ABSTRACT
Coccidiosis is a major gastrointestinal disease caused by several Eimeria species in floor raised chickens. Feeding an antibody to interleukin 10 (aIL-10) ameliorates the negative symptoms of coccidiosis in broilers, i.e., lack of weight gain, decreased feed conversion, and mortality. IL-10 signals by forming a ligand-receptor complex with IL-10 Receptor 1 (IL-10 R1) and IL-10 Receptor 2 (IL-10 R2). In this study, we hypothesize oral antibodies to the IL-10 receptors will neutralize the IL-10 signaling pathway equal to or better than aIL-10 to act as an oral anti-coccidiosis immunotherapy.
A total of 5 sequential feed trials, set up as a 4 (diet antibody) × 2 (Eimeria challenge) factorial design, tested oral egg yolk antibodies to a total of 6 IL-10 R1 epitopes and 3 IL-10 R2 epitopes compared to a control antibody diet. A total of 10 pens of 5 chicks/pen/diet antibody/Eimeria challenge were housed for 21 d. On day 3 of age, chicks were either infected or not infected with a 10× dose of an Eimeria vaccine containing Eimeria acervulina, Eimeria tenella, and Eimeria maxima. Pen feed consumption and mean body weights were assessed weekly (d1, d7, d14, and d21); fecal oocyst shedding was assessed on day 10. Data were analyzed using a 2-way ANOVA. No significant interaction on chick weight was observed in chicks fed IL-10 R1 antibodies compared to chicks fed the control antibody was observed. In studies evaluating aIL-10 R2 oral antibodies, infected chicks fed aIL-10 R2: epitope 1 overcame the negative effects of Eimeria infection and had similar 21-d body weight to uninfected chicks (P4 = 0.07).
We hypothesized that feeding oral antibodies to the IL-10 receptors would result in equivalent anti-coccidial benefits to aIL-10. However, none of the 6 antibodies to IL-10 R1 epitopes yielded any benefits during Eimeria infection compared to controls. A total of 2 oral antibodies to IL-10 R2 showed promising results equivalent to the aIL-10 immunotherapeutic. Immunofluorescence staining shows that the IL-10R2 significantly increases in abundance in response to Eimeria infection, whereas IL-10R1 does not.
Keywords: Eimeria, coccidia, broiler, interleukin-10, interleukin-10 receptor
INTRODUCTION
Coccidiosis is a ubiquitous intestinal parasite in floor raised chickens, costing the broiler industry approximately 1.5 billion dollars each year (Hong et al., 2006b). Coccidiosis is caused by several Eimeria species, commonly referred to as coccidia, and is transmitted fecal-oral from bird to bird. After oral ingestion of an Eimeria oocyst, Eimeria sporozoites invade, develop and rupture intestinal epithelial cells to undergo asexual and sexual replication. Eimeria associated damage to the intestinal barrier results in impaired nutrient absorption, lack of weight gain, hemorrhagic diarrhea, sepsis, and mortality.
Economic losses due to inefficient feed utilization, decreased carcass weights, and mortality necessitate management and treatment of coccidiosis. Commonly used preventatives include attenuated Eimeria vaccines and the use of chemicals, ionophores, and antibiotics. Due to the implementation of the Veterinary Feed Directive and consumer preference for antibiotic free chicken, many producers are minimizing the prophylactic use of ionophores and antibiotics. Decreased ionophore and antibiotic usage result in increased use of vaccines and chemicals. Eimeria are beginning to mount a resistance to anti-coccidial chemicals (Arabkhazaeli et al., 2013). Vaccination against Eimeria species to elicit immunological protection is dependent on a low dose exposure as ingesting large numbers of oocysts causes decreased weight gain (Vermeulen et al., 2001; Price et al., 2016). Improved alternative methods of Eimeria therapeutics are needed.
One potential method commonly used in cancer and chronic disease treatment is immunotherapy. Recently, Sand et al. (2016) discovered that feeding an oral antibody to interleukin 10 (aIL-10) acts as an effective preventative for coccidiosis symptoms. aIL-10 has been shown to improve growth rate, feed conversion, and mortality losses associated with Eimeria infection (Sand et al., 2016; Arendt et al., 2016). Interleukin-10 (IL-10) is an anti-inflammatory cytokine, typically associated with regulating immune homeostasis by acting as a stand down signal (Moore et al., 2001). IL-10 has been shown to be upregulated during a variety of pathogenic infections (Cyktor and Turner, 2011). It is hypothesized that parasites such as Eimeria upregulate IL-10 to dampen and evade the host immune response. Eimeria increases IL-10 within the lumen of the gut and systemically days 4 to 7 post infection (Arendt et al., 2016; Hong et al., 2006a,b). The underlying mechanism of aIL-10 is not fully understood, and it is hypothesized feeding an oral antibody to neutralize anti-inflammatory IL-10 enables the chicken to mount a proper immune response and clear Eimeria without detrimental effects to its growth rate.
In this manuscript, we investigate targeting the IL-10 receptor to improve the efficacy of aIL-10 technology, because while aIL-10 ameliorates the negative symptoms of coccidiosis, it would be more cost effective if it could be used at a lower dose. IL-10 exerts its anti-inflammatory effects through a signaling cascade. First, an IL-10 homodimer binds to IL-10 Receptor 1 (IL-10 R1) with high affinity, and then IL-10 Receptor 2 (IL-10 R2) with low affinity on the cell surface to form a tetrameric heterodimer IL-10 receptor (Josephson et al., 2002; Verma et al., 2016). IL-10 receptors have been found to be present on the surface of intestinal epithelial cells, making the receptors ideal targets for oral antibody administration (Kominsky et al., 2014). We hypothesize that targeting the IL-10 receptors will require a lower dose of antibody and have increased efficacy over aIL-10.
MATERIALS AND METHODS
All experimental procedures involving chickens were approved by the College of Agricultural and Life Sciences Animal Care and Use Committee at the University of Wisconsin-Madison.
Antibody Selection, Preparation and Specificity
Antibody to avian IL-10 was prepared using a procedure previously described (Bobeck et al., 2015; Arendt et al., 2016). The sequences for chicken IL-10, IL-10 R1, and IL-10 R2 were sourced from pubmed.gov protein: CAF18432, CAJ15791.1, and NP_990188.1 (Rothwell et al., 2004; Reboul et al., 1999). A total of 8 amino acid peptide sequences were evaluated for their antigenicity using Bepipred 2.0 software and crystallography was utilized to determine antibody accessibility (Jespersen et al., 2017). Briefly, selected 8 amino acid peptide sequences for IL-10 (vlpramqt); IL-10 R1: 1 (pgrdapsd), 2 (gtnspwta), 3 (tnafspqe), 4 (rtvkyddi), 5 (isssgstd), and 6 (hhrhspat); and IL-10 R2: 1; (vpkprnar), 2 (ppgvrkgn), 3 (adtvigpp); were synthesized by GenScript (Piscatawy, NJ), and conjugated to bovine gamma globulin (BGG, Sigma, St. Louis, MO) using a glutaraldehyde procedure (Bobeck et al., 2012). The control vaccine and booster consisted of glutaraldehyde treated BGG and Freund's complete and incomplete adjuvants (Difco Laboratories, Detroit, MI), the same adjuvants used for making aIL-10 antibody. Hens were injected as previously described and eggs containing the antibody were collected beginning 21 d after the first injection, yolks separated, and dried by lyophilization (Cook and Trott, 2010). Presence of the antibody was determined using ELISA, where the coating peptide was attached to ovalbumin (Bobeck et al., 2012).
Chick Experimentation
A total of 5 chick experiments were conducted to determine the neutralization efficacy of oral IgY antibodies to IL-10 R1 and IL-10 R2. Day-old Cobb 500 Cornish Rock broiler pullets from Welp Hatchery, Bancroft, Iowa, were divided into 5 chicks/pen and housed in a battery brooder with raised wire floors. A total of 10 pens of chicks were assigned to each of the treatments in an antibody × Eimeria infection factorial arrangement in a complete randomized design. Diets consisted of a standard broiler starter diet supplemented with either control dried egg yolk antibody (from hens injected with BGG carrier in adjuvant) or an aIL-10/aIL-10 R1/aIL-10 R2 peptide dried egg yolk antibody (3.41 g/Kg diet), and were fed continuously from the day of chick placement to the end of each study (Table 1). The nutrient content of all diets were identical and came from the same lot of feed because peptide antibody replaced control antibody containing the exact same nutrient profiles (nutrient profiles of dried egg yolk powder). The dietary level of anti-IL-10 antibody was based on the level used by Sand et al. (2016), and was a level that prevented Eimeria-induced growth depression in chicks. Chicks assigned each diet treatment were either orally gavaged with a saline solution or an Advent coccidiosis vaccine at 3 d (10 × vaccine dose consisting of a proprietary blend of live nonattenuated Eimeria acervulina, Eimeria maxima, and Eimeria tenella oocysts, Huvepharma, Sofia, Bulgaria). The 10× dose of vaccine causes a reproducible growth depression, but no significant mortality compared to uninfected chicks.
Table 1.
Oral antibodies evaluated in each experiment.
| Experiment number | Dates | Antibodies evaluated |
|---|---|---|
| Study 1 | 1/7 to 1/28/2016 | Control, aIL-10 R1:2, aIL-10 R1:4, and aIL-10 R1:5 |
| Study 2 | 2/10 to 3/2/2016 | Control, aIL-10 R1:1, aIL-10 R1:3, and aIL-10 R1:6 |
| Study 3 | 3/17 to 4/7/2016 | Control, aIL-10 R1:5, and aIL-10 R1:6 |
| Study 4 | 1/19 to 2/9/2017 | Control, aIL-10 R2:1, aIL-10 R2:2, and aIL-10 R2:3 |
| Study 5 | 6/14 to 7/5/2017 | Control, aIL-10, aIL-10 R2:1, and aIL-10 R2:2 |
Chicks and feed were weighed on days 1, 7, 14, and 21. Feed conversion was calculated by dividing feed consumption by body weight gain over the 21-d period. Mean body weights were calculated for days 1, 7, 14, and 21 as well as feed conversion ratio for days 1 to 7, 1 to 14, and 1 to 21. For all treatment groups, mean chick body weights were not statistically different from each other on day 1 (data not shown). Feed conversion ratio was calculated for days 7 to 14 because those days correspond to the peak infection period. Fresh, moist fecal samples were collected from clean fecal trays on day 7 post infection. Approximately 10 grams were collected and mixed together until feces appeared homogenous. Oocysts per gram of feces were quantified using the McMaster technique (Haug et al., 2006).
Statistical Analysis
The experiments were set up in a completely randomized factorial design and analyzed as a 2-way ANOVA using ANOVAs PROC MIXED of SAS 9.4 (SAS Institute Inc., Cary, NC). The LSD test was used for multiple treatment comparisons using the LSMEANS statement of SAS 9.4 with letter grouping obtained using the SAS pdmix800 macro. For the different statistical tests, significance was declared at a P-value of ≤0.05, and a statistical trend was noted at a P-value of ≤0.10. Post hoc analyses for treatment differences were conducted if interactions were significant.
Immunohistochemistry Staining
Previously collected paraffin sections with known mucosal IL-10 elevation compared to uninfected birds were used to demonstrate IL-10 R1 and IL-10 R2 presence. Slides were of the duodenum of 26-day-old birds either infected with Eimeria or a control saline gavage on day 21 of age as described above. Slides were incubated overnight at 60°C before being deparaffinized with 2 changes of xylene for 10 min, and rehydrated with isopropyl alcohol at 2 changes of 100% alcohol, 2 changes of 95% alcohol, 1 change of 75% alcohol, and 1 change of distilled water at 1 min per change. Slides underwent heat induced epitope-retrieval in tris urea solution. After rinsing with tris buffered saline solution, an ImmEdge pen (Vector Laboratories, Inc., Burlingame, CA) was used to isolate tissue sections and were submerged with blocking buffer for 1 h in a humidified chamber (Bobeck et al., 2015).
To evaluate IL-10 R1, tissues were coated in rabbit anti-IL-10 R1 polyclonal antibody (GeneTex, Irvine, CA) at 1:300 dilution in blocking buffer overnight at 4°C in a humidified dark enclosure. Slides were then stained with 1:100 diluted donkey anti-rabbit Dylight594 (Bethyl, Montgomery, TX) for 1 h in a humidified chamber. Nuclei were highlighted by fluorogel with tris buffer and 4',6-diamidino-2-phenylindole (DAPI) solution.
For IL-10 R2 staining, a contiguous intestinal section was coated in rabbit anti-IL-10 R2 polyclonal antibody (GeneTex, Irvine, CA) at 1:100 dilution in blocking buffer overnight at 4°C in a humidified dark enclosure. Slides were then stained with 1:100 diluted donkey anti-rabbit Dylight594 (Bethyl, Montgomery, TX) for 1 h in a humidified chamber. Nuclei were highlighted by fluorogel with tris buffer and DAPI solution. Due to Eimeria oocyst wall autofluorescence at 495 nm, only the red channel was used for immunofluorescence and the blue channel was used for cell nuclei. Slides were imaged using a Nikon Eclipse E600 with Y-FL fluorescence attachment microscope.
RESULTS
Broiler Performance with Oral IL-10 R1 Antibody Administration
None of the 6 IL-10 R1 antibodies tested recovered the losses associated with Eimeria infection or reduced oocyst shedding. In study 1, aIL-10 R1:2, and 5 diets had a negative impact on feed conversion at time points day 7 to 14 and day 1 to 21 compared to control antibody fed birds (P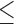 0.05, Table 2). aIL-10 R1:5 had a 69% increase in oocyst shedding and a 12% reduction in average chick weight compared to control fed chicks (P < 0.0001, Table 2). Study 2 showed no effects of aIL-10 R1:1, 3, and 6 (Table 3). Due to the observed improvement of aIL-10 R1:6 fed, Eimeria infected birds with a 15% improvement of feed conversion, compared to control antibody fed, Eimeria infected birds, aIL-10R1:6 treatment was repeated in study 3.
0.05, Table 2). aIL-10 R1:5 had a 69% increase in oocyst shedding and a 12% reduction in average chick weight compared to control fed chicks (P < 0.0001, Table 2). Study 2 showed no effects of aIL-10 R1:1, 3, and 6 (Table 3). Due to the observed improvement of aIL-10 R1:6 fed, Eimeria infected birds with a 15% improvement of feed conversion, compared to control antibody fed, Eimeria infected birds, aIL-10R1:6 treatment was repeated in study 3.
Table 2.
Study 1, IL-10 Receptor 1 antibodies 2, 4, and 5.
| Eimeria challenge Antibody treatment | Uninfected | Eimeria infected | P values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | aIL-10 R1:2 | aIL-10 R1:4 | aIL-10 R1:5 | Control | aIL-10 R1:2 | aIL-10 R1:4 | aIL-10 R1:5 | SEM | Antibody | Coccidiosis | Antibody × Coccidiosis | |
| Day | Chick body weight1 (g) | |||||||||||
| 7 | 160w | 157w | 153w,x | 145x,y | 134z | 137y,z | 133z | 137y,z | 4 | 0.25 | <0.0001 | 0.09 |
| 14 | 397 | 384 | 388 | 349 | 328 | 317 | 318 | 303 | 10 | 0.0045 | <0.0001 | 0.62 |
| 14 Antibody main effect | 363A | 350A | 353A | 326B | ||||||||
| 21 | 790 | 762 | 780 | 677 | 663 | 636 | 657 | 593 | 21 | <0.0001 | <0.0001 | 0.70 |
| 21 Antibody main effect | 726A | 699A | 718A | 635B | ||||||||
| Days | Feed conversion ratio2 | |||||||||||
| 1 to 7 | 1.242z | 1.296z | 1.306y,z | 1.386x,y | 1.413w,x | 1.414w,x | 1.478 w | 1.424w,x | 0.028 | 0.06 | <0.0001 | 0.10 |
| 7 to 14 | 1.378 | 1.476 | 1.374 | 1.518 | 1.534 | 1.704 | 1.606 | 1.678 | 0.057 | 0.03 | <0.0001 | 0.85 |
| 7 to 14 Antibody main effect | 1.456b | 1.590a | 1.490a,b | 1.598a | ||||||||
| 1 to 14 | 1.326 | 1.415 | 1.351 | 1.471 | 1.507 | 1.601 | 1.575 | 1.579 | 0.045 | 0.09 | <0.0001 | 0.62 |
| 1 to 14 Antibody main effect | 1.417z | 1.508y | 1.463y,z | 1.525y | ||||||||
| 1 to 21 | 1.452 | 1.531 | 1.522 | 1.644 | 1.572 | 1.723 | 1.730 | 1.746 | 0.055 | 0.02 | 0.0003 | 0.74 |
| 1 to 21 Antibody Main Effect | 1.512b | 1.627a | 1.626a,b | 1.695a | ||||||||
| Eimeria oocysts/g feces3 | 316,236B | 307,109B | 388,711B | 535,654A | 32,377 | 0.0003 | <0.0001 | 0.0003 | ||||
A,BMeans with different superscripts within a row are significantly different (P 0.01).
0.01).
a,bMeans with different superscripts within a row are significantly different (P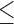 0.05).
0.05).
w-zMeans with different superscripts within a row are significantly different (P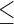 0.10). SEM = Standard error of the mean.
0.10). SEM = Standard error of the mean.
1All treatment groups mean chick body weights were not statistically different from each other at the start of the study.
2Feed conversion ratio was calculated by dividing feed consumption by average pen body weight gain (n = 10).
3All uninfected pens had oocyst counts of 0 oocysts per gram of feces. Only Eimeria infected groups are shown in the table.
Table 3.
Study 2, IL-10 Receptor 1 antibodies 1, 3, and 6.
| Eimeria challenge Antibody treatment | Uninfected | Eimeria infected | P values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | aIL-10 R1:1 | aIL-10 R1:3 | aIL-10 R1:6 | Control | aIL-10 R1:1 | aIL-10 R1:3 | aIL-10 R1:6 | SEM | Antibody | Coccidiosis | Antibody × Coccidiosis | |
| Day | Chick body weight1 (g) | |||||||||||
| 7 | 158 | 158 | 161 | 160 | 140 | 149 | 148 | 140 | 5 | 0.57 | <0.0001 | 0.62 |
| 14 | 373 | 364 | 359 | 376 | 319 | 321 | 338 | 317 | 11 | 0.95 | <0.0001 | 0.31 |
| 21 | 735 | 710 | 711 | 731 | 639 | 655 | 676 | 656 | 21 | 0.94 | <0.0001 | 0.53 |
| Days | Feed conversion ratio2 | |||||||||||
| 1 to 7 | 1.327 | 1.314 | 1.290 | 1.344 | 1.508 | 1.485 | 1.479 | 1.437 | 0.040 | 0.84 | <0.0001 | 0.58 |
| 7 to 14 | 1.518 | 1.523 | 1.543 | 1.508 | 1.736 | 1.647 | 1.670 | 1.573 | 0.060 | 0.51 | 0.0021 | 0.64 |
| 1 to 14 | 1.450 | 1.445 | 1.443 | 1.451 | 1.651 | 1.580 | 1.598 | 1.521 | 0.041 | 0.51 | <0.0001 | 0.482 |
| 1 to 21 | 1.678 | 1.682 | 1.674 | 1.685 | 1.877 | 1.740 | 1.751 | 1.593 | 0.071 | 0.17 | 0.14 | 0.13 |
| Eimeria oocysts/g feces3 | 657,688 | 327,232 | 360,146 | 522,890 | 93,221 | 0.26 | <0.0001 | 0.26 | ||||
SEM = Standard error of the mean.
1All treatment groups mean chick body weights were not statistically different from each other at the start of the study.
2Feed conversion ratio was calculated by dividing feed consumption by average pen body weight gain (n = 10).
3All uninfected pens had oocyst counts of 0 oocysts per gram of feces. Only Eimeria infected groups are shown in the table.
Study 3 aimed to retest IL-10 R1 antibodies 5 and 6 due to the profound negative effect of aIL-10R1:5 and the potential positive effect of aIL-10 R1:6 on feed conversion during Eimeria infection. Similar to study 1, aIL-10 R1:5 had a negative impact on feed conversion compared to control during days 1 to 14 and 1 to 21; however, the negative effect on body weight did not repeat (Table 4). In re-examining the ability of aIL-10 R1:6 to improve feed conversion, aIL-10 R1:6 failed to improve feed conversion at days 1 to 21, and instead had a negative effect on feed conversion at day 7 to 14 and day 1 to 14. In summary, IL-10 R1 oral antibody administration did not ameliorate negative Eimeria symptoms; and in the case of aIL-10 R1:2, aIL-10 R1:5 and aIL-10 R1:6 had a negative impact on the growth rate or feed conversion of the bird.
Table 4.
Study 3, IL-10 Receptor 1 Antibodies 5 and 6.
| Eimeria challenge Antibody treatment | Uninfected | Eimeria infected | P values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | aIL-10 R1:5 | aIL-10 R1:6 | Control | aIL-10 R1:5 | aIL-10 R1:6 | SEM | Antibody | Coccidiosis | Antibody × Coccidiosis | |
| Day | Chick body weight1 (g) | |||||||||
| 7 | 149 | 152 | 144 | 140 | 139 | 134 | 4 | 0.22 | 0.0014 | 0.91 |
| 14 | 386 | 372 | 361 | 341 | 341 | 338 | 12 | 0.49 | 0.0009 | 0.65 |
| 21 | 698 | 645 | 631 | 634 | 657 | 650 | 27 | 0.51 | 0.57 | 0.13 |
| Days | Feed conversion ratio2 | |||||||||
| 1 to 7 | 1.302 | 1.320 | 1.225 | 1.314 | 1.400 | 1.389 | 0.071 | 0.70 | 0.14 | 0.56 |
| 7 to 14 | 1.359 | 1.502 | 1.581 | 1.420 | 1.458 | 1.458 | 0.049 | 0.029 | 0.38 | 0.17 |
| 7 to 14 Antibody main effect | 1.390b | 1.480a,b | 1.519a | |||||||
| 1 to 14 | 1.358 | 1.453 | 1.467 | 1.384 | 1.438 | 1.431 | 0.033 | 0.023 | 0.85 | 0.54 |
| 1 to 14 Antibody main effect | 1.366b | 1.446a | 1.449a | |||||||
| 1 to 21 | 1.411 | 1.473 | 1.469 | 1.430 | 1.469 | 1.431 | 0.017 | 0.018 | 0.58 | 0.23 |
| 1 to 21 Antibody main effect | 1.421b | 1.471a | 1.450a,b | |||||||
| Eimeria oocysts/g feces3 | 309,184z | 317,882z | 449,404y | 34,718 | 0.087 | <0.0001 | 0.087 | |||
a,bMeans with different superscripts within a row are significantly different (P < 0.05).
y,zMeans with different superscripts within a row have a P value of 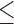 0.10. SEM = Standard error of the mean.
0.10. SEM = Standard error of the mean.
1All treatment groups mean chick body weights were not statistically different from each other at the start of the study.
2Feed conversion ratio was calculated by dividing feed consumption by average pen body weight gain (n = 10).
3All uninfected pens had oocyst counts of 0 oocysts per gram of feces. Only Eimeria infected groups are shown in the table.
Broiler Performance with Oral IL-10 R2 Antibody Administration
A total of 3 antibodies were tested against IL-10 R2. In study 4, infected chicks fed aIL-10 R2:1 or aIL-10 R2:2 overcame the Eimeria infection and had similar 21-d body weight to uninfected chicks (trend, P = 0.07, Table 5). aIL-10 R2:1 and aIL-10R2:2 fed birds did not demonstrate an improvement in body weight until day 21, indicating that the birds fed the antibody are still affected by Eimeria infection, but have an improved recovery time. On day 14, aIL-10 R2:2 had a negative effect on body weight with uninfected, aIL-10 R2:2 fed birds mean body weight at 394 g compared to control fed uninfected birds with a mean body weight of 429 g. Additionally, during the peak infection period, day 7 to 14, aIL-10 R2:2 fed birds trended towards an improved feed conversion by 0.089 (P = 0.08). Oral antibody administration had no effect on oocyst shedding in Eimeria infected treatment groups.
Table 5.
Study 4, IL-10 Receptor 2 Antibodies 1, 2, and 3.
| Eimeria challenge Antibody treatment | Uninfected | Eimeria infected | P values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | aIL-10 R2:1 | aIL-10 R2:2 | aIL-10 R2:3 | Control | aIL-10 R2:1 | aIL-10 R2:2 | aIL-10 R2:3 | SEM | Antibody | Coccidiosis | Antibody × Coccidiosis | |
| Day | Chick body weight1 (g) | |||||||||||
| 7 | 187 | 179 | 173 | 176 | 154 | 155 | 154 | 152 | 4 | 0.31 | <0.0001 | 0.42 |
| 14 | 429a | 415a,b | 394b | 405a,b | 326c,d | 337c,d | 352c | 317d | 11 | 0.45 | <0.0001 | 0.05 |
| 21 | 684x | 679x | 659x | 668x | 585z | 663x | 646x,y | 604y,z | 19 | 0.17 | 0.0003 | 0.069 |
| Days | Feed conversion ratio2 | |||||||||||
| 1 to 7 | 1.171 | 1.238 | 1.262 | 1.239 | 1.392 | 1.348 | 1.396 | 1.398 | 0.028 | 0.25 | <0.0001 | 0.21 |
| 7 to 14 | 1.455 | 1.548 | 1.396 | 1.451 | 1.607 | 1.610 | 1.487 | 1.663 | 0.060 | 0.08 | 0.004 | 0.61 |
| 1.531y | 1.579y,z | 1.442z | 1.557y,z | |||||||||
| 1 to 14 | 1.343 | 1.435 | 1.343 | 1.367 | 1.519 | 1.508 | 1.452 | 1.545 | 0.045 | 0.29 | <0.0001 | 0.55 |
| 1 to 21 | 1.468 | 1.511 | 1.405 | 1.459 | 1.533 | 1.516 | 1.495 | 1.530 | 0.034 | 0.27 | 0.04 | 0.62 |
| Eimeria oocysts/g feces3 | 622,918 | 614,536 | 486,347 | 866,860 | 132,053 | 0.73 | <0.0001 | 0.73 | ||||
a–dMeans with different superscripts within a row are significantly different (P 0.05).
0.05).
x–zMeans with different superscripts within a row have a P value of  0.10.
0.10.
SEM = Standard error of the mean.
1All treatment groups mean chick body weights were not statistically different from each other at the start of the study.
2Feed conversion ratio was calculated by dividing feed consumption by average pen body weight gain (n = 10).
3All uninfected pens had oocyst counts of 0 oocysts per gram of feces. Only Eimeria infected groups are shown in the table.
In study 5, uninfected, aIL-10 fed chicks gained significantly less weight compared to uninfected, control antibody fed chicks (Table 6). Infected control fed birds had a 131-gram difference in mean body weight compared to uninfected control fed birds. In comparison to control fed birds, aIL-10 had a significantly less difference of 67 grams in mean body weight between Eimeria treatments, indicating aIL-10 had a positive effect on growth rate during Eimeria infection (Figure 1). aIL-10 also trended towards having an improved feed conversion of 1.491 compared to control antibody fed birds with a feed conversion of 1.546 (P 0.10, Table 6). In studies 4 and 5, birds infected with Eimeria gained significantly less weight and had an increased feed conversion ratio compared to uninfected birds at days 7, 14, and 21 time points, indicating the Eimeria infection model was effective (P
0.10, Table 6). In studies 4 and 5, birds infected with Eimeria gained significantly less weight and had an increased feed conversion ratio compared to uninfected birds at days 7, 14, and 21 time points, indicating the Eimeria infection model was effective (P 0.05, Tables 5 and 6). Overall, aIL-10 R2:1 and aIL-10 R2:2 showed promising results similar to aIL-10.
0.05, Tables 5 and 6). Overall, aIL-10 R2:1 and aIL-10 R2:2 showed promising results similar to aIL-10.
Table 6.
Study 5, IL-10 Receptor 2 Antibodies 1 and 2.
| Eimeria challenge Antibody treatment | Uninfected | Eimeria infected | P values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | aIL-10 | aIL-10 R2:1 | aIL-10 R2:2 | Control | aIL-10 | aIL-10 R2:1 | aIL-10 R2:2 | SEM | Antibody | Coccidiosis | Antibody × Coccidiosis | |
| Day | Chick body weight1 (g) | |||||||||||
| 7 | 153 | 152 | 155 | 154 | 129 | 131 | 128 | 127 | 2 | 0.93 | <0.0001 | 0.49 |
| 14 | 395 | 389 | 400 | 401 | 298 | 303 | 300 | 302 | 11 | 0.81 | <0.0001 | 0.71 |
| 21 | 709a | 662b | 681a,b | 701a | 578c | 595c | 598c | 606c | 11 | 0.24 | <0.0001 | 0.048 |
| Days | Feed conversion ratio2 | |||||||||||
| 1 to 7 | 1.405 | 1.275 | 1.228 | 1.222 | 1.347 | 1.415 | 1.435 | 1.425 | 0.067 | 0.86 | 0.01 | 0.14 |
| 7 to 14 | 1.514 | 1.466 | 1.484 | 1.419 | 1.735 | 1.650 | 1.645 | 1.662 | 0.039 | 0.14 | <0.0001 | 0.71 |
| 1 to 14 | 1.477 | 1.402 | 1.401 | 1.355 | 1.592 | 1.568 | 1.572 | 1.580 | 0.032 | 0.19 | <0.0001 | 0.38 |
| 1 to 21 | 1.523 | 1.484 | 1.508 | 1.446 | 1.569 | 1.498 | 1.564 | 1.548 | 0.028 | 0.10 | 0.004 | 0.43 |
| 1.546y | 1.491z | 1.536y,z | 1.497y,z | |||||||||
| Eimeria oocysts/g feces3 | 195,031 | 374,626 | 245,589 | 166,483 | 35,037 | 0.46 | <0.0001 | 0.46 | ||||
a–cMeans with different superscripts within a row are significantly different (P 0.05).
0.05).
x–zMeans with different superscripts within a row have a P value of less than  0.10. SEM = Standard error of the mean.
0.10. SEM = Standard error of the mean.
1All treatment groups mean chick body weights were not statistically different from each other at the start of the study.
2Feed conversion ratio was calculated by dividing feed consumption by average pen body weight gain (n = 10).
3All uninfected pens had oocyst counts of 0 oocysts per gram of feces. Only Eimeria infected groups are shown in the table.
Figure 1.

Study 5 differences in uninfected vs. infected mean chick weight at day 21 of age. a-b: Means with different labels were significantly different (P < 0.05). The difference in mean chick weight was calculated by subtracting, uninfected − Eimeria infected mean chick weights, and were measured in grams.
Intestinal Mucosal IL-10 Receptor 1 and 2 Presence
To further elucidate why aIL-10 R2 is more beneficial than aIL-10 R1, immunohistochemistry was used to evaluate IL-10 R1 and IL-10 R2 presence during Eimeria infection. Both IL-10 R1 and R2 are of low abundance in uninfected chicks. On day 5 post infection in the duodenum, IL-10 R1 is visually lower in presence compared to the IL-10 R2 shown in red (Figure 2). The increased presence of IL-10 R2 staining illustrates that it is more prominent in the intestinal mucosa and surrounding Eimeria infected regions when compared to IL-10 R1. IL-10 R2 presence was objectively higher in birds infected with Eimeria compared to control birds. This result indicates that IL-10R2 may be playing an additional role during Eimeria infection in chickens.
Figure 2.
Duodenal intestinal mucosa 5 d post Eimeria infection IL-10 Receptor immunofluorescent histochemistry in duodenum 5 d post infection. One representative image per treatment is shown. A primary polyclonal rabbit aIL-10 R1 and IL-10 R2 antibody and secondary goat anti-rabbit IgY-Dylight 594 antibody on separate, but contiguous slides was used to visualize IL-10 R1 and IL-10 R2 (red fluorescence). DAPI was applied to intestinal slides to achieve nuclear staining (blue fluorescence). Blue and red were merged to allow the observation of IL-10 R1 and R2 in context of intestinal cell nuclei. Each image focuses on the area between 2 villi, where Eimeria infection would typically occur. The intestinal lumen is at the top left and the submucosa is at the bottom right.
DISCUSSION
Feeding Eimeria infected chicks aIL-10 has previously been shown to prevent reduced body weight compared to challenged chicks fed control antibody (Arendt et al., 2016; Sand et al., 2016). In studies 1, 2, and 3, we did not see the prevention of reduced growth rate when chicks were fed an antibody to IL-10 Receptor 1. However, we did see improvement with administration of an oral antibody to IL-10 Receptor 2 in study 4 indicating IL-10 receptor complex–IL-10 R2 binding is critical for the anti-inflammatory signaling pathway potentially upregulated by Eimeria. The binding site of aIL-10 (peptide vlpramqt) is not located near the IL-10 Receptor binding region so direct allosteric hindrance of the aIL-10 antibody to IL-10 R1 does not explain the ability of aIL-10 to neutralize IL-10. In Josephson et al. (2002), a monoclonal antibody to a noncontiguous peptide sequence on IL-10, which overlaps with the vlpramqt region, also showed that antibody binding to this specific IL-10 region resulted in a noncompetitive binding to the IL-10 R1 receptor. Even through this noncompetitive binding, the monoclonal antibody was able to neutralize IL-10 function. The monoclonal binding was capable of interfering with conformational changes in the IL-10/IL-10 R1 complex (Josephson et al., 2002). We hypothesize that the aIL-10 binding peptide region does not interfere with IL-10R1 binding, but may inhibit the ability of the IL-10/IL-10 R1 complex binding IL-10 R2 (Yoon et al., 2010).
IL-10 R2 presence was greater than IL-10 R1 in the Eimeria infected duodenum (Figure 2). The prominence of IL-10 R2 expression during Eimeria infection may indicate it has an additional role during Eimeria invasion and replication. IL-10R1 is faithful to IL-10, whereas IL-10R2 is promiscuous and interacts in other cytokine signaling pathways, including IL-22, IL-26, IL-28A, IL-28B, and IL-29 (Yoon et al., 2010; Williams et al., 2004). Feeding an oral antibody to IL-10 R2 may have an effect on these related cytokine pathways. IL-22 has been shown to drive intestinal immunopathology associated with many related apicomplexan parasites, including the closely related Eimeria falciformis (Stange et al., 2012). Chicken IL-26 was cloned by Truong et al. (2016) and shown to induce proinflammatory cytokines. The role of IL-26 during Eimeria infection has not been well studied, but it has been associated with exacerbation of pathology in a parasitic disease in humans, lymphatic filariasis (Anuradha et al., 2014). IL-28A, IL-28B, and IL-29 are all part of the interferon λ (IFNλ) signaling cascade. The role of IFNλ during Eimeria infection has not been elucidated, but IFNλ is critical for the control of related parasites. IL-10R2 neutralization by aIL-10R2:1 and aIL-10R2:2 may have an effect on these pro-inflammatory cytokine pathways and reduce Eimeria associated inflammation and immunopathology resulting in the therapeutic effects of aIL-10R2:1 and aIL-10R2:2.
aIL-10 R1:5 was observed to have a negative effect on growth rate and feed conversion, and resulted in a larger number of oocysts shed per gram of feces (Tables 2 and 4). The negative effect on intestinal absorptive function is likely due to the importance of IL-10 signaling to maintain normal intestinal mucosal immune homeostasis and increased parasite burden reflected by the increased number of oocysts shed per gram of feces. IL-10 knockout mice are used as a model organism to study Crohn's disease and exhibit colitis associated with the absence of functional IL-10 within the intestine (Keubler et al., 2015). Neutralization of IL-10 receptor 1 by feeding aIL-10 R1:5 exerts similar effects on intestines exhibited by lack of weight gain in this study. A possible reason for the effects of aIL-10 R1:5 on weight gain and feed conversion in both infected and uninfected birds could be an overdose of the antibody, resulting in the inability to properly mediate the intestinal host––microbiota relationship.
aIL-10 R2:1 and aIL-10R2:2 were shown to potentially ameliorate negative coccidiosis symptoms. In study 4, these antibodies demonstrated positive effects on weight gain equivalent to previous aIL-10 studies (Sand et al., 2016). To evaluate the effect of aIL-10R2:1 and aIL-10R2:2 in direct comparison to aIL-10, study 5 was performed. In study 5, the Eimeria vaccine was less than 1-mo old resulting in a greater virulence and had more detrimental effects on chick weight at day 21 of age than in previous studies 1 to 4. Despite having the greatest impact on day 21 mean body weight, study 5 had the lowest amount of Eimeria oocysts/g of feces shed in the infected birds fed the control antibody. The low rate of shedding was likely due to study 5 occurring in the summer, a season when Eimeria oocyst shedding is typically the lowest; whereas, studies 1 to 4 occurred during the winter/spring (Krassner, 1963). In addition, high titer aIL-10 was fed at a dose 10 times that currently commercially used to be equivalent in dose to aIL-10R2 antibodies. The high dose of aIL-10 likely resulted in an overdose of antibody evident in the 70 g mean chick weight difference between uninfected control and uninfected aIL-10 fed chicks. Nevertheless, aIL-10 fed birds had a significantly decreased difference in weight loss due to Eimeria infection compared to control (Figure 1), indicating aIL-10 was protective against Eimeria related lack of weight gain despite its confounding negative effects on uninfected bird weight.
In conclusion, IL-10 R2:1 is a promising alternative anti-coccidial immunotherapeutic. Study 5 results indicate that aIL-10 is better than either of the IL-10 R2 antibodies at reducing the effect of Eimeria infection on weight gain. In future studies, the dose of aIL-10 R2:1 will be honed and birds will be fed aIL-10 and aIL-10 R2:1 in combination to evaluate if the combination therapy improves efficacy and decreases dose.
ACKNOWLEDGMENTS
The authors would like to thank Jessica Muhlenbeck, Zhouzheng Ren, Jonathan Elissa, Natalie Schmidt, Emily Michael, Taylor Marcone, Zachary Simons, and Dan Butz for aiding in sample collection and analysis, as well as the UW Animal Care Staff (Dawn Irish, John Kemper, and Terry Jobsis) for animal care. The authors would also like to thank the Chad Vezina laboratory for use of the Nikon Eclipse E600 microscope. Laying hens used for making antibodies in these studies were kindly donated by S&R Farms, Whitewater, WI. Eimeria vaccine was donated by Huvepharma, Sofia, Bulgaria. Funding for the studies was provided by the NIH T32OD010423 training grant, Poultry Science Association Foundation Merck Animal Health Graduate Poultry Research Fellowship and the University of Wisconsin – Madison Food Research Institute.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Anuradha R., Parakkal J. G., Hanna L. E., Kumaran P., Chandrasekaran V., Nutman T. B., Babu S.. 2014. Expansion of parasite-specific CD4+ and CD8+ T cells expressing IL-10 superfamily cytokine members and their regulation in human lymphatic filariasis. PLoS Negl. Trop. Dis. 8:e2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabkhazaeli F., Modrisanei M., Nabian S., Mansoori B., Madani A.. 2013. Evaluating the resistance of Eimeria spp. field isolates to anticoccidial drugs using three different indices. Iran J. Parasitol. 8:234. [PMC free article] [PubMed] [Google Scholar]
- Arendt M. K., Sand J. M., Marcone T. M., Cook M. E.. 2016. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 95:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck E. A., Burgess K. S., Jarmes T. R., Piccione M. L., Cook M. E.. 2012. Maternally-derived antibody to fibroblast growth factor-23 reduced dietary phosphate requirements in growing chicks. Biochem. Biophys. Res. Commun. 420:666–670. [DOI] [PubMed] [Google Scholar]
- Bobeck E. A., Hellestad E. M., Sand J. M., Piccione M. L., Bishop J. W., Helvig C., Petkovich M., Cook M. E.. 2015. Oral peptide specific egg antibody to intestinal sodium-dependent phosphate co-transporter-2b is effective at altering phosphate transport in vitro and in vivo. Poult. Sci. 94:1128–1137. [DOI] [PubMed] [Google Scholar]
- Cook M. E., Trott D. L.. 2010. IgY – Immune component of eggs as a source of passive immunity for animals and humans. World. Poult. Sci. J. 66:215–226. [Google Scholar]
- Cyktor J. C., Turner J.. 2011. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect. Immun. 79:2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A., Williams R. B., Larsen S.. 2006. Counting coccidial oocysts in chicken faeces: a comparative study of a standard McMaster technique and a new rapid method. Vet. Parasitol. 136:233–242. [DOI] [PubMed] [Google Scholar]
- Hong Y. H., Lillehoj H. S., Lillehoj E. P., Lee S. H.. 2006. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 114:259–272. [DOI] [PubMed] [Google Scholar]
- Hong Y. H., Lillehoj H. S., Lee S. H., Dalloul R. A., Lillehoj E. P.. 2006. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 114:209–223. [DOI] [PubMed] [Google Scholar]
- Jespersen M. C., Peters B., Nielsen M., Marcatili P.. 2017. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 45:W24–W29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson K., Jones B. C., Walter L. J., DiGiacomo R., Indelicato S. R., Walter M. R.. 2002. Noncompetitive antibody neutralization of IL-10 revealed by protein engineering and x-ray crystallography. Structure 10:981–987. [DOI] [PubMed] [Google Scholar]
- Keubler L. M., Buettner M., Häger C., Bleich A.. 2015. A multihit model. Inflamm. Bowel Dis. 21:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominsky D. J., Campbell E. L., Ehrentraut S. F., Wilson K. E., Kelly C. J., Glover L. E., Collins C. B., Bayless A. J., Saeedi B., Dobrinskikh E., Bowers B. E., MacManus C. F., Müller W., Colgan S. P., Bruder D.. 2014. IFN- γ-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J. Immunol. 192:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassner S. M. 1963. Factors in host susceptibility and oocyst infectivity in Eimeria acervulina infections. J. Protozool. 10:327–333. [DOI] [PubMed] [Google Scholar]
- Moore K., deWaalMalefyt R., Coffman3 R. L., O’Garra3 A.. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- Price K. R., Hafeez M. A., Bulfon J., Barta J. R.. 2016. Live Eimeria vaccination success in the face of artificial non-uniform vaccine administration in conventionally reared pullets. Avian Pathol. 45:82–93. [DOI] [PubMed] [Google Scholar]
- Reboul J., Gardiner K., Monneron D., Uzé G., Lutfalla G.. 1999. Comparative genomic analysis of the interferon/interleukin-10 receptor gene cluster. Genome Res. 9:242–50. [PMC free article] [PubMed] [Google Scholar]
- Rothwell L., Young J. R., Zoorob R., Whittaker C. A., Hesketh P., Archer A., Smith A. L., Kaiser P.. 2004. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 173:2675–2682. [DOI] [PubMed] [Google Scholar]
- Sand J. M., Arendt M. K., Repasy A., Deniz G., Cook M. E.. 2016. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poult. Sci. 95:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange J., Hepworth M. R., Rausch S., Zajic L., Kühl A. A., Uyttenhove C., Renauld J.-C., Hartmann S., Lucius R.. 2012. IL-22 Mediates host defense against an intestinal intracellular parasite in the absence of IFN-γ at the cost of Th17-driven immunopathology. J. Immunol. 188:2410–2418. [DOI] [PubMed] [Google Scholar]
- Truong A. D., Park B., Ban J., Hong Y. H.. 2016. The novel chicken interleukin 26 protein is overexpressed in T cells and induces proinflammatory cytokines. Vet. Res. 47:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Balakrishnan L., Sharma K., Khan A. A., Advani J., Gowda H., Tripathy S. P., Suar M., Pandey A., Gandotra S., Prasad T. S. K., Shankar S.. 2016. A network map of interleukin-10 signaling pathway. J. Cell Commun. Signal. 10: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., Schaap D. C., Schetters P. M.. 2001. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 100:13–20. [DOI] [PubMed] [Google Scholar]
- Williams L. M, Ricchetti G., Sarma U., Smallie T., Foxwell B.. 2004. Interleukin-10 suppression of myeloid cell activation–a continuing puzzle. Immunology 113:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. I., Jones B. C., Logsdon N. J., Harris B. D., Deshpande A., Radaeva S., Halloran B. A., Gao B., Walter M. R.. 2010. Structure and mechanism of receptor sharing by the IL-10R2 common chain. Structure 18:638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]



