Abstract
Purpose
Percutaneous ablation techniques, including microwave ablation (MWA) and radiofrequency ablation (RFA), have become important minimally invasive treatment options for liver cancer. This systematic review compared MWA with RFA for treatment of liver cancer.
Methods
The systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search of MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials was conducted for randomized and observational studies published from 2006 onwards. A random-effects model was used for meta-analyses and local tumor progression (LTP), technique efficacy, overall survival (OS), disease-free survival (DFS), intrahepatic de novo lesions (IDL), extrahepatic metastases (EHM), length of stay (LOS), and complications were analyzed. Subgroup and sensitivity analyses were also conducted.
Results
Of 1379 studies identified, 28 randomized and observational studies met inclusion criteria. The main analysis demonstrated that LTP was significantly reduced by 30% with MWA versus RFA (RR=0.70; P=0.02) (all studies) and by 45% with MWA versus RFA (RR=0.55; P=0.007) (randomized studies only). There were no significant differences between MWA and RFA for other efficacy and safety outcomes. Higher frequency (2450 MHz) and larger tumor size (≥2.5 cm) are amongst variables that may be associated with improved outcomes for MWA. Sensitivity analyses were generally congruent with the main results.
Conclusion
MWA is at least as safe and effective as RFA for treating liver cancer and demonstrated significantly reduced LTP rates. Future studies should assess time and costs associated with these two treatment modalities.
Keywords: microwave ablation, radiofrequency ablation, hepatocellular carcinoma, meta-analysis, liver cancer
Introduction
Primary liver cancer is the second leading cause of cancer-related deaths and accounted for 788,000 mortalities in 2015.1 Surgical resection is considered the gold standard of treatment for curative intent but is often only possible in the early stages of hepatocellular carcinoma (HCC) and among those with limited cirrhosis.2 During the past ten years, percutaneous ablation has become an important minimally invasive alternative to surgery for liver cancer.3
Radiofrequency ablation (RFA) is currently the most widely used thermal ablation modality for unresectable, early-stage, hepatic malignancy.2 Microwave ablation (MWA), which was first introduced in 1994,4 has recently increased in use as a result of several significant advancements in the technology and improvements in the clinical application. These advancements include improvements in microwave applicator tissue attachment, spatially and synchronously distributed power to multiple antennas,5,6 and the development of internally cooled applicators with distal energy control.7–9
The primary clinical advantages these advancements in MWA have provided are higher temperatures and faster heating than RFA, shorter ablation times, larger ablation volumes, and less heat sink effect.8 Although MWA and RFA both destroy tissue via thermally induced coagulative necrosis and the frequencies of microwaves used in MWA are from the radiofrequency spectrum,10 the two ablative modalities differ in their mechanisms of energy deposition.11 RFA has limited effectiveness in tissues with low electrical conductivity (eg adipose tissue)8 since it requires an electrically conductive route through which to transfer resistive heat. RFA applies frequencies from 450 to 500 kHz to destroy tissues in the proximity of the electrode by causing friction that results in heating. The heating produced by RFA is maintained from 50°C to 100°C to avoid charring the tissue and rendering it electrically non conductive.8,11 Charred tissue acts as an insulator that prevents radiofrequency energy transfer to surrounding tissue, thus limiting ablation volumes. The optimal protocols for maximizing RFA ablation volumes involve using slow and methodical energy deposition. In contrast, MWA applies an electromagnetic field of either 915 or 2450 MHz to the tissue surrounding the antenna, heating it to >150°C via dielectric polarization11 and is most effective in tissues with high water content.8 The MWA direct heating mechanism leads to larger, more homogenous ablation zones than RFA, that are created more quickly.8,12 MWA is less susceptible to the heat sink effect11,13,14 which is the dissipation of heat via blood vessel perfusion.11,12 Reduced heat sink effect susceptibility may enable MWA to produce larger ablation zones. Larger and more uniform ablation zones with MWA may destroy neoplastic cells more effectively compared with RFA, potentially impacting local tumor progression (LTP).
Meta-analyses have compared MWA with RFA for the treatment of HCC.15–18 Generally, these studies have shown similar efficacy and safety between these modalities, with some benefit in LTP for MWA in larger hepatic tumors.17,18 However, several clinical studies, not included within these meta-analyses, have been published recently.19–29 Moreover, these meta-analyses have been limited by the type and number of outcomes included. The aim of this meta-analysis is to compare the efficacy and safety of MWA to RFA for the treatment of patients with HCC or liver metastasis. The meta-analysis included both randomized and observational studies; the outcomes were the rate of LTP, technique efficacy, overall survival (OS), disease-free survival (DFS), intrahepatic de novo lesions (IDL), extrahepatic metastasis (EHM), length of stay (LOS), and complications.
Methods
Search strategy
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.30 A systematic search of MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials was conducted for systematic reviews, randomized controlled trials (RCTs), and observational studies (prospective or retrospective cohort and case–control studies) using a search strategy developed by a medical information specialist that involved controlled vocabulary and keywords related to our research question (eg, “Liver Neoplasms”, “Microwave”, “Ablation Techniques”) (Appendix A). The search strategy was not limited by time or language; however, only English language articles published on or after January 1, 2006, were screened. The strategy was peer-reviewed by another senior information specialist prior to execution using the PRESS Checklist.31 Searches for the systematic reviews and RCTs were performed on October 29, 2017. A supplementary search for non-RCTs was performed on November 24, 2017. Reference lists of retrieved articles and relevant reviews were hand-searched. As this meta-analysis examined existing data from published studies, it was exempted from Institutional Review Board (IRB) approval.
Study selection
Specific inclusion criteria were defined according to PICOS (ie, population, intervention, comparator, outcomes, and study design). Studies were considered for inclusion in the meta-analysis if they were RCTs or observational studies comparing MWA with RFA in adults (≥18 years) with confirmed HCC or liver metastasis who either refused or were ineligible for surgery. Based on the inclusion criteria, the eligibility of each publication was evaluated in the title and abstract review. If the title and abstract suggested potential eligibility, a full-text screening followed. Reviewers were not blinded to the names of the authors or the institutions of the studies considered for inclusion, but no criteria were applied to include or exclude studies based on these parameters. Systematic reviews and meta-analyses were reviewed for insight and reference retrieval. Articles published before January 1, 2006, were excluded given the high likelihood that outdated technologies were used. Records were evaluated for eligibility by two independent reviewers and discrepancies were resolved either through consensus or by adjudication from a third reviewer.
Data extraction
Details (ie, baseline characteristics and outcomes) from the included studies were extracted using a standardized data extraction form developed in Microsoft Excel. The following study details were retrieved: study authors, publication year, study time frame, study design, country of origin, sample size, key patient characteristics (eg, age, diagnosis, average tumor size, average number of ablated tumors, duration of follow-up, etc.), intervention and comparator details [eg, microwave system, frequency, utilization of transarterial chemoembolization (TACE), transarterial embolization (TAE) etc.], and detailed outcomes data. Some studies did not report percentages in text for LTP, OS, and DFS, but did present Kaplan–Meier curves, which were utilized instead (extracted using DigitizeIt 2.2.2, Braunschweig, Germany). If studies did not report the number at risk at each time point, the denominator for number treated was assumed to be the initial sample size. Data were extracted by one reviewer and then checked for accuracy by a second reviewer with discrepancies resolved by consensus, or by adjudication from a third reviewer.
Study outcomes
LTP was defined as reappearance of tumors within or adjacent areas to the ablation zone.10 This definition aligned with many study definitions of “local recurrence”. If a study reported “recurrence” but did not specify whether it was local or distant, it was assumed to be local (only two studies).21,32 If more than one LTP value was reported, the latest value in the study was used in the meta-analysis. Other outcomes were 1) technique efficacy (typically defined as complete tumor ablation), measured at one week to three months post ablation;10 2) one-, three-, and five-year OS; 3) one-, three-, and five-year DFS; 4) IDL, defined as appearance of a new tumor at a new focus within liver (sometimes referred to in studies as “de novo lesions,” “intrahepatic metastasis,” or “distant recurrence”); 5) EHM, defined as appearance of a new tumor outside the liver; 6) length of hospital stay (days); and 7) overall complications, including any major or minor adverse events that were reported by the studies. For outcomes that were not typically measured at defined time points within the study (ie, LTP, IDL, EHM, and complications), study data were excluded if the study reported a large difference in mean follow-up time (ie, ≥25% difference) between treatment arms.
Risk of bias assessment
The quality of studies included in the meta-analyses was assessed using the Cochrane Risk of Bias (RoB) tool33 for RCTs and the Newcastle–Ottawa quality assessment Scale (NOS)34 for observational studies. The RoB tool assessed the following domains: sequence generation, allocation concealment, blinding, selective reporting, and other sources of bias. Each study was assigned a rating for each domain (ie, low, unclear, or high risk of bias).
The NOS assessed the following categories: selection (ie, representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study), comparability (comparability based on design and analysis), and outcome (assessment of the outcome, follow-up long enough for the outcome to occur, and adequacy of follow-up of cohorts). When assessing cohort representativeness, the studies received a star if they were representative of the HCC population: BCLC stage 0 or A (Child–Pugh A or B, single tumor ≤3 cm, or up to three nodules ≤3 cm).2 For studies of liver metastasis, no restrictions were placed on the source of the primary tumor. For comparability, studies received a star if treatment groups were balanced on the potential effect modifiers of Child–Pugh class (HCC studies) and tumor size and primary origin (metastatic studies). Studies also received a star if they used a matching design, or regression analyses indicated that Child–Pugh class or tumor size and primary origin were not predictors of outcomes. Studies received an additional star if they were balanced on additional potential effect modifiers [ie, age, gender, tumor size (for HCC), surgical approach, tumor location], or they used a matching design, or regression analyses indicated that these variables were not predictors of outcomes. For outcome assessment, a study received a star if the follow-up time was at least six months. Studies also received a star if loss to follow-up was less than 20%.33 In total, each observational study could obtain a maximum of nine stars. The quality of included studies was assessed by two reviewers and reconciled by a third reviewer, if required.
Data synthesis and statistical methods
The DerSimonian–Laird random-effects model was used for the meta-analyses and forest plots were generated. For continuous outcomes (ie, LOS), the weighted mean difference (WMD) and its corresponding 95% CI were calculated. For dichotomous outcomes (all outcomes except LOS), the relative risk (RR) and the corresponding 95% CIs were calculated. As follow-up times varied across studies, a random-effects meta-regression analysis was completed to assess the association between mean study follow-up time and the treatment effect for LTP. All analyses were conducted for RCTs alone, observational studies alone, and the combination of RCTs and observational studies.
An I2 value was generated to describe the percentage of variance attributable to heterogeneity among studies.33 The following ranges were used to interpret I2 values: 0–40% represented minimal heterogeneity, 30–60% represented moderate heterogeneity, 50–90% represented substantial heterogeneity, and 75–90% represented considerable heterogeneity.33 The following subgroup analyses were conducted for LTP, one-year OS, complications, and technique efficacy outcomes: 1) tumor size (<2.5 versus ≥2.5 cm); 2) type of liver tumor (HCC versus metastasis); 3) impact of adding another treatment to both arms (MWA and RFA versus MWA+TACE and RFA+TACE); and 4) MWA frequency (915 versus 2450 MHz). For the subgroup analysis assessing the effects of the type of liver tumor, any study that included patients with metastatic tumors was classified as a metastatic study. Additionally, several sensitivity analyses were performed to assess the impact of alternative methods (ie, fixed-effects model), study quality (ie, exclusion of lower quality studies, defined as any RCT with high risk for any domain of the RoB tool or any observational study with ≤7 stars on the NOS), and surgery type (ie, exclusion of open surgical approach). Publication bias was examined using funnel plots for outcomes reported by 10 or more studies.35 Data were analyzed using STATA (Version 15.1, Texas, USA).
