Abstract
Background:
Evidence supporting the efficacy of stereotactic body radiotherapy (SBRT) for localized prostate cancer is accumulating, but comparative studies of patient-reported quality of life (QOL) following SBRT versus conventionally-fractionated external beam radiotherapy (EBRT) or active surveillance (AS) are limited.
Objective:
To compare QOL of patients pursuing SBRT and EBRT vs. AS.
Design, Setting, and Participants:
Population-based cohort of 680 men with newly-diagnosed localized prostate cancer was prospectively enrolled from 2011–2013.
Intervention:
SBRT, EBRT without androgen deprivation therapy, or AS
Outcome Measurements and Statistical Analysis:
QOL was prospectively assessed before treatment (baseline), and at 3, 12, and 24 months after treatment using the validated Prostate Cancer Symptom Indices (PCSI), which contains 4 domains: sexual dysfunction, urinary obstruction/irritation, urinary incontinence, and bowel problems. Propensity weighting via logistic regression models was used to balance baseline characteristics, and the mean QOL scores of EBRT and SBRT patients were compared against AS as the control group.
Results and Limitations:
Compared to AS, EBRT patients had worse urinary obstructive/irritative symptoms and sexual dysfunction at 3 months, and worse bowel symptoms at 3 and 24 months. SBRT patients had similar scores as AS in all domains across at all time points; however, due to small sample size, worse sexual function and urinary incontinence in SBRT patients cannot be ruled out. Further research is needed to assess long-term outcomes.
Conclusions:
In a non-randomized cohort of men with localized prostate cancer, SBRT appeared to result in favorable QOL results through 2 years of follow-up, but worse sexual function and urinary incontinence compared to AS cannot be completely ruled out. Larger studies with longer follow-up are needed to confirm these findings.
Patient Summary:
SBRT and AS appear to have similar QOL outcomes through 2 years, although worse sexual function and urinary incontinence from SBRT cannot be completely ruled out.
Keywords: Stereotactic body radiotherapy, External beam radiotherapy, Intensity-modulated radiotherapy, Active surveillance, Localized prostate cancer, Patient-reported quality of life
Introduction
Patients with localized prostate cancer often have excellent survival outcomes. As a result, quality of life (QOL) is an important factor in the patient’s decision-making process concerning treatment options. One option is active surveillance (AS), which is surveillance without immediate treatment, and delays treatment-related adverse effects without compromising long-term survival in select patients [1]. For patients receiving radiotherapy (RT), it is most commonly delivered using small daily doses of RT over several weeks (termed “conventional fractionation”). Continued technological developments have more recently allowed the use of stereotactic body RT (SBRT) to deliver extremely hypofractionated treatment using large daily doses and completing RT within 5 treatments.
As an evolving treatment option, SBRT comparative outcomes versus other modalities are limited but of substantial clinical interest. While some studies have reported that SBRT is safe and effective [2,3], others have raised concerns regarding its toxicity profile. A Phase I trial of dose escalation from 45 Gy to 50 Gy in five fractions reported 18% and 31% of ≥ grade 2 gastrointestinal (GI) and genitourinary (GU) toxicity, respectively [4]; and another dose escalation study reported a 10% ≥ grade 3 rectal toxicity in the 50 Gy cohort with many requiring a diverting colostomy [5]. A claims data-based analysis suggested increased GU toxicity following SBRT compared to intensity-modulated radiotherapy (IMRT) at 6 and 24 months [6]. It is well-recognized that in prostate cancer, patient-reported QOL provides valid and more comprehensive data regarding treatment-related side effects than physician assessments and claims data [7,8]. To inform patients and physicians about treatment-related side effects related to SBRT, the goal of this study was to compare QOL of SBRT patients versus those who received conventional fractionation RT and AS.
Patients and Methods
Patient cohort
Population-based prospective cohort of patients with newly diagnosed prostate cancer was enrolled in collaboration with the Rapid Case Ascertainment system of the North Carolina Central Cancer Registry. From January 2011 to June 2013, patients with newly-diagnosed localized prostate cancer were identified from across all 100 counties of North Carolina by the Cancer Registry within a median of 1–2 weeks of diagnosis, and contacted by the study team for enrollment on a prospective observational cohort. Patient enrollment details were described previously [9]. All patients were enrolled and baseline data collected prior to any treatment.
