Abstract
Background
Binocular amblyopia treatments promote visual acuity recovery and binocularity by rebalancing the signal strength of dichoptic images. Most require active participation by the amblyopic child to play a game or perform a repetitive visual task. The purpose of this study was to investigate a passive form of binocular treatment with contrast-rebalanced dichoptic movies.
Methods
A total of 27 amblyopic children, 4–10 years of age, wore polarized glasses to watch 6 contrast-rebalanced dichoptic movies on a passive 3D display during a 2-week period. Amblyopic eye contrast was 100%; fellow eye contrast was initially set to a lower level (20%−60%), which allowed the child to overcome suppression and use binocular vision. Fellow eye contrast was incremented by 10% for each subsequent movie. Best-corrected visual acuity, random dot stereoacuity, and interocular suppression were measured at baseline and at 2 weeks.
Results
Amblyopic eye best-corrected visual acuity improved from 0.57 ± 0.22 at baseline to 0.42 ± 0.23 logMAR (t26 = 8.09; P < 0.0001; 95% CI for improvement, 0.11–0.19 logMAR). Children aged 3–6 years had more improvement (0.21 ± 0.11 logMAR) than children aged 7–10 years (0.11 ± 0.06 logMAR; t25 = 3.05; P = 0.005). Children with severe amblyopia (≥0.7 logMAR) at baseline experienced greater improvement (0.24 ± 0.12 logMAR) than children with moderate amblyopia at baseline (0.12 ± 0.06 logMAR; t25 = 3.49; P = 0.002).
Conclusions
In this cohort, passive viewing of contrast-rebalanced dichoptic movies effectively improved visual acuity in amblyopic subjects. The degree of improvement observed was similar to that previously reported for 2 weeks of binocular games treatment and with 3–4 months of occlusion therapy.
Patching improves visual acuity in amblyopic children; however, there is substantial variability in response to monocular treatment, with only 50%−85% achieving normal visual acuity.1–4 Residual amblyopia is associated with lifelong limitations in visuomotor tasks,5,6 slow reading,7,8 fixation instability,9–11 and altered self-perception.12–14
Our evolving understanding of the role of interocular suppression as the primary factor interfering with normal visual development in amblyopia15,16 has led to the recent development of clinical therapies that aim to alleviate interocular suppression, restore binocular combination, and rehabilitate visual acuity. Binocular amblyopia treatments promote simultaneous use of both eyes by rebalancing the strength of each eye’s image with high-contrast or high-luminance input to the amblyopic eye and low-contrast or low-luminance input to the fellow eye.17–22
Currently, most binocular treatments require active participation by the amblyopic child: playing a game or performing a repetitive psychophysical task. We recently reported our results of a passive binocular treatment, where the subject watches contrast-rebalanced dichoptic movies in which reciprocal blob-shaped parts of the image are presented to each eye to promote binocular combination.23 After 2 weeks (6 movies; approximately 9 hours), mean amblyopic eye best-corrected visual acuity (with standard error) improved from 0.72 ± 0.08 logMAR at baseline to 0.52 ± 0.09 logMAR (P = 0.003), that is, 2 logMAR lines of improvement at the 2-week outcome visit. These results suggested that passive viewing of dichoptic animated feature films is a feasible and effective amblyopia treatment. However, the sample size was small (n = 8), limiting generalizability and our ability to assess whether treatment effectiveness was associated with baseline factors. In the current study, a larger cohort of 27 amblyopic children participated in a 2-week intervention with contrast-rebalanced dichoptic movies. We investigated whether any baseline factors were associated with response to this passive binocular intervention.
Subjects and Methods
The study was approved by the Institutional Review Board of University of Texas Southwestern Medical Center and complied with regulations of the US Health Insurance Portability and Accountability Act of 1996. Written informed consent was obtained from a parent of each participant and the child’s written assent was obtained in accordance with the Institutional Review Board’s regulations. A total of 27 amblyopic children, 4–10 years of age, were enrolled. Eligibility criteria included a diagnosis of strabismic, anisometropic, or combined mechanism amblyopia by the referring pediatric ophthalmologist, and best-corrected visual acuity in the amblyopic eye of ≥0.3 logMAR and in the fellow eye of ≤0.2 logMAR, with an interocular difference of ≥0.2 logMAR. Strabismic children were eligible to participate only after correction of strabismus with glasses or surgery to <5∆ residual strabismus. Eligible children had to have been wearing their current spectacle correction for at least 3 months prior to the baseline visit, and the child’s referring pediatric ophthalmologist had to be willing to forgo other amblyopia treatment during the study period. Exclusion criteria were gestational age at birth of ≤32 weeks, developmental delay, and coexisting ocular or systemic diseases. Medical records were obtained from the referring pediatric ophthalmologist to extract diagnosis, cycloplegic refraction, and treatment history.
The movies and protocol were the same as previously reported in our pilot study of 8 amblyopic children.23 Briefly, children wore glasses fitted with polarized film over their habitual glasses to watch 6 dichoptic movies shown on a passive 3D display (LG Electronics USA, Englewood, NJ) in our laboratory. Odd lines on the 3D display were visible to one eye, and the even lines were visible to the other eye. Dichoptic versions of 18 popular animated feature films were created.23 Using a customized MatLab program, a patterned image mask composed of irregularly shaped blobs was multiplied with the images seen by the amblyopic eye, and the inverse patterned mask was multiplied with the images seen by the fellow eye, so that different parts of the display were seen by each eye. Blobs of the movie seen by the amblyopic eye always had high contrast (100%), whereas the complementary blobs were seen by the fellow eye with reduced contrast. Because the blobs had Gaussian edges, the edges of the blobs overlapped and were seen by both eyes with differing contrasts. The shape and location of the blobs were varied dynamically every 10 seconds.
Children watched 6 movies during the 2-week period. A 2-week study duration was chosen as adequate to evaluate whether dichoptic movies were effective in improving visual acuity.18,20,23 Previous binocular amblyopia treatments have been shown to improve visual acuity with 8–10 hours of treatment, and we needed to minimize the demand on the family for study-required visits to the laboratory to view each movie. Fellow-eye contrast was initially set at a reduced level that allowed binocular vision, based on the child’s dichoptic motion coherence contrast ratio (CR) minus 0.10, with a minimum of 0.20 and a maximum setting of 0.60.19,23,24 Fellow eye contrast was incremented by 10% of the previous contrast for each subsequent movie. With a maximum initial fellow eye contrast of 0.60 and a 10% increment, we ensured that a contrast imbalance would be present for all 6 movies. A parent accompanied their child during the movie sessions to ensure compliance (polarized glasses wear and attention to the movie). Compliance was also confirmed by study personnel at 15- to 30-minute intervals.
Best-corrected visual acuity, random dot stereoacuity, and interocular suppression were measured at baseline and outcome visits. Best-corrected visual acuity was obtained for each eye with the ATS-HOTV for children <7 years old or E-ETDRS for children ≥7 years. Retrospective visual acuity data from visits 6 months, 3 months, and 1 month prior to the baseline visit were obtained from medical records for 20, 23, and 27 of the 27 participants, respectively. Random dot stereoacuity was evaluated using the Randot Preschool Stereoacuity Test (Stereo Optical Co Inc, Chicago, IL), the Stereo Butterfly Test (Stereo Optical Co Inc), and the Lang-Stereotest I (Lang-Stereotest AG; Küsnacht, Switzerland). Nil stereoacuity was arbitrarily assigned a value of 4.0 log arcsec. Severity of interocular suppression, measured by CR, was quantified using a dichoptic motion coherence test that determines the maximum contrast of randomly moving dots in the fellow eye that still allows the child to discriminate the direction of coherent motion dots in the amblyopic eye.19,23,24
Sample Size and Data Analysis
The pilot study reported a mean (± standard deviation) improvement of 0.23 ± 0.14 logMAR.23 However, the inclusion criteria for the pilot study restricted baseline best-corrected visual acuity to ≥0.5 logMAR and, likely because of a ceiling effect, worse baseline best-corrected visual acuity is associated with more improvement with amblyopia treatment.25 In the current study we included visual acuity of ≥0.3 logMAR and estimated a more conservative mean effect of 0.1 line improvement, to be evaluated by paired t test, with α = 0.025 and 1-β = 0.90, requiring a sample size of 24.26
The primary outcome, amblyopic eye best-corrected visual acuity at 2 weeks, was compared with best-corrected visual acuity at baseline using a paired t test. Stereoacuity and suppression at the 2-week visit were also compared from baseline using a paired t test. Secondary group analyses of amblyopic visual acuity improvement were conducted on 6 dichotomized baseline factors using t tests: 3–6 years versus 7–10 years old, moderate versus severe amblyopia, history of patching treatment present versus absent, history of binocular amblyopia treatment present versus absent, random dot stereoacuity present versus nil, and initial dichoptic CR 1.0–2.9 (no or mild suppression) versus ≥3.0 (moderate to severe suppression). Because 6 t tests were conducted on the same data set, Bonferroni correction was used to reduce the chance of type 1 error; that is, only P values of ≤0.008 were considered statistically significant. Pearson r correlations were conducted to determine associations of baseline variables with amblyopic best-corrected visual acuity improvement at the outcome visit.
Results
Baseline data of the 27 subjects are provided in Table 1. Overall, 48% were female and 59% were non-Hispanic white. Mean age (with standard deviation) was 7.3 ± 1.8 years. Children had strabismic (7%), anisometropic (59%), or combined mechanism (33%) amblyopia. Mean (± standard deviation) best-corrected visual acuity was 0.57 ± 0.22 logMAR in the amblyopic eye; 0.02 ± 0.12 logMAR, in the fellow eye. Visual acuity data extracted from medical records showed that mean best-corrected visual acuity in the amblyopic eye varied little on multiple visits prior to the baseline visit (mean range, 0.50–0.54 logMAR) and was similar to the mean baseline value (0.57 logMAR; Figure 1A).
Table 1.
Baseline characteristics
| Characteristic | No. (%) |
|---|---|
| Female | 13 (48) |
| Race/ethnicity, no. (%) | |
| Non-Hispanic white | 16 (59) |
| Hispanic white | 2 (7) |
| African American | 3 (11) |
| Asian | 4 (15) |
| More than one | 2 (7) |
| Age | |
| 3–6 years | 11 (41) |
| 7–10 years | 16 (59) |
| Cause of amblyopia | |
| Strabismus | 2 (7) |
| Anisometropia | 16 (59) |
| Combined mechanism | 9 (33) |
| Prior amblyopia treatment | |
| Glasses | 27 (100) |
| Patching | 23 (85) |
| Binocular iPad game | 15 (56)a |
| Baseline Amblyopic Eye BCVA (logMAR), n (%) | |
| 0.30−0.60 | 19 (70) |
| 0.70−1.30 | 8 (30) |
| Nil baseline stereoacuity | 21 (78) |
| Baseline suppression (CR) | |
| 1.0−3.0 | 14 (52) |
| >3.0 | 13 (48) |
BCVA, best-corrected visual acuity; CR, contrast ratio.
Includes 5 children who were assigned to binocular iPad games but never played, 4 children who played <5 hours, and 6 children who played up to 20 hours but still had residual amblyopia with BCVA ≥0.4 logMAR (20/50).
FIG 1.
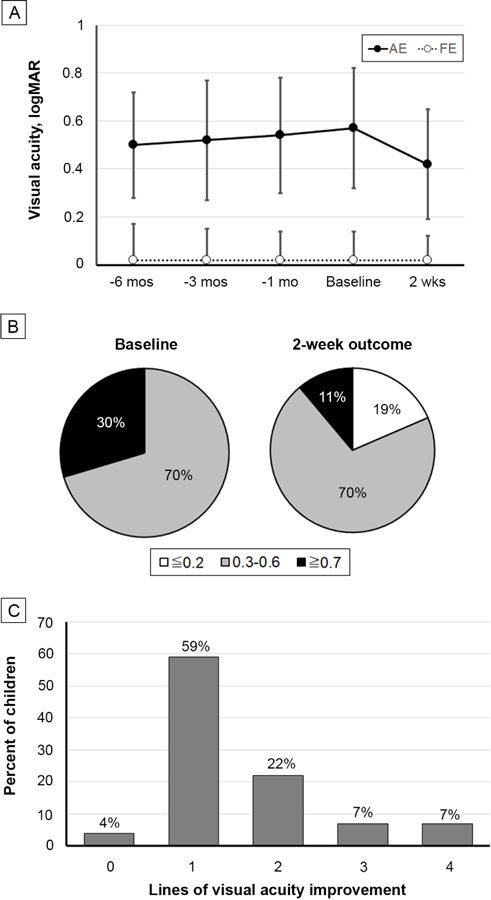
A, Best-corrected visual acuity (mean with standard deviation) of the amblyopic and fellow eyes for the baseline and 2-week primary outcome visits. Also shown are retrospective data at 6 months, 3 months, and 1 month prior to baseline, obtained from medical records for 20, 23, and 27 of the 27 participants, respectively. B, Percentages of children with severe (≥0.7 logMAR), moderate (0.3–0.6 logMAR), and mild or no (≤0.2 logMAR) amblyopia at baseline and after treatment. C, Number of lines of best-corrected visual acuity improvement from baseline at the outcome examination.
At the outcome visit, mean amblyopic eye visual acuity improved from baseline by 0.15 ± 0.10 logMAR, from 0.57 ± 0.22 to 0.42 ± 0.23 logMAR (t26 = 8.09; P < 0.0001; 95% CI for improvement, 0.11–0.19 logMAR). Fellow eye visual acuity was stable throughout all 5 visits, at 0.02 logMAR. Figure 1B shows that the percentage of children with severe amblyopia (≥0.7 logMAR) was reduced from 30% at baseline to 11% and that 19% of children had mild or no amblyopia after 2 weeks of treatment. Most children (81%) had an improvement of 1–2 lines (0.1–0.2 logMAR) in best-corrected visual acuity, whereas 14% had 3–4 lines improvement (Figure 1C). Only one child failed to show any improvement.
Mean stereoacuity showed no significant improvement between baseline (3.57 ± 0.77 log arcsec) and the outcome visit (3.50 ± 0.76 log arcsec; t26 = 1.37; P = 0.18). Severity of suppression, as indexed by the mean CR was significantly reduced between baseline (4.1 ± 3.2) and the outcome visit (3.0 ± 2.6; t26 = 3.10, P = 0.01). Reduced suppression (improvement in CR) was correlated with improvement in amblyopic eye visual acuity (r = 0.39; P = 0.04; 95% CI, 0.01–0.67). Only two baseline factors were associated with amblyopic best-corrected visual acuity improvement (Figure 2). Children 3–6 years of age had a mean improvement of 0.21 ± 0.11 logMAR, whereas children 7–10 years of age improved by 0.11 ± 0.06 logMAR (t25 = 3.05; P = 0.005). Also, children with severe amblyopia (≥0.7 logMAR) had improvement of 0.24 ± 0.12 logMAR, whereas children with moderate amblyopia improved by 0.12 ± 0.06 logMAR (t25 = 3.49; P = 0.002). None of the other baseline variables examined (history of prior patching treatment, history of prior binocular treatment, stereoacuity, CR) had a significant association with amblyopic eye best-corrected visual acuity improvement (t25 < 1.64; P > 0.1 for all comparisons).
FIG 2.
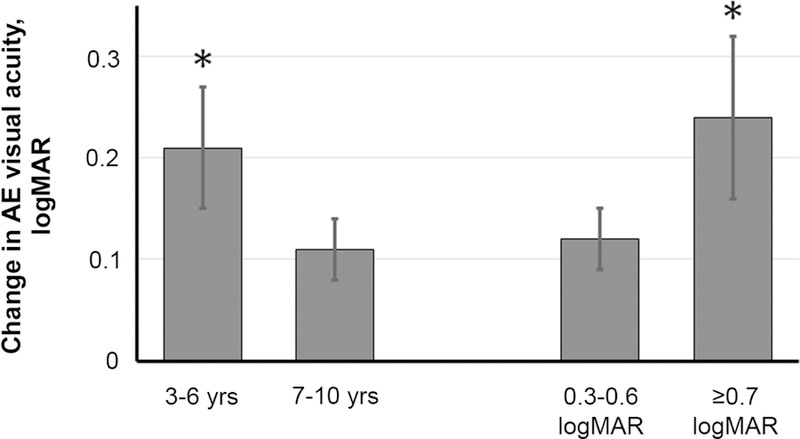
Improvement in amblyopic eye best-corrected visual acuity in the younger (3–6 years) and the older (7–10 years) age subgroups and in subgroups with moderate (0.3–0.6 logMAR) or severe (≥0.7 logMAR) amblyopia at baseline.
Although we did not have a formal plan to conduct long-term follow-up of participants, visual acuity data were available at 6–11 months (n = 10) or 12–24 months (n = 6) later for children who had no treatment other than spectacles following completion of the study. On average, there was 0.00 ± 0.07 logMAR change between the outcome visit and the follow-up examination. Four children had 0.10 logMAR deterioration, 8 had no change, and 4 had an improvement of 0.1 logMAR. Other participants were lost to follow-up (n = 4) or were excluded from the follow-up data because they were patching for residual amblyopia following participation in this study (n = 7).
Discussion
Passive binocular amblyopia treatment of watching 6 contrast-rebalanced dichoptic movies (approximately 9 hours total) over a 2-week period resulted in 0.15 logMAR mean improvement in amblyopic eye best-corrected visual acuity. Retrospective data from medical records showed stable visual acuity was present on multiple visits prior to the baseline visit. Thus, although we did not have a sham movie comparison group, it is unlikely that the observed visual acuity improvement was due simply to repeated testing. A similar improvement in visual acuity has been observed with 2 weeks of active binocular amblyopia treatment with binocular games20 and with 3–4 months of occlusion therapy in children with stable visual acuity in spectacle correction prior to baseline.27–30
Accompanying the improvement in amblyopic eye visual acuity, there was also a significant reduction in the severity of suppression at the outcome visit, and this reduction was correlated with improved visual acuity. This relationship is consistent with a correlation between amblyopic eye visual acuity and depth of suppression.31,32 Converging evidence implicates interocular suppression in the etiology of amblyopia,15,16 and binocular treatment that reduces or eliminates suppression may be the key to successful amblyopia treatment.
A variety of binocular, dichoptic, and virtual reality perceptual learning tasks and games have been developed as potential treatments for amblyopia.17–22 Some authors have hypothesized that action video games may provide the best approach because they are not only highly engaging, requiring attention to identify and track potential targets, but they also trigger arousal via time constraints, decision making, and task performance and provide immediate feedback on success or failure.22,33 The current study provides evidence for visual acuity improvement as a result of passive exposure to dichoptic contrast-rebalanced video content, in the absence of the requirement to perform any task and without any feedback.
Only two baseline variables in the current study were associated with the amount of visual acuity improvement observed at the outcome visit: age and severity of amblyopia. There was greater improvement in amblyopic eye visual acuity in children 3–6 years of age compared with those 7–10 years of age. Randomized clinical trials conducted by the Pediatric Eye Disease Investigator Group (PEDIG) also show that, although patching treatment is effective in older children, the response tends to be slower, with less gain.34–36 Our finding of greater visual acuity improvement in children with severe amblyopia at baseline is similar to the larger improvement reported for patching treatment by PEDIG.25 The finding of a 0.24 logMAR improvement in the severe amblyopia group is consistent with our pilot study of 8 amblyopic children, with baseline visual acuity of 0.72 ± 0.24 logMAR who achieved 0.23 ± 0.14 logMAR improvement at the end of 2 weeks.23 The lack of association with other baseline variables (prior treatment, stereoacuity, and severity of suppression) suggests that the potential benefit of binocular amblyopia treatment is generally applicable to children with amblyopia.
This study had several limitations. Although 8–10 hours of treatment yielded significant visual acuity improvement, we were not able to assess whether a longer period of treatment might result in additional benefit. The short duration of the intervention was chosen as a trade-off based on prior short-term binocular amblyopia treatment studies that demonstrated visual acuity improvement with 8–10 hours of treatment and the demand placed on participating families to travel to the laboratory. In addition, there was no randomized comparison to patching or other amblyopia treatments. To address these limitations, we are currently conducting a randomized trial of at-home dichoptic movies versus patching for the treatment of amblyopia (). Lastly, we did not have a formal plan to evaluate long-term stability of visual acuity. Because of the short treatment duration, many participants had residual amblyopia at the outcome visit, and some opted to immediately begin patching treatment. As a result, we were unable to assess the stability of the visual acuity gain achieved with dichoptic movie treatment. Nonetheless, we did find visual acuity stability within 0.1 logMAR among the 16 children who had no additional treatment, other than continued spectacle wear, within the expectation for visual acuity test–retest reliability.37,38
Acknowledgments
This research was supported in part by a grant from the National Eye Institute (EY022313).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Repka MX, Beck RW, Holmes JM, et al. ; Pediatric Eye Disease Investigator Group. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol 2005;123:149–57. [DOI] [PubMed] [Google Scholar]
- 2.Pediatric Eye Disease Investigator Group. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol 2003;121:603–11. [DOI] [PubMed] [Google Scholar]
- 3.Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci 2004;45:3048–54. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DK, Edwards AR, Cotter SA, et al. ; Pediatric Eye Disease Investigator Group. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology 2006;113:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus 2011;19:119–28. [DOI] [PubMed] [Google Scholar]
- 6.Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci 2008;49:594–603. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KR, Jost RM, De La Cruz A, Birch EE. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J AAPOS 2015;19:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanonidou E, Proudlock FA, Gottlob I. Reading strategies in mild to moderate strabismic amblyopia: an eye movement investigation. Invest Ophthalmol Vis Sci 2010;51:3502–8. [DOI] [PubMed] [Google Scholar]
- 9.Birch EE, Subramanian V, Weakley DR. Fixation instability in anisometropic children with reduced stereopsis. J AAPOS 2013;17:287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly KR, Cheng-Patel CS, Jost RM, Wang YZ, Birch EE. Fixation instability during binocular viewing in anisometropic and strabismic children. Epub ahead of print, July 10, 2018. Exp Eye Res 2018 [DOI] [PMC free article] [PubMed]
- 11.Chung ST, Kumar G, Li RW, Levi DM. Characteristics of fixational eye movements in amblyopia: limitations on fixation stability and acuity? Vision Res 2015;114:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webber AL, Wood JM, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optom Vis Sci 2008;85:1074–81. [DOI] [PubMed] [Google Scholar]
- 13.Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. Epub ahead of print, November 15, 2018. JAMA Ophthalmol [DOI] [PMC free article] [PubMed]
- 14.Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception in amblyopic children age 3–7 years. JAMA Ophthalmol In press. [DOI] [PMC free article] [PubMed]
- 15.Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res 2013;33:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vision Res 2015;114:4–16. [DOI] [PubMed] [Google Scholar]
- 17.Herbison N, Cobb S, Gregson R, et al. ; I-BiT study group. Interactive binocular treatment (I-BiT) for amblyopia: results of a pilot study of 3D shutter glasses system. Eye (Lond) 2013;27:1077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch EE, Li SL, Jost RM, et al. Binocular iPad treatment for amblyopia in preschool children. J AAPOS 2015;19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo CX, Babu RJ, Black JM, et al. Binocular treatment of amblyopia using videogames (BRAVO): study protocol for a randomised controlled trial. Trials 2016;17:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, Birch EE. Binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol 2016;134:1402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes JM, Manny RE, Lazar EL, et al. ; Pediatric Eye Disease Investigator Group. A randomized trial of binocular Dig Rush game treatment for amblyopia in children aged 7 to 12 Years. Ophthalmology 2019;126:456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambacorta C, Nahum M, Vedamurthy I, et al. An action video game for the treatment of amblyopia in children: a feasibility study. Vision Res 2018;148:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SL, Reynaud A, Hess RF, et al. Dichoptic movie viewing treats childhood amblyopia. J AAPOS 2015;19:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen AM, Cotter SA. The amblyopia treatment studies: Implications for clinical practice. Adv Ophthalmol Optom 2016;1:287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansouri B, Thompson B, Hess RF. Measurement of suprathreshold binocular interactions in amblyopia. Vision Res 2008;48:2775–84. [DOI] [PubMed] [Google Scholar]
- 26.Rosner B Fundamentals of Biostatistics 4th ed. Pacific Grove CA: Duxbury Press; 1995:221. [Google Scholar]
- 27.Holmes JM, Manh VM, Lazar EL, et al. ; Pediatric Eye Disease Investigator Group. Effect of a binocular iPad game vs part-time patching in children aged 5 to 12 years with amblyopia: a randomized clinical trial. JAMA Ophthalmol 2016;134:1391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheiman MM, Hertle RW, Kraker RT, et al. ; Pediatric Eye Disease Investigator Group. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a randomized trial. Arch Ophthalmol 2008;126:1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Neely DE, Galli J, et al. A pilot randomized clinical trial of intermittent occlusion therapy liquid crystal glasses versus traditional patching for treatment of moderate unilateral amblyopia. J AAPOS 2016;20:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tailor VK, Glaze S, Khandelwal P, et al. Prescribed computer games in addition to occlusion versus standard occlusion treatment for childhood amblyopia: a pilot randomised controlled trial. Pilot Feasibility Stud 2015;1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birch EE, Morale SE, Jost RM, et al. Assessing suppression in amblyopic children with a dichoptic eye chart. Invest Ophthalmol Vis Sci 2016;57:5649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narasimhan S, Harrison ER, Giaschi DE. Quantitative measurement of interocular suppression in children with amblyopia. Vision Res 2012;66:1–10. [DOI] [PubMed] [Google Scholar]
- 33.Li RW, Young KG, Hoenig P, Levi DM. Perceptual learning improves visual performance in juvenile amblyopia. Invest Ophthalmol Vis Sci 2005;46:3161–8. [DOI] [PubMed] [Google Scholar]
- 34.Holmes JM, Lazar EL, Melia BM, et al. ; Pediatric Eye Disease Investigator Group. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol 2011;129:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pediatric Eye Disease Investigator Group. The course of moderate amblyopia treated with patching in children: experience of the amblyopia treatment study. Am J Ophthalmol 2003;136:620–29. [DOI] [PubMed] [Google Scholar]
- 36.Pediatric Eye Disease Investigator Group. A prospective, pilot study of treatment of amblyopia in children 10 to <18 years old. Am J Ophthalmol 2004;137:581–3. [DOI] [PubMed] [Google Scholar]
- 37.Cotter SA, Chu RH, Chandler DL, et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol 2003;136:655–61. [DOI] [PubMed] [Google Scholar]
- 38.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol 2001;132:903–9. [DOI] [PubMed] [Google Scholar]