Results
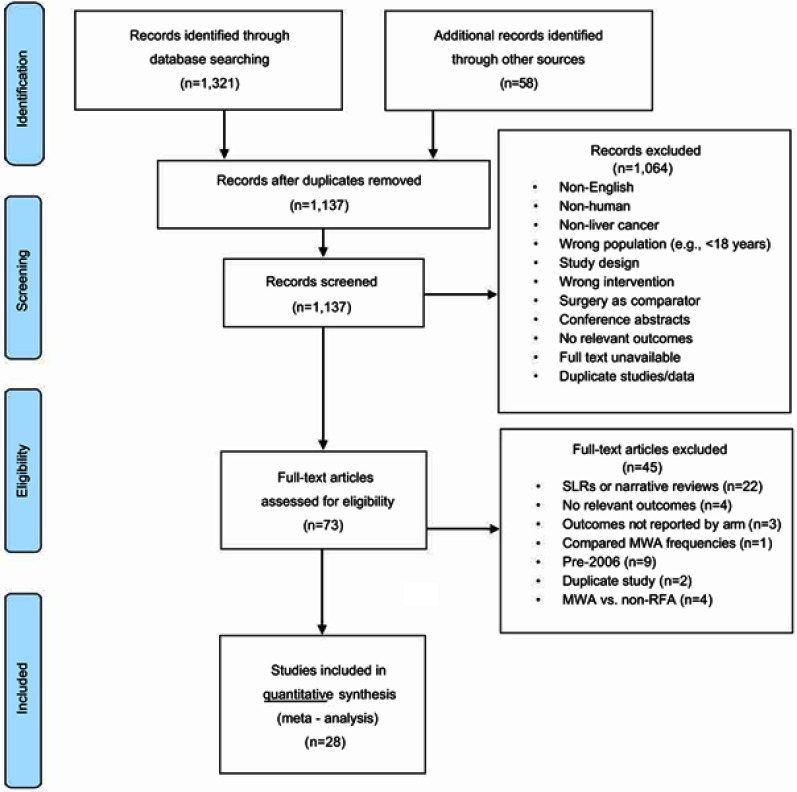
A total of 1379 citations were identified from database searching. After removing duplicates, 1137 unique records were screened. Of these, 1064 studies were excluded at the abstract screening phase for several reasons (eg, non-human, not English, not liver cancer, etc.) (Figure 1). Seventy-three articles were screened at the full-text stage. Of these, 45 articles were further excluded if the studies were systematic or narrative reviews (n=22), were published prior to 2006 (n=9), reported irrelevant outcomes (n=4), utilized non surgical modalities other than RFA as the comparator (n=4), did not report outcomes by treatment arm (n=3), were duplicates (n=2), and were comparing MWA frequencies (n=1). The four studies that utilized non surgical modalities other than RFA (not included in the meta-analysis) compared MWA to TACE,36 IRE,37 RFA+TACE (did not include TACE in the intervention arm),38 and systemic chemotherapy.39 Twenty-eight studies, consisting of a total of 3531 patients, that compared MWA versus RFA (24 studies) or MWA+TACE/TAE versus RFA+TACE/TAE (4 studies) were included in the meta-analyses.19–29,32,40–55 The demographics of the included studies followed historical patterns for gender56 and geography.57
Figure 1.
PRISMA flow diagram.Abbreviations: PRISM, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR, systematic literature review; MWA, microwave ablation; RFA, radiofrequency ablation.
Study characteristics are presented in Table 1. The sample size of the included studies (four RCTs and 24 observational studies) ranged from 19 to 460 patients and study follow-up duration ranged from five to 62 months. The average age across studies ranged from 50.4 to 69.4 years. In total, most studies (n=10) originated from China. Other regions included USA (n=7), Egypt (n=3), Italy (n=3), Japan (n=2), Australia (n=1), Belgium (n=1), and the Netherlands (n=1). Retreatment after the initial ablation session was reported by 16 studies (Table S1).19,23–29,42,48–51,53–55 Of these 16 studies, ten reported retreatment with the same type of thermal ablation that the patient initially received,26,42,50,51,54,55 but only seven of them reported the number of patients that received retreatment.19,25,26,29,51,54,55 Other types of retreatments with chemotherapy,28,49 TACE,19,23,29,48 resection,19,25 MWA or RFA,19 and radiotherapy19,25 were reported by eight studies. The number of retreated patients were generally similar for MWA and RFA across most studies (n=14), with a few notable exceptions.26,54 Four studies reported patients receiving liver transplants after thermal ablation.24,26,27,53
Table 1.
Study and baseline characteristics for studies included in the meta-analysis
| First author | Year | Study design | Region | Population | Intervention | Comparator | Patients (N) | Mean age | Male (%) | Follow-up duration (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWA | RFA | MWA | RFA | |||||||||
| Abdelaziz | 2014 | RCT | Egypt | HCC | MWA | RFA | 66 | 45 | 54.90 | 71 | NR | NR |
| Chinnaratha | 2015 | Retrospective cohort | Australia | HCC | MWA | RFA | 25 | 101 | 62.10 | 78 | 14 | 14 |
| Cillo | 2014 | Prospective observational; propensity score matched | Italy | HCC | MWA | RFA | 28 | 28 | 64.00 | 82 | 24 | NR |
| Correa-Gallego | 2014 | Retrospective matched cohort | USA | Metastatic | MWA | RFA | 67 | 67 | 55.50 | NR | 18 | 31 |
| Di Vece | 2014 | RCT | Italy | HCC/Metastatic | MWA | RFA | 20 | 20 | 61.00 | 73 | NR | NR |
| Ding | 2013 | Retrospective cohort | China | HCC | MWA | RFA | 113 | 85 | 58.82 | 77 | 18 | 28 |
| Ginsburg | 2015 | Retrospective cohort | USA | HCC | MWA+TACE | RFA+TACE | 51 | 38 | 65.29 | 38 | 28 | 56 |
| Hompes | 2010 | Retrospective cohort case-matched | Belgium | Metastatic | MWA | RFA | 6 | 13 | 59.89 | 47 | 6 | NR |
| Kuanga | 2011 | Prospective observational | China | HCC | MWA | RFA | 19 | 31 | 55.00 | 94 | 45 | 45 |
| Lee | 2017 | Retrospective cohort case-matched | China | HCC | MWA | RFA | 26 | 47 | 59.60 | 71 | 48 | 53 |
| Liu | 2013 | Retrospective cohort | China | Metastatic | MWA | RFA | 35 | 54 | 53.20 | 61 | 32 | 32 |
| Ohmoto | 2009 | Retrospective cohort | Japan | HCC | MWA | RFA | 49 | 34 | 65.23 | 80 | 34 | 26 |
| Potretzke | 2016 | Retrospective cohort | USA | HCC | MWA | RFA | 99 | 55 | 61.36 | 79 | 24 | 31 |
| Qian | 2012 | Prospective observational | China | HCC | MWA | RFA | 22 | 20 | 53.90 | 93 | 5 | 5 |
| Sakaguchi | 2009 | Retrospective cohort | Japan | HCC | MWA | RFA | 142 | 249 | 65.35 | 71 | NR | NR |
| Santambrogio | 2017 | Retrospective cohort | Italy | HCC | MWA | RFA | 60 | 94 | 69.39 | 73 | 27 | 27 |
| Shady | 2017 | Retrospective cohort | USA | Metastatic | MWA | RFA | 48 | 62 | NR | 66 | 29 | 56 |
| Shetaa | 2016 | RCT | Egypt | HCC | MWA+TACE | RFA+TACE | 10 | 20 | NR | NR | 6 | 6 |
| Simo | 2011 | Retrospective cohort | USA | HCC | MWA | RFA | 13 | 22 | 58.61 | 74 | 7 | 19 |
| Thornton | 2017 | Retrospective cohort | USA | HCC | MWA+TAE/TACE | RFA+TAE/TACE | 20 | 15 | 64.63 | 83 | 14 | 18 |
| van Tilborg | 2016 | Retrospective cohort | Netherlands | Metastatic | MWA | RFA | 15 | 96 | 61.27 | 65 | 31 | NR |
| Vasnani | 2016 | Retrospective cohort | USA | HCC | MWA+TACE | RFA+TACE | 31 | 11 | 58.31 | 64 | NR | NR |
| Vogl | 2015 | Retrospective cohort | Egypt | HCC | MWA | RFA | 28 | 25 | 58.58 | 79 | NR | NR |
| Xu | 2017 | Retrospective cohort | China | HCC | MWA | RFA | 301 | 159 | 54.13 | 80 | 53 | 62 |
| Yang | 2017 | Retrospective cohort | China | Metastatic | MWA | RFA | 71 | 108 | 50.40 | 65 | 39 | 39 |
| Yin | 2009 | Prospective observational | China | HCC | MWA | RFA | 50 | 59 | 53.00 | 87 | 22 | 22 |
| Yu | 2017 | RCT | China | HCC | MWA | RFA | 203 | 200 | NR | NR | 35 | 35 |
| Zhang | 2013 | Prospective observational | China | HCC | MWA | RFA | 77 | 78 | 54.00 | 85 | 25 | 26 |
Note: aThree-armed study.
Abbreviations: MWA, microwave ablation; NR, not reported; RCT, randomized control trial; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TAE, transarterial embolization.
Table S1.
