (Bradburn et al., 2017)]. In addition, IL-1β was the only cytokine to have a single finding that is consistent with previous studies, showing that higher levels had an adverse effect in older adults (>50y) on cognitive change in the domain of executive function. This is in line with the findings from the meta-analysis previously described (Lai et al., 2017). In addition, both IL-6 and IL-1β gene polymorphisms were shown to linked with increased risk for AD (Ravaglia et al., 2006). Specifically, IL-1β polymorphism was shown to be associated with higher total homocysteine (tHcy) in that report (Ravaglia et al., 2006). tHcy has been linked with poor cognitive outcomes in numerous cohort studies (Beydoun et al., 2014).
In contrast, our findings with IL-18 were largely inconsistent, suggesting that higher IL-18 was linked to a poorer performance on a test of attention at baseline (age≤50y, γ01=−0.007±0.0025, p=0.004) though a slower rate of decline with higher IL-18 was detected for a test of psychomotor speed in older adults (age>50y, γ11=+0.0010±0.0004, p=0.008). While the first finding is in line with the previous meta-analysis (Lai et al., 2017) for the younger group in its acute effect on attention, this was not the case among the older group in terms of rate of change in psychomotor speed. As is the case for IL-6, more studies are needed to examine the association between IL-1β, IL-18 and cognitive performance or change in cognition over time.
Despite a growing body of work linking cytokines and other inflammatory markers to cognitive health and deterioration, the biological pathways remain unclear (Meyer, 2011). Some studies suggest that the deposition of beta-amyloid in the brain sets off a cascade of effects including the secretion of IL-1β from microglia ultimately resulting in neuroinflammation, neuronal dysfunction, and accelerated neurogenerative processes (Teixeira et al., 2008). There is also evidence that IL-1β induces the expression of VCAM-1, a cell adhesion molecule involved in the regulation of microvasculature permeability, which in turn plays a role in the transmission of leukocytes along with other signaling cascades which have been found in AD patients (Guerriero et al., 2017). A recent review (McAfoose and Baune, 2009) provides evidence that IL-1β and IL-6 may operate at the molecular level via synaptic plasticity, neurogenesis, or neuromodulation as well as at the cellular level affecting learning, memory, and cognitive functioning. In addition to their association with beta-amyloid accumulation, IL-1β and IL-6 have been associated with neurofibrillary tangles in AD patients (Da Mesquita et al., 2016). Elevated IL-6 has also been linked with reduced hippocampal volume in dementia-free patients suggesting that IL-6 may be useful in early AD detection and with staging the severity of the disease (Satizabal et al., 2012). Despite IL-6 being the most frequently studied interleukin, others such as IL-18 and IL-10 play important roles in regulating inflammatory processes. For example, IL-18 plays a key role in signaling during an inflammatory response via the recruitment of leukocytes, lymphocytes, and macrophages (Dinarello et al., 2013). There is further evidence suggesting that the anti-inflammatory IL-10 operates through deactivation of macrophage proinflammatory cytokine synthesis processes (Iyer and Cheng, 2012).
Several strengths are notable in our study, including the use of a comprehensive cognitive test battery and the adequate statistical power allowing for stratification by key socio-demographic factors. With up to two waves of cognitive data, it was possible to ascertain temporal relationships using longitudinal design and analyses. Furthermore, the cognitive battery available consistently between the two waves was extensive allowing to assess change in various cognitive domains in relation to cytokine levels. Additionally, advanced techniques were used to adjust for potential confounding and sample selectivity, while providing population estimates using sampling weights for baseline covariates for the descriptive part of the analyses.