Because SBRT was a newer modality with relatively lower use, the study also collaborated with three institutions outside of North Carolina to enroll additional patients receiving SBRT to enrich this cohort. Eligibility criteria and study methodology were identical between North Carolina patients and additional SBRT patients in this study. SBRT patients were treated with the Accuray CyberKnife system.
The study was approved by the University of North Carolina institutional review board. All patients enrolled on the study provided written informed consent.
Data collection
Patient’s demographic information, including age, race, health insurance status, education level, household income, and marital status were collected by patient report at baseline. Medical records were collected from all patients, and abstracted to determine treatment received; if medical record was not available, cancer registry data were used to determine treatment.
Quality of life (QOL) assessment
Quality of life was assessed prospectively using the validated Prostate Cancer Symptom Indices (PCSI) [10]. PCSI assesses 4 domains including sexual dysfunction, urinary obstruction and irritation, urinary incontinence, and bowel problems with each domain scored from 0 to 100, where a higher score represents worse dysfunction. All surveys were conducted by telephone in a similar process as previously described [11] at baseline (pre-treatment), and at 3, 12, and 24 months after completion of treatment. For patients on AS, timing of follow-up surveys was calculated from an anchor date of 3 months after initial diagnosis.
Statistical analysis
The primary goal of this study was to compare patients who received SBRT and conventionally-fractionated RT to those who pursued AS as the “control” group. None of the SBRT patients received androgen deprivation therapy (ADT), and therefore only EBRT patients who did not receive ADT were included. Among EBRT patients, 79% received intensity-modulated RT (IMRT).
In order to adjust for potential differences in baseline characteristics, propensity score weighting was used as previously described [12] contrasting AS against each of the RT groups. In brief, propensity scores were estimated using logistic regression models incorporating age, race, health insurance status, education level, household income, marital status, year of diagnosis, baseline 12-Item Short Form (SF-12) QOL, and baseline PCSI domain scores. Propensity score odds were used to assign weights relative to AS to balance potential confounders [13], and standardized differences [14] were calculated to assess and verify that the balancing was adequate. Missing data were multiply imputed using the fully conditional specification approach as previously described [12]. The imputation model included as many relevant baseline characteristics as possible (including age, race, education, household income, health insurance, employment status, and QOL scores at baseline or at the preceding time point) in order to make the data most likely to satisfy the missing at random assumption [15].
Propensity score-weighted PCSI domain scores were calculated for each time point, and the mean difference of each of the RT groups was assessed in comparison to the AS group. More specifically, the PCSI domain score of a treatment group was compared to the AS group by conducting a simple regression, in which the treatment type was entered as a binary indicator. In these regression analyses, inverse probability of treatment-weighted estimates were used for the respective treatment types and robust standard errors were used for the computation of confidence intervals (CI).
In addition to the primary analysis described above, we also report PCSI scores without imputation or propensity weighting, in order to examine consistency in results and our overall conclusions.
All tests used a 2-sided p < 0.05 for statistical significance. All statistical analyses were performed using SAS (SAS Institute, version 9.4).
Results
Baseline characteristics
The cohort includes 387 patients who pursued AS, 189 patients who received EBRT without ADT, and 104 patients who underwent SBRT. Among 680 total patients, median age was 65–66 years in all 3 groups and 72–82% were married (Table 1). Propensity score weighting was used to balance baseline patient characteristics. A majority of patients on AS had low risk disease (76%), while 57% of EBRT patients and 41% of SBRT patients had intermediate risk disease (Supplemental Table 1). Characteristics of patients who reported data only at baseline and those who reported follow-up data are summarized in Supplemental Table 6. In active surveillance and EBRT groups, there appears to be more missing data in racial minority patients; there are also more missing data in non-married patients within the EBRT group.
Table 1.
Patient demographics and baseline characteristics across different treatment groups
| Before propensity weighting, No. (%) | After propensity weighting*, % | |||||
|---|---|---|---|---|---|---|
| Active Surveillance (n = 387) |
EBRT without ADT (n = 189) |
SBRT (n = 104) |
Active Surveillance |
EBRT without ADT |
SBRT | |
| Age at diagnosis, mean (SD) | 66 (7.5) | 66 (6.9) | 65 (6.8) | 66 (7.5) | 67 (7.2) | 65 (7.8) |
| Race White Black/Other |
286 (74) 101 (26) |
124 (66) 65 (34) |
100 (96) 4 (4) |
74 26 |
75 25 |
72 28 |
| Health Insurance Medicare Private Medicaid/None |
187 (48) 123 (32) 77 (20) |
98 (52) 51 (27) 40 (21) |
52 (50) 45 (43) 7 (7) |
48 32 20 |
48 32 20 |
44 39 16 |
| Education ≤High school Some college College graduate |
126 (33) 103 (27) 158 (41) |
70 (37) 53 (28) 66 (35) |
9 (9) 30 (29) 65 (63) |
33 27 41 |
32 28 40 |
29 24 46 |
| Household income, $/year <40,000 40,000–70,000 70,001–90,000 >90,000 |
164 (42) 97 (25) 49 (13) 77 (20) |
92 (49) 47 (25) 19 (10) 31 (16) |
19 (18) 22 (21) 18 (17) 45 (43) |
42 25 13 20 |
41 27 13 20 |
51 16 13 21 |
| Marital status Married Not married |
313 (81) 74 (19) |
136 (72) 53 (28) |
85 (82) 19 (18) |
81 19 |
80 20 |
87 13 |
| Baseline QOL Scores | ||||||
| SF-12, mean (SD) Physical Mental |
47.7 (11.3) 54.6 (8.4) |
47.6 (11.4) 53.6 (9.3) |
52.1 (8.4) 53.2 (7.6) |
47.7 (11.3) 54.6 (8.4) |
47.8 (11.6) 54.8 (8.4) |
49.9 (11.8) 55.1 (6.2) |
| PCSI, mean (SD) Sexual dysfunction Urinary obstruction/irritation Urinary incontinence Bowel problems |
44.7 (38.0) 23.4 (14.0) 11.1 (20.7) 6.1 (8.3) |
49.9 (39.1) 22.6 (14.7) 12.5 (22.3) 6.9 (10.2) |
42.0 (38.0) 22.9 (13.2) 8.3 (16.3) 5.5 (6.9) |
44.7 (38.0) 23.4 (14.0) 11.1 (20.7) 6.1 (8.3) |
43.6 (39.2) 22.9 (15.2) 10.7 (21.1) 5.8 (8.6) |
52.0 (39.5) 20.9 (12.9) 12.0 (18.2) 4.4 (6.7) |
Sample sizes after propensity weighting are not provided because they often involve decimal points (non-whole numbers).
Abbreviations: EBRT, external beam radiation treatment; ADT, androgen deprivation therapy; SBRT, stereotactic body radiotherapy; QOL, quality of life; PCSI, Prostate Cancer Symptom Indices.
Sexual dysfunction
Propensity score-weighted mean QOL domain scores of each group and the mean difference score vs. AS are shown in Table 2. For the sexual dysfunction domain, patients on AS had a baseline mean score of 44.7 (standard deviation [SD] 38.0) with a gradual worsening to 56.7 (SD 38.1) by 24 months. At 3 months, patients who received EBRT without ADT had statistically significantly worse sexual dysfunction compared to those on AS with a mean difference of 8.0 (95% CI 0.5–15.6). Otherwise, there was no statistically significant difference at baseline, 12 months, or 24 months between the two groups. For patients who received SBRT, there was no statistically significant difference in sexual dysfunction scores compared to those on AS at all times points of follow-up. However, with an upper bound of the 95% CI ranging from 8.0–14.2, the possibility of SBRT resulting in worse sexual dysfunction compared to active surveillance cannot be completely ruled out. We performed a subgroup analysis of EBRT patients who received IMRT in comparison to those electing for AS, which is summarized in Supplemental Table 2.
Table 2.
Propensity-weighted prostate cancer symptoms indices scores for sexual, urinary, and bowel symptoms across different treatment groups at baseline, and at 3, 12, and 24 months
| Active surveillance | EBRT without ADT | SBRT | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Mean score (SD) |
No. of patients | Mean score (SD) |
Mean difference score vs active surveillance (95% CI) |
No. of patients | Mean score (SD) |
Mean difference score vs active surveillance (95% CI) |
|
| Sexual dysfunction | ||||||||
| Baseline | 382 | 44.7 (38.0) |
182 | 43.6 (55.6) |
−1.0 (−8.1, 6.1) |
100 | 44.2† (67.3) |
−0.5 (−12.1, 11.1) |
| 3 months | 299 | 45.5 (38.9) |
134 | 53.5 (55.2) |
8.0* (0.5, 15.6) |
95 | 43.2† (65.7) |
−2.2 (−13.6, 9.1) |
| 12 months | 272 | 48.3 (37.5) |
128 | 51.3 (52.9) |
3.0 (−4.3, 10.3) |
92 | 51.2† (65.2) |
2.8 (−8.5, 14.2) |
| 24 months | 233 | 56.7 (38.1) |
117 | 55.5 (52.4) |
−1.2 (−8.7, 6.3) |
74 | 52.9† (62.5) |
−3.9 (−15.7, 8.0) |
| Urinary obstruction/irritation | ||||||||
| Baseline | 379 | 23.4 (14.0) |
182 | 22.9 (21.5) |
−0.5 (−3.4, 2.3) |
102 | 20.9 (23.9) |
−2.5 (−6.7, 1.6) |
| 3 months | 298 | 23.6 (13.1) |
140 | 34.4 (27.3) |
10.8* (7.5, 14.2) |
95 | 20.5 (33.0) |
−3.1 (−7.1, 1.0) |
| 12 months | 278 | 26.6 (15.5) |
129 | 24.5 (20.3) |
−2.0 (−5.0, 0.9) |
94 | 26.1 (38.6) |
−0.4 (−5.9, 5.1) |
| 24 months | 225 | 25.7 (14.3) |
120 | 24.4 (21.4) |
−1.3 (−4.6, 2.1) |
78 | 25.6 (28.0) |
0 (−6.6, 6.5) |
| Urinary incontinence | ||||||||
| Baseline | 379 | 11.1 (20.7) |
182 | 10.7 (29.9) |
−0.3 (−4.0, 3.3) |
102 | 12.0 (33.9) |
1.0 (−5.4, 7.4) |
| 3 months | 301 | 12.6 (20.8) |
136 | 16.7 (34.5) |
4.0 (−0.5, 8.6) |
95 | 12.3 (31.3) |
−0.4 (−5.5, 4.8) |
| 12 months | 276 | 14.5 (22.6) |
130 | 15.1 (33.8) |
0.7 (−4.2, 5.5) |
94 | 17.3 (43.6) |
2.8 (−5.3, 11.0) |
| 24 months | 230 | 17.8 (23.8) |
120 | 17.8 (35.0) |
−0.1 (−5.2, 5.1) |
79 | 11.9 (36.9) |
−5.9 (−12.5, 0.7) |
| Bowel problems | ||||||||
| Baseline | 387 | 6.1 (8.3) |
182 | 5.8 (12.1) |
−0.3 (−1.8, 1.1) |
102 | 4.4 (12.5) |
−1.7 (−3.8, 0.4) |
| 3 months | 302 | 7.3 (9.8) |
140 | 11.9 (21.2) |
4.6* (2.0, 7.3) |
97 | 4.9 (11.8) |
−2.4* (−4.4, −0.5) |
| 12 months | 279 | 7.5 (10.4) |
130 | 9.5 (19.0) |
2.0 (−0.5, 4.5) |
95 | 4.7 (13.5) |
−2.8* (−5.1, −0.5) |
| 24 months | 233 | 6.5 (7.2) |
120 | 9.7 (21.4) |
3.2* (0.2, 6.2) |
79 | 4.0 (9.3) |
−2.5* (−4.3, −0.7) |
Domain scores range from 0 to 100, with higher score indicating worse symptoms.
Statistically significant difference vs active surveillance.
Analysis of the Sexual Dysfunction score for SBRT patients excluded 2 outliers who required very high propensity weights if included in the analysis.
Abbreviations: EBRT, external beam radiation therapy; ADT, androgen deprivation therapy; SBRT, stereotactic body radiotherapy; SD, standard deviation; CI, confidence interval
Urinary obstruction and irritation
For the urinary obstruction and irritation domain, patients on AS had a baseline mean score of 23.4 (SD 14.0), which remained relatively stable throughout the 24-month follow-up. Patients who received EBRT had worse urinary obstruction and irritation at 3 months compared to those on AS with a mean difference of 10.8 (95% CI 7.5–14.2) (Table 2; Figure 1B). Otherwise, there was no difference at baseline, 12 months, or 24 months between the two groups. For patients who received SBRT, there was no difference in urinary obstruction and irritation score compared to those on AS at all times points.
Figure 1.
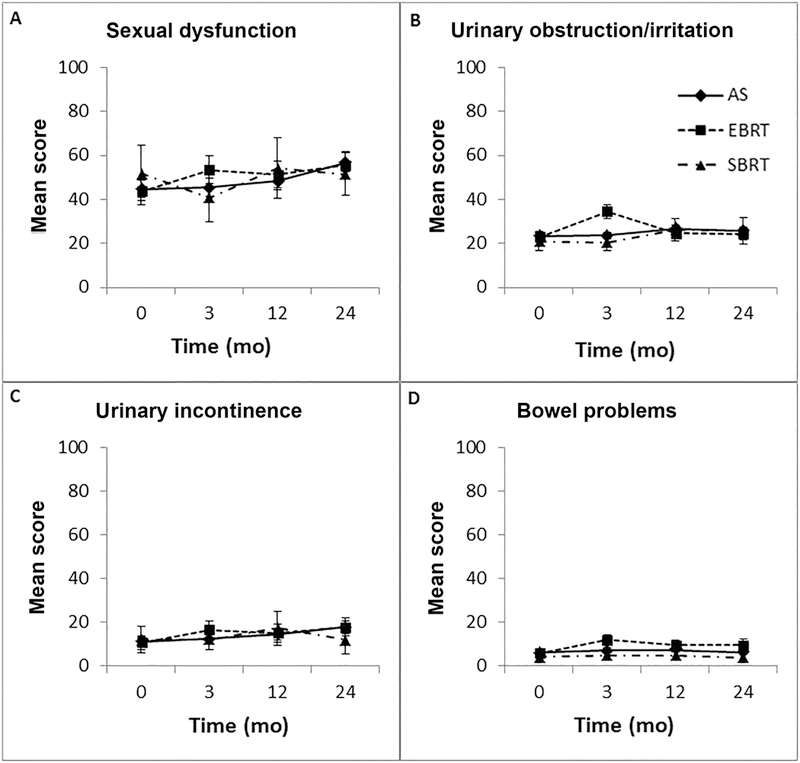
Patient-reported quality of life (with 95% confidence intervals) over time for A) sexual dysfunction, B) urinary obstruction/irritation, C) urinary incontinence, and D) bowel problems. Abbreviations: AS, active surveillance; EBRT, conventionally-fractionated external-beam radiotherapy; SBRT, stereotactic body radiotherapy
Urinary incontinence
Patients on AS had a baseline mean score of 11.1 (SD 20.7) on the urinary incontinence domain with increase over time to 17.8 (SD 23.8) by 24 months. Patients receiving EBRT without ADT and SBRT had no statistically significant difference in urinary incontinence at all time points assessed compared to those on AS. However, with an upper bound of the 95% CI as high as 11.0, the possibility of SBRT resulting in worse urinary incontinence compared to active surveillance cannot be completely ruled out.
Bowel problems
Overall, patients had minimal bowel problems at baseline with mean scores of 6.1 (SD 8.3), 5.8 (SD 12.1), and 4.4 (SD 12.5) for patients on AS, EBRT without ADT, and SBRT, respectively. Compared to AS, those who received EBRT had statistically significantly worse bowel scores at 3 months with a mean difference score of 4.6 (95% CI 2.0–7.3), and at 24 months with a mean difference score of 3.2 (95% CI 0.2–6.2). Patients who received SBRT had statistically lower (better) bowel problem scores at 3, 12, and 24 months compared to those on AS, although the magnitudes of these score differences are small.
Sensitivity Analysis
Supplemental Table 4 summarizes QOL scores without propensity score weighting or imputation, for patients with complete data throughout all assessment time points. Supplemental Table 5 summarizes QOL scores without propensity score weighting or imputation for all patients with completed data at each time point. These results are consistent with the data reported in Table 2.
Discussion
There is intense research interest in shortening the RT course in prostate cancer due to the improved patient convenience of fewer treatments, associated cost-savings, and potential radiobiological advantages of delivering high doses per fraction [16]. Nine randomized trials have been published comparing conventionally-fractionated RT (8–9 weeks) to moderately hypofractionated RT (4–5 weeks), demonstrating similar cancer-control outcomes, though some trials have shown increased toxicity from hypofractionation [17]. There are multiple ongoing trials now comparing longer duration RT with extreme hypofractionation (1–2 weeks) including HYPO-RT-PC (ISRCTN45905321), HEAT (NCT01794403), NRG-GU005 (NCT03367702), and PACE (NCT01584258). Results of the Scandinavian non-inferiority Phase III trial (HYPO-RT-PC) randomizing 1200 patients with intermediate risk disease to 42.7 Gy in 7 fractions vs. 78 Gy in 39 fractions were recently reported, showing non-inferior freedom from biochemical or clinical failure at 5 years with hypofractionation (83.7% vs. 83.8%) [18]. In addition, a large pooled-analysis of multiple Phase II trials including 1100 patients receiving 35–40 Gy in 4–5 fractions showed 5-year biochemical relapse-free survival rates of 95% and 84% for low and intermediate risk patients, respectively [19]. As published evidence accumulates demonstrating the efficacy of extreme hypofractionation including SBRT (≤ 5 fractions), its use has continued to increase [20,21], with demand driven by the convenience of a 1–2 week treatment compared to a conventional 8–9 week course.
On the other hand, some published studies have raised concerns about SBRT delivering large doses of RT with each treatment. Medicare claims-based study reported higher rates of GU toxicity (as determined by diagnoses and diagnostic procedures performed) with SBRT vs. IMRT [6], while a Phase I/II trial reported 6 cases of ≥ grade 3 rectal toxicity among 61 patients treated at the 50 Gy in 5 fractions dose level, 2 of whom suffered a severe rectourethral fistula and 5 required a diverting colostomy [5]. However, the limitations of using claims data as surrogates for treatment-related toxicity is well-recognized [22,23], and the toxicity observed in a dose-escalation trial is likely explained by the dose-finding nature of that study. To date, there is a paucity of studies comparing QOL for patients receiving SBRT vs. conventionally-fractionated EBRT or AS. This is important information in the patient’s decision-making process.
To fulfill this knowledge gap, we prospectively enrolled patients with newly-diagnosed prostate cancer to assess QOL changes from before to after treatment. To our knowledge, this is the first comparative study between SBRT, EBRT, and AS. AS serves as an important “control” group often lacking in previous studies. A prior study by Evans et al. assessed QOL using the Expanded Prostate Cancer Index Composite (EPIC)-26 questionnaire in 381 SBRT patients, 160 IMRT patients, and 262 brachytherapy patients [24].This study showed better bowel QOL for SBRT patients compared to IMRT. Another study by Johnson et al. compared QOL between SBRT and moderately hypofractionated RT in 912 men [25]. The latter had worse urinary symptoms, and there was no difference in bowel or sexual domains. Our results are consistent with these prior studies in that EBRT caused worse urinary and bowel QOL compared to AS, but SBRT was not worse than AS. These findings require further validation through randomized studies, and multiple on-going trials including HYPO-RT-PC, HEAT, NRG-GU005, and PACE are collecting QOL data. However, it may take several years for these data to be reported, and again, these randomized studies do not have an AS arm for comparison.
Improved QOL for SBRT compared to EBRT may stem from underlying radiobiology. Prostate cancer has a relatively low alpha-beta ratio (α/β) compared to other malignancies and even in relation to dose-limiting adjacent normal tissues including rectum and bladder [17]. This suggests that the therapeutic ratio can be augmented with larger doses per fraction, i.e. prostate cancer cells are more sensitive to hypofractionation than the surrounding organs at risk. In addition, SBRT by definition uses higher-precision patient immobilization, organ motion tracking and radiation targeting than conventional RT [26], which may be translating to a clinical QOL benefit.
There are several strengths and limitations of this study. The population-based cohort is a strength. However, because patient recruitment was statewide, details of specific RT dosimetry such as total dose, fractionation, and seminal vesicle coverage were not available. Further, we did not assess QOL of patients who received moderately hypofractionated RT, as this was not commonly used during 2011–2013 when patients were enrolled. Although we used propensity score weighting to account for potential differences in baseline characteristics, the study is not randomized and cannot account for uncontrolled confounders. Further, missing data is a limitation and appeared to occur more often in racial minority (active surveillance and EBRT) and non-married (EBRT) patients, which can introduce bias. Another strength is that all data were collected prospectively (including all baseline QOL collected prior to treatment); to our knowledge, this is the only population-based prostate cancer cohort for which this is true. However, individual patients received treatment as deemed appropriate by the radiation oncologist and/or urologist, and it is not possible to distinguish the role of the natural course of radiation effects vs. symptom-directed therapy on QOL in this study. Finally, while we report QOL results through 2 years after treatment, long-term outcomes may differ and requires further research. However, prior studies have demonstrated little QOL change after 2 years for EBRT [27] and SBRT [28]. Specifically, a single-institutional cohort of 230 patients treated with SBRT demonstrated that urinary and bowel QOL assessed by the EPIC questionnaire changed little from 1 year out to 8 years [28]. More studies are needed to confirm these findings.
Conclusions
In a prospective, non-randomized cohort of men with prostate cancer, patients treated with conventionally-fractionated EBRT experienced worse sexual dysfunction and urinary obstruction/irritation compared to AS at 3 months. They also experienced worse bowel symptoms at 3 and 24 months, although the magnitudes of differences in the bowel domain were small. Patients who received SBRT appeared to have favorable outcomes similar to AS in all domains and across all time points through 2 years, although a difference in sexual dysfunction and urinary incontinence cannot be completely ruled out. Larger studies with longer follow-up are needed to confirm these findings.
Supplementary Material
Acknowledgments
This research was funded by Patient-Centered Outcomes Research Institute (PCORI) grant CER-1310–06453; the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the DEcIDE program, contract HHSA29020050040I; and Accuray Inc. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee. Accuray did not participate in the study design, analysis, or manuscript.
References
- [1].Chen RC, Rumble RB, Loblaw DA, Finelli A, Ehdaie B, Cooperberg MR, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:2182–90. doi: 10.1200/JCO.2015.65.7759. [DOI] [PubMed] [Google Scholar]
- [2].Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol Lond Engl 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pan HY, Jiang J, Hoffman KE, Tang C, Choi SL, Nguyen Q-N, et al. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer. J Clin Oncol Off J Am Soc Clin Oncol 2018:JCO2017755371. doi: 10.1200/JCO.2017.75.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie X-J, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol 2011;29:2020–6. doi: 10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim DWN, Cho LC, Straka C, Christie A, Lotan Y, Pistenmaa D, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1–2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;89:509–17. doi: 10.1016/j.ijrobp.2014.03.012. [DOI] [PubMed] [Google Scholar]
- [6].Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, Gross CP. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol Off J Am Soc Clin Oncol 2014;32:1195–201. doi: 10.1200/JCO.2013.53.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ávila M, Patel L, López S, Cortés-Sanabria L, Garin O, Pont À, et al. Patient-reported outcomes after treatment for clinically localized prostate cancer: A systematic review and meta-analysis. Cancer Treat Rev 2018;66:23–44. doi: 10.1016/j.ctrv.2018.03.005. [DOI] [PubMed] [Google Scholar]
- [8].Lardas M, Liew M, van den Bergh RC, De Santis M, Bellmunt J, Van den Broeck T, et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur Urol 2017;72:869–85. doi: 10.1016/j.eururo.2017.06.035. [DOI] [PubMed] [Google Scholar]
- [9].Chen RC, Carpenter WR, Kim M, Hendrix LH, Agans RP, Meyer A-M, et al. Design of the North Carolina Prostate Cancer Comparative Effectiveness and Survivorship Study (NC ProCESS). J Comp Eff Res 2015;4:3–9. doi: 10.2217/cer.14.67. [DOI] [PubMed] [Google Scholar]
- [10].Clark JA, Talcott JA. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care 2001;39:1118–30. [DOI] [PubMed] [Google Scholar]
- [11].Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- [12].Chen RC, Basak R, Meyer A-M, Kuo T-M, Carpenter WR, Agans RP, et al. Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men With Localized Prostate Cancer. JAMA 2017;317:1141–50. doi: 10.1001/jama.2017.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med 2014;275:570–80. doi: 10.1111/joim.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shafer J Analysis of Incomplete Multivariate Data. 1st ed. Chapman and Hall; 1997. [Google Scholar]
- [16].Moon DH, Efstathiou JA, Chen RC. What is the best way to radiate the prostate in 2016? Urol Oncol 2017;35:59–68. doi: 10.1016/j.urolonc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- [17].Benjamin LC, Tree AC, Dearnaley DP. The Role of Hypofractionated Radiotherapy in Prostate Cancer. Curr Oncol Rep 2017;19:30. doi: 10.1007/s11912-017-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultrahypofractionation for prostate cancer: Outcome from the Scandinavian phase 3 HYPO-RT-PC trial. Radiother Oncol J Eur Soc Ther Radiol Oncol 2018;127:S314. [Google Scholar]
- [19].King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol J Eur Soc Ther Radiol Oncol 2013;109:217–21. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- [20].Baker BR, Basak R, Mohiuddin JJ, Chen RC. Use of stereotactic body radiotherapy for prostate cancer in the United States from 2004 through 2012. Cancer 2016;122:2234–41. doi: 10.1002/cncr.30034. [DOI] [PubMed] [Google Scholar]
- [21].Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: A National Cancer Database analysis. Pract Radiat Oncol 2017;7:270–8. doi: 10.1016/j.prro.2017.03.011. [DOI] [PubMed] [Google Scholar]
- [22].King CR, Steinberg MS, Kupelian P. Perils of comparing toxicities between stereotactic body radiation and intensity-modulated radiation therapy for prostate cancer on the basis of incomplete demographic registries. J Clin Oncol Off J Am Soc Clin Oncol 2014;32:3453. doi: 10.1200/JCO.2014.56.4229. [DOI] [PubMed] [Google Scholar]
- [23].Arcangeli S, De Bari B, Alongi F. Toxicity of stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: a potential comparison bias. J Clin Oncol Off J Am Soc Clin Oncol 2014;32:3454. doi: 10.1200/JCO.2014.56.3213. [DOI] [PubMed] [Google Scholar]
- [24].Evans JR, Zhao S, Daignault S, Sanda MG, Michalski J, Sandler HM, et al. Patient-reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol 2015;116:179–84. doi: 10.1016/j.radonc.2015.07.016. [DOI] [PubMed] [Google Scholar]
- [25].Johnson SB, Soulos PR, Shafman TD, Mantz CA, Dosoretz AP, Ross R, et al. Patient-reported quality of life after stereotactic body radiation therapy versus moderate hypofractionation for clinically localized prostate cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol 2016;121:294–8. doi: 10.1016/j.radonc.2016.10.013. [DOI] [PubMed] [Google Scholar]
- [26].Folkert MR, Timmerman RD. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv Drug Deliv Rev 2017;109:3–14. doi: 10.1016/j.addr.2016.11.005. [DOI] [PubMed] [Google Scholar]
- [27].Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2016;375:1425–37. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Katz A Stereotactic Body Radiotherapy for Low-Risk Prostate Cancer: A Ten-Year Analysis. Cureus 2017;9:e1668. doi: 10.7759/cureus.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