Summary of patient retreatment after initial thermal ablation with MWA or RFA
| First Author | Year | Study Design | Region | Population | Intervention | Comparator | Retreatment Methods | Number of Retreated Patients |
|---|---|---|---|---|---|---|---|---|
| Abdelaziz, A | 2014 | RCT | Egypt | HCC | MWA | RFA | - | - |
| Chinnaratha, MA | 2015 | Retrospective cohort | Australia | HCC | MWA | RFA | Repeat thermal ablation until complete radiological ablation. | - |
| Cillo, U | 2014 | Prospective observational; propensity score matched | Italy | HCC | MWA | RFA | - | - |
| Correa-Gallego, C | 2014 | Retrospective matched cohort | USA | Metastatic | MWA | RFA | - | - |
| Di Vece, F | 2014 | RCT | Italy | HCC/Metastatic | MWA | RFA | - | - |
| Ding, J | 2013 | Retrospective cohort | China | HCC | MWA | RFA | - | - |
| Ginsburg, M | 2015 | Retrospective cohort | USA | HCC | MWA+TACE | RFA+TACE | - | - |
| Hompes, R | 2010 | Retrospective cohort case-matched | Belgium | Metastatic | MWA | RFA | - | - |
| Kuang, Ma | 2011 | Prospective observational | China | HCC | MWA | RFA | Repeat ablation with initially received treatment for cases with late failure | 1 MWA and 1 RFA patient treated with resection or TACE |
| Lee, KF | 2017 | Retrospective cohort case-matched | China | HCC | MWA | RFA | Patients with residual tumors were retreated | Patients received RFA (n=2) or TACE (n=2) |
| Liu, Y | 2013 | Retrospective cohort | China | Metastatic | MWA | RFA | Post ablation systemic chemotherapy | 72 patients (34 colorectal mets, 38 others) received chemotherapy |
| Ohmoto, K | 2009 | Retrospective cohort | Japan | HCC | MWA | RFA | Additional MWA or RFA was performed whenever possible on detection of recurrence | - |
| Potretzke, TA | 2016 | Retrospective cohort | USA | HCC | MWA | RFA | Liver transplants | 18 RFA and 20 MWA patients received transplants |
| Qian, GJ | 2012 | Prospective observational | China | HCC | MWA | RFA | Additional MWA or RFA was performed if complete ablation was not achieved | 1 RFA and 1 MWA patient received repeat ablation |
| Sakaguchi, H | 2009 | Retrospective cohort | Japan | HCC | MWA | RFA | - | - |
| Santambrogio, R | 2017 | Retrospective cohort | Italy | HCC | MWA | RFA | Additional ablation or TACE for incomplete local response | - |
| Shady, W | 2017 | Retrospective cohort | USA | Metastatic | MWA | RFA | - | - |
| Sheta, Ea | 2016 | RCT | Egypt | HCC | MWA+TACE | RFA+TACE | - | - |
| Simo, KA | 2011 | Retrospective cohort | USA | HCC | MWA | RFA | Liver transplant post ablation | 2 MWA and 5 RFA received liver transplants. |
| Thornton, LM | 2017 | Retrospective cohort | USA | HCC | MWA+TAE/TACE | RFA+TAE/TACE | Alternative treatment for field progression or multifocal disease | Alternate treatment received by 2 RFA and 4 MWA patients. 2 RFA patients and 5 MWA patients received transplants. |
| van Tilborg, AA | 2016 | Retrospective cohort | Netherlands | Metastatic | MWA | RFA | Retreatment or additional treatment for recurrence | Successful retreatments. RFA patients: 9 re-RFA, 3 MWA, 2 resection, 1 stereotactic radiotherapy. MWA patients: 5 re-MWA, 3 RFA, 2 resection |
| Vasnani, R | 2016 | Retrospective cohort | USA | HCC | MWA+TACE | RFA+TACE | Retreatment for patients without complete response with initial combination. Liver transplantation. | 4 RFA patients and 9 MWA patients had repeat treatment. 1 RFA patient had liver transplant and 8 MWA patients had liver transplant before follow-up imaging. 6 MWA patients had LT before complete response |
| Vogl, TJ | 2015 | Retrospective cohort | Egypt | HCC | MWA | RFA | - | - |
| Xu, Y | 2017 | Retrospective cohort | China | HCC | MWA | RFA | Retreatment for LTP and distant recurrence based on disease stage | MWA LTP patients n=29: 27 re-MWA, 4 resection. RFA LTP patients n=16: 14 re-RFA, 2 resection. MWA distant recurrence patients n=122: 91 re-MWA, 18 TACE, 11 resection, and 2 radiation therapy. RFA distant recurrence patients n=76: 58 re-RFA, 9 TACE, 8 resection, and 1 radiation therapy. |
| Yang, B | 2017 | Retrospective cohort | China | Metastatic | MWA | RFA | Repeat ablation or chemotherapy for patients without complete response | - |
| Yin, XY | 2009 | Prospective observational | China | HCC | MWA | RFA | Retreatment of local and distant recurrence with RFA or MWA if applicable | 12 RFA patients and 4 MWA patients received repeat ablation |
| Yu, J | 2017 | RCT | China | HCC | MWA | RFA | - | - |
| Zhang, L | 2013 | Prospective observational | China | HCC | MWA | RFA | Retreatment with MWA or RFA for incomplete ablation | 15 RFA and 14 MWA patients received repeat ablation |
Abbreviations: LTP, local tumor progression; MWA, microwave ablation; -, not reported; RCT, randomized control trial; RFA, radiofrequency ablation; TACE; transarterial chemoembolization; TAE; transarterial embolization.
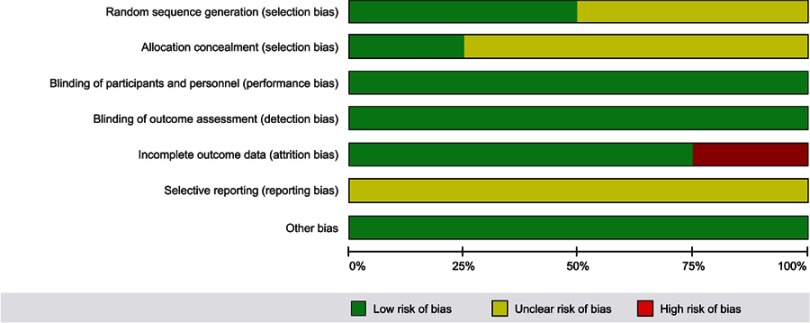
Quality assessment
RCTs
Risk of bias assessments and individual study quality assessments are presented in Table S2 and Figure S1. Overall, the quality of studies was acceptable, with most studies having low or unclear risk of bias across most domains. Two studies reported the methods for random sequence generation (ie, coin flip and computer-generated)40,41 and the other two studies20,21 were assigned an unclear risk of selection bias as no information was provided. The methods for allocation concealment were defined by one study (centralized computer-generated allocation list)41 and the other three studies20,21,40 were assigned an unclear risk of selection bias. The risk of bias associated with blinding of patients or outcome assessors was considered to be low for outcomes because of their objectivity. For three studies, patient withdrawals, loss to follow-up, and missing data were minimal. However, one study reported a loss to follow-up of greater than 20% and was assigned high risk for attrition bias due to incomplete OS data.40 The bias associated with selective reporting was unclear for all studies.
Table S2.
Methodological quality assessment of RCTs using the Cochrane RoB tool
| Study | Random Sequence Generation | Allocation Concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Abdelaziz 2014 | Low | Unclear | Low | Low | High | Unclear | Low |
| Di Vece 2014 | Low | Low | Low | Low | Low | Unclear | Low |
| Sheta 2016 | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Yu 2017 | Unclear | Unclear | Low | Low | Low | Unclear | Low |
Abbreviations: RoB, risk of bias; RCT, randomized control trial.
Observational studies
The NOS scores for observational studies are presented in Table S3. The 24 observational studies were given scores that ranged from 7 to 9; the studies varied in terms of comparability and adequacy of follow-up. All studies were either truly or somewhat representative of the exposed cohort, drew the non exposed cohort from the same community as the exposed cohort, and used secure records for exposure ascertainment. Most studies received two stars for comparability; however, seven studies received one star as they did not report Child–Pugh classification,24,51 or reported differences in patient age, sex, ablation approach, tumor size, or primary origin between treatment arms. These variables were either not controlled for, or adjusted analyses showed that one or more of them affected outcomes.23,25,28,47,53 Only one study received no stars for comparability because it did not report Child–Pugh classification and reported significant differences between treatment arms for patient age and tumor size.32 Additionally, one study did not receive a star for the adequacy of the follow-up period as it did not report the follow-up time for the RFA group.47 There were no studies that had a loss to follow-up that could potentially impact results (ie, >20%).
Table S3.
Methodological quality assessment of observational studies using the NOS scalea
| Study | Representativeness of Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration that outcome of interest was not present at the start of the study | Comparability of the cohorts on the basis of design or analysis | Assessment of outcome | Was the follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|
| Chinnaratha 2015 | * | * | * | * | ** | * | * | * | 9 |
| Cillo 2014 | * | * | * | * | ** | * | * | * | 9 |
| Correa-Gallego 2014 | * | * | * | * | ** | * | * | * | 9 |
| Ding 2013 | * | * | * | * | ** | * | * | * | 9 |
| Ginsburg 2015 | * | * | * | * | ** | * | * | * | 9 |
| Hompes 2010 | * | * | * | * | * | * | * | 7 | |
| Kuang 2011 | * | * | * | * | ** | * | * | * | 9 |
| Lee 2017 | * | * | * | * | * | * | * | * | 8 |
| Liu 2013 | * | * | * | * | ** | * | * | * | 9 |
| Ohmoto 2009 | * | * | * | * | ** | * | * | * | 9 |
| Potretzke 2016 | * | * | * | * | * | * | * | * | 8 |
| Qian 2012 | * | * | * | * | * | * | * | * | 8 |
| Sakaguchi 2009 | * | * | * | * | ** | * | * | * | 9 |
| Santambrogio 2017 | * | * | * | * | * | * | * | * | 9 |
| Shady 2017 | * | * | * | * | ** | * | * | * | 9 |
| Simo 2011 | * | * | * | * | * | * | * | * | 8 |
| Thornton 2017 | * | * | * | * | ** | * | * | * | 9 |
| van Tilborg 2016 | * | * | * | * | * | * | * | * | 8 |
| Vasnani 2016 | * | * | * | * | ** | * | * | * | 9 |
| Vogl 2015 | * | * | * | * | * | * | * | 7 | |
| Xu 2017 | * | * | * | * | ** | * | * | * | 9 |
| Yang 2017 | * | * | * | * | * | * | * | * | 8 |
| Yin 2009 | * | * | * | * | ** | * | * | * | 9 |
| Zhang 2013 | * | * | * | * | ** | * | * | * | 9 |
Notes: a The NOS scale has eight categories in which stars may be awarded for the study meeting the quality assessment criterion. For the comparability of cohorts on the basis of design or analysis category, up to two stars may be awarded (**); all other categories can be awarded a maximum of one star (*). The maximum number of stars awarded per study is nine and the total stars awarded to each study are summarized in the total column.
Abbreviation: NOS, Newcastle Ottawa Scale.
Analysis
LTP
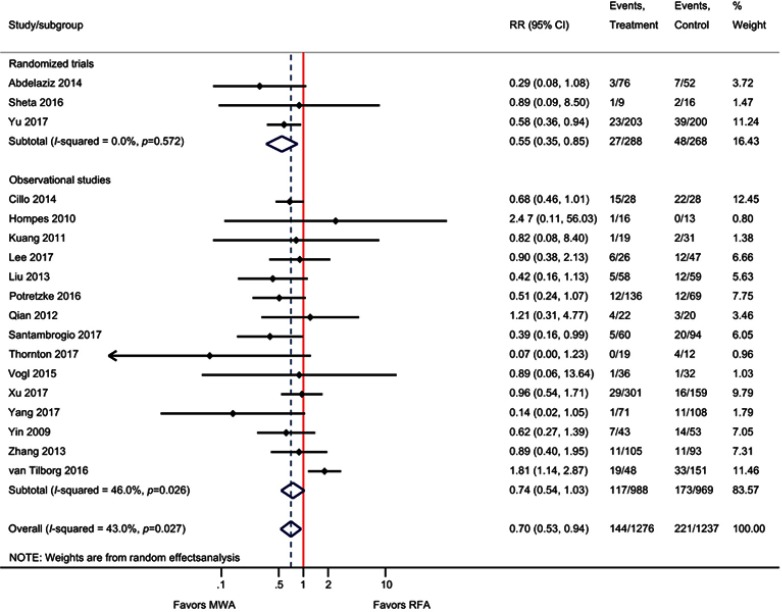
For the outcome of LTP, three RCTs and 15 observational studies were included. The meta-analysis demonstrated that the risk of LTP was significantly reduced by 30% with MWA compared with RFA (RR=0.70; P=0.02) (Table 2, Figure 2). For the three RCTs only, LTP was significantly reduced by 45% with MWA (RR=0.55; P=0.007). Meta-regression indicated that average study follow-up duration did not impact LTP outcomes (P=0.78). A sensitivity analysis excluding studies that reported “recurrence,” but did not specify “local recurrence”21,32 was also performed; results remained consistent with the main analysis (RR=0.69, 95% CI 0.51–0.94, P=0.02).
Table 2.
