ABSTRACT
Heat stress has a large negative impact on poultry around the world in both intensive and small-scale production systems. Better understanding of genetic factors contributing to response to high ambient temperatures would provide a basis to develop strategies for alleviating negative impacts of heat on poultry production. The objective of this work was to characterize the genetic control (heritability estimate and quantitative trait loci (QTL)) of blood chemistry components before and after exposure to acute and chronic high ambient temperature in a commercial egg laying line Hy-Line W-36 female parent line mature hens were exposed to 4 wk of daily cyclic heat exposure. Blood was collected pre-heat, on the first day of heat, and 2 and 4 wk post heat initiation and analyzed immediately using an i-STAT® hand-held blood analyzer. Thirteen blood components were quantified at the 4 time points: pH, pCO2, pO2, HCO3, TCO2, sO2, iCa, Na, K, base excess, glucose, “hematocrit” (estimated from blood electrical conductivity, BEC), and “hemoglobin” (calculated from BEC). Heritabilities were estimated using genomic relationship information obtained from 600k SNP chip data. All 13 parameters exhibited a significant change after 5 h of heat exposure and most did not return to pre-heat levels throughout the duration of the study. Eight parameters (base excess, glucose, hemoglobin, HCO3, hematocrit, K, pCO2, TCO2) had heritability estimates differing from zero at one or more time points (0.21 to 0.45). The traits with significant heritability would be good candidates for use as biomarkers in a selection program if they are correlated with traits of economic importance that are more difficult to measure. QTL were identified for nine of the traits at one or more time point. These nine traits, however, did not have significant heritability estimates suggesting that while some QTL have been identified their effects are generally small.
Keywords: chronic heat stress, acute heat stress, i-STAT®, GWAS, laying hen
INTRODUCTION
Heat stress has a large negative impact on poultry around the world in both intensive and small-scale production systems (Lara and Rostagno, 2013; Nyoni et al., 2018). It is well documented that heat stress results in decreased egg production (Daniel and Balnave, 1981; Deng et al., 2012), decreased egg quality (Bollengier-Lee et al., 1999; Franco-Jimenez et al., 2007), and eventual mortality (Wolc et al., 2018) in laying hens. A better understanding of physiological changes and identification of genetic factors contributing to response to high ambient temperatures would provide a basis for alleviating the negative impact of heat on poultry production by management or genetic selection strategies.
Hand-held blood analyzers, initially developed for human bedside diagnostics, provide a quick and convenient method for measuring multiple blood gas and chemistry components. This is an emerging technology for quantifying health and welfare status of poultry because of its accuracy, speed, and ease of use. Previously the i-STAT® device (Abbott Labs) has been used in other animal species (Tinkey et al., 2006; Harter et al., 2014; Rettenmund et al., 2014; Yildirim et al., 2015) and to establish reference ranges for broiler breeders and laying hens (Steinmetz et al., 2007; Martin et al., 2010; Schaal et al., 2015). Van Goor et al. was the first report using the i-STAT® blood analyzer to evaluate chickens under high ambient temperature (Van Goor et al., 2016).
The objectives of the current work was to characterize the genetic control of blood chemistry parameters before and after exposure to acute and chronic high ambient temperature in mature hens of a commercial egg laying line Blood components measured rapidly with a pen-side device would be ideal phenotypes to use as biomarkers for genetic improvement. This is the first report of pen-side blood component genetic analysis in a commercial egg laying line under heat stress.
MATERIALS AND METHODS
Animals, Husbandry, and Heat Treatment
Hy-Line W-36 female parent line chicks were reared at Hy-Line International (Dallas Center, IA). At 18 wk of age, 400 pullets were transferred to Virginia Tech (Blacksburg, VA) and placed in individual cages for a 6-wk acclimation period within a climate-controlled room that was maintained at approximately 23°C. Heat treatment began at 24 wk of age, lasting 4 wk and continued until 28 wk of age. The daily cyclic heat cycle, beginning at 9:00 a.m., was 7 h at 35°C and then 30°C for the remaining 17 h. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Virginia Tech (log # 14–093).