Despite those strengths, several limitations are also worth noting. First, residual confounding cannot be ruled given the observational nature of the study, though key covariates were adjusted for, particularly socio-demographic factors and selected covariates that were shown to be related to each of the cytokines. A second limitation is the availability of only up to 2 time points of cognitive data, limited individual-level decline with time due to a younger baseline age range (30-64y), the restricted ability to create reliable cognitive domains due to poor factorial invariance across age groups, sex, race and poverty status among others. These are among several limitations detailed in previous studies (e.g.(Beydoun et al., 2018b)). Moreover, though statistical power was adequate in our current analysis, studying non-linear relationships using tertiles or clinical cut-points of cytokines was not readily possible, nor was it possible to stratifying by imbalanced factors such as chronic conditions. Furthermore, since our sample population was limited to the African-American and White urban adults who are relatively young and healthy, the results may have insufficient power to generalize to older populations. Finally, the opportunity to control for important confounding and to study other related phenotypes (e.g. MRI data) was limited. In fact, MRI data on HANDLS participants was limited to ~10% of MRV participants which would have reduced our sample substantially. Additionally, since genotype data was only available on a sub-sample of African-American participants, adjustment for or stratification by ApoE4 status was precluded (Watanabe et al., 2016).
In sum, there were substantial longitudinal and cross-sectional associations between cytokine levels (IL-1β, IL-6 and IL-18) with cognitive performance, generally indicative of poorer performance and faster decline with higher systemic inflammation. Those associations were found for domains of executive function in older adults (IL-1β), verbal memory in the total population (IL-6), attention for younger adults (IL-18, at baseline). Nevertheless, IL-18 had an inconsistent relationship with a measure of psychomotor speed and was found to be potentially protective over time among older individuals. Finally, among Whites, unlike among African-Americans, IL-6 was associated with a better baseline performance on two tests of verbal and working memory. Further longitudinal studies are needed to replicate our findings and mediation through relevant biological and psychosocial factors need to be studied as well.
Supplementary Material
FIGURE S1. Participant flow chart
KEY: AF=Animal Fluency test; BTA=Brief Test of Attention; BVRT=Benton Visual Retention Test; CDT=Clock Drawing Test; CVLT-DFR= California Verbal Learning Test, Delayed Free Recall (List A); CVLT-List A= California Verbal Learning Test, immediate recall (List A); DS-B= Digit Span Backwards; DS-F=Digit Span Forward; HANDLS=Healthy Aging in Neighborhoods of Diversity Across the Life Span; Trails A=Trailmaking test, Part A; Trails B=Trailmaking test, Part B.
Note: Sample 1 is the initial visit 1, phase 1 selected HANDLS sample of adults aged 30-64y, Whites and African-Americans. Samples 1a-1c as the sub-samples with complete exposure measurements. Samples 2a through 4k are the sub-samples with complete exposure, outcome (at either of two visits) and covariate data.
Supplemental method 1: Description of cognitive tests, literacy and the CES-D
Supplemental Method 2: Description of mixed-effects regression models
Table 4.
Cognitive performance test scores by IL-6, stratified by age group, sex and race, for HANDLS participants with complete and reliable baseline and/or follow-up cognitive scores: mixed-effects regression modelsa
| All | Younger (≤50y) |
Older (>50y) |
Women | Men | Whites | African- Americans |
|
|---|---|---|---|---|---|---|---|
| Mini-Mental State Exam, total score | |||||||
| IL-6 | −0.001±0.009 | −0.015±0.013 | +0.003±0.012 | +0.012±0.012 | −0.011±0.012 | +0.038±0.013b | −0.012±0.011 |
| IL-6 ×TIME | +0.000±0.003 (N=204; k=1.6) |
−0.002±0.005 (N=88; k=1.7) |
+0.004±0.004 (N=116; k=1.6) |
−0.001±0.004 (N=137; k=1.7) |
−0.004±0.006 (N=67; k=1.6) |
−0.005±0.005 (N=59; k=1.7) |
+0.001±0.004 (N=145; k=1.6) |
| California Verbal Learning Test (CVLT), List A | |||||||
| IL-6 | +0.053±0.028 | +0.092±0.045b | +0.03±0.034 | +0.079±0.038b | +0.075±0.039 | +0.098±0.059 | +0.040±0.032 |
| IL-6 ×TIME | −0.018±0.009b
(N=195; k=1.6) |
−0.024±0.015 (N=86; k=1.6) |
−0.008±0.011 (N=109; k=1.5) |
−0.019±0.009b
(N=129; k=1.6) |
−0.037±0.022 (N=66; k=1.5) |
+0.004±0.016 (N=57; k=1.5) |
−0.014±0.011 (N=138; k=1.6) |
| CVLT, Free Delayed Recall | |||||||
| IL-6 | +0.035±0.012c | +0.048±0.018b | +0.033±0.016b | +0.041±0.017b | +0.033±0.015b | +0.069±0.023c | +0.028±0.014 |
| IL-6 ×TIME |
−0.011±0.004d
(N=194; k=1.5) |
−0.013±0.007 (N=86; k=1.5) |
−0.008±0.005 (N=108; k=1.5) |
−0.01±0.005b
(N=130; k=1.6) |
−0.02±0.009b
(N=64; k=1.4) |
−0.007±0.006 (N=55; k=1.5) |
−0.011±0.006b
(N=139; k=1.5) |
| Benton Visual Retention Test | |||||||
| IL-6 | +0.039±0.023 | +0.012±0.033 | +0.070±0.030b | +0.060±0.033 | −0.009±0.031 | −0.025±0.031 | +0.078±0.0315b |
| IL-6 ×TIME | −0.006±0.008 (N=205; k=1.7) |
+0.000±0.009 (N=88; k=1.8) |
−0.011±0.014 (N=117; k=1.6) |
−0.011±0.011 (N=135; k=1.7) |
+0.021±0.012 (N=70; k=1.6) |
−0.007±0.014 (N=45; k=1.7) |
−0.014±0.012 (N=120; k=1.7) |
| Brief Test of Attention | |||||||
| IL-6 | −0.010±0.011 | −0.019±0.015 | +0.000±0.016 | −0.036±0.015 | +0.03±0.014 | −0.016±0.019 | −0.006±0.014 |
| IL-6 ×TIME | −0.003±0.004 (N=200; k=1.6) |
−0.005±0.005 (N=87; k=1.7) |
−0.004±0.005 (N=113; k=1.6) |
+0.002±0.004 (N=136; k=1.6) |
−0.016±0.009 (N=64; k=1.6) |
−0.004±0.005 (N=60; k=1.7) |
−0.006±0.005 (N=140; k=1.6) |
| Animal Fluency | |||||||
| IL-6 | +0.003±0.022 | +0.006±0.034 | −0.007±0.026 | +0.027±0.028 | −0.016±0.031 | +0.092±0.043 | −0.022±0.024 |
| IL-6 ×TIME | −0.001±0.007 (N=209; k=1.7) |
−0.020±0.012 (N=89; k=1.7) |
+0.012±0.008 (N=120; k=1.6) |
−0.007±0.007 (N=139; k=1.7) |
+0.008±0.017 (N=70; k=1.6) |
+0.011±0.013 (N=61; k=1.7) |
−0.010±0.008 (N=148; k=1.6) |
| Digits Span, Forward | |||||||
| IL-6 | −0.007±0.010 | +0.027±0.016 | −0.031±0.012 | −0.009±0.014 | +0.002±0.013 | −0.008±0.020 | −0.002±0.012 |
| IL-6 ×TIME | +0.000±0.003 (N=206; k=1.6) |
−0.008±0.005 (N=87; k=1.7) |
+0.007±0.004 (N=119; k=1.6) |
+0.001±0.004 (N=137; k=1.7) |
−0.001±0.007 (N=69; k=1.6) |
+0.001±0.004 (N=60; k=1.7) |
+0.000±0.004 (N=146; k=1.6) |
| Digits Span, Backward | |||||||
| IL-6 | +0.005±0.009 | +0.008±0.012 | +0.000±0.012 | +0.029±0.012b | −0.013±0.010 | +0.053±0.017c | −0.008±0.010 |
| IL-6 ×TIME | +0.004±0.003 (N=206; k=1.6) |
+0.003±0.004 (N=87; k=1.7) |
+0.006±0.005 (N=119; k=1.6) |
−0.001±0.004 (N=137; k=1.7) |
+0.012±0.006 (N=69; k=1.6) |
−0.005±0.006 (N=60; k=1.7) |
+0.006±0.004 (N=146; k=1.6) |
| Clock, Command | |||||||
| IL-6 | +0.009±0.006 | +0.008±0.009 | +0.008±0.009 | +0.011±0.009 | +0.001±0.008 | +0.012±0.009 | +0.007±0.008 |
| IL-6 ×TIME | −0.002±0.002 (N=206; k=1.7) |
−0.007±0.003b
(N=89; k=1.7) |
+0.000±0.003 (N=117; k=1.6) |
−0.002±0.003 (N=139; k=1.7) |
+0.004±0.004 (N=67; k=1.6) |
−0.002±0.003 (N=62; k=1.7) |
−0.002±0.003 (N=144; k=1.7) |
| Trailmaking Test, PartA | |||||||
| IL-6 | −0.002±0.251 | +0.057±0.467 | −0.086±0.322 | +0.007±0.263 | −0.022±0.602 | −0.091±0.109 | +0.035±0.367 |
| IL-6 ×TIME | −0.047±0.102 (N=331) |
−0.076±0.182 (N=152) |
+0.011±0.134 (N=179) |
−0.063±0.100 (N=227) |
+0.079±0.318 (N=104) |
+0.034±0.047 (N=103) |
−0.117±0.147 (N=228) |
| Trailmaking Test, Part B | |||||||
| IL-6 | +0.763±0.766 | +1.400±1.009 | −0.235±1.132 | −0.664±1.065 | +3.499±1.209b | −1.464±0.625b | +1.589±1.094 |
| IL-6 ×TIME | +0.485±0.232b
(N=197; k=1.7) |
−0.008±0.270 (N=86; k=1.8) |
+1.138±0.392b
(N=111; k=1.6) |
+0.738±0.407 (N=221) |
−0.050±0.638 (N=105) |
+0.282±0.276 (N=101) |
+0.572±0.445 (N=225) |
Key: CES-D=Center for Epidemiologic Studies-Depression; IL=Interleukin; k=number of observations/participant; MMSE=Mini-Mental State Examination; NSAIDs=Non-steroidal anti-inflammatory drugs; PIR=poverty income ratio; N=number of participants; N’=Number of observations (when ordinary least square linear regression is used instead of mixed-effects regression models); WRAT=Wide Range Achievement Test.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds). Models were controlled for socio-demographic factors, namely age (centered at 50y), sex, race, poverty status, education, marital status, literacy, and employment status and the inverse mills ratio. Additional covariates were considered for inclusion namely current smoking status, current drug use, body mass index (BMI, centered at 30), CES-D total score (centered at 15), HEI-2010 (centered at 40), self-reported diabetes, hypertension, high cholesterol, cardiovascular disease, inflammatory conditions, NSAIDs. Only those that were associated with IL-6 in a separate bivariate OLS regression model were included (current smoking, cardiovascular disease). All covariates were interacted with TIME. All inverse mills ratios were centered at zero.
P<0.05 for null hypothesis that γ=0;
P<0.004 for null hypothesis that γ=0 for main effect IL-6;
P<0.009 for null hypothesis that γ=0 for interaction between IL-6 and TIME.
p<0.05 for null hypothesis of no difference by Age group, sex or race, based on 2-way and 3-way interaction terms with IL-6 and TIME.
Highlights.
IL1β was positively associated with a faster rate of decline on a test of executive functioning, among older adults (age>50y, γ11=+2.49±0.89, p=0.005).
In the total population, IL-6 was linked to a faster decline on a test of verbal memory (γ11=−0.011±0.004, p=0.009).
Among younger participants, IL-18 was linked to a poorer performance on a test of attention at baseline (age≤50y, γ01=−0.007±0.0025, p=0.004) though a slower rate of decline with higher IL-18 was detected for a test of psychomotor speed in older adults (age>50y, γ11=+0.0010±0.0004, p=0.008).
Finally, among Whites, unlike among African-Americans, IL-6 was associated with a better baseline performance on two tests of verbal and working memory.
ACKNOWLEDGMENTS
The authors would like to thank Salman Tajuddin and Ola Rostant for their internal review of the manuscript.
Sources of funding: This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
ABBREVIATIONS
- AD
Alzheimer’s Disease
- AF
Animal Fluency test
- BTA
Brief Test of Attention
- BVRT
Benton Visual Retention Test
- CDT
Clock Drawing Test
- CES-D
Center for Epidemiologic Studies-Depression
- CVLT-DFR
California Verbal Learning Test, Delayed Free Recal; (List A)
- CVLT-List A
California Verbal Learning Test, immediate recall (List A)
- DS-B
Digit Span Backwards
- DS-F
Digit Span Forward
- HANDLS
Healthy Aging in Neighborhoods of Diversity Across the Life Span
- HS
High School
- OLS
Ordinary Least Square
- PIR
Poverty Income Ratio
- Trails A
Trailmaking test, Part A
- Trails B
Trailmaking test, Part B
- WRAT
Wide Range Achievement Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure statement: All authors declare no conflict of interest.
REFERENCES
- Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, 2006. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of the American College of Cardiology 48, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Fleischman DA, Grisot G, Barth CM, Varentsova A, Morris MC, Barnes LL, Bennett DA, 2013. Systemic inflammation in non-demented elderly human subjects: brain microstructure and cognition. PLoS One 8, e73107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulock SV, Deininger S, Draing C, Gueinzius K, Dehus O, Hermann DC, 2006. Gender Difference in Cytokine Secretion on Immune Stimulation with LPS and LTA. Journal of Interferon & Cytokine Research 26, 887–892. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, 2011. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 10, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL, Kramer JH, 2012. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun 26, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y, 2014. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Kitner-Triolo MH, Kaufman JS, Evans MK, Zonderman AB, 2013. Thyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. The Journal of clinical endocrinology and metabolism 98, 3470–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Canas JA, Dore GA, Beydoun HA, Rostant OS, Fanelli-Kuczmarski MT, Evans MK, Zonderman AB, 2016. Serum Uric Acid and Its Association with Longitudinal Cognitive Change Among Urban Adults. J Alzheimers Dis 52, 1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Dore GA, Canas JA, Liang H, Beydoun HA, Evans MK, Zonderman AB, 2018a. Systemic Inflammation Is Associated With Longitudinal Changes in Cognitive Performance Among Urban Adults. Front Aging Neurosci 10, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Hossain S, Fanelli-Kuczmarski MT, Beydoun HA, Canas JA, Evans MK, Zonderman AB, 2018b. Vitamin D Status and Intakes and Their Association with Cognitive Trajectory in A Longitudinal Study of Urban Adults. J Clin Endocrinol Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Hossain S, Tajuddin SM, Canas JA, Kuczmarski M, Beydoun HA, Evans MK, Zonderman AB, 2018c. Vitamin D Metabolism-Related Gene Haplotypes and Their Association with Metabolic Disturbances Among African-American Urban Adults. Sci Rep 8, 8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Kushner I, Samols D, 2004. C-reactive Protein. J Biol Chem 279, 48487–48490. [DOI] [PubMed] [Google Scholar]
- Bradburn S, Sarginson J, Murgatroyd CA, 2017. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front Aging Neurosci 9, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canon ME, Crimmins EM, 2011. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. The journal of nutrition, health & aging 15, 695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Khan A, Harper M, Stockman D, Fiscella K, Walton J, Duberstein P, Talbot N, Lyness JM, Moynihan J, 2009. Gender, race/ethnicity, personality, and interleukin-6 in urban primary care patients. Brain, behavior, and immunity 23, 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlier F, Hafzalla G, Faskowitz J, Kuller LH, Becker JT, Lopez OL, Thompson PM, Braskie MN, 2018. Systemic inflammation as a predictor of brain aging: Contributions of physical activity, metabolic risk, and genetic risk. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F, 2016. Insights on the pathophysiology of Alzheimer's disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev 68, 547–562. [DOI] [PubMed] [Google Scholar]
- Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A, 2018a. Inflammatory markers and the risk of dementia and Alzheimer's disease: A meta-analysis. Alzheimers Dement. [DOI] [PubMed] [Google Scholar]
- Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A, 2018b. Inflammatory markers and the risk of dementia and Alzheimer's disease: A meta-analysis. Alzheimers Dement 14, 1450–1459. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Kim S, Kaplanski G, 2013. Interleukin-18 and IL-18 binding protein. Front Immunol 4, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A, 2012. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am J Geriatr Psychiatry 20, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB, 2010. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & disease 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL, 2005. The origins of age-related proinflammatory state. Blood 105, 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P, Kiejna A, Beszlej JA, 2015. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci 265, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG, Ferrie JE, 2009. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med 39, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein FC, Zhao L, Steenland K, Levey AI, 2015. Inflammation and cognitive functioning in African Americans and Caucasians. International journal of geriatric psychiatry 30, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, 2010. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 1207, 155–162. [DOI] [PubMed] [Google Scholar]
- Gu Y, Manly JJ, Mayeux RP, Brickman AM, 2018. An inflammation-related nutrient pattern is associated with both brain and cognitive measures in a multiethnic elderly population. Curr Alzheimer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero F, Sgarlata C, Francis M, Maurizi N, Faragli A, Perna S, Rondanelli M, Rollone M, Ricevuti G, 2017. Neuroinflammation, immune system and Alzheimer disease: searching for the missing link. Aging Clin Exp Res 29, 821–831. [DOI] [PubMed] [Google Scholar]
- Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, Teerlink T, Scheffer PG, van den Hurk K, Kappelle LJ, Dekker JM, Biessels GJ, 2014. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population - the Hoorn Study. Psychoneuroendocrinology 40, 108–118. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Tamhane AC,, 1987. Multiple comparison procedures. Wiley, New York. [Google Scholar]
- Hoshi T, Yamagami H, Furukado S, Miwa K, Tanaka M, Sakaguchi M, Sakoda S, Kitagawa K, 2010. Serum inflammatory proteins and frontal lobe dysfunction in patients with cardiovascular risk factors. Eur J Neurol 17, 1134–1140. [DOI] [PubMed] [Google Scholar]
- Hull M, Fiebich BL, Lieb K, Strauss S, Berger SS, Volk B, Bauer J, 1996. Interleukin-6-associated inflammatory processes in Alzheimer's disease: new therapeutic options. Neurobiol Aging 17, 795–800. [DOI] [PubMed] [Google Scholar]
- Ibrahim JG, Molenberghs G, 2009. Missing data methods in longitudinal studies: a review. Test 18, 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Cheng G, 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32, 23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF Jr., Lipinska I, Kathiresan S, Benjamin EJ, DeCarli C, 2007. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology 68, 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanova V, Stewart R, Davies E, Sherwood R, Prince M, 2007. Markers of inflammation and cognitive decline in an African-Caribbean population. Int J Geriatr Psychiatry 22, 966–973. [DOI] [PubMed] [Google Scholar]
- Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M, Carvalho AF, Herrmann N, 2017. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 88, 876–882. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhou S, Dreyer RP, Spatz ES, Geda M, Lorenze NP, D'Onofrio G, Lichtman JH, Spertus JA, Ridker PM, Krumholz HM, 2017. Sex Differences in Inflammatory Markers and Health Status Among Young Adults With Acute Myocardial Infarction: Results From the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) Study. Circ Cardiovasc Qual Outcomes 10, e003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB, 2006. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosomatic Medicine 68, 895–903. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT, 2009. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 33, 355–366. [DOI] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM, 2016a. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging 31, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM, 2016b. Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging 31, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Yeo GW, 2017. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 13, 72–83. [DOI] [PubMed] [Google Scholar]
- Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, Harris TB, Ayonayon HN, Rosano C, Cauley JA, Health ABCS, 2014. Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol Aging 35, 2785–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, 2011. Anti-inflammatory signaling in schizophrenia. Brain Behav Immun 25, 1507–1518. [DOI] [PubMed] [Google Scholar]
- Miles TP, Froehlich TE, Bogardus ST Jr., Inouye SK, 2001. Dementia and Race: Are There Differences Between African Americans and Caucasians? Journal of the American Geriatrics Society 49, 477–484. [DOI] [PubMed] [Google Scholar]
- Mooijaart SP, Sattar N, Trompet S, Lucke J, Stott DJ, Ford I, Jukema JW, Westendorp RG, de Craen AJ, Group PS, 2013. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J Intern Med 274, 77–85. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N, Ejiogu N, Zonderman AB, Evans MK, 2012. Association of oxidative DNA damage and C-reactive protein in women at risk for cardiovascular disease. Arterioscler Thromb Vasc Biol 32, 2776–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Fitzpatrick M, Wood WH 3rd, De S, Ejiogu N, Zhang Y, Mattison JA, Becker KG, Zonderman AB, Evans MK, 2013. Age-related changes in microRNA levels in serum. Aging (Albany NY) 5, 725–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR, 2007. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol 293, R145–151. [DOI] [PubMed] [Google Scholar]
- Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC, 2015. Interleukin-6 and C-Reactive Protein Levels and 9-Year Cognitive Decline in Community-Dwelling Older Women: The Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci 70, 873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez Roux AV, Shea S, Cushman M, Ni H, Seeman T, 2007. Socioeconomic position, race/ethnicity and inflammation in the Multi-Ethnic Study of Atherosclerosis. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Paola F, Maioli F, Martelli M, Montesi F, Bastagli L, Bianchin M, Chiappelli M, Tumini E, Bolondi L, Licastro F, 2006. Interleukin-1beta and interleukin-6 gene polymorphisms as risk factors for AD: a prospective study. Exp Gerontol 41, 85–92. [DOI] [PubMed] [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C, 2012. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 78, 720–727. [DOI] [PubMed] [Google Scholar]
- Selvin S, 2004. Statistical Analysis of Epidemiologic Data. Oxford University Press. [Google Scholar]
- Simpson EE, Hodkinson CF, Maylor EA, McCormack JM, Rae G, Strain S, Alexander HD, Wallace JM, 2013. Intracellular cytokine production and cognition in healthy older adults. Psychoneuroendocrinology 38, 2196–2208. [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR, 2010. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic medicine 72, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STATA, 2017. Statistics/Data Analysis: Release 15.0. Stata Corporation, Texas. [Google Scholar]
- Stepanikova I, Bateman LB, Oates GR, 2017. Systemic inflammation in midlife: race, socioeconomic status, and perceived discrimination. American journal of preventive medicine 52, S63–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Wu K, Kakizaki M, Tsuji I, Kawashima R, Fukuda H, 2013. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum Brain Mapp 34, 2418–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler C, O'Sullivan JL, Bucholtz N, Goldeck D, Pawelec G, Steinhagen-Thiessen E, Demuth I, 2016. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol Aging 38, 112–117. [DOI] [PubMed] [Google Scholar]
- Teixeira AL, Reis HJ, Coelho FM, Carneiro DS, Teixeira MM, Vieira LB, Mukhamedyarov MA, Zefirov AL, Janka Z, Palotas A, 2008. All-or-nothing type biphasic cytokine production of human lymphocytes after exposure to Alzheimer's beta-amyloid peptide. Biol Psychiatry 64, 891–895. [DOI] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, Samaras K, Crawford J, Lux O, Kochan NA, Brodaty H, Sachdev P, 2012. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 34, 1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, Crawford J, Brodaty H, Sachdev P, 2010. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord 30, 569–578. [DOI] [PubMed] [Google Scholar]
- Wada M, Takahashi Y, Iseki C, Kawanami T, Daimon M, Kato T, 2011. Plasma fibrinogen, global cognitive function, and cerebral small vessel disease: results of a cross-sectional study in community-dwelling Japanese elderly. Intern Med 50, 999–1007. [DOI] [PubMed] [Google Scholar]
- Walker KA, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Selvin E, Jack CR Jr., Gottesman RF, 2017. Midlife Systemic Inflammation, Late-Life White Matter Integrity, and Cerebral Small Vessel Disease: The Atherosclerosis Risk in Communities Study. Stroke 48, 3196–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KN, Beason-Held LL, Carlson O, Egan JM, An Y, Doshi J, Davatzikos C, Ferrucci L, Resnick SM, 2018. Elevated Markers of Inflammation Are Associated With Longitudinal Changes in Brain Function in Older Adults. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Kitamura K, Nakamura K, Sanpei K, Wakasugi M, Yokoseki A, Onodera O, Ikeuchi T, Kuwano R, Momotsu T, Narita I, Endo N, 2016. Elevated C-Reactive Protein Is Associated with Cognitive Decline in Outpatients of a General Hospital: The Project in Sado for Total Health (PROST). Dement Geriatr Cogn Dis Extra 6, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S, 2010. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, Hebert LE, Bennett DA, Wilson RS, Evans DA, 2018. Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology (Cambridge, Mass.) 29, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E, 2013. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ : British Medical Journal 347, f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T, 2003. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61, 76–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Participant flow chart
KEY: AF=Animal Fluency test; BTA=Brief Test of Attention; BVRT=Benton Visual Retention Test; CDT=Clock Drawing Test; CVLT-DFR= California Verbal Learning Test, Delayed Free Recall (List A); CVLT-List A= California Verbal Learning Test, immediate recall (List A); DS-B= Digit Span Backwards; DS-F=Digit Span Forward; HANDLS=Healthy Aging in Neighborhoods of Diversity Across the Life Span; Trails A=Trailmaking test, Part A; Trails B=Trailmaking test, Part B.
Note: Sample 1 is the initial visit 1, phase 1 selected HANDLS sample of adults aged 30-64y, Whites and African-Americans. Samples 1a-1c as the sub-samples with complete exposure measurements. Samples 2a through 4k are the sub-samples with complete exposure, outcome (at either of two visits) and covariate data.
Supplemental method 1: Description of cognitive tests, literacy and the CES-D
Supplemental Method 2: Description of mixed-effects regression models
Data Availability Statement
Data are available upon request to researchers with valid proposals who agree to the confidentiality agreement as required by our Institutional Review Board. We publicize our policies on our website https://handls.nih.gov. Requests for data access may be sent to the PIs or the study manager, Jennifer Norbeck at norbeckje@mail.nih.gov. These data are owned by the National Institute on Aging at the National Institutes of Health. The Principal Investigators, have restricted public access to these data because (1) the study collects medical, psychological, cognitive, and psychosocial information on racial and poverty differences that could be misconstrued or willfully manipulated to promote racial discrimination; and (2) although the sample is fairly large, there are sufficient identifiers that the PIs cannot guarantee absolute confidentiality for every participant as we have stated in acquiring our confidentiality certificate.