Summary of analyses for MWA compared with RFA
| Outcome | Number of studies included in meta-analysis | Summary effecta (95% CI); P value | Heterogeneity (I2 value) |
|---|---|---|---|
| LTP | 18 | 0.70 (0.53, 0.94); P=0.02 | 43% |
| Technique efficacy | 18 | 1.01 (0.99, 1.03); P=0.25 | 13% |
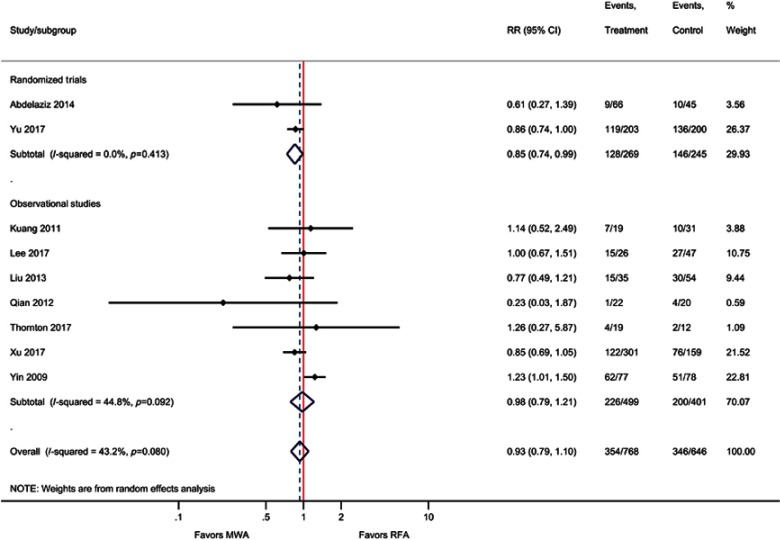
| IDL | 9 | 0.93 (0.79, 1.10); P=0.40 | 43% |
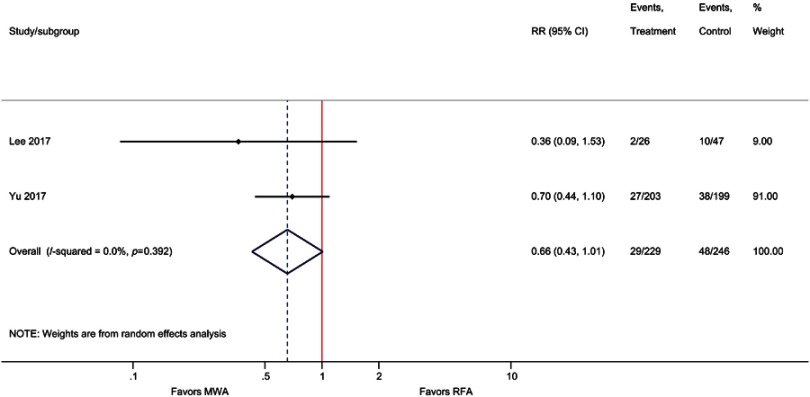
| EHM | 2 | 0.66 (0.43, 1.01); P=0.06 | 0% |
| OS (1-Year) | 16 | 1.00 (0.98, 1.02); P=0.80 | 26% |
| OS (3-Year) | 14 | 1.03 (0.97, 1.09); P=0.40 | 37% |
| OS (5-Year) | 9 | 1.03 (0.93, 1.13); P=0.60 | 33% |
| DFS (1-Year) | 8 | 1.00 (0.96, 1.04); P=0.93 | 13% |
| DFS (3-Year) | 7 | 1.05 (0.96, 1.14); P=0.27 | 0% |
| DFS (5-Year) | 5 | 0.97 (0.71, 1.33); P=0.86 | 71% |
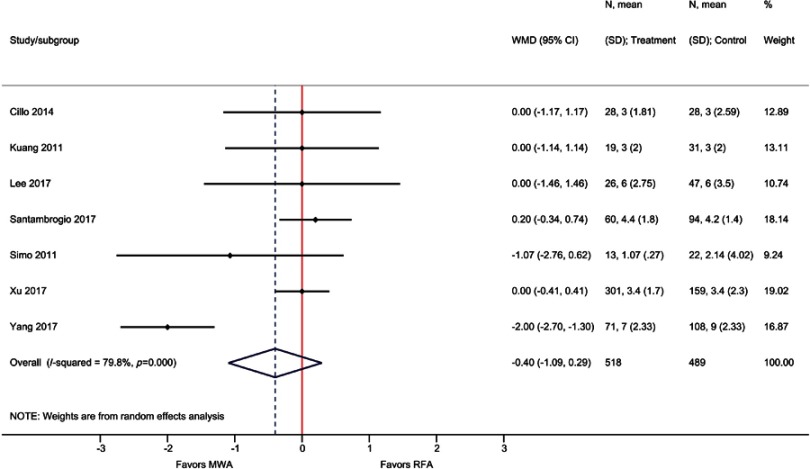
| Length of hospital stay (days) | 7 | −0.40 (−1.09, 0.29); P=0.26 | 80% |
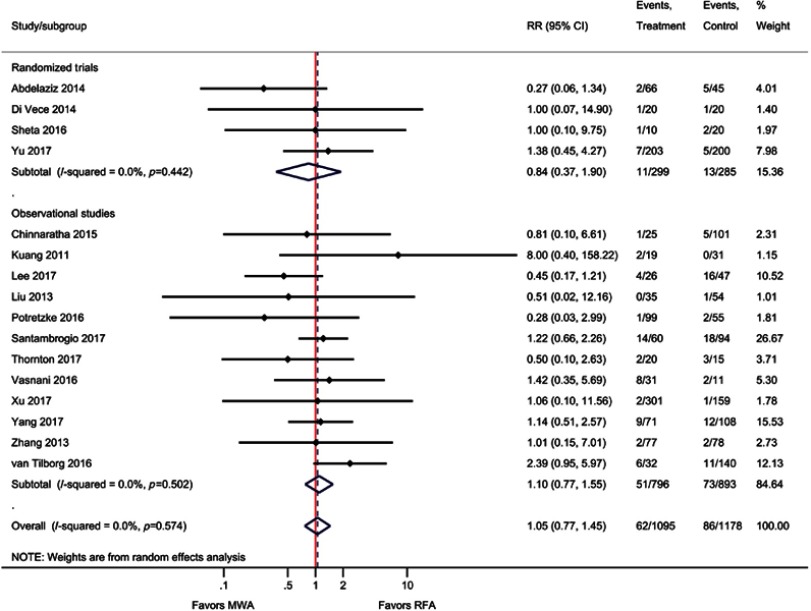
| Complications | 16 | 1.05 (0.77, 1.45); P=0.75 | 0% |
Notes: aRR for MWA versus RFA for all outcomes except length of hospital stay, which is reported as the WMD. Point estimates and CIs were calculated using a random-effects model.
Abbreviations: DFS, disease-free survival; EHM, extrahepatic metastasis; IDL, intrahepatic de novo lesions; LTP, local tumor progression; MWA, microwave ablation; OS, overall survival; RFA, radiofrequency ablation; RR, risk ratio; WMD, weighted mean difference.
Figure 2.
Forest plot of random-effects meta-analysis results for LTP (P=0.02), stratified by RCTs (P=0.01) versus observational studies (P=0.07).
Abbreviations: LTP, local tumor progression; RCT, randomized control trial.
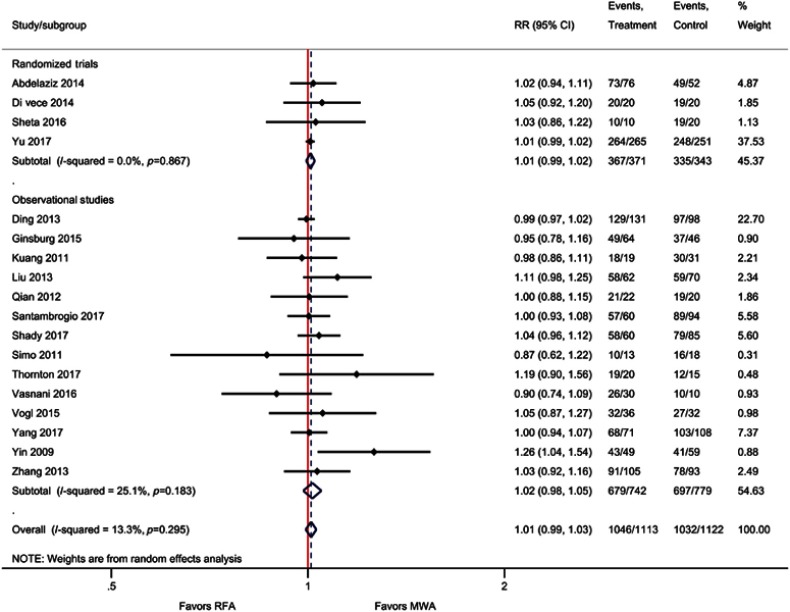
Technique efficacy
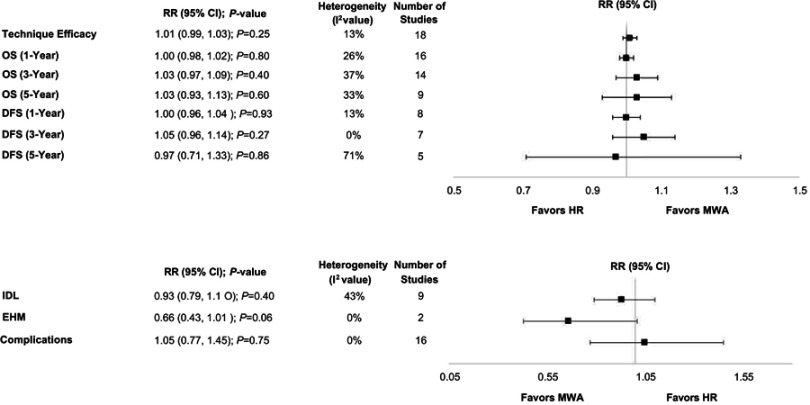
Technique efficacy measured at one week to three months post ablation was reported by four RCTs and 14 observational studies. One RCT reported technique efficacy at one week40 and three studies did not specify the time point at which technique efficacy was assessed.20,26,51 The meta-analysis demonstrated that technique efficacy was not significantly different between MWA and RFA (RR=1.01; P=0.25). For the four RCTs only, there were also no significant differences (RR=1.01; P=0.23) (Table 2, Figure 4, Figure S2). A sensitivity analysis excluding studies that reported technique efficacy at one-week40 or an unspecified time point20,26,51 was also performed; results remained consistent with the main analyses (RR=1.02, 95% CI 0.99–1.05; P=0.20).
Figure 4.
Summary of meta-analyses.
Abbreviations: DFS, disease-free survival; EHM, extrahepatic metastasis; IDL, intrahepatic de novo lesions; OS, overall survival.
Overall survival
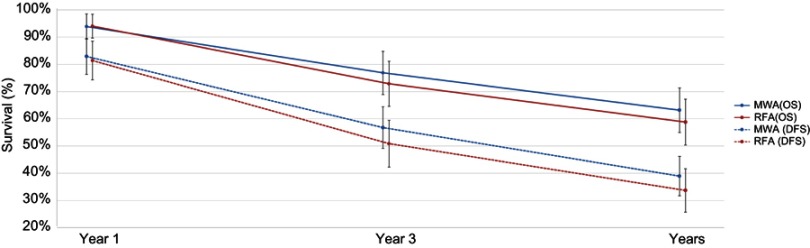
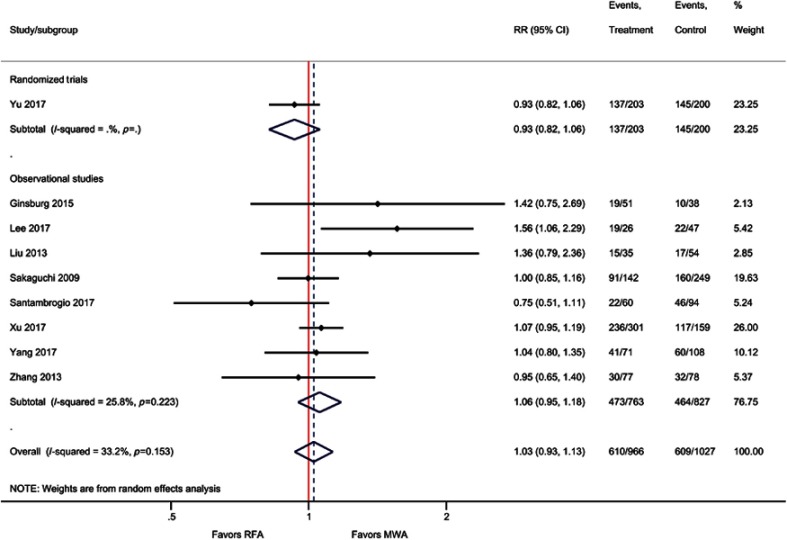
Based on sample size, weighted averages demonstrated that OS at three years (77% versus 73%) and five years (63% versus 59%) was higher with MWA compared with RFA, respectively (Figure 3), although the meta-analyses indicated no significant differences [one-year OS (RR=1.00; P=0.80), three-year OS (RR=1.03; P=0.40), and five-year OS (RR=1.03; P=0.60)]. For the two RCTs only, one-year OS was not significantly different between MWA and RFA (RR=1.17; P=0.43) (Table 2, Figure 4, Figure S3–S5).
Figure 3.
Weighted one-, three-, and five-year OS and DFS for MWA and RFA.
Notes: The error bars represent the 95% CIs for each estimate.
Abbreviations: DFS, disease-free survival; MWA, microwave ablation; OS, overall survival; RFA, radiofrequency ablation.
DFS
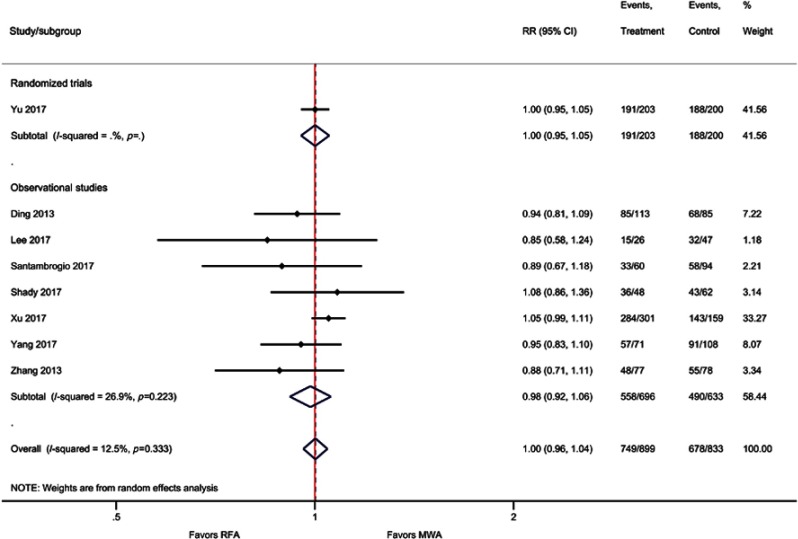
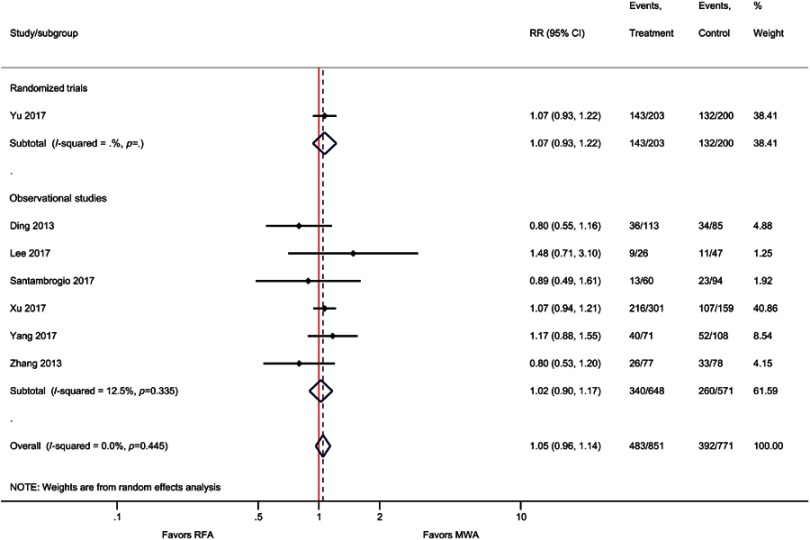
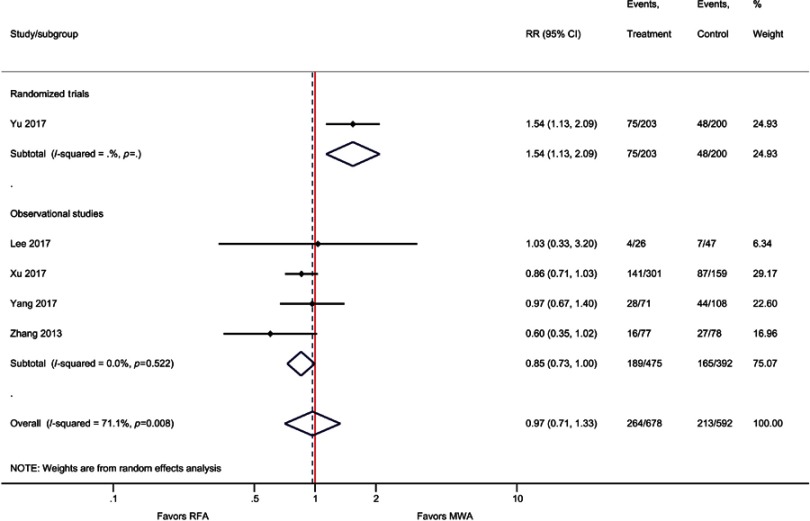
Similar to OS, weighted averages showed that DFS at one year (83% versus 81%), three years (57% versus 51%), and five years (39% versus 34%) was higher with MWA as compared with RFA (Figure 3); however, the meta-analysis indicated these differences were not statistically significant [one-year (RR=1.00; P=0.93), three-year (RR=1.05; P=0.27), and five-year (RR=0.97; P=0.86) DFS] (Table 2, Figure 4, Figure S6–S8).
IDL and EHM
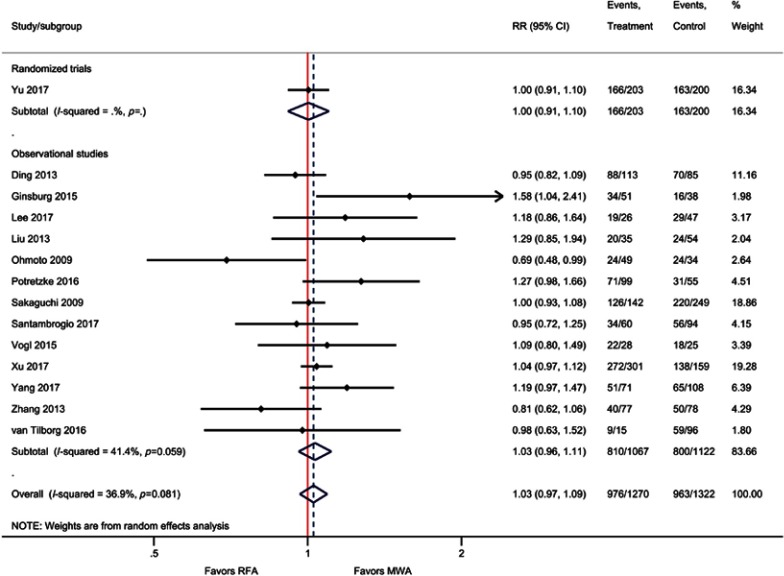
Although not statistically significant, the results of the meta-analysis for EHM favored MWA compared with RFA, that is, the incidence of EHM was 34% lower with MWA compared with RFA (RR=0.66; P=0.06). Similarly, for the outcome of IDL, MWA risk was non significantly lower compared with RFA (RR=0.93; P=0.40). When only pooling RCTs, IDL was significantly reduced by 15% with MWA vs RFA (RR=0.85, P=0.03) (Table 2, Figure 4, Figure S9–S10).
LOS
Although not statistically significant, LOS was lower by 0.4 days with MWA compared with RFA based on seven observational studies (WMD=−0.40 days; P=0.26) (Table 2, Figure S11).
Overall complications
The main reported complications for both study groups included bleeding, hematoma, portal vein thrombosis, pleural effusions, pneumothorax, ascites, infections, and fever. The risk of complications was not significantly different between MWA and RFA overall (RR=1.05; P=0.75) or for the four RCTs (RR=0.84; P=0.68) (Table 2, Figure 4, Figure S12).
Subgroup analyses
Tumor size (<2.5 versus >2.5 cm)
Results showed that there were no statistically significant differences between MWA and RFA regardless of tumor size for one-year OS, complications, and technique efficacy outcomes (Table 3). However, for the outcome of LTP, MWA was associated with a significant reduction of 37% (RR=0.63; P=0.001) compared with RFA, among patients with tumor sizes ≥2.5 cm (Table 3).
Table 3.
Summary of subgroup analyses for MWA compared with RFA
| Subgroup | LTP [RR (95% CI); P value; studies (N); I2] | OS (1-Year) [RR (95% CI); P value; studies (N); I2] | Complications [RR (95% CI); P value; studies (N); I2] | Technique efficacy [RR (95% CI); P value; studies (N); I2] |
|---|---|---|---|---|
| Tumor size | ||||
| <2.5 cm | 0.72 (0.41,1.29); P=0.27; 8; 67% | 0.98 (0.95,1.01); P=0.26; 9; 61% | 1.27 (0.84,1.91); P=0.26; 7; 0% | 1.01 (0.98,1.04); P=0.53; 6; 14% |
| ≥2.5 cm | 0.63 (0.49,0.82); P=0.001; 8; 0% | 1.06 (0.97,1.16); P=0.22; 6; 53% | 0.71 (0.42,1.23); P=0.22; 7; 0% | 1.02 (0.97,1.08); P=0.38; 10; 35% |
| Type of tumor | ||||
| HCC | 0.67 (0.54,0.82); P<0.001; 14; 0% | 1.00 (0.98,1.02); P=0.87; 13; 19% | 0.91 (0.62,1.33); P=0.62; 12; 0% | 1.01 (0.99,1.02); P=0.41; 14; 2% |
| Liver metastasis | 0.71 (0.19,2.67); P=0.61; 4; 78% | 0.90 (0.82,0.99); P=0.04; 3; 0% | 1.49 (0.83,2.66); P=0.18; 4; 0% | 1.03 (0.99,1.08); P=0.13; 4; 0% |
| Intervention and comparator | ||||
| MWA and RFA | 0.72 (0.54,0.96); P=0.02; 16; 44% | 1.00 (0.98,1.02); P=0.80; 15; 31% | 1.06 (0.75,1.51); P=0.74; 13; 3% | 1.01 (0.99,1.04); P=0.22; 14; 30% |
| MWA+TACE and RFA+TACE | 0.29 (0.02,3.66); P=0.34; 2; 49% | Too few studies (<2) to inform | 0.94 (0.36,2.46); P=0.90; 3; 0% | 0.99 (0.89,1.10); P=0.85; 4; 2% |
| MWA frequency | ||||
| 2450 MHz | 0.67 (0.53,0.84); P<0.001; 13; 0% | 1.00 (0.97,1.03); P=0.94; 10; 34% | 0.82 (0.54,1.24); P=0.345; 12; 0% | 1.03 (0.99,1.07); P=0.13; 12; 42% |
| 915 MHz | 1.79 (1.14,2.81); P=0.01; 3; 0% | 0.92 (0.78,1.08); P=0.31; 2; 9% | 2.04 (0.95,4.38); P=0.07; 2; 0% | 0.96 (0.86,1.06); P=0.41; 4; 0% |
Abbreviations: HCC, hepatocellular carcinoma; MWA, microwave ablation; RFA, radiofrequency ablation; RR, risk ratio; TACE, transarterial chemoembolization.
Type of tumor (HCC versus metastasis)
No statistically significant differences were reported between MWA and RFA for complications and technique efficacy regardless of tumor type (Table 3). However, LTP was statistically significantly reduced by 33% with MWA compared with RFA among patients with HCC (RR=0.67; P<0.001). Although LTP was also lower in patients with liver metastasis with MWA versus RFA, the difference was not significant (RR=0.71; P=0.61). Also, among patients with liver metastasis, MWA was associated with lower survival at one-year compared with MWA (RR=0.90; P=0.04); however, differences were not statistically significant in HCC patients (RR=1.00; P=0.87). (Table 3).
Adding another treatment to both arms (MWA and RFA versus MWA + TACE and RFA + TACE)
There were no significant differences between MWA and RFA for one-year OS, complications, and technique efficacy, regardless of whether TACE was added to ablation treatment arms or not. For LTP, MWA was associated with a lower risk compared with RFA for both comparisons; however, results were only significant for the comparison of MWA and RFA only (RR=0.72; P=0.02) (Table 3).
MWA frequency (915 versus 2450 MHz)
For the outcomes of one-year OS, complications, and technique efficacy, there were no significant differences between MWA and RFA, irrespective of MWA frequency. For LTP, the 2450 MHz MWA frequency was associated with a significant reduction of 33% compared with RFA (RR=0.67; P<0.001); however, the 915 MHz MWA frequency was associated with an increase in the risk of LTP (RR=1.79; P=0.01) (Table 3).
Sensitivity analyses
Results of sensitivity analyses on alternative methods (ie, fixed-effects model), study quality (ie, exclusion of poor-quality studies), and surgery type (ie, exclusion of studies involving open surgery23,25,44) were similar in magnitude and direction to the main analysis with some exceptions. MWA was associated with a significant reduction in LTP for all sensitivity analyses. Additionally, when fixed-effects models were used instead of the random-effects model, MWA was associated with significant improvements in technique efficacy (RR=1.02; P=0.04), reductions in EHM (RR=0.64; P<0.05), and hospital LOS (WMD=−0.27 days; P<0.05) compared with RFA. The results of the meta-analysis were not sensitive to the exclusion of studies involving open surgery (Table 4).
Table 4.
Summary of sensitivity analyses for MWA compared with RFA
| LTP | OS (1-Year) | OS (3-Year) | OS (5-Year) | DFS (1-Year) | DFS (3-Year) | DFS (5-Year) | Technique efficacy | EHM | IDL | LOS | Comp. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary analysis | 0.70 (0.53,0.94); P=0.02; 18; 43% | 1.00 (0.98,1.02); P=0.80; 16; 26% | 1.03 (0.97,1.09); P=0.40; 14; 37% | 1.03 (0.93,1.13); P=0.60; 9; 33% | 1.00 (0.96,1.04); P=0.93; 8; 13% | 1.05 (0.96,1.14); P=0.27; 7; 0% | 0.97 (0.71,1.33); P=0.86; 5; 71% | 1.01 (0.99,1.03); P=0.25; 18; 13% | 0.66 (0.43,1.01); P=0.06; 2; 0% | 0.93 (0.79,1.10); P=0.40; 9; 43% | −0.40 (−1.09,0.29); P=0.26; 7; 80% | 1.05 (0.77,1.45); P=0.75; 16; 0% |
| Sensitivity analysis | ||||||||||||
| Fixed effects | 0.71 (0.59,0.85); P<0.001; 18; 43% | 0.99 (0.97,1.02); P=0.59; 16; 26% | 1.03 (0.98,1.08); P=0.20; 14; 37% | 1.02 (0.95,1.09); P=0.65; 9; 33% | 0.98 (0.94,1.03); P=0.49; 8; 13% | 1.03 (0.94,1.12); P=0.50; 7; 0% | 0.99 (0.86,1.14); P=0.90; 5; 71% | 1.02 (1.00,1.05); P=0.04; 18; 25% | 0.64 (0.42,0.99); P<0.05; 2; 0% | 0.91 (0.82,1.00); P=0.05; 9; 43% | −0.27 (−0.54,0.00); P<0.05; 7; 80% | 1.01 (0.75,1.38); P=0.93; 16; 0% |
| Exclusion of studies with poor qualitya | 0.72 (0.53,0.97); P=0.03; 15; 49% | 1.00 (0.98,1.01); P=0.74; 14; 16% | 1.03 (0.96,1.09); P=0.45; 13; 41% | 1.03 (0.93,1.13); P=0.60; 9; 33% | 1.00 (0.96,1.04); P=0.93; 8; 13% | 1.05 (0.96,1.14); P=0.27; 7; 0% | 0.97 (0.71,1.33); P=0.86; 5; 71% | 1.01 (0.99,1.03); P=0.30; 16; 21% | 0.66 (0.43,1.01); P=0.06; 2; 0% | 0.95 (0.80,1.12); P=0.52; 8; 46% | −0.40 (−1.09,0.29); P=0.26; 7; 80% | 1.11 (0.80,1.54); P=0.52; 15; 0% |
| Exclusion of studies involving open surgeryb | 0.64 (0.52,0.80); P<0.001; 14; 0% | 1.00 (0.98,1.02); P=0.82; 12; 30% | 1.01 (0.95,1.07); P=0.77; 10; 36% | 1.00 (0.93,1.07); P=0.90; 6; 0% | 1.00 (0.96,1.05); P=0.82; 7; 11% | 1.04 (0.96,1.13); P=0.33; 6; 1% | 0.96 (0.68,1.36); P=0.84; 4; 78% | 1.01 (0.99,1.03); P=0.39; 14; 15% | Too few studies (<2) to inform | 0.94 (0.77,1.16); P=0.56; 7; 55% | −0.45 (−1.21,0.31); P=0.25; 6; 83% | 1.05 (0.72,1.53); P=0.79; 11; 0% |
Notes: Table reports the effect estimate (RR for all outcomes except hospital LOS, which is presented as the WMD); 95% CIs; P value; number of studies; and heterogeneity (I2 value). A random-effects model was applied if not specified. aExcludes Abdelaziz (2014), Hompes (2010), and Vogl (2015). bExcludes Correa-Gallego (2014), Lee (2017), and van Tilborg (2016).
Abbreviations: Comp., complications; DFS, disease-free survival; EHM, extrahepatic metastases; IDL, intrahepatic de novo lesions; LOS, length of stay; LTP, local tumor progression; OS, overall survival; RR, risk ratio; WMD, weighted mean difference.
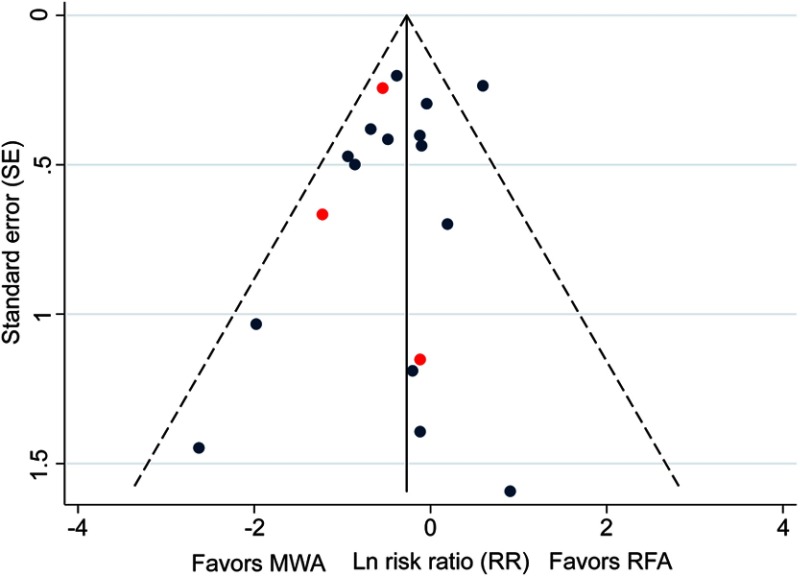
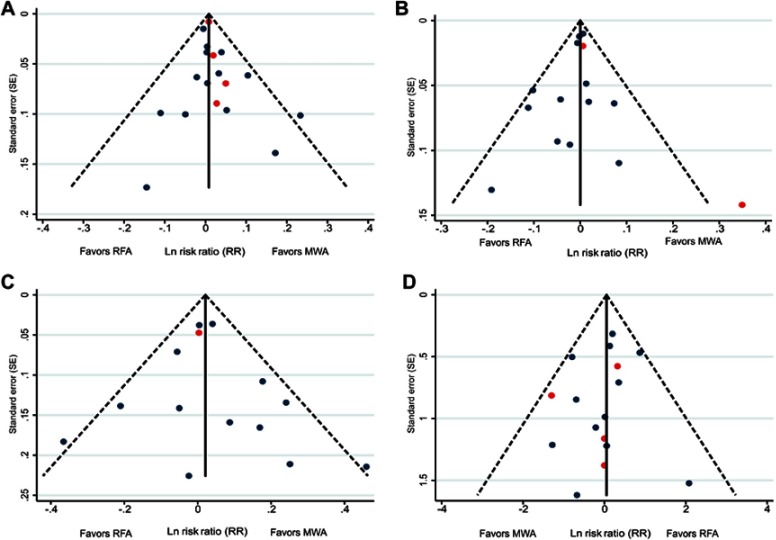
Publication bias
Outcomes reported by ≥10 studies (LTP, technique efficacy, one- and three-year OS, and complications) were examined for publication bias using funnel plots. Results demonstrated a low risk of publication bias for the outcomes assessed. The funnel plot for LTP is presented in Figure 5, and those for technique efficacy, one- and three-year OS, and complications are presented in Figure S13.
Figure 5.
Funnel plot assessing publication bias for LTP in 18 studies. Red points indicate RCTs and blue points indicate observational studies.
Abbreviation: LTP, local tumor progression; MWA, microwave ablation; RCT, randomized control trial; RFA, radiofrequency ablation.
Discussion
This study represents, to the authors’ knowledge, the first documentation of statistically significant improvements in LTP for patients with liver tumors treated with MWA in comparison to RFA based on the analysis of 3531 patients in 28 studies. Specifically, the risk of LTP was significantly reduced by 30% with MWA compared with RFA when analyzing all included studies. Furthermore, if only the three RCTs are considered, the LTP was significantly reduced by 45%. Additional analyses were performed using the fixed-effects model, with exclusion of poor-quality studies, and those using an open surgical approach for ablation. These analyses demonstrated results that were congruent with the main analyses.
OS and DFS were not significantly different between the MWA and RFA treatment arms. This finding is not unexpected given the presence of underlying liver disease in the HCC population and the multimodal therapy required for colorectal liver metastasis. In fact, LTP is generally considered a better indicator of treatment effectiveness for ablative therapies than OS or DFS because of the aforementioned factors. There was one notable exception, in that one-year OS significantly favored RFA over MWA in patients with metastatic disease. This effect is driven by the results from the van Tilborg et al, 2016 study, in which patients were assigned to MWA based on tumor proximity to blood vessels or to RFA based on tumor proximity to the biliary tract, diaphragm, or intestine.25 This allocation bias may have affected our results. In addition to the current meta-analysis, Huo performed a subgroup analysis on tumor type (HCC and metastases) and found conflicting results.16 Notably, only a few studies were included in metastases populations; thus, future studies are required to further assess this subgroup effect.
Results of the subgroup analyses indicated significant differences between treatment modalities based on tumor size and tumor type. LTP was the same between MWA and RFA for tumors less than 2.5 cm, but MWA had a significant reduction of 37% in LTP when compared with RFA in tumors ≥2.5 cm. This finding is consistent with a previous meta-analysis (discussed below).18 This is also consistent with the physics of radiofrequency and microwave energies. The penetration with RFA is variable because of the heat sink effect and the insulative effect of charred tissue.8,11,58 MWA will achieve penetration of 2.0 cm, leading to larger ablation volumes than RFA.8 Thus, tumors approximately 2.0 cm in diameter should be adequately covered with margin if the tumor is precisely targeted in the center and the margin is kept to 5 mm, as is the case for HCC. Conversely, the advantages of MWA should be observed for tumors greater than 2–2.5 cm because of the greater tissue penetration. These findings raise the question of whether the overall significant results seen for LTP with MWA over RFA are solely the result of the large tumor effect. However, the present study does not completely exclude a benefit of MWA for smaller tumors since a nonsignificant reduction of 28% was observed for tumors less than 2.5 cm.
Despite the overall improvement in LTP for MWA over RFA, the risk of complications was not significantly different between groups. This finding is important since larger ablations could be perceived to have a higher risk of complications. The results of this study refute that viewpoint. Technical efficacy was also not significantly different between MWA and RFA, nor for any subgroup analysis. This result is not surprising given the limitations associated with measurement of technique efficacy by the inability of imaging to detect whether neoplastic cells within the ablation zone have been sufficiently ablated,11 by variability between assessors in such evaluations, the high rates (>80%) reported for techniques efficacy in both arms, and by continuing ablation until an adequate margin is determined. Unfortunately, this analysis was unable to assess the number of treatment courses per procedure to achieve similar technique efficacy in both treatment arms. However, in the RCT performed by Yu et al, the number of treatment courses per session (or procedure) was significantly lower in the MWA treatment arm versus the RFA arm.20 This suggests that despite similar technical efficacy rates, MWA is more efficient than RFA.
Three systematic reviews and meta-analyses have recently been published of the comparison between MWA and RFA for the treatment of HCC and/or liver metastases.15,16,18 Huo and colleagues included metastatic liver cancer studies,16 whereas they were not included by Luo and Facciorusso. Luo found that MWA and RFA had similar rates of one- and three-year survival, complete tumor ablation, local tumor recurrence, and major complications,15 which generally aligns with the results reported here. Huo and colleagues reported similar findings in their meta-analysis of both HCC and metastatic patients.16 However, our meta-analysis showed that MWA was also associated with significantly lower LTP compared with RFA. In comparison to these two systematic reviews, the current meta-analysis included several additional studies. Facciorusso and colleagues,18 whose findings were generally aligned with the other two published reviews, included only seven studies in their meta-analysis (two RCTs and 5 observational studies) and only five were in common with the 28 included in our study.32,40,45,50,55 Regarding local recurrence, Facciorusso reported significantly lower odds with MWA over RFA (OR=0.46; P=0.02) in patients with high tumor burden (>1.2 tumors per patient and/or large tumors >2.5 cm in diameter).18
This meta-analysis had broad inclusion criteria allowing for a wide variety of subgroup analyses other than tumor size and type. The results of the frequency subgroup analysis showed that the benefit of MWA over RFA was most apparent for the 2450 MHz versus the 915 MHz frequency. These results may be explained by the ability of 2450 MHz MWA systems to deliver greater amounts of power and achieve larger ablation volumes.11 A recent observational study comparing 915 and 2450 MHz MWA for ablation of lung metastases found that ablation margin size was significantly associated with the local progression rate and that 2450 MHz MWA demonstrated a significantly better local progression-free survival curve than 915 MHz MWA (P=0.048).59 Liu and colleagues did not report any differences in treatment effect when comparing these two MWA frequencies.60 The results of the tumor type subgroup analysis on HCC and metastases showed that there were no differences between MWA and RFA in HCC or metastases for technique efficacy and complications.
There are a few limitations of this meta-analysis. First, most of the included studies are observational (primarily retrospective cohort studies) and present a potential for selection bias. RFA was often used for a longer time at institutions than MWA and had a longer follow-up duration than MWA. Despite this, most studies were well balanced for baseline covariates, and some of the studies used matching to control for differences or reported regression analyses that showed the minimal impact of potential effect modifiers on treatment outcome. To control for the effect of poor study quality, studies receiving lower quality assessment scores were excluded in a sensitivity analysis. The results of these sensitivity analyses remained aligned with the main analyses. Second, there was often variability between and within studies for follow-up time; this was handled in two ways: 1) a meta-regression was performed to determine whether follow-up time had a significant effect on LTP (results showed there was no effect) and 2) studies with ≥25% difference in mean follow-up time between arms were excluded from analyses of outcomes potentially impacted by follow-up time. Third, significant heterogeneity was observed for certain outcomes, such as five-year DFS and LOS, which may have been due to variability in patient baseline characteristics, treatment parameters, and study designs across studies. As such, random-effects model, which accounts for heterogeneity, was used; sensitivity analyses were performed with a fixed-effects model. Fourth, due to the broad inclusion of this meta-analysis, there was some variability in the definitions of outcomes, particularly technique efficacy and LTP. Reporting guidelines recommend that technique efficacy (often called complete tumor ablation or complete response) should be assessed by imaging ideally one week to one month after the procedure and no later than three months afterwards.10 Thus, studies were included regardless of the terminology used to describe the outcome, as long as it was reported from one week to three months after treatment. It is unclear whether all the studies assessed treatment efficacy (ie, effective ablation of tumors) or if some assessed technical success (ie, tumors treated according to protocol),10 since these terms are sometimes used interchangeably. As well, the terminology used to define local tumor recurrence or LTP were sometimes variable. Here, we used the following definition for LTP: reappearance of tumors, within or adjacent to the ablation zone, based on that of Ahmed et al.10 This definition often allowed the inclusion of studies which labeled the outcome as LTP and studies which labeled it as local tumor recurrence (LTR). However, it is unclear whether minor variations in definition could have impacted overall results. Finally, 16 studies reported that patients underwent retreatment after initial ablation; however, it is difficult to assess how retreatment could have impacted outcomes due to lack of data availability (ie, outcomes were not reported separately for patients that did or did not receive retreatment or by type of retreatment).
Despite these limitations, this meta-analysis is strengthened by its broad inclusion of 28 studies in liver cancer which enrolled over 3500 patients. The time period for study inclusion was limited from 2006 to 2017 to control for the use of outdated ablation devices. A broad range of outcomes such as IDL and EHM were included, which have not been meta-analyzed previously. This meta-analysis also used several methods to control for heterogeneity and study quality including use of a random-effects model, subgroup and sensitivity analyses, study quality assessment, assessment of publication bias, and meta-regression analysis.
Conclusions
In conclusion, results indicated that MWA is just as safe and effective as RFA for the treatment of HCC or liver metastases. Compared with RFA, MWA is associated with statistically significantly lower rates of LTP across analyses. Subgroup analyses showed that higher frequency (ie, 2450 MHz MWA) and larger tumor size (ie, >2.5 cm) may be associated with improved outcomes for MWA versus RFA. Further studies are required to assess the cost and time savings associated with MWA versus RFA as well as the comparison of MWA with non ablative strategies (eg, resection).
Supplementary materials
Methodological quality assessment of RCTs using the Cochrane RoB tool.
Abbreviations: RCT, randomized control trial; RoB, Risk of Bias.
Forest plot of random effects meta-analysis results for technique efficacy (P=0.25), stratified by RCTs (P=0.23) versus observational studies (P=0.34).
Abbreviation: RCT, randomized control trial.
Forest plot of random effects meta-analysis results for one-year OS (P=0.80), stratified by RCTs (P=0.43) versus observational studies (P=0.57).
Abbreviations: OS, overall survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for three-year OS (P=0.40), stratified by RCTs (P=0.94) versus observational studies (P=0.38).
Abbreviations: OS, overall survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for five-year OS (P=0.60), stratified by RCTs (P=0.27) versus observational studies (P=0.32).
Abbreviations: OS, overall survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for one-year DFS (P=0.93), stratified by RCTs (P=0.97) versus observational studies (P=0.67).
Abbreviations: DFS, disease-free survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for three-year DFS (P=0.27), stratified by RCTs (P=0.34) observational studies (P=0.73).
Abbreviations: DFS, disease-free survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for five-year DFS (P=0.86), stratified by RCTs (P=0.006) versus observational studies (P=0.045).
Abbreviations: DFS, disease-free survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for EHM (P=0.056).
Abbreviation: EHM, extrahepatic metastasis.
Forest plot of random effects meta-analysis results for IDL (P=0.40), stratified by RCTs (P=0.034) versus observational studies (P=0.83).
Abbreviations: IDL, intrahepatic de novo lesions; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for LOS (P=0.26).
Abbreviation: LOS, length of stay.
Forest plot of random effects meta-analysis results for complications (P=0.75), stratified by RCTs (P=0.68) versus observational studies (P=0.60).
Abbreviation: RCT, randomized control trial.
Publication Bias Assessments.
Notes: Funnel plots assessing publication bias for (A) technique efficacy (n=18), (B) one-year OS (n=15), (C) three-year OS (n=14), and (D) complications (n=16). Red points indicate RCTs and blue points indicate observational studies.
Abbreviations: MWA, microwave ablation; OS, overall survival; RCT, randomized control trial; RFA, radiofrequency ablation.
Acknowledgments
This paper was presented at the Society of Interventional Oncology 2019 conference as a poster presentation with interim findings. The poster’s abstract was published online: https://www.eventscribe.com/2019/SIO2019/fsPopup.asp?efp=Q0JTUENDVUY4MDE1&PosterID=200029&rnd=0.7269141&mode=posterinfo. This work was sponsored by Ethicon, Inc., who provided funding to conduct the analysis and prepare the manuscript.
Data availability
The dataset supporting the conclusions of this article is included in the article (and its supplementary data).
Abbreviation list
DFS, disease free survival; EHM, extrahepatic metastasis; HCC, hepatocellular carcinoma; IDL, intrahepatic de novo lesions; IRB, institutional review board; LOS, length of stay; LTP, local tumor progression; LTR, local tumor recurrence; MWA, microwave ablation; NOS, Newcastle–Ottawa quality assessment scale; OS, overall survival; PICOS, population intervention comparator outcomes study design; PRESS, peer review of electronic search strategies; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized control trial; RFA, radiofrequency ablation; RoB, Cochrane risk of bias; RR, relative risk; TACE, transarterial chemoembolization; TAE, transarterial embolization; WMD, weighted mean difference.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
SG, JWC, MBG, and JFA are employees of Ethicon, Inc. (manufacturer of Neuwave microwave ablation instrumentation). RAQ, NCF, BS, and GWJW are employees of Cornerstone Research Group, who were sponsored to perform this study by Ethicon, Inc. MBG reports stocks and stock options from Johnson & Johnson during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organization. Cancer fact sheet. [Updated February 2018] Available from: http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed April3, 2018.
- 2.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 3.Verslype C, Rosmorduc O, Rougier P, Group EGW. Hepatocellular carcinoma: ESMO-ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii41–vii48. [DOI] [PubMed] [Google Scholar]
- 4.Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74(3):817–825. doi: [DOI] [PubMed] [Google Scholar]
- 5.Laeseke PF, Lee FT Jr., van der Weide DW, Brace CL. Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol. 2009;2(2):65–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Harari CM, Magagna M, Bedoya M, et al. Microwave ablation: comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology. 2016;278(1):95–103. doi: 10.1148/radiol.2015142151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. AJR Am J Roentgenol. 2012;198(3):W260–W265. doi: 10.2214/AJR.11.7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(8):1054–1063. doi: 10.4254/wjh.v7.i8.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison P. Devices and equipment in interventional oncology and their operation In: Dupuy DFY, McMullen W, editors. Image-Guided Cancer Therapy. New York (NY): Springer; 2013: 179-199. [Google Scholar]
- 10.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260. doi: 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C. Understanding the nuances of microwave ablation for more accurate post-treatment assessment. Future Oncol. 2018;14(17):1755–1764. doi: 10.2217/fon-2017-0736 [DOI] [PubMed] [Google Scholar]
- 12.Pathak S, Jones R, Tang JM, et al. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis. 2011;13(9):e252–e265. doi: 10.1111/j.1463-1318.2011.02695.x [DOI] [PubMed] [Google Scholar]
- 13.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19(7):1087–1092. doi: 10.1016/j.jvir.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9(1 Pt 1):101–111. [DOI] [PubMed] [Google Scholar]
- 15.Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15(1):126. doi: 10.1186/s12957-017-1196-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huo YR, Eslick GD. Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol. 2015;26(8):1139–1146 e1132. [DOI] [PubMed] [Google Scholar]
- 17.Chinnaratha MA, Chuang MY, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(2):294–301. doi: 10.1111/jgh.13028 [DOI] [PubMed] [Google Scholar]
- 18.Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32(3):339–344. doi: 10.3109/02656736.2015.1127434 [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Shen Q, Wang N, et al. Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma. Chin J Cancer. 2017;36(1):14. doi: 10.1186/s40880-017-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66(6):1172–1173. doi: 10.1136/gutjnl-2016-312629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheta E, El-Kalla F, El-Gharib M, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28(10):1198–1203. doi: 10.1097/MEG.0000000000000688 [DOI] [PubMed] [Google Scholar]
- 22.Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2017;29(2):268–275.e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KF, Wong J, Hui JW, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: a retrospective comparative study. Asian J Surg. 2017;40(4):301–308. doi: 10.1016/j.asjsur.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Potretzke TA, Ziemlewicz TJ, Hinshaw JL, et al. Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol. 2016;27(5):631–638. doi: 10.1016/j.jvir.2016.01.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39(10):1438–1446. doi: 10.1007/s00270-016-1413-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasnani R, Ginsburg M, Ahmed O, et al. Radiofrequency and microwave ablation in combination with transarterial chemoembolization induce equivalent histopathologic coagulation necrosis in hepatocellular carcinoma patients bridged to liver transplantation. Hepatobiliary Surg Nutr. 2016;5(3):225–233. doi: 10.21037/hbsn.2016.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton LM, Cabrera R, Kapp M, Lazarowicz M, Vogel JD, Toskich BB. Radiofrequency vs microwave ablation after neoadjuvant transarterial bland and drug-eluting microsphere chembolization for the treatment of hepatocellular carcinoma. Curr Probl Diagn Radiol. 2017;46(6):402–409. doi: 10.1067/j.cpradiol.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Li Y. A comparative study of laparoscopic microwave ablation with laparoscopic radiofrequency ablation for colorectal liver metastasis. J Buon. 2017;22(3):667–672. [PubMed] [Google Scholar]
- 29.Santambrogio R, Chiang J, Barabino M, et al. Comparison of laparoscopic microwave to radiofrequency ablation of small hepatocellular carcinoma (</=3 cm). Ann Surg Oncol. 2017;24(1):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 32.Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging. 2015;40(6):1829–1837. doi: 10.1007/s00261-015-0355-6 [DOI] [PubMed] [Google Scholar]
- 33.Higgins J, Green S, (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0. [Updated March 2011]. Available from: http://handbook.cochrane.org. Accessed April 2018.
- 34.Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomized studies in metaanalysis. [Updated 2014] Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed November 2017.
- 35.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 36.Abdelaziz AO, Nabeel MM, Elbaz TM, et al. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: prospective analysis. Scand J Gastroenterol. 2015;50(4):479–484. doi: 10.3109/00365521.2014.1003397 [DOI] [PubMed] [Google Scholar]
- 37.Bhutiani N, Philips P, Scoggins CR, McMasters KM, Potts MH, Martin RC. Evaluation of tolerability and efficacy of irreversible electroporation (IRE) in treatment of Child-Pugh B (7/8) hepatocellular carcinoma (HCC). HPB (Oxford). 2016;18(7):593–599. doi: 10.1016/j.hpb.2016.03.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veltri A, Gazzera C, Calandri M, et al. Percutaneous treatment of Hepatocellular carcinoma exceeding 3 cm: combined therapy or microwave ablation? Preliminary results. Radiol Med. 2015;120(12):1177–1183. doi: 10.1007/s11547-014-0465-1 [DOI] [PubMed] [Google Scholar]
- 39.Zhou F, Yu XL, Liang P, et al. Microwave ablation is effective against liver metastases from gastric adenocarcinoma. Int J Hyperthermia. 2017;33(7):830–835. [DOI] [PubMed] [Google Scholar]
- 40.Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28(12):3429–3434. doi: 10.1007/s00464-013-3343-3 [DOI] [PubMed] [Google Scholar]
- 41.Di Vece F, Tombesi P, Ermili F, Maraldi C, Sartori S. Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: a prospective pilot study. Cardiovasc Intervent Radiol. 2014;37(3):723–729. [DOI] [PubMed] [Google Scholar]
- 42.Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol. 2015;27(3):349–354. doi: 10.1097/MEG.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 43.Cillo U, Noaro G, Vitale A, et al. Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB (Oxford). 2014;16(11):979–986. doi: 10.1111/hpb.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21(13):4278–4283. doi: 10.1245/s10434-014-3817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82(9):1379–1384. doi: 10.1016/j.ejrad.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 46.Ginsburg M, Zivin SP, Wroblewski K, Doshi T, Vasnani RJ, Van Ha TG. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26(3):330–341. doi: 10.1016/j.jvir.2014.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hompes R, Fieuws S, Aerts R, Thijs M, Penninckx F, Topal B. Results of single-probe microwave ablation of metastatic liver cancer. Eur J Surg Oncol. 2010;36(8):725–730. doi: 10.1016/j.ejso.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 48.Kuang M, Xie XY, Huang C, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg. 2011;15(12):2165–2171. doi: 10.1007/s11605-011-1716-2 [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Li S, Wan X, et al. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol. 2013;25(4):442–446. doi: 10.1097/MEG.0b013e32835cb566 [DOI] [PubMed] [Google Scholar]
- 50.Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol. 2009;24(2):223–227. doi: 10.1111/j.1440-1746.2008.05596.x [DOI] [PubMed] [Google Scholar]
- 51.Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22(9):1983–1990. doi: 10.1007/s00330-012-2442-1 [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi H, Seki S, Tsuji K, et al. Endoscopic thermal ablation therapies for hepatocellular carcinoma: a multi-center study. Hepatol Res. 2009;39(1):47–52. doi: 10.1111/j.1872-034X.2008.00410.x [DOI] [PubMed] [Google Scholar]
- 53.Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104(7):822–829. doi: 10.1002/jso.21933 [DOI] [PubMed] [Google Scholar]
- 54.Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(9):1914–1923. doi: 10.1002/cncr.24196 [DOI] [PubMed] [Google Scholar]
- 55.Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One. 2013;8(10):e76119. doi: 10.1371/journal.pone.0076119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hefaiedh R, Ennaifer R, Romdhane H, et al. Gender difference in patients with hepatocellular carcinoma. Tunis Med. 2013;91(8–9):505–508. [PubMed] [Google Scholar]
- 57.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016;10(3):332–339. doi: 10.5009/gnl15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171. doi: 10.1097/01.sla.0000171032.99149.fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogl TJ, Roman A, Nour-Eldin NA, Hohenforst-Schmidt W, Bednarova I, Kaltenbach B. A comparison between 915 MHz and 2450 MHz microwave ablation systems for the treatment of small diameter lung metastases. Diagn Interv Radiol. 2018;24(1):31–37. doi: 10.5152/dir.2018.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia. 2010;26(5):448–455. doi: 10.3109/02656731003717574 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methodological quality assessment of RCTs using the Cochrane RoB tool.
Abbreviations: RCT, randomized control trial; RoB, Risk of Bias.
Forest plot of random effects meta-analysis results for technique efficacy (P=0.25), stratified by RCTs (P=0.23) versus observational studies (P=0.34).
Abbreviation: RCT, randomized control trial.
Forest plot of random effects meta-analysis results for one-year OS (P=0.80), stratified by RCTs (P=0.43) versus observational studies (P=0.57).
Abbreviations: OS, overall survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for three-year OS (P=0.40), stratified by RCTs (P=0.94) versus observational studies (P=0.38).
Abbreviations: OS, overall survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for five-year OS (P=0.60), stratified by RCTs (P=0.27) versus observational studies (P=0.32).
Abbreviations: OS, overall survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for one-year DFS (P=0.93), stratified by RCTs (P=0.97) versus observational studies (P=0.67).
Abbreviations: DFS, disease-free survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for three-year DFS (P=0.27), stratified by RCTs (P=0.34) observational studies (P=0.73).
Abbreviations: DFS, disease-free survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for five-year DFS (P=0.86), stratified by RCTs (P=0.006) versus observational studies (P=0.045).
Abbreviations: DFS, disease-free survival; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for EHM (P=0.056).
Abbreviation: EHM, extrahepatic metastasis.
Forest plot of random effects meta-analysis results for IDL (P=0.40), stratified by RCTs (P=0.034) versus observational studies (P=0.83).
Abbreviations: IDL, intrahepatic de novo lesions; RCT, randomized control trial.
Forest plot of random effects meta-analysis results for LOS (P=0.26).
Abbreviation: LOS, length of stay.
Forest plot of random effects meta-analysis results for complications (P=0.75), stratified by RCTs (P=0.68) versus observational studies (P=0.60).
Abbreviation: RCT, randomized control trial.
Publication Bias Assessments.
Notes: Funnel plots assessing publication bias for (A) technique efficacy (n=18), (B) one-year OS (n=15), (C) three-year OS (n=14), and (D) complications (n=16). Red points indicate RCTs and blue points indicate observational studies.
Abbreviations: MWA, microwave ablation; OS, overall survival; RCT, randomized control trial; RFA, radiofrequency ablation.
Data Availability Statement
The dataset supporting the conclusions of this article is included in the article (and its supplementary data).