Phenotypes
Blood was collected at four time points. Collection occurred one day prior to heat initiation, and at 4 to 6 h post heat initiation during the first day of heat exposure (hereafter referred to as the 5-h time point), and 2 and 4 wk post heat initiation. Blood was collected from the ulnar vein with syringe and needle, then immediately transferred to the chamber of an i-STAT® hand-held blood analyzer (Abbott Laboratories, San Diego, CA). Thirteen blood components were quantified using the CG8+ cartridge: pH, pCO2, pO2, HCO3, TCO2, sO2, iCa, Na, K, BE, glucose, “hematocrit”, and “hemoglobin”. The i-STAT® analyzer and cartridges are designed and optimized for use in human medicine. Because of this, there are some relevant concerns for the direct application of this technology to avian biology, as discussed in the results and discussion section.
Other phenotypes including egg production, egg weight, Haugh units, yolk weight, shell quality, feed intake, digestibility, and 6 more were also recorded pre and post heat exposure. These traits are discussed in a companion manuscript to be submitted to Genomics Selection and Evolution.
Genotyping and Quality Control
Genomic DNA was isolated by GeneSeek, Neogen Genomics (Lincoln, NE, United States) from whole blood collected at 17 wk of age. Genotyping was done using the Axiom Chicken 600k Genotyping Array (Kranis et al., 2013) and genotyping array annotation files were based on galGal genome version 5.0 (Thermo Fisher Scientific). Quality filtering requirements included: CR ≥ 95, MinorAlleleFrequency ≥ 0.01, FLD ≥ 4, HomRO ≥ −0.6, BB.varX ≤ 0.9, BB.varY ≤ 0.45, AB.varX ≤ 0.55, AB.varY ≤ 0.5, AA.varX ≤ 0.6, HomFLD ≥ 9, HetSO ≥ −0.2, ConversionType ≠ “OTV”. Quality metrics are described in the Axiom Analysis Suite User Guide obtained from Thermo Fisher Scientific (Applied Biosystems, 2017). After filtering, 261,509 SNPs and 374 animals remained for analysis.
Data analysis
Heritabilities and variance components were estimated using ASReml 4.0 (Gilmour et al., 2015) with a univariate animal model:
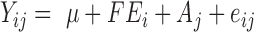 |
Y is the dependent variable of phenotype. Fixed effect was cage row within room (FEi). Animal genetic effects (Aj) with a genomic relationship matrix computed from SNP genotypes, as described by (VanRaden, 2008), and residuals (eij) were the two random effects.
Quantitative trait loci (QTL) identification was performed using a hierarchical generalized linear model (Rönnegård et al., 2010), with the same effects described for heritability estimation, in R—GenABEL (Aulchenko, 2015). The association analysis method used in GenABEL, polygenic_hglm, and mmscore, functions similarly to the FASTA method for related individuals described by (Chen and Abecasis, 2007). A modified Bonferroni multiple test correction was used to determine the number of independent tests, as previously described (Rowland et al., 2018). The number of independent tests was determined to be 16,085 and a 20% genomewide threshold was calculated to be 1.2 × 10−5.
RESULTS AND DISCUSSION
Use of i-STAT® Technology in Chickens
The data generated by i-STAT® technology has not generally been validated against “gold standard” techniques for measuring blood chemistry and other components in chickens. Of special concern about using the i-STAT® for measuring blood chemistry components in chickens are the values reported for “hematocrit” and “hemoglobin”. Because the structure of chicken erythrocytes (elongated and nucleated) differs greatly from that of most mammalian cells, the blood electrical conductance assay and the algorithms used by the i-STAT® to generate “hematocrit” and “hemoglobin” values from chicken blood samples may not be equivalent to the standard packed-cell volume method of measuring hematocrit. However, even though the i-STAT®-generated numbers for “hematocrit” and “hemoglobin” do not represent the same biologically relevant measurements as reported from standard assays, they do represent heritable measurements as shown in Table 1 and therefore are of potential value in a selection program.
Table 1.
Heritability estimates and standard errors for 13 blood gas and chemistry components across 4 time points. Bold indicates estimate differs from 0.
| Trait | pre | 5 h | 2 wk | 4 wk |
|---|---|---|---|---|
| Base Excess (BE) | 0.01 (0.09) | 0.21 (0.10) | 0.17 (0.11) | 0.26 (0.12) |
| Glucose (Glu) | 0.08 (0.11) | NC1 | 0.37 (0.12) | 0.10 (0.10) |
| Hemoglobin (Hb) | 0.44 (0.11) | 0.04 (0.09) | 0.22 (0.11) | 0.31 (0.11) |
| Bicarbonate (HCO3) | 0.01 (0.09) | 0.19 (0.10) | 0.29 (0.12) | 0.42 (0.12) |
| Hematocrit (Hct) | 0.45 (0.11) | 0.05 (0.09) | 0.20 (0.11) | 0.32 (0.11) |
| Ionized calcium (iCa) | 0.01 (0.09) | NC | 0.13 (0.11) | 0.05 (0.10) |
| Potassium (K) | 0.04 (0.10) | 0.07 (0.10) | 0.06 (0.09) | 0.29 (0.12) |
| Sodium (Na) | NC | NC | 0.12 (0.11) | 0.003 (0.08) |
| Partial pressure of CO2 (PCO2) | 0.03 (0.09) | 0.19 (0.11) | NC | 0.26 (0.11) |
| pH | 0.05 (0.09) | 0.09 (0.10) | 0.001 (0.08) | 0.12 (0.11) |
| Partial pressure of O2 (PO2) | 0.02 (0.08) | NC | NC | 0.01 (0.09) |
| Oxygen saturation (sO2) | 0.05 (0.09) | 0.05 (0.10) | NC | 0.02 (0.09) |
| Total carbon dioxide (TCO2) | NC | 0.13 (0.10) | 0.34 (0.12) | 0.33 (0.12) |
1Does not converge
An additional consideration for this study is that the measurements of the blood chemistries were done on venous blood. Composition of venous and arterial blood differ greatly, and blood gas and pH balances are regulated at a systemic level by the arterial, not venous, blood composition (Wideman et al., 2003). For the purposes of the current study, however, the researchers chose to draw venous blood, which presents fewer difficulties in staunching blood leakage from the site, an important consideration in testing an assay for potential application in the field. Also, other reports on use of the iSTAT in chickens used venous blood (Schaal et al., 2015; Van Goor et al., 2016). Thus, there was interest in determining the genetics of venous blood chemistries.
Heritability
Eight of the blood parameters had heritability estimates differing from zero (defined as an estimate greater than two times the standard error) at one or more time point (0.21 to 0.45, Table 1). The traits with significant heritability would be good candidates for use as indicator traits in a selection program if they are correlated with traits of economic importance that may be more difficult to measure.
In the first and only other report of h2 for traits measured using an i-STAT® device, Van Goor, et al. measured blood parameters in a heat stressed research chicken line (Van Goor et al., 2016). From pre and post heat exposure measurements, only one parameter, HCO3 post heat, was found to have non-zero heritability in both studies. The estimates were similar between the two studies, 0.23 reported by Van Goor et al. (2016) and 0.29 reported here. Closter et al. reported h2 for HCO3 to be 0.19 in cold-stressed broilers. All 3 studies report moderate h2 for HCO3 in different lines and under different environmental conditions. (Closter et al. (2009) used the GEM Premier 3000 system to quantify pH, pvCO2, pvO2, HCO3, TCO2, and sO2 in cold stressed broilers at 22 d of age. The GEM Premier is a comparable technology to the i-STAT® system. Closter et al. (2009) additionally estimated significant h2 for pCO2 and TCO2. Our estimate of pCO2 after 4 wk of heat exposure was 0.26, higher than that of Closter et al. (2009) in cold stressed broilers (0.15). Our h2 estimates of TCO2 (0.34 after 2 wk of heat and 0.33 after 4 wk of heat) were also higher than that of Closter et al. (2009) (0.19).
QTL Associations
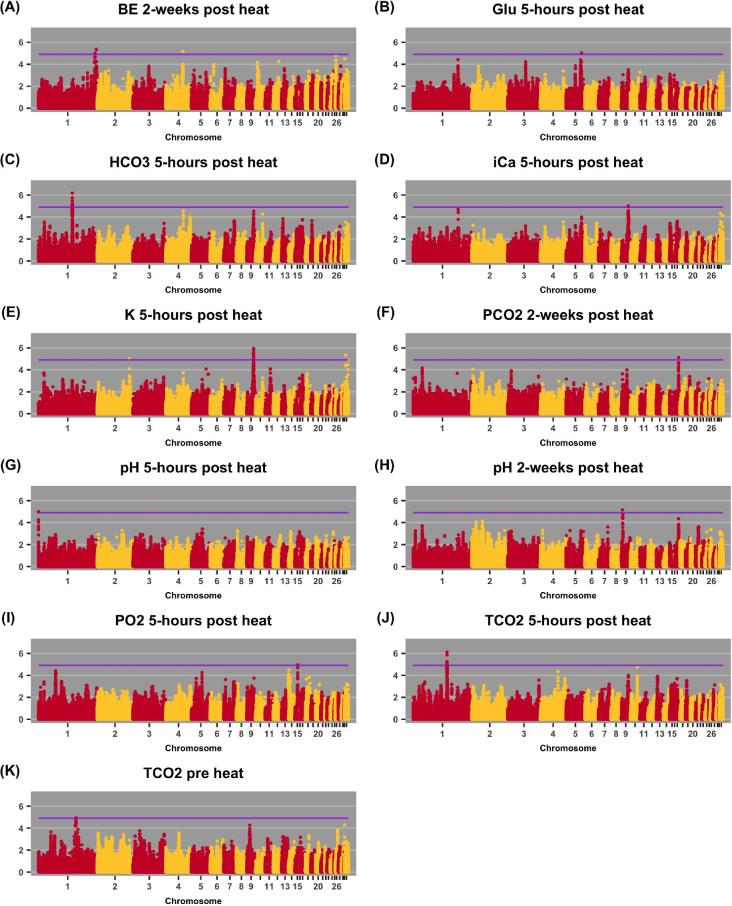
QTL were identified for nine of the blood components at one or more time point (Figure 1, Table 2), although it is important to add a word of caution—these 9 traits did not have significant heritability estimates. Heritability not differing from 0 indicates a lack of detectable genetic control due to insufficient statistical power or a lack of genetic variation overall in the trait. Therefore, identification of QTL for a trait without significant h2 suggests that some genomic regions are significant but overall the effects are very small. Many association studies are reported without presenting the estimated heritability of analyzed traits, so interpretation of the reported QTL should be guarded. Heritability estimation is an important first step for genetic/genomic studies. Determining the existence of significant total genetic variation to phenotypic variability indicates that genes are involved in the phenotypic expression and therefore can be interrogated by, for example, association analyses. Reports of QTL in studies that do not present evidence of significant h2 should be viewed with caution, as while individual genomic regions may be identified the effect size is likely small.
Figure 1.
Manhattan plots for traits with significant QTL. The purple line indicates a 20% genome wide threshold of 1.2 × 10−5. The y-axis represents the -log10(P-value) of SNP association.
Table 2.
Details of significant QTL: position, P-value of association, and number of SNPs in the QTL.
| Trait | Time | Position (Chr:Mb) | P-value | Number of SNPs |
|---|---|---|---|---|
| TCO2 | pre heat | 1:129 | 1.19 × 10−5 | 4 |
| 5 h | 1:116 | 7.73 × 10−7 | 8 | |
| PO2 | 5 h | 15:7.5 | 1.10 × 10−5 | 2 |
| pH | 2 wk | 9:3.6 | 6.91 × 10−6 | 1 |
| 5 h | 1:0.2 | 1.02 × 10−5 | 2 | |
| PCO2 | 2 wk | 17:8 | 7.62 × 10−6 | 1 |
| iCa | 5 h | 9:19 | 9.79 × 10−6 | 3 |
| HCO3 | 5 h | 1:116 | 6.71 × 10−7 | 12 |
| Glu | 5 h | 5:49 | 9.25 × 10−6 | 1 |
| BE | 2 wk | 1:193 | 4.53 × 10−6 | 5 |
| 4:63 | 6.73 × 10−6 | 1 | ||
| K | 5 h | 9:19 | 1.14 × 10−6 | 71 |
| 2:137 | 9.60 × 10−6 | 1 |
In summary, all 13 parameters exhibited a significant change due to high ambient temperature exposure at one or more time point. Eight of the blood parameters (base excess, glucose, hemoglobin, HCO3, hematocrit, K, pCO2, TCO2) had heritability estimates differing from zero at one or more time point (0.21 to 0.45). The traits with significant heritability would be good candidates for use as indicator traits in a selection program if they are correlated with traits of economic importance that may be more difficult to measure. QTL were identified for nine of the traits at one or more time point, however, these nine traits did not have significant heritability estimates.
ACKNOWLEDGMENTS
This research was supported by United States Department of Agriculture (USDA)-National Institute of Food and Agriculture (NIFA)-Agriculture and Food Research Initiative (AFRI) Climate Change Award #2011–67003-30228. KR was partly supported by a USDA National Needs Fellowship (2013–38420-20496). The authors thank Chris M. Ashwell for his contribution to the conception and design of this experiment, and Claire Andreasen for discussion regarding clinical chemistries and the i-STAT® technology.
REFERENCES
- Applied Biosystems 2017. Axiom Analysis Suite 3.1 User Guide. Available at https://www.thermofisher.com/us/en/home/life-science/microarray-analysis/microarray-analysis-instruments-software-services/microarray-analysis-software/axiom-analysis-suite.html. Accessed May 5, 2017. [Google Scholar]
- Aulchenko Y. 2015. Package GenABEL - R package reference manual. Available at https://cran.r-project.org/web/packages/GenABEL/GenABEL.pdf%5Cnhttps://cran.r-project.org/web/packages/GenABEL/index.html. Accessed May 1, 2016. [Google Scholar]
- Bollengier-Lee S., Williams P. E. V., Whitehead C. C.. 1999. Optimal dietary concentration of vitamin E for alleviating the effect of heat stress on egg production in laying hens. Br. Poult. Sci. 40:102–107. [DOI] [PubMed] [Google Scholar]
- Chen W. M., Abecasis G. R.. 2007. Family-based association tests for genomewide association scans. Am. J. Human Gen. 81:913–926. Available at http://linkinghub.elsevier.com/retrieve/pii/S0002929707638695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closter A. M., van As P., Groenen M. A. M., Vereijken A. L. J., van Arendonk J. A. M., Bovenhuis H.. 2009. Genetic and phenotypic relationships between blood gas parameters and ascites-related traits in broilers. Poult. Sci. 88:483–490. [DOI] [PubMed] [Google Scholar]
- Daniel M., Balnave D.. 1981. Responses of laying hens to gradual and abrupt increases in ambient temperature and humidity. Aust. J. Exp. Agric. 21:189–195. [Google Scholar]
- Deng W., Dong X. F., Tong J. M., Zhang Q.. 2012. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 91:575–582. [DOI] [PubMed] [Google Scholar]
- Franco-Jimenez D. J., Scheideler S. E., Kittok R. J., Brown-Brandl T. M., Robeson L. R., Taira H., Beck M. M.. 2007. Differential effects of heat stress in three strains of laying hens. J. Appl. Poult. Res. 16:628–634. [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., Welham S. J., Thompson R.. 2015. Asreml User Guide Release 4.1 Functional Specification. Available at www.vsni.co.uk. Accessed November 12, 2016. [Google Scholar]
- Van Goor A., Ashwell C. M., Persia M. E., Rothschild M. F., Schmidt C. J., Lamont S. J.. 2016. Quantitative trait loci identified for blood chemistry components of an advanced intercross line of chickens under heat stress. BMC Genomics 17:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter T. S., Shartau R. B., Brauner C. J., Farrell A. P.. 2014. Validation of the i-STAT system for the analysis of blood parameters in fish. Conserv. Physiol. 2:cou037–cou037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranis A., Gheyas A. A., Boschiero C., Turner F., Yu L., Smith S., Talbot R., Pirani A., Brew F., Kaiser P., Hocking P. M., Fife M., Salmon N., Fulton J., Strom T. M., Haberer G., Weigend S., Preisinger R., Gholami M., Qanbari S., Simianer H., Watson K. A., Woolliams J. A., Burt D. W.. 2013. Development of a high density 600 K SNP genotyping array for chicken. BMC Genomics 14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L. J., Rostagno M. H.. 2013. Impact of heat stress on poultry production. Animals 3:356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. P., Wineland M., Barnes H. J.. 2010. Selected blood chemistry and gas reference ranges for broiler breeders using the i-STAT® handheld clinical analyzer. Avian Dis. 54:1016–1020. [DOI] [PubMed] [Google Scholar]
- Nyoni N. M. B., Grab S., Archer E. R. M.. 2018. Heat stress and chickens: climate risk effects on rural poultry farming in low-income countries. Clim. Dev 11:1–18. [Google Scholar]
- Rettenmund C. L., Heatley J. J., Russell K. E.. 2014. Comparison of two analyzers to determine selected venous blood analytes of quaker parrots (Myiopsitta Monachus). J. Zoo Wildl. Med. 45:256–262. [DOI] [PubMed] [Google Scholar]
- Rönnegård L., Shen X., Alam M.. 2010. hglm: A package for fitting hierarchical generalized linear models. R J. 2:20–28. [Google Scholar]
- Rowland K., Wolc A., Gallardo R. A., Kelly T., Zhou H., Dekkers J. C. M., Lamont S. J.. 2018. Genetic analysis of a commercial egg laying line challenged with newcastle disease virus. Front. Genet. 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal T. P., Arango J., Wolc A., Brady J. V, Fulton J. E., Rubinoff I., Ehr I. J., Persia M. E., Sullivan N. P. O.. 2015. Commercial Hy-Line W-36 pullet and laying hen venous blood gas and chemistry profiles utilizing the portable i-STAT analyzer. Poult. Sci. 00:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H. W., Vogt R., Kästner S., Riond B., Hatt J. M.. 2007. Evaluation of the i-STAT portable clinical analyzer in chickens (Gallus gallus). J. Vet. Diagn. Invest. 19:382–388. [DOI] [PubMed] [Google Scholar]
- Tinkey P., Lembo T., Craig S., West C., Van Pelt C.. 2006. Use of the i-STAT portable clinical analyzer in mice. Lab. Anim. 35:45–50. [DOI] [PubMed] [Google Scholar]
- VanRaden P. M. 2008. Efficient methods to compute genomic predictions. J. Dairy Sci. 91:4414–4423. [DOI] [PubMed] [Google Scholar]
- Wideman R. F., Hooge D. M., Cummings K. R.. 2003. Dietary sodium bicarbonate, cool temperatures, and feed withdrawal: Impact on arterial and venous blood-gas values in broilers. Poult. Sci. 82:560–570. [DOI] [PubMed] [Google Scholar]
- Wolc A., Arango J., Settar P., Fulton J. E., O’Sullivan N. P., Dekkers J. C. M.. 2018. Genome wide association study for heat stress induced mortality in a white egg layer line. Poult. Sci. 98:1–5. [DOI] [PubMed] [Google Scholar]
- Yildirim E., Karapinar T., Hayirli A.. 2015. Reliability of the i-STAT for the determination of blood electrolyte (K+, Na+, and CI-) concentrations in cattle. J. Vet. Intern. Med. 29:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]