Abstract
Background:
Patients with peripheral arterial disease (PAD) often have walking impairment due to insufficient oxygen supply to skeletal muscle. In aged rats, we have shown that daily stretching of calf muscles improves endothelium-dependent dilation of arterioles from the soleus muscle and increases capillarity and muscle blood flow during exercise. Therefore, we hypothesized that daily muscle stretching of calf muscles would improve endothelium-dependent vasodilation of the popliteal artery and walking function in PAD patients.
Methods:
We performed a randomized, non-blinded, crossover study whereby 13 patients with stable symptomatic PAD were randomized to undergo either 4 weeks of passive calf muscle stretching (ankle dorsiflexion applied 30 min/d, 5 days/wk) followed by 4 weeks of no muscle stretching and vice versa. Endothelium-dependent flow-mediated dilation (FMD) and endothelium-independent nitroglycerin-induced dilation of the popliteal artery and 6 minute walk test (6MWT) were evaluated at baseline and after each 4 week interval.
Results:
After 4 weeks of muscle stretching, FMD and 6MWT improved significantly in the muscle stretching group vs. the control (FMD: 5.1 ± 0.5 % vs. 3.7 ± 0.3 %, P=0.005; 6MWT continuous walking distance: 304 ± 43m vs. 182 ± 34m; P=0.0006). No difference in nitroglycerin-induced dilation was found between groups (10.9 ± 1.2 vs. 9.9 ± 0.4 %, P=0.48). Post-stretching, 6MWT total walking distance was positively correlated with normalized FMD (R=0.645, P=0.02).
Conclusions:
Passive calf muscle stretching enhanced vascular endothelial function and improved walking function in elderly patients with stable symptomatic PAD. These findings merit further investigation in a prospective randomized trial.
Keywords: Endothelial function, Peripheral artery disease, Muscle stretching
Introduction
Lower extremity peripheral artery disease (PAD) afflicts 8.5 million Americans and is associated with significant morbidity and mortality.1 Reduced arterial blood flow to the lower extremities causes considerable impairment in the quality of life of PAD patients.2–4 Claudication, due to insufficient oxygen supply, often results in impaired walking, physical inactivity and reduced mobility of patients with PAD.5–7 McDermott, et al.5, 8 reported PAD patients have poorer physical function, and greater annual decline in 6-minute walk performance compared to patients without PAD.
In a report of the ACC and AHA Joint Task Force on Practice Guidelines for treating patients with PAD,9 a program of supervised exercise training is strongly recommended as an initial treatment modality for patients with intermittent claudication; however, the rate of compliance with supervised exercise training programs is low in elderly PAD patients. Approximately 15% of these patients drop out of exercise programs because of disinterest or inconvenience,10, 11 whereas others find the program too intense to maintain over time. To promote long-term adherence of participants to exercise training regimes, home-based programs are appealing for elderly PAD patients. The Inter-Society Consensus for the Management of PAD (TASK II),12 suggests that the most effective programs tend to employ treadmill or track walking that is of sufficient intensity to precipitate claudication, followed by rest, over the course of a 30–60 minute session. However, it is often difficult for elderly PAD patients to routinely achieve and maintain a claudication-inducing walking program, due to patient discomfort, sedentary state, poor physical conditioning, or arthritis. Therefore, there remains an unmet need for simple non-pharmacological, non-surgical, and non-exercise therapies that increase blood flow and reduce symptoms in elderly patients with PAD.
In an aged rat model, we recently showed that daily passive stretching of the calf muscles, achieved by applying an ankle dorsiflexion splint, resulted in improvement of vascular endothelium-dependent vasodilation and increased capillarity and blood flow during exercise13. We hypothesized that daily passive stretching of the calf muscles would improve endothelium-dependent vasodilation and walking function in elderly patients with PAD. To test this hypothesis, we constructed the following prospective randomized cross-over study.
Methods
Patient Sample
The study sample consisted of patients with stable symptomatic PAD. Inclusion criteria included reproducible claudication with walking associated with an ankle brachial index (ABI) of less than 0.90,14 and/or computed tomographic (CT) angiographic evidence of at least a 60% stenosis of the iliac, femoral, or popliteal artery deemed to be the source of claudication. Individuals with critical limb ischemia or who had severe comorbid conditions limiting their walking ability were excluded.
Study Design
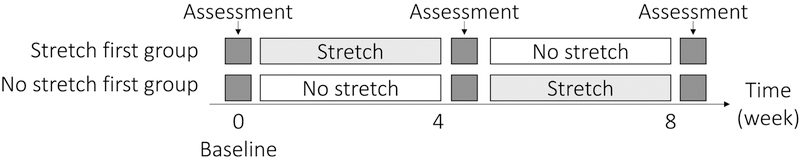
This study was a prospective randomized, non-blinded, crossover trial conducted at Tallahassee Memorial HealthCare (TMH), Tallahassee, FL, USA between October 2015 and May 2016. The TMH Institutional Review Board and the Human Subject Committee at the Florida State University approved the study. Informed consent was provided by all patients. Participants were initially randomized to undergo either 4 weeks of passive stretching or 4 weeks of no stretching, respectively, followed by cross-over to the other intervention (Figure 1). Patients were evaluated at baseline, after the first 4 weeks of initial intervention (stretching or no stretching) and then again after 4wks following the cross-over intervention (stretching or no stretching). Participants were taught how to apply a simple dorsiflexion splint and during the 4 weeks of daily stretching intervention took the splints home. Endothelium-dependent flow-mediated dilation (FMD) and endothelium-independent nitroglycerin-induced dilation (NID) of the popliteal artery, and 6 minute walk test (6MWT) were evaluated at baseline and after each 4 week treatment interval.
Figure 1. Study design.
A randomized, non-blinded, cross over study was performed to test the effects of muscle stretching. Participants were initially randomized to undergo either 4 weeks of passive stretching or 4 weeks of no stretching, followed by cross-over to the other intervention. Participants visited hospital at 0 (baseline), 4 and 8 weeks for evaluation of vascular function and administration of walking tests.
Muscle Stretching Protocol.
To passively stretch each patient’s calf muscle, dorsiflexion splints (Healwell Plantar Fasciitis Night Splint®, FLA Orthopedics, Charlotte, NC, USA) (Figure 2) were applied. Using the splint, ankle joints were kept at 15° of dorsiflexion for 30 min/day, 5 days/week, for the 4 weeks. The splint has a strap to adjust the angle of the joint. Participants learned how to wear the splint correctly from a trained physical therapist who initially fitted and adjusted the splint. Splints were sent home with the patient and a physical therapist was available for questions by phone. Using the splint, both the gastrocnemius and soleus muscles were effectively stretched. If participants complained of pain during muscle stretching, the physical therapist assisted in adjusting the straps, but efforts were made to maintain the angle of dorsiflexion.
Figure 2. Calf muscle stretching.

Ankle dorsiflexion splint (Healwell Plantar Fasciitis Night Splint®, FLA Orthopedics, NC, USA) was used to induce static stretch the calf muscles of patients with PAD. Muscle stretching was performed for 30 min/day, 5 days/week, for 4 weeks at home.
Flow-mediated Dilation (FMD) of the Popliteal Artery.
Vascular functional testing was performed in a temperature controlled (~72°F), quiet and dark laboratory. To exclude the effects of acute exercise or medicine on vascular endothelial function, participants were asked to refrain from taking sildenafil, tadalafil and vardenafil for 48 hours prior to the vascular assessment, and not to take any beta blocker or calcium channel blocker medication for at least 24 hours prior to the assessment. Participants were also asked to refrain from any form of exercise and not to eat or drink for 3 hours prior to the assessment.
Systolic and diastolic blood pressure was measured using an automatic brachial sphygmomanometer applied to the upper arm after at least 10 minutes of quiet rest with the patient seated in the vascular laboratory. Pulse rate was recorded. Participants then assumed a prone position on a bed and a blood pressure cuff applied to the lower thigh of the most symptomatic lower extremity. Using vascular ultrasound and Doppler (GE Vivid Q ultrasound with 12L-RS Linear Vascular Probe, GE, Milwaukee, WI, USA), flow-mediated dilation of the popliteal artery was measured in the following manner. The measurement was conducted on the affected leg (leg with greater symptoms or lower ABI). First, popliteal artery diameter and blood flow velocity were recorded after resting for 10 min. A lower thigh blood pressure cuff was then inflated up to 250 mmHg for 5 minutes. During the 5 minutes of arterial occlusion, participants were asked to remain as still as possible. The blood pressure cuff was then quickly deflated, and popliteal artery diameter and blood flow re-measured in the same location for another 10 minutes. FMD was obtained by referencing the peak popliteal artery diameter to the baseline measurement.
Endothelium-independent dilation of the popliteal artery.
At least 30 minutes after the assessment of FMD, nitroglycerin-induced dilation of the popliteal artery was assessed as a measure of endothelium-independent dilation. Baseline popliteal artery diameter was recorded after resting for 10 minutes. The recording was done continuously for 10 minutes after the administration of 400 µg of sublingual nitroglycerin (NTG, Wilshire, Atlanta, Georgia). The peak diameter relative to baseline served as the endothelium-independent dilation.
Hemodynamic analyses.
Vessel diameter and blood flow velocity were recorded at 30 Hz of frequency, and converted into DICOM (DICOM, Rosslyn, VA, USA) format to perform further analysis. Since peripheral arterial diameter and blood flow velocity vary during the cardiac cycle,15 popliteal artery diameter was measured at the end of the diastolic phase (defined as the point just before the popliteal arterial Doppler waveform rises). Blood flow velocity was measured at the peak of the systolic phase. Using two-dimensional B mode images, end-diastolic popliteal artery diameter and peak velocity were obtained from three consecutive cardiac cycles, averaged and the same technique used to measure arterial diameter for FMD and NID. FMD was calculated as follows.
Because the magnitude of a given stimulus on popliteal artery created with reactive hyperemia may be widely different between participants,16 the FMD was normalized to shear rate by using following equations.
Six-minute Walk Test (6MWT)
The Six-minute walk test (6MWT) was performed after vascular function testing according to the American Thoracic Society guidelines.17 Using a straight 30m corridor, participants were asked to walk back and forth over 6 minutes. Participants were asked to report any symptoms, including leg pain or cramping, tiredness and/or chest discomfort. Participants were allowed to rest if they felt it necessary and then asked to resume walking as soon as able. Symptom-free walking distance was measured as the distance covered without symptoms. Continuous walking distance was measured as the distance covered without stopping. Total 6-minute walking distance was measured as the distance walked for 6 minutes. Immediately after the 6-minute walk, participants were seated and again asked to report any chest and lower-extremity symptoms. Chest and lower-extremity exertional symptoms were assessed using a modified Borg scale.18 The modified Borg scale is scored from zero (no symptoms of exertion) to 10 (maximal sensation of symptoms). Participants answered the chest and lower-extremity symptoms by indicating a number from zero to 10. Systolic and diastolic blood pressure and heart rate were then measured at approximately 1-min after 6-min walk termination.
Sample size calculation.
The primary endpoint for which the sample size was derived was popliteal artery FMD. From published data,19 we estimated that the FMD of popliteal artery would improve by approximately 40% with muscle stretching. In a pilot study, we observed a baseline FMD of 3.87% (SD, 0.62%) in 13 patients with stable PAD. Assuming an alpha of 0.05, and a cross-over design we predicted that we would need at least 12 patients per group to have 90% power to detect a 20% increase in FMD. Allowing for an approximately 20% drop out rate (3 patients), the targeted sample size was adjusted to 15 in this study. However, recruitment was to continue until at least 12 patients were enrolled and completed the protocol.
Statistical analysis.
Baseline clinical characteristics are reported as means and standard deviations. A histogram of vascular function and walking distance was made to assess their distribution. If non-normal distribution was suggested by the histogram, a Kolmogorov-Smirnov test was performed to assess the distribution of obtained data. On the basis of the distribution test, paired t-test (normal distribution) or Wilcoxon signed-rank test (non-normal distribution) were used to compare between those data obtained after 4 weeks of stretching and after 4 weeks of no stretching with a 2-sided level of significance of 0.05. In seven patients who completed 4 weeks of passive stretching followed by 4 weeks of no stretching, one-way repeated measures analysis of variance was used to compare FMD and 6MWT results at baseline (pre-stretching), post-stretching, and washout (4 weeks of no stretching) time points. Data were presented as mean ± standard error.
Results
Fifteen participants (60–85 years old, 6 female) provided written informed consent, and were enrolled in this study. All patients found the splint tolerable and adhered to the splinting regimen. During the study period, two participants dropped out; one due to coronary artery bypass surgery and the other due to disinterest. Therefore, statistical analysis was performed with the data obtained from the 13 participants who completed the study.
Baseline Patient characteristics.
Table 1 shows the baseline characteristics of the patients. The mean age was 71 ± 2 years and all patients but one were of Caucasian race. Atherosclerotic risk factors were prevalent, including diabetes mellitus (31%), hypertension (85%), dyslipidemia (92%), current or former smoking (38%) and obesity (62%). Established coronary artery disease was present in 54% of patients and 31% had a history of prior stroke. Statin and aspirin use were high (85% and 92%, respectively) and the mean baseline ABI was 0.73 ± 0.07. All patients were either Rutherford Class IIa or IIb. The majority had at least stenosis of the superficial femoral artery and most had had either prior percutaneous or surgical revascularization. There was no significant difference in the baseline characteristics between patients randomized to no-stretching first and stretching-first groups.
Table 1.
Patient Characteristics (Baseline)
| Age (years old) | 71 ± 2 |
| Race: White/Black/Other | 12/1/0 |
| Male/Female | 7/6 |
| No-stretching/Stretching first group | 7/6 |
| Height (m) | 1.68 ± 0.03 |
| Body weight (kg) | 92 ± 9.5 |
| Body mass index (kg/m2) | 33 ± 3.3 |
| Risk factors n (%) | |
| Diabetes | 4 (31) |
| Hypertension | 11 (85) |
| Dyslipidemia | 12 (92) |
| Obesity | 8 (62) |
| Current or former smoker | 5 (38) |
| Comorbid cardiovascular disease n (%) | |
| Coronary artery disease | 7 (54) |
| Prior Stroke | 4 (31) |
| Medication use n (%) | |
| Statin | 11 (85) |
| Aspirin | 12 (92) |
| PAD status | |
| Rutherford Classification (IIa/IIb) | 7/6 |
| Ankle-brachial index | 0.73 ± 0.07 |
| PAD location n (%)* | |
| Iliac artery | 1 (7.6) |
| Common femoral artery | 1 (7.6) |
| Superficial femoral artery | 9 (69) |
| Popliteal artery | 2 (15) |
| Infrapopliteal vessels | 5 (38) |
| Prior PTA | 8 (62) |
| Prior surgical revascularization | 2 (15) |
Abbreviations: PTA: percutaneous transluminal angioplasty
percentages are not mutually exclusive.
Hemodynamic responses to NTG and exercise.
Systolic, diastolic and mean blood pressure and pulse rate measured at rest, 10-min after NTG sublingual administration and after 6-min walk, were similar between stretching and no-stretching (Table 2). Systolic blood pressure measured at 10-min after NTG sublingual administration was significantly lower than at rest in both stretching and no-stretching (P<0.05, respectively).
Table 2.
Hemodynamic responses to nitroglycerin, exercise
| No stretching | Stretching | |
|---|---|---|
| Systolic BP at rest (mmHg) | 126 ± 3 | 133 ± 5 |
| Systolic BP at 10 min after NTG (mmHg) | 116 ± 3 § | 118 ± 5 § |
| Systolic BP after 6-min walk (mmHg) | 138 ± 9 | 147 ± 9 |
| Diastolic BP at rest (mmHg) | 65 ± 3 | 67 ± 2 |
| Diastolic BP at 10 min after NTG (mmHg) | 65 ± 4 | 66 ± 2 |
| Diastolic BP after 6-min walk (mmHg) | 64 ± 4 | 64 ± 2 |
| Mean BP at rest (mmHg) | 85 ± 2 | 89 ±2 |
| Mean BP at 10 min after NTG (mmHg) | 82 ± 3 | 84 ± 3 |
| Mean BP after 6-min walk (mmHg) | 89 ± 5 | 95 ± 2 |
| Pulse rate at rest (mmHg) | 60 ± 2 | 61 ± 2 |
| Pulse rate at 10 min after NTG (mmHg) | 59 ± 2 | 62 ± 2 |
| Heart rate after 6-min walk (mmHg) | 64 ± 3 | 67± 3 |
BP and pulse rate were measured at rest before vascular function test, 10 min after NTG administration, and immediately after 6 min walk. No significant difference was found in hemodynamic responses between stretching and no stretching. Systolic BP after NTG administration was significantly lower than at rest in both stretching and no stretching. BP, blood pressure; NTG, nitroglycerin
P<0.05 vs. at rest.
Data are mean ± SE.
Vascular Testing Results
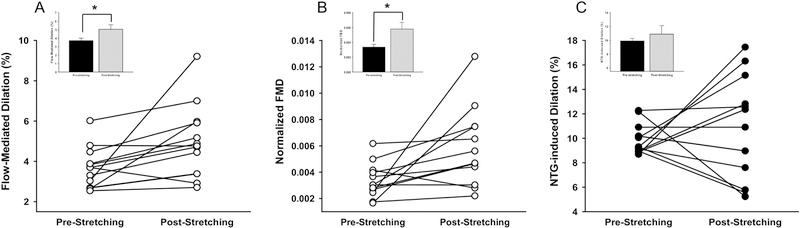
At baseline, resting popliteal arterial diameter and maximum dilation were similar between no-stretching first and stretching-first groups (Table 3). Peak shear rate, FMD and normalized FMD were also not different between no-stretching first and stretching first groups at baseline. Figure 3-A shows FMD of the popliteal artery pre- and post-stretching. The FMD of the popliteal artery was significantly higher post-stretching compared to pre-stretching (5.1 ± 0.5% vs. 3.7 ± 0.3%; P=0.005). After normalizing FMD with peak shear rate, the difference in FMD between pre-stretching and post-stretching remained (5.8×10−3 ± 3.8×10−4 vs. 3.4×10−3 ± 9.0×10−4; P=0.03, Figure 3-B). The vasodilatory response to sublingual administration of NTG is shown in Figure 3-C. There was no significant difference in NTG-induced dilation between pre- and post-stretching.
Table 3.
Vascular function and walking tests at baseline
| No-stretching first | Stretching first | |
|---|---|---|
| Vessel diameter (mm) | 5.78±0.30 | 5.61±0.32 |
| Peak vessel diameter (mm) | 5.92±0.29 | 5.91±0.35 |
| Peak shear rate (1/s) | 1108±127 | 1138±97 |
| FMD (%) | 3.65±0.36 | 3.62±0.19 |
| Normalized FMD | 0.0037±0.0007 | 0.0036±0.0009 |
| NID (%) | 9.54±0.35 | 9.75±0.47 |
| Symptom-free walking distance (m) | 178±41 | 152±28 |
| Continuous walking distance (m) | 265±31 | 263±50 |
| Total walking distance (m) | 309±27 | 311±34 |
Vascular function and walking tests were performed at baseline immediately after randomization. There was no difference in vessel characteristics or walking performance between stretching-first and no stretching-first groups. FMD, flow-mediated dilation; NID, nitroglycerin-induced dilation. Data are mean ± SE.
Figure 3. Stretching-induced vascular adaptation.
FMD (A) and FMD normalized by peak shear rate of the popliteal artery (B). Figure C shows NID of the popliteal artery. FMD and normalized FMD were significantly higher in post-stretching as compared to pre-stretching. The NID was not changed with muscle stretching. *P<0.05 post-stretching vs. pre-stretching; FMD, flow-mediated dilation; NID, nitroglycerin-induced dilation; n=13, Inset data are shown as mean ± SE.
Results of 6MWT.
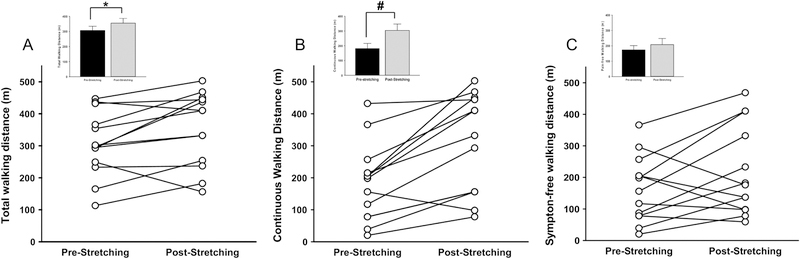
Symptom-free, continuous and total walking distance are shown in Figure 4. Both total walking distance (355.0 ± 32.1m vs. 306.3 ± 28.3m; P=0.023) and continuous walking distance (355.0 ± 32.1m vs. 306.3 ± 28.3m; P=0.0006) were significantly higher after the period of stretching compared to no stretching (Figure 4-A and 4-B, respectively); however, symptom-free walking distance was not significantly altered by muscle stretching (P=0.30; Fig 4-C).
Figure 4. 6MWT.
Six-minute walk test (6MWT) was performed to measure total walking distance (A), continuous walking distance (B), and symptom-free walking distance (C). Continuous and total walking distance were significantly higher post-stretching as compared to pre-stretching. *P<0.05 post-stretching vs. pre-stretching; #P<0.01 post-stretching vs. pre-stretching; n=13, Inset data are shown as mean ± SE.
Relationship between endothelial and walking function.
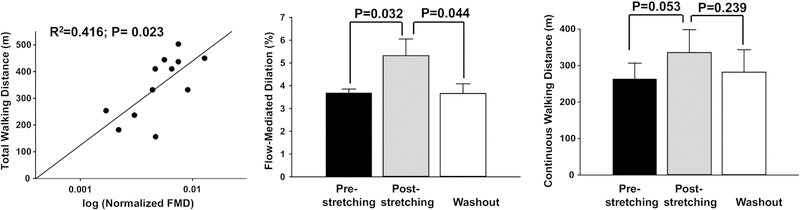
Figure 5-A shows the coupling between endothelial function and walking function in all patients after 4 weeks of muscle stretching. Post-stretching continuous walking distance was positively correlated with post-stretching normalized FMD (R2 = 0.416; P = 0.023). In seven patients that stretched in the first 4 weeks, followed by 4 weeks without stretching, a washout effect was also assessed. The muscle stretching-induced improvement in FMD in these seven patients (5.3 ± 0.7% vs. 3.7 ± 0.2%; P=0.032, post-stretching vs. pre-stretching) was not maintained 4 weeks after cessation of muscle stretching (3.7 ± 0.4% vs. 5.3 ± 0.7%; P=0.044 washout vs. post-stretching). In contrast, the muscle stretching-induced improvement in continuous walking distance in the same seven patients (335.7 ± 62.7m vs. 263.4 ± 43.4m; P=0.053, post-stretching vs. pre-stretching) was maintained 4 weeks after cessation of stretching (282.1 ± 61.4m vs. 335.7 ± 62.7m; P=0.239 washout vs. post-stretching).
Figure 5. Relationship between endothelial function and muscle function post-stretching and effects of washout.
Post-stretching, total walking distance was significantly and positively correlated with normalized FMD (A; R2=0.42, P=0.023); n=13. In patients who stretched first (n=7), post-stretching FMD (B) and post-stretching continuous walking distance (C) were significantly increased compared to pre-stretching values. After 4 weeks of washout (cessation of muscle stretching), FMD (B) was reduced significantly as compared to post-stretching (P=0.044) and was not different from pre-stretching (P=0.97; washout vs. pre-stretching). After washout (cessation of muscle stretching), continuous walking distance (C) was not different compared to post-stretching (P=0.239) and also no different from pre-stretching (P=0.49; washout vs. pre-stretching).
Discussion
This study is the first to our knowledge to comprehensively evaluate the physiologic and clinical effects of passive calf muscle stretching on patients with established stable symptomatic PAD. We report the following findings: (1) regular passive calf muscle stretching can be accomplished using a simple patient-administered dorsiflexion brace, (2) passive stretching is associated with significant improvements in endothelium-dependent vasodilatation (FMD) and continuous and total walking distance on the 6MWT, and (3) passive stretching does not affect endothelium-independent vasodilation.
Although the physiologic mechanisms underlying the functional limitation of PAD patients are not fully understood, alterations in skeletal muscle blood flow and insufficient oxygen supply to active muscle appear to be key determinants.20 Skeletal muscle blood flow is regulated by the balance between vasodilation and vasoconstriction of resistance arteries through the interaction of smooth muscle cells, endothelial cells and neuroendocrine signaling.21 Impaired endothelium-dependent vasodilation has been shown to be present in patients with lower extremity PAD compared to those without PAD.22 Although it is unknown if endothelial dysfunction is the primary contributor to the functional limitation seen in PAD, exercise training has been shown to enhance FMD and mobility in patients with PAD, suggesting a major role for endothelial function.23, 24 We hypothesize that improved endothelium-dependent vasodilation (FMD), with a related increase in calf muscle blood flow during exercise, is the primary mechanism through which continuous and total walking distance improved in the passive stretching group. The positive correlation between post-stretching FMD and post-stretching total 6-min walking distance supports this notion.
Our previous study showed that 4 weeks of passive muscle stretching in old sedentary rats enhanced endothelial function of soleus muscle arterioles, increased capillarity, and increased skeletal muscle blood flow during treadmill exercise13. In healthy young humans, passive leg movement increases muscle blood flow and augments endothelial nitric oxide synthase mRNA and endothelial proliferation in muscle, independent of changes in metabolism or central hemodynamics.25, 26 Data obtained in our study of old rats indicates that the muscles which are stretched during ankle dorsiflexion, including the soleus muscle, experience relative ischemia. Histological assessment of levels of HIF-1α and VEGF-A in the soleus muscle after 4 weeks of stretching via ankle dorsiflexion indicated increased levels of both of these angiogenic factors that are known to be induced by ischemia13. Further studies will be needed to identify metabolic and microvascular mechanisms that contribute to the improved walking function that occurs with muscle stretching and to determine whether a dose-response relationship exists between muscle stretching and improvement of endothelial function.
Our regression analysis in all patients indicated a significant correlation between post-stretching endothelium-dependent dilation and post-stretching walking function, suggesting coupling between stretching-induced improvement of endothelial function and muscle function. Interestingly, in the seven patients that underwent 4 weeks of muscle stretching, followed by 4 weeks without muscle stretching, our evaluation of FMD and administration of the 6MWT after the washout period indicated that the improvement in muscle function was sustained, whereas the improvement in endothelial function was not. Four weeks after cessation of muscle stretching, endothelial function was not different than pre-stretching values (3.7 ± 0.4% vs. 3.7 ± 0.2%; P=0.97, washout vs. pre-stretching). These results suggest that the improvement of endothelial function may contribute to improvements in blood flow and muscle function, but once the muscle function increases, the improved walking ability is sustained, even without a continuous increase in endothelial function. It should be recognized that we evaluated the washout period in only 7 patients, and there was a trend toward a decline in muscle function at the end of 4 weeks (washout continuous walking distance was not significantly different from pre-stretching continuous walking distance in these patients (P=0.49). This suggests that with a longer washout period, muscle function could continue to decline. A study performed in more patients with measurements made at shorter time intervals during the stretching intervention, and a longer washout period will need to be studied to determine whether there is a time-dependent coupling of endothelial function and muscle function, i.e., whether improvement and decline of endothelial function precedes improvement and decline in muscle function.
We observed a mean difference of 44m in total walking distance between the stretching and no stretching groups. This degree of change represents a clinically meaningful change for elderly patients with PAD.27 Treadmill testing has also been used in clinical trials to assess changes in functional status in PAD patients;28, 29 however, the 6MWT has been shown to more closely correlate with physical activity levels in the community than treadmill testing.30 Furthermore, treadmill testing is associated with test-related learning phenomenon in people with PAD, whereas the 6MWT is not.11, 31 Changes in 6MWT have been linked to clinically meaningful outcomes such as mortality and loss of mobility in patients with PAD.32 Therefore, the 6MWT has emerged as the preferred functional assessment for PAD patients.33, 34. Further studies will be required to determine if the treatment effect observed remains robust across other measures of functional status in PAD patients.
Limitations
There are several limitations of this study. This was a small study involving 13 patients but with a cross-over design which enhanced our ability to evaluate treatments effects. Although the vascular technician performing the vascular duplex studies was blinded to treatment allocation, the clinician performing the 6MWT was not. There were strict efforts to not influence the patient during the 6MWT; however, the potential for observer bias remains. The patient was also not blinded as we did not perform a sham stretching procedure such as applying a brace to keep the ankle joint at a neutral position; therefore, a placebo effect is also possible. However, a placebo effect would not explain the improvements in FMD or its correlation with improvements in 6MWT. We did not evaluate metabolic changes in skeletal muscle of our PAD patients. Oxygen supply and utilization can be assessed non-invasively in the future using near-infrared spectroscopy.35 Furthermore, we cannot exclude a change in muscle strength or function as the cause of the improvement in walking distance. It should be noted that our purpose was to test the hypothesis that muscular stretching leads to improved endothelial and muscle function in a patient population in which these outcomes are known to be impaired by vascular disease. Finally, although all patients were encouraged to walk at least 5 days per week for at least 10–15 minutes at a time, no patients were engaged in a formal ongoing supervised exercise program. Given these limitations, our results should only be interpreted as hypothesis generating and not conclusive. However, they do provide ample impetus for a larger prospective randomized controlled trial in the future.
Conclusion
We performed a randomized, crossover pilot study to determine whether passive stretching of the calf muscles improves lower extremity endothelial function and walking performance in elderly patients with stable symptomatic PAD. Passive calf muscle stretching was found to enhance vascular endothelial function and improve walking function. If confirmed by randomized clinical trials, these results might represent a novel safe and simple therapeutic intervention for symptomatic PAD patients.
Acknowledgements:
This work was supported by funds from Florida State University (to Judy Muller-Delp), from a grant the 27th Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad (to Kazuki Hotta), and a grant from National Institutes of Health Grants R21 AG-044858 (Judy M. Muller-Delp).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR and Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med 2007;32:328–33. [DOI] [PubMed] [Google Scholar]
- 2.Lindgren H, Qvarfordt P, Akesson M, Bergman S, Gottsater A and Swedish Endovascular Claudication Stenting T. Primary Stenting of the Superficial Femoral Artery in Intermittent Claudication Improves Health Related Quality of Life, ABI and Walking Distance: 12 Month Results of a Controlled Randomised Multicentre Trial. Eur J Vasc Endovasc Surg 2017;53:686–694. [DOI] [PubMed] [Google Scholar]
- 3.Wu AZ, Coresh J, Selvin E, Tanaka H, Heiss G, Hirsch AT, Jaar BG and Matsushita K. Lower Extremity Peripheral Artery Disease and Quality of Life Among Older Individuals in the Community. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long J, Modrall JG, Parker BJ, Swann A, Welborn MB and Anthony T. Correlation between ankle-brachial index, symptoms, and health-related quality of life in patients with peripheral vascular disease. Journal of Vascular Surgery 2004;39:723–727. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D and Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001;286:1599–606. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM and Clark E. The ankle brachial index is associated with leg function and physical activity: The walking and leg circulation study. Ann Intern Med 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y and Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol 2007;50:974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ and Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 2004;292:453–61. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW and Shen WK. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:1425–43. [DOI] [PubMed] [Google Scholar]
- 10.Gardner AW, Parker DE, Montgomery PS, Scott KJ and Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR and Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc 2001;49:755–62. [DOI] [PubMed] [Google Scholar]
- 12.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG and Group TIW. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45 Suppl S:S5–67. [DOI] [PubMed] [Google Scholar]
- 13.Hotta K, Behnke BJ, Arjmandi B, Ghosh P, Chen B, Brooks R, Maraj JJ, Elam ML, Maher P, Kurien D, Churchill A, Sepulveda JL, Kabolowsky MB, Christou DD and Muller-Delp JM. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J Physiol 2018;596:1903–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D, American Heart Association Council on Peripheral Vascular D, Council on E, Prevention, Council on Clinical C, Council on Cardiovascular N, Council on Cardiovascular R, Intervention, Council on Cardiovascular S and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 15.Chen HC, Patel V, Wiek J, Rassam SM and Kohner EM. Vessel diameter changes during the cardiac cycle. Eye (Lond) 1994;8 (Pt 1):97–103. [DOI] [PubMed] [Google Scholar]
- 16.Pyke KE and Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol-London 2005;568:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RC and Jones PW. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin Sci (Lond) 1989;76:277–82. [DOI] [PubMed] [Google Scholar]
- 19.Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ and MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 2008;295:R236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR and Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol 2011;111:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behringer EJ and Segal SS. Spreading the signal for vasodilatation: implