Abstract
The mechanisms underlying nutrient-induced diversity–stability relationships have been examined extensively. However, the effects of nutrient-induced shifts of dominant species on ecosystem stability have rarely been evaluated. We compiled a dataset from a long-term nitrogen (N) and phosphorus (P) enrichment experiment conducted in an alpine grassland on the Tibetan Plateau to test the effects of nutrient-induced shifts of dominant species on stability. Our results show that N enrichment increased synchrony among the dominant species, which contributed to a significant increase in synchrony of the whole community. Meanwhile, N-induced shifts in dominant species composition significantly increased population variability. Increases in species synchrony and population variability resulted in a decline in ecosystem stability. Our study has important implications for progress in understanding the role of plant functional compensation in the stability of ecosystem functions, which is critical for better understanding the mechanisms driving both community assembly and ecosystem functions.
Keywords: Nutrient enrichment, Selection effect, Species richness, Alpine grassland, Tibetan Plateau
1. Introduction
Knowledge of the mechanisms underlying the stability of ecosystem functions is important in understanding how to maintain and improve ecosystem functions in the face of global changes. Coexisting species can maintain relatively stable community compositions and their functional stability through asynchronous responses to certain levels of environmental fluctuations or anthropogenic perturbations (Grime, 1998; Ernest and Brown, 2001; Hooper et al., 2005; Grman et al., 2010). However, frequent and intense perturbations can often induce alterations of species composition, lead to community turnover, and increase ecosystem variability (Bobbink et al., 2010; Hautier et al., 2014; Payne et al., 2017; Stevens et al., 2004; Yang et al., 2012; Zhang et al., 2016). Numerous studies have empirically tested and compared several stabilizing mechanisms simultaneously (Craven et al., 2018; Grman et al., 2010; Ma et al., 2018; Song and Yu, 2015; Zhang et al., 2016). Many of the mechanisms are largely overlapping, e.g., the insurance effect, overyielding, compensatory dynamics, and species asynchrony (Yachi and Loreau, 1999; Grman et al., 2010; Hector et al., 2010; Loreau and de Mazancourt, 2013). Meanwhile mean–variance scaling is a good method for showing time series abundance and variances scaled from rare to dominant species within communities, rather than treating these as among the mechanisms (Loreau and de Mazancourt, 2013). An approach that combines temporal complementarity between species, functional complementarity, selection effects, and behavioral changes (Loreau and de Mazancourt, 2013), to organizing and distinguishing among the various mechanisms can lead to clear conceptual understanding and help to accurately quantify the contributions of these various mechanisms in different ecosystems.
Nitrogen (N) and phosphorus (P) are essential elements for organisms, and they are usually limiting nutrients for plant productivity in most terrestrial ecosystems. N enrichment by atmospheric deposition or agricultural fertilization is a common anthropogenic perturbation of ecosystems (Hooper and Vitousek, 1997; Hooper et al., 2005). N enrichment can reduce plant richness (Suding et al., 2005; Tilman et al., 2006; Xia and Wan, 2008), shift plant dominance (Hillebrand et al., 2008; Suding et al., 2005;), alter plant interactions (Grman et al., 2010; Yang et al., 2012), and weaken the stabilizing effect of diversity (Hautier et al., 2014; Song and Yu, 2015). The effect of nutrient-induced decreases in species richness on ecosystem stability have been extensively tested. For instance, N enrichment has been found to have decreased the stability of the grasslands in Minnesota and Inner Mongolia by decreasing species richness (Romanuk et al., 2006; Tilman, 1996; Yang et al., 2012). A meta-analysis on the diversity–stability relationships of 41 grasslands on five continents showed that nutrient enrichment weakened the positive effect of diversity on stability, not because of species loss after eutrophication but rather because of an increase in the temporal variation of productivity, in combination with a decrease in species asynchrony (Hautier et al., 2014). Moreover, other studies have demonstrated the weak direct effect of nutrient-induced decrease in species richness on ecosystem stability (Song and Yu, 2015; Zhang et al., 2016). Therefore, it is critical to differentiate the specific effect of diversity on stability from the confounding effect of nutrient availability on diversity and stability (Duffy, 2009; Huston, 1997; Lepš, 2004; Loreau, 1998; Wardle et al., 2000).
Dominant species in natural communities contribute a great deal to community biomass and other properties (Polley et al., 2007). Ecosystem stability largely depends on whether biomass varies more or less in dominant species than in other species. When dominance is high, aggregate species synchrony can be mostly determined by dominant species in some cases (Lepš, 2004; Polley et al., 2007). There are at least two different ways in which dominance could influence ecosystem stability. Firstly, dominant species can have a disproportionate effect on population and ecosystem stability through a selection effect, if they are either more or less stable than other species (Loreau and de Mazancourt, 2013). Secondly, species asynchrony can be more or less pronounced among dominant species. The latter contributes to the classical mechanism of asynchrony, which is a form of temporal complementarity among species, even though the mechanism is mostly driven by only some species in the community (Loreau and de Mazancourt, 2013). However, little is known about the effect of nutrient enrichment-induced shifts of dominant species on ecosystem stability. It is critical to differentiate effects of nutrient-induced shifts of dominant species from the general effects of nutrient-induced changes of species richness on stability.
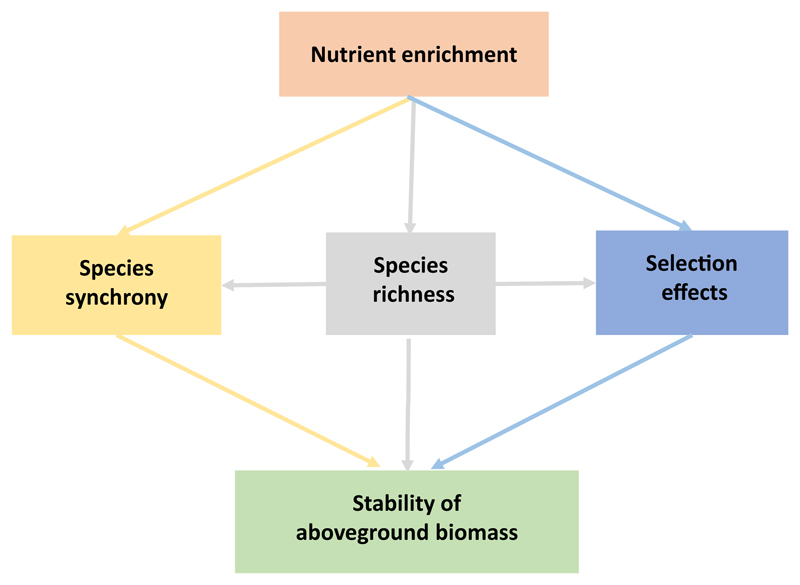
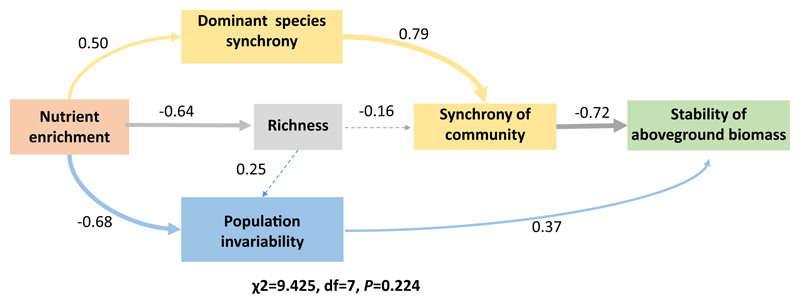
In this study, our objectives were to test (1) whether only dominant species or all species within communities drive ecosystem stability, and (2) whether these effects are driven by selection effects and/or by changes in synchrony. We hypothesized that nutrient-induced shifts of dominant species could contribute to variation in ecosystem stability via changes in species synchrony, i.e., temporal complementarity among species, and functional complementarity. We also hypothesized that nutrient-induced shifts of dominant species could contribute to ecosystem stability via selection effects, i.e., whether communities contain more or less stable species, as indicated by high or low population stability. To integrate the knowledge on mechanisms of ecosystem stability and test our hypotheses, we developed a causal diagram (Fig. 1) and compiled a dataset from long-term nitrogen (N) and phosphorous (P) enrichment experiment conducted in an alpine grassland on the Tibetan Plateau.
Fig. 1.
A causal diagram showing potential mechanisms underlying the effects of nutrient enrichment on ecosystem stability via species synchrony and selection effect. Nutrient enrichment-mediated changes in species richness may influence ecosystem stability directly or via species synchrony and selection effects indirectly.
2. Materials and methods
We compiled a dataset from a long-term nutrient enrichment experiment conducted in an alpine grassland ecosystem on the Tibetan Plateau. The site is located in Damxung County (30°51′N, 91°05′E), in the mid-south of the Tibetan Plateau, in the Tibet autonomous region.
2.1. Experimental site
A nutrient enrichment experiment was conducted in an alpine grassland in Damxung County (30°51′N, 91°05′E), located in the midsouth of the Tibetan Plateau. The average altitude of this area is 4320 m above sea level. The climate is continental and semiarid, with dry and frigid winters and springs. The mean annual temperature is 1.3 °C, with the lowest mean monthly temperature of −10.4 °C corresponding to January and the highest mean monthly temperature of 10.7 °C corresponding to July (see Supplementary Fig. S1). The average annual precipitation is 380 mm, and approximately 85% of this falls during the summer monsoon season, which runs from June through August. The vegetation was originally dominated by the perennial sedge Kobresia pygmaea C.B. Clarke, accompanied by the sedge species Carex montis-everestii Kükenth., the grass species Stipa capillacea Keng, and the forb species Anaphalis xylorhiza Sch.-BiP. and Potentilla bifurca L. However, the less palatable perennial herb Anaphalis xylorhiza Sch.-Bip. ex Hook. f., which belongs to a composite family, usually occupies a position of high dominance in communities that experiencing winter grazing within this semiarid grassland. Palatable graminoids, such as the clonal species S. capillacea and C. montis-everestii, are subdominant species because of the suppression of grazing herbivores, while the clonal species K. pygmaea acts as a keystone species and maintains relatively stable abundance in the alpine meadow. In the summer, the average height of the canopy is <10 cm, and the degree of vegetation cover ranges from 30 to 60%, depending on the annual precipitation. The soil is classified as alpine meadow soil and characterized as sandy loam. Plant roots are mainly distributed within soil layer of 10–15 cm where soil organic matter is relative high ranging from 0.9 to 2.79%. Total nitrogen concentration is ranging from 0.05 to 0.19%, and total phosphorus concentration is ranging from 0.03 to 0.07%.
2.2. Experimental design
In 2008, an area of 40 m × 40 m, with uniform vegetation cover, in an area where the vegetation had never been fertilized, was selected for a nutrient enrichment experiment. The experiment was fractional factorial design with N addition rates of 5 and 10 g N m−2 yr−1 and phosphorus (P) addition rates of 5 g P m−2 yr−1, and the combination of N at the two levels with phosphorus at rate of 5 g P m−2 yr−1, respectively. Treatment without addition of N and P was treated as control. The six treatments were coded as LN (low N: 5 g N m−2 yr−1), HN (high N: 10 g N m−2 yr−1), P (5 g P m−2 yr−1), LNP ((5 g N + 5 g P) m−2 yr−1), HNP ((10 g N + 5 g P) m−2 yr−1), and C (control: without addition of N and P). We chose the N rates based on the knowledge that the N critical load for plant growth is around 5 g m−2 yr−1 in the alpine grassland, and N rate of around 10 g m−2 yr−1 is the relative high rate usually used for fertilization by grassland managers (Zong et al., 2016; Zhang et al., 2019). P rate of 5 g m−2 yr−1 was referred to the N addition rates and also considered that plant growth in the grassland is not limited by soil available P. A completely random block design was developed for the experiment. Six blocks were established, and five 5 m × 5 m plots were set up in each block for each of the six treatments. Therefore, there were 30 plots in total. The plots were separated from each other by 2-m aisles that served as buffer zone. CO(NH2)2 and (NH4)2HPO4 were applied in the middle of June in each year.
2.3. Sampling and measurements
A 1 m × 1 m quadrat was established at the center of each plot. The occurrence of each vascular species in each quadrat was recorded in the middle of August each year from 2010 to 2018, and species richness was then calculated. The coverage and aboveground biomass of each species was measured in August of each year from 2010 to 2018. For the P treatment plots, measurements were carried out from 2013 to 2018. For the cover measurements, a 1 m × 1 m frame with 100 grid cells (10 cm × 10 cm) was placed above the canopy in each quadrat, and the cover of each species was visually estimated in each grid cell. For biomass measurements, we clipped aboveground shoots within a 0.25 m × 0.25 m quadrat outside the central 1 m × 1 m quadrat but within the plot, in the middle of August every year, when biomass peaked. The quadrats for clipping were shifted each year within the plot to avoid harvesting the same area in successive years. Shoots were clipped at ground level and sorted by species. All shoots were oven-dried at 60 °C for 48 h and then weighed for biomass. All the above measurements were performed in randomly selected three out of the five replicate plots in each treatment.
2.4. Variable calculations
We used the data of species richness and aboveground biomass collected from each plot in the Damxung alpine grassland from 2013 to 2018 for P treatment and from 2010 to 2018 for other five treatments. Ecosystem stability was measured by calculating the temporal mean in each plot over multiple years divided by the temporal standard deviation (Lehman and Tilman, 2000; Tilman et al., 2002). Population stability was calculated as the sum of the stability of each species weighted by the species biomass in each plot (Haegeman et al., 2016; Thibaut and Connolly, 2013). Species relative abundance was calculated as the aboveground biomass of species divided by the aboveground biomass of the community. We calculated the mean species richness in each plot over time. Values of Simpson's dominance indices (Smith and Wilson, 1996) were calculated based on the species relative biomass in each plot. The mean dominance was calculated as the average over the years spanned by the data.
We calculated the community-wide synchrony of species as follows (Loreau and de Mazancourt, 2008; Loreau, 2010):
where φc is the community-wide synchrony of species based on species biomass, is the variance of aboveground biomass at the community level (i.e., the sum of all species in a community), σci is the standard deviation of the aboveground biomass of species i in the community, and S is the species number (richness) in the community (Yang et al., 2012). The value of the synchrony is equal to one if species fluctuate synchronously, which indicates that there is no significant compensatory effect, and the value is less than one if there is a significant compensatory effect (Hautier et al., 2014; Loreau and de Mazancourt, 2008). We treated the species those their relative aboveground biomass were higher than 30% within the sampling plots in the six blocks at the initial of nutrient addition treatment as dominant species. In addition, the species those their aboveground biomass showed increasing trends and their relative aboveground biomass were higher than 30% in the most of years from 2010 to 2018 were also treated as dominant species. The synchrony of dominant and non-dominant species was calculated.
2.5. Statistical analysis
One-way analyses of variance (ANOVAs), followed by Tukey tests, were used to compare the differences in mean richness, mean dominance, species synchrony, and community stability among the nutrient treatments. We also used one-way ANOVAs to compare the differences in synchrony among dominant species, non-dominant species, and the community as a whole. One-way analyses of variance (ANOVAs), followed by Tukey tests, were used to examine effects of nutrient addition on relative abundance and their biomass stability of common species within the communities. We calculated the temporal mean and temporal variance of each species' aboveground biomass within each replicate plot and conducted linear regressions to examine the relationships between log(variance of species biomass) and log(mean species biomass) for nutrient treatments. Mean–variance scaling relationships were also analyzed among the dominant and non-dominant species. We conducted analyses of covariance (ANCOVAs), and used nutrient treatments, log(mean), and treatment by log(mean) to predict log(variance). Differences in slopes was judged by the significant interactions between nutrient treatments and log(mean) to suggest that N enrichment affects the mean–variance scaling relationship (Grman et al.,2010). To differentiate between the effects of nutrient enrichment and those of nutrient-induced changes in species richness on ecosystem stability, we used the generalized linear model (GLM) to test the main effects of nutrient enrichment and species richness and their interactions. In this analysis, nutrient treatment was factor, and richness was covariate variable. We detected significant effects of nutrient enrichment (F5,18 = 8.67, P = 0.010) and interaction between nutrient enrichment and species richness (F5,18 = 8.46, P = 0.011) on stability. The effect of species richness on stability was not significant (F1,18 = 1.71, P = 0.239). We conducted linear regressions to examine the relationships of stability to mean richness, mean dominance, and species synchrony. Before all the above analyses, assumption of normal distribution and homogeneity of variance were tested. Richness and aboveground biomass were log transformed. In the study, we disregarded the block effect during data analyses due to small sample size. In addition, we employed completely random block design by considering the homogeneity of vegetation in the alpine grassland. Structural equation models (SEMs) were used to integrate our results and test the contributions of species synchrony and selection effect to ecosystem stability proposed in our causal diagram (Fig. 1; Grace et al., 2012). Based on the above univariate regression analyses, nutrient enrichment could influence ecosystem stability via species synchrony and population invariability (selection effect, whether more or less stable species were selected or filtered out), as well as species richness. Nutrient-induced changes in species richness may influence ecosystem stability through effects either on species synchrony or on population invariability. We set up the structural equations to firstly consider the effects of nutrient enrichment on ecosystem stability via the paths, i.e. synchrony of dominant species contributed significantly to species synchrony of community which further influenced ecosystem stability. Then second paths of the structural equations were set up to assess nutrient enrichment-driven selection effect via the paths, i.e. the contribution of nutrient-mediated population invariability to ecosystem stability. Finally, the third paths were set up to test whether nutrient enrichment-induced changes in species richness might indirectly influence ecosystem stability via association with species synchrony and population invariability. We also tried to fit the other models by considering selection effect via paths from in variability of dominant species to population invariability, then to ecosystem stability. But the results did not fit well (see Supplementary Fig. S4). The model fit was evaluated using the model chi-square and its associated P value. The SEMs were evaluated using the lavaan R package for SEM in R 3.2.5 (Rosseel, 2012; R Core Team, 2012). All of the other statistical analyses were performed using SPSS 16 (SPSS Inc. Chicago, Illinois, USA).
3. Results
3.1. Nutrient enrichment-induced changes in aboveground biomass and temporal stability and species synchrony
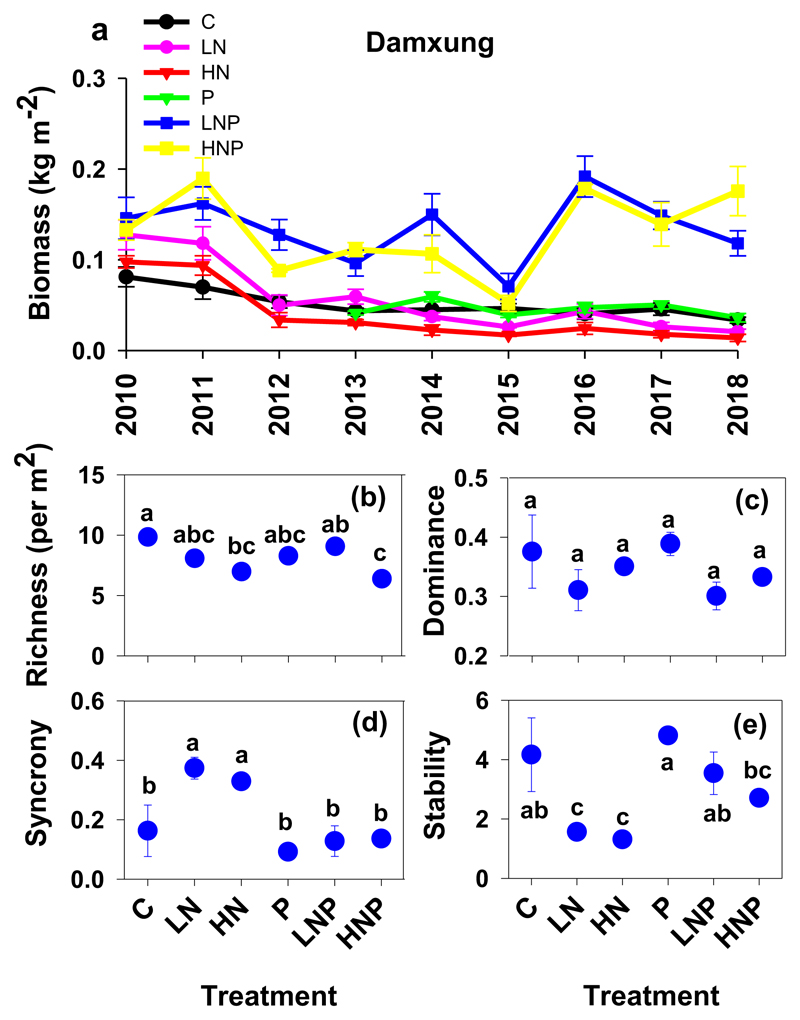
Low or high N enrichment did not alter aboveground biomass over the eight years of the experiment, with fluctuating decreasing trends in aboveground biomass after the N enrichment was performed (Fig. 2a). The combination of P with low and high N increased the aboveground biomass by 158% and 135%, respectively, over the eight years of the experiment (Fig. 2a). In comparison to the control, the high N enrichment decreased the species richness by 29.3%, and the combination of P and high N decreased the species richness by 35.3% (Fig. 2b). However, neither the low-N nor the P additions alone nor the combination of P with low N significantly altered species richness (Fig. 2b). Nutrient treatment did not alter species dominance (Fig. 2c). The low- and high-N treatments increased species synchrony and decreased the stability of the aboveground biomass, as a result of the marked increase in the variance of the aboveground biomass (Fig. 2d, e).
Fig. 2.
Time series variation of aboveground biomass in Damxung (a) alpine grasslands. Mean richness, mean dominance, species synchrony, and stability of aboveground biomass in Damxung (b-e) nitrogen (N) and phosphorous (P) enrichment were calculated. In Damxung nutrient enrichment experiment, N and P addition were performed in a winter grazing alpine grassland. LN, HN, P, LNP, and HNP represent low N (5 g N m−2 yr−1), high N (10 g N m−2 yr−1), constant P (5 g P m−2 yr−1), low N combined with P ((5 g N + 5 g P) m−2 yr−1), and high N combined with P ((10 g N + 5 g P) m−2 yr−1), respectively. In the control treatment (C), neither N nor P was added. Symbols with different letters represent significant differences among the treatments at the P < 0.05 level (based on Tukey's test). The nutrient treatments were carried out starting in 2008. We used data collected in P treatment ranging from 2013 to 2018, in other five treatments ranging from 2010 to 2018.
3.2. Nutrient enrichment-induced shifts of dominant species and changes of population stability
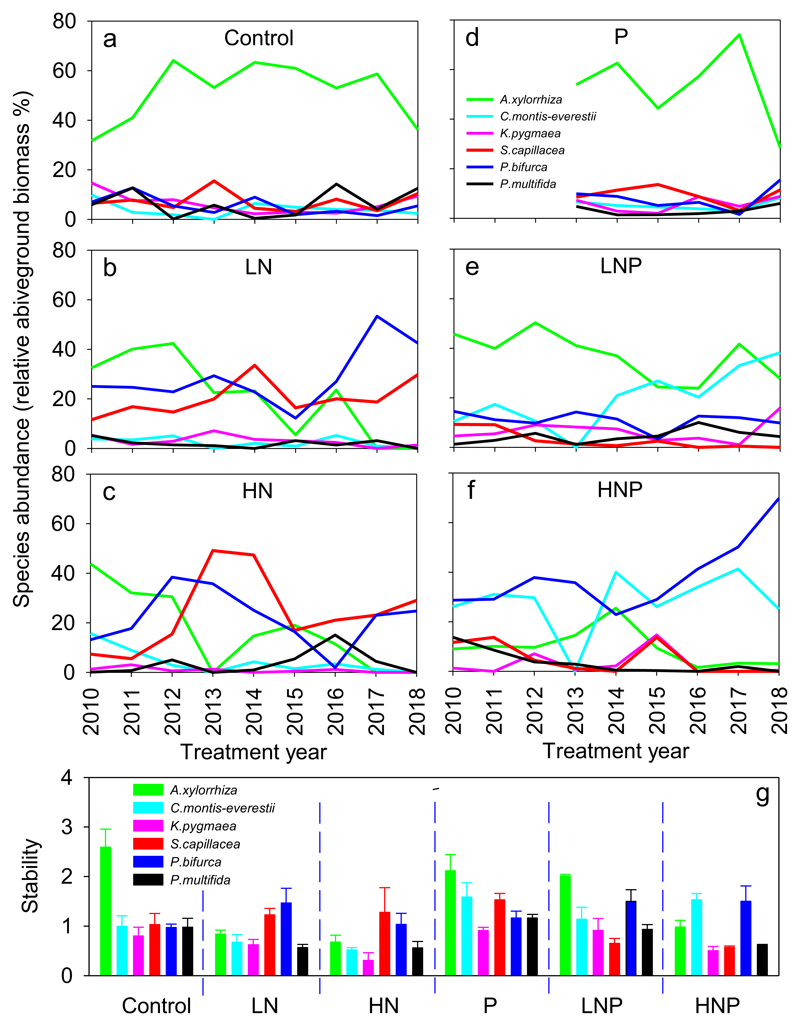
The relative aboveground biomass of Anaphalis xylorhiza amounted to over 54% in the control, and five other species with high frequencies contributed approximately 29% to the aboveground biomass (Fig. 3a and also see Supplementary Table S1). P addition alone did not significantly alter the relative aboveground biomass of A. xylorhiza or the other five species (Fig. 3d). The aboveground biomass of A. xylorhiza was significantly decreased by low and high N additions, and A. xylorhiza have been locally extinct in both the low- and high-N plots since 2017 (Fig. 3b, c). In contrast, low and high N tended to increase the aboveground biomass of the grass Stipa capillacea and the forb Potentilla bifurca (Fig. 3b, c). The combination of P with low N tended to decrease the aboveground biomass of A. xylorhiza over time, but the aboveground biomass of the community as a whole was compensated by the increase in the aboveground biomass of Carex montiseverestii (Fig. 3e). The combination of P with high N significantly decreased the aboveground biomass of A. xylorhiza over time but increased the aboveground biomass of P. bifurca and C. montis-everestii (Fig. 3f). Meanwhile, LN, HN, and HNP significantly decreased the stability of A. xylorhiza, primarily due to a decrease in the aboveground biomass of A. xylorhiza over time (Fig. 3g). LNP and HNP increased both the aboveground biomass over time and the variance of C. montis-everestii but did not change the stability in comparison with the control (Fig. 3g). Similarly, HNP increased the aboveground biomass and the variance of P. bifurca significantly over time, although the stability was not altered significantly in comparison to the control (Fig. 3g).
Fig. 3.
Time series variation in species dominance in nutrient treatments indicated by their relative aboveground biomass (a–f) and stability of species aboveground biomass in nutrient treatments (g). Treatment codes are the same as in Fig. 2. For each treatment, replicate plots n = 3.
3.3. Nutrient enrichment-induced alterations in mean–variance scaling relationships
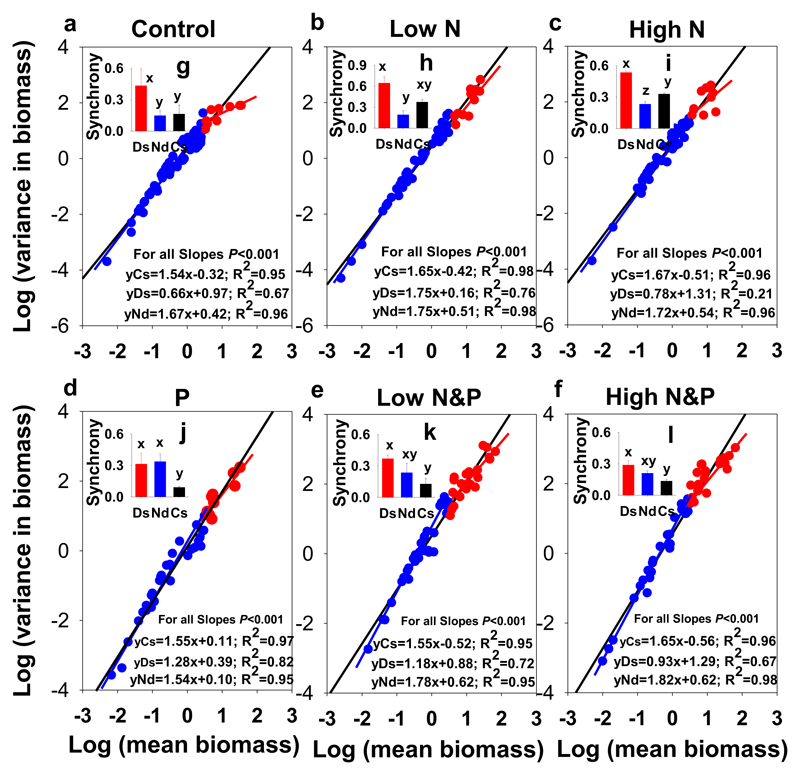
The log-transformed species mean biomass over time was linearly scaled with respect to the log-transformed variance of the biomass (Fig. 4). We also found a lower scaling coefficient (lower slope) in the dominant species than in the non-dominant species for the control (Fig. 4a). Low and high N enrichment increased the slope of the mean–variance scaling relationship (see Supplementary Fig. S2) to marginally significant degrees. These increases are attributed primarily to amplified increases in the slopes of the relationships among dominant species for low and high N (Fig. 4b, c). P addition alone or the combination of P with low or high N did not significantly alter the mean–variance scaling relationships across all species or among dominant or non-dominant species (Fig. 4d, e, f). In addition, the significantly lower synchrony of the community as a whole than the synchrony among dominant or non-dominant species for P, LNP, and HNP indicates that compensatory dynamics are generated between dominant and non-dominant species (Fig. 4j, k, l).
Fig. 4.
Mean–variance scaling relationships in N and P enrichment communities in Damxung alpine grassland (a–f; black lines). Mean–variance scaling relationships among dominant and non-dominant species are also shown separately (red and blue dots and lines). Bar figures (g–l) show the synchrony of dominant (Ds) and non-dominant (Nd) species and the species synchrony of the community (Cs). Bars with different letters represent significant differences among the categories at the P < 0.05 level (based on Tukey's test). Treatment codes are the same as in Fig. 2. For each treatment, replicate plots n = 3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
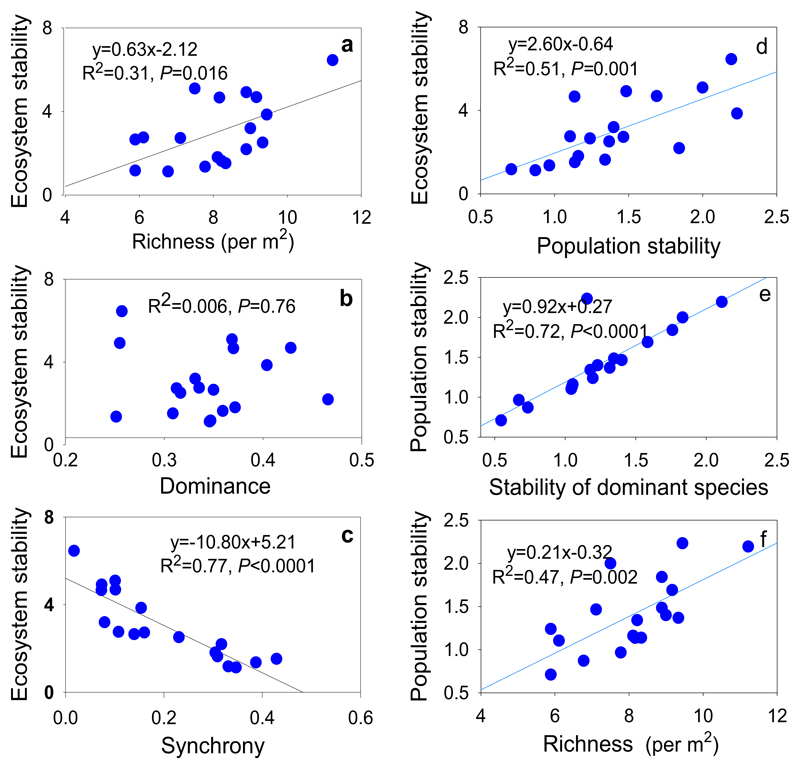
3.4. Contributions of the stabilizing mechanisms driven by nutrient enrichment
The stability of the aboveground biomass was found to be positively correlated with species richness but not correlated to community dominance (Fig. 5a, b). Stability was found to be negatively correlated to species synchrony (Fig. 5c). A significant positive correlation was found between population invariability and ecosystem stability (Fig. 5d). Population stability was positively correlated with both stability of dominant species and species richness (Fig. 5e, f). Structural equation modelling revealed that nutrient enrichment amplified the variance of the dominant species, which generated high synchrony among dominant species and contributed to the increase in species synchrony of community as a whole. Meanwhile, nutrient enrichment induced shifts in the dominant species, which contributed to significant increases in population variability. In addition, the nutrient-induced decrease in species richness contributed to a decrease in ecosystem stability via an increase of population variability and species synchrony. The nutrient-induced increase in both the aggregate species synchrony and population variability ultimately resulted in the decline of stability in the Damxung grassland ecosystem (Fig. 6).
Fig. 5.
Bivariant regression analyses were performed to test relationships between stability and species richness, Simpson's dominance indices, and species synchrony (a–c) and relationships between population stability and ecosystem stability, species richness, and stability of dominant species (d–f) in Damxung grasslands. Species richness and dominance are values in each plot averaged across years. For each treatment, replicate plots n = 3.
Fig. 6.
Structural equation model analysis of the contributions of species synchrony (paths colored yellow) and selection effect (paths colored blue) driven by nutrient enrichment to stability of aboveground biomass. Synchrony of dominant species contributed significantly to species synchrony of community which further contributed to ecosystem stability. Nutrient enrichment drove selection on more or less stable population which further contributed significantly to ecosystem stability. Nutrient enrichment-induced changes in species richness indirectly influence ecosystem stability via association with species synchrony and population invariability. The thicknesses of the arrows indicate the relative contributions of the variables. χ2, df, and P of the model fit are given. Values alongside arrow lines are standardized regression coefficients, indicating the contribution of each variable to the response. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We compiled a dataset from a long-term nutrient enrichment experiment in an alpine grassland to test the hypotheses that nutrient-induced shifts of dominant species could contribute to ecosystem stability via changes of species synchrony and could contribute to ecosystem stability via selection effects. We found that nutrient-induced decreases in species richness can contribute to ecosystem stability via regulation of population invariability and species synchrony. Thus, our results confirmed the hypotheses. Interestingly, we found that a combination of P and high N decreased the stability of dominant species A. xylorhiza as a result of a decrease in the aboveground biomass over time. However, the decreased stability of A. xylorhiza did not induce a decline in ecosystem stability. The effect of selection on a relatively stable species as the co-dominant species could facilitate the maintenance of ecosystem stability in HNP treatment.
In regions with severe climates, communities are shaped more by climate filtering than by species competition for soil nutrients or light resources (Callaway et al., 2002). Relatively strong climate filtering would allow fewer species to coexist and domination by a smaller number of species. In such communities, synchrony could be generated primarily by consistent responses among dominant species to environmental fluctuations and/or perturbations (Polley et al., 2008), while asynchronous responses among rare species could compensate for synchronous responses among dominant species. In the semiarid grassland in Damxung, low precipitation and low available nutrient concentration in the soil are two critical factors that restrict plant growth and productivity (Fu et al., 2018; Zong et al., 2016), although soil microorganisms, such as arbuscular mycorrhizal fungi, alleviate the extent of nutrient limitation to plant growth, especially for dominant species (Wei et al., 2013; Zheng et al., 2014). Coexisting species within 1 m2 belong to approximately four different functional groups, such as grasses, sedges, legumes, and forbs. Species are characterized by various leaf and root traits and exhibit niche partitioning and resource compensation (Song et al., 2015; Yin et al., 2017). As a dominant species in the winter grazing grassland, A. xylorhiza develops a large and deep root system that allows it to utilize water deep in the soil during the summer drought. The keystone sedge species Kobresia pygmaea and common species Carex montis-everestii are characterized by shallow root systems and utilize surficial soil water in the summer. The forb species Potentilla bifurca is characterized by a large and deep root system. In addition, mutualistic organisms, such as arbuscular mycorrhizal fungi, can facilitate some plants to obtain the nutrient, which contributed to functional differences of some plant species in preference for N and P, such as Graminoids are, in general, nitrophilous, whereas P. bifurca and C. montis-everestii and S. capillacea are phosphophilous in the alpine grassland (Yang et al., 2014; Zong et al., 2016; Zong and Shi, 2019). Our results provide evidence that the release of N limitation induces amplified synchrony among dominant species, which plays a key role in the species synchrony of the aggregate community (Hautier et al., 2014; Polley et al., 2008; Song and Yu, 2015; Zhang et al., 2016). In addition, N enrichment induces rare species losses, which reduces the possibility of generating compensatory dynamics among dominant and non-dominant species via asynchronous responses (Fig. 3g–l). Therefore, perturbation-induced species losses could regulate the species synchrony of aggregate communities.
The significant contributions of dominant species to stability were observed in the grassland of Damxung. Low and high N additions induced shifts in dominant species. The large population variability caused by N enrichment ultimately contributed to a significant decline in ecosystem stability, which also indicated by the marginally significant differences in the slopes of the mean-variance scaling (Fig. S2). Ecosystem stability largely depends on whether biomass varies more or less in dominant species than in other species (Grman et al., 2010; Polley et al., 2007). Several studies have found that dominant species contribute greatly to ecosystem stability (Grman et al., 2010; Lepš, 2004; Polley et al., 2007; Steiner et al., 2005). In the Damxung grassland, less variance in the dominant species A. xylorhiza contributed primarily to the stability of communities without nutrient perturbations or P addition alone. Low and high N additions drove dominance replacement by the grass species Stipa capillacea and forb species Potentilla bifurca. Both of these species are characterized by relatively low population stability (Fig. 3g). N enrichment significantly decreased the population stability of the dominant species A. xylorhiza, primarily as a results of a decrease in aboveground biomass over time. As a consequence, shifts in the dominant species resulted in a decline in ecosystem stability. In our study, a combination of P with low N tended to decrease the dominance of A. xylorhiza. However, the relative smooth increase in the sedge species C. montis-everestii and P. bifurca compensated for the decrease in dominance of A. xylorhiza (Fig. 4g), which maintained ecosystem stability. Interestingly, a combination of P and high N induced obvious shifts in the dominant species, with P. bifurca and C. montis-everestii taking the place of A. xylorhiza (Fig. 4f). Selection of these new, relatively stable co-dominant species and compensatory dynamics among the dominant and non-dominant species could explain the absence of an effect of HNP on ecosystem stability. Thus, population stability appears to be more sensitive to nutrient enrichment than the stability of the community as a whole. In our study, nutrient enrichment did not affect the stability of rare species (F5, 17 = 1.18, P = 0.373; also indicated by none difference in slopes of mean-variance scaling among non-dominant species in Fig. 4); rather, variability of the dominant species was the primary contributor to variation in ecosystem stability. Interestingly, the nutrient-induced decrease in the stability of the dominant species was primarily due to a decrease in aboveground biomass over time, whereas the nutrient-induced decrease in the stability of the community as a whole mainly was due to an increase in the variation in aboveground biomass over time. These results suggest that nutrient-induced decreases in the aboveground biomass of dominant species can be compensated for by other species and that large variations in the aboveground biomass of species over time contribute to ecosystem variability.
N-induced loss of community stability is usually linked to nutrient-induced loss of plant species richness, which could lead to both a decline in population stability and species asynchrony (Hautier et al., 2015; Yang et al., 2012; Zhang et al., 2016). However, nutrient enrichmentrelated decline in species asynchrony and population stability are not necessary causally related to changes in species richness (Grman et al., 2010; Xu et al., 2015). In addition, when communities are dominated by a small number of species, N-induced reductions in the stability of dominant species could also translate into decreases in community stability (Xu et al., 2015). In this study, we did not detect significant differences in the synchrony and stability of rare species for different nutrient treatments. We therefore conclude that nutrient-induced rare species loss has a limited effect on population and ecosystem stability. The contribution of nutrient-induced decreases in species richness to ecosystem stability is mainly attributable to losses of the dominant or highfrequency species. Relations among species, such as competition and facilitation, could also contribute to the large variability of species populations (Song et al., 2006; Loreau and de Mazancourt, 2013). Increasing the strength of interspecific species competition could synchronize population size in the first period due to coupling strong density dependence between species (Loreau and de Mazancourt, 2013). Then ecological drift rather than competition could play a major role as species become increasing equivalent (Loreau and de Mazancourt, 2013). Species responses to climate fluctuations, competition or facilitation, and perturbations are largely determined by their traits (Loreau and de Mazancourt, 2013). Although we cannot provide more specific information on the effect of plant functional compensation on ecosystem stability on the basis of the results of this study, our results do indeed demonstrate synergistic effects between N and P on productivity (i.e. low and high N decreased aboveground biomass of community over years, and P addition did not alter community biomass, but combination of N and P increased aboveground biomass of community over years) and synergistic responses of species (i.e. biomass of C. montis-everestii did not significantly respond to N or P alone, but biomass of C. montiseverestii was significantly increased in combination of N and P). The task of incorporating plant functional compensation into the stability of ecosystem functions is critical for better understanding of the mechanisms that drive both community assembly and ecosystem functions, and this remains an important challenge for future research.
5. Conclusions
The integrative approach adopted in this study demonstrates a clear conceptual understanding of how changes in dominant species and species richness can generate compensatory dynamics and changes in species synchrony and how the selection effect is important in determining ecosystem functions and stability. Moreover, our results also indicate the potential effects of plant functional compensation on ecosystem stability through the synergistic responses of plant species those are characterized as different preference to N and P. Our findings are equally applicable to and have implications for evaluating the effects of other environmental changes and quantifying the contributions of species richness, dominant species, and environmental fluctuations on ecosystem stability.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2019.07.266.
Highlights.
The effects of nutrient-induced shifts of dominant species on ecosystem stability have rarely been evaluated.
Data from a long-term experiment were compiled to test the effects of nutrient-induced shifts of dominant species on stability.
Nutrient-induced increases in species synchrony and population variability resulted in a decline in ecosystem stability.
Plant functional compensation is critical for driving both community assembly and ecosystem functions.
Acknowledgements
We thank the anonymous reviewers for their valuable comments. The study was supported by the National Key Research and Development Program (2016YFC0501803, 2016YFC0502001), the National Natural Science Foundation of China (41671263, 41703079, 31870406, 31600431), and the Qinghai Innovation Platform Construction Project (2017-ZJ-Y20). ML was supported by the TULIP Laboratory of Excellence (ANR-10-LABX-41), the BIOSTASES Advanced Grant, and the European Research Council, under the European Union's Horizon 2020 Research and Innovation Program (Grant Agreement No. 666971).
Footnotes
The authors declare no competing financial interests.
References
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Craven D, Eisenhauer N, Pearse WD, Hautier Y, Isbell F, Rosch C, Bahn M, Beierkuhnlein C, Bönisch G, Buchmann N, Byun C, et al. Multiple facets of biodiversity drive the diversity-stability relationship. Nature Ecology & Evolution. 2018;2:1579–1587. doi: 10.1038/s41559-018-0647-7. [DOI] [PubMed] [Google Scholar]
- Duffy JE. Why biodiversity is important to the functioning of real-world ecosystems. Front Ecol Environ. 2009;7:437–444. [Google Scholar]
- Ernest SKM, Brown JH. Homeostasis and compensation: the role of species and resources in ecosystem stability. Ecology. 2001;82:2118–2132. [Google Scholar]
- Fu G, Shen Z, Zhang X. Increased precipitation has stronger effects on plant production of an alpine meadow than does experimental warming in the Northern Tibetan Plateau. Agric For Meteorol. 2018;249:11–21. [Google Scholar]
- Grace JB, Schoolmaster DR, Jr, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere. 2012;3(8):1–44. [Google Scholar]
- Grime JP. Plant classification for ecological purposes: is there a role for genome size? Ann Bot. 1998;82:117–120. [Google Scholar]
- Grman E, Lau JA, Schoolmaster DR, Jr, Gross KL. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol Lett. 2010;13:1400–1410. doi: 10.1111/j.1461-0248.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- Haegeman B, Arnoldi JF, Wang S, de Mazancourt C, Montoya JM, Loreau M. Resilience, Invariability, and Ecological Stability Across Levels of Organization. bioRxiv. 2016 085852. [Google Scholar]
- Hautier Y, Seabloom EW, Borer ET, Adler PB, Harpole WS, Hillebrand H, Lind EM, MacDougall AS, Stevens CJ, Bakker JD, Buckley YM, et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348:336–340. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- Hector A, Hautier Y, Saner P, Wacker L, Bagghi R, Joshi J, Scherer-Lorenzen M, Spehn EM, Bazeley-White E, Weilenmann M, Caldeira MC, et al. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology. 2010;91:2213–2220. doi: 10.1890/09-1162.1. [DOI] [PubMed] [Google Scholar]
- Hillebrand H, Bennett DM, Cadotte MW. Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology. 2008;89:1510–1520. doi: 10.1890/07-1053.1. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Vitousek PM. The effects of plant composition and diversity on ecosystem processes. Science. 1997;277:1302–1305. [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton J, Lodge D, Loreau M, Naeem S, Schmid B, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- Huston MA. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- Lehman CL, Tilman D. Biodiversity, stability, and productivity in competitive communities. Am Nat. 2000;156:534–552. doi: 10.1086/303402. [DOI] [PubMed] [Google Scholar]
- Lepš J. Variability in population and community biomass in a grassland community affected by environmental productivity and diversity. Oikos. 2004;107:64–71. [Google Scholar]
- Loreau M. Biodiversity and ecosystem functioning: a mechanistic model. Proceedings of the National Academy of Sciences USA. 1998;95:5632–5636. doi: 10.1073/pnas.95.10.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M. Linking biodiversity and ecosystems: towards a unifying ecological theory. Philosophical Transactions Royal Society B-Biological Sciences. 2010;365:49–60. doi: 10.1098/rstb.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Species synchrony and its drivers: neutral and non-neutral community dynamics in fluctuating environments. Am Nat. 2008;172:E48–E66. doi: 10.1086/589746. [DOI] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Ma Z, Liu H, Mi Z, Zhang Z, Wang Y, Xu W, He J. Climate warming reduces the temporal stability of plant community biomass production. Nat Commun. 2018 doi: 10.1038/ncomms15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RJ, Dise NB, Field CD, Dore AJ, Caporn SJM, Stevens CJ. Nitrogen deposition and plant biodiversity: past, present, and future. Front Ecol Environ. 2017;15:431–436. [Google Scholar]
- Polley HW, Wilsey BJ, Derner JD. Dominant species constrain effects of species diversity on temporal variability in biomass production of tallgrass prairie. Oikos. 2007;116:2044–2052. [Google Scholar]
- Polley HW, Frank AB, Sanabria J, Phillips RL. Inter-annual variability in carbon dioxide fluxes and flux-climate relationships on grazed and ungrazed northern mixed-grass prairie. Glob Chang Biol. 2008;14:1620–1632. [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria: 2012. ISBN 3-900051-07-0 http://www.R-project.org/ [Google Scholar]
- Romanuk TN, Vogt RJ, Kolasa J. Nutrient enrichment weakens the stabilizing effect of species richness. Oikos. 2006;114:291–302. [Google Scholar]
- Rosseel Y. Lavaan: An R package for structural equation modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- Smith B, Wilson JB. A consumer's guide to evenness indices. Oikos. 1996;76:70–82. [Google Scholar]
- Song MH, Yu FH. Reduced compensatory effects explain the nitrogen-mediated reduction in stability of an alpine meadow on the Tibetan Plateau. New Phytol. 2015;207:70–77. doi: 10.1111/nph.13329. [DOI] [PubMed] [Google Scholar]
- Song MH, Tian YQ, Xu XL, Hu QW, Ouyang H. Interactions between root and shoot competition among four plant species in an alpine meadow on the Tibetan Plateau. Acta Oecol. 2006;29:214–220. [Google Scholar]
- Song MH, Zheng LL, Suding KN, Yin TF, Yu FH. Plasticity in nitrogen form uptake and preference in response to long-term nitrogen fertilization. Plant Soil. 2015;394:215–224. [Google Scholar]
- Steiner CF, Long ZT, Krumins JA, Morin PJ. Temporal stability of aquatic food webs: partitioning the effects of species diversity, species composition and enrichment. Ecol Lett. 2005;8:819–828. [Google Scholar]
- Stevens CJ, Dise NB, Mountford JO, Gowing DJ. Impact of nitrogen deposition on the species richness of grasslands. Science. 2004;303:1876–1879. doi: 10.1126/science.1094678. [DOI] [PubMed] [Google Scholar]
- Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Pennings S. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proceedings of the National Academy of Sciences USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut LM, Connolly SR. Understanding diversity-stability relationships: towards a unified model of portfolio effects. Ecol Lett. 2013;16:140–150. doi: 10.1111/ele.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D. Biodiversity: population versus ecosystem stability. Ecology. 1996;77:350–363. [Google Scholar]
- Tilman D, Knops J, Wedin D, Reich P. Experimental and observational studies of diversity, productivity, and stability. The Functional Consequences of Biodiversity. 2002;33:42–70. [Google Scholar]
- Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314:1598–1600. doi: 10.1126/science.1133306. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Bonner KI, Barker GM. Stability of ecosystem properties in response to above-ground functional group richness and composition. Oikos. 2000;89:11–23. [Google Scholar]
- Wei CZ, Yu Q, Bai E, Lü XT, Li Q, Xia JY, Kardol P, Liang WJ, Wang ZW, Han XG. Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Glob Chang Biol. 2013;19:3688–3697. doi: 10.1111/gcb.12348. [DOI] [PubMed] [Google Scholar]
- Xia J, Wan S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 2008;179:428–439. doi: 10.1111/j.1469-8137.2008.02488.x. [DOI] [PubMed] [Google Scholar]
- Xu ZW, Ren HY, Li MH, van Ruijven J, Han XG, Wan SQ, Jiang L. Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J Ecol. 2015;103:1308–1316. [Google Scholar]
- Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Sciences USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Jiang L, Li L, Li A, Wu M, Wan S. Diversity-dependent stability under mowing and nutrient addition: evidence from a 7-year grassland experiment. Ecol Lett. 2012;15:619–626. doi: 10.1111/j.1461-0248.2012.01778.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Ren F, Zhou H, He J. Responses of plant community biomass to nitrogen and phosphorus additions in an alpine meadow on the Qinghai-Xizang Plateau. Chinese Journal of Plant Ecology. 2014;15:159–166. [Google Scholar]
- Yin TF, Zheng LL, Cao GM, Song MH, Yu FH. Species-specific phenological responses to long-term nitrogen fertilization in an alpine meadow. J Plant Ecol. 2017;10:301–309. [Google Scholar]
- Zhang Y, Loreau M, Lu X, He N, Zhang G, Han X. Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Glob Chang Biol. 2016;22:1445–1455. doi: 10.1111/gcb.13140. [DOI] [PubMed] [Google Scholar]
- Zhang C, Willis CG, Ma Z, Ma M, Csontos P, Baskin CC, Baskin JM, Li J, Zhou H, Zhao X, Yao B, et al. Direct and indirect effects of long-term fertilization on the stability of the persistent seed bank. Plant Soil. 2019;438:239–250. [Google Scholar]
- Zheng Y, Kim YC, Tian XF, Chen L, Yang W, Gao C, Song MH, Xu XL, Guo LD. Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS Microbiol Ecol. 2014;89:594–605. doi: 10.1111/1574-6941.12361. [DOI] [PubMed] [Google Scholar]
- Zong N, Shi P. Enhanced community production rather than structure improvement under nitrogen and phosphorus addition in severely degraded alpine meadows. Sustainability. 2019;11:2023. [Google Scholar]
- Zong N, Shi PL, Song MH, Zhang XZ, Jiang J, Chai X. Nitrogen critical loads for an alpine meadow ecosystem on the Tibetan Plateau. Environ Manag. 2016;57:531–542. doi: 10.1007/s00267-015-0626-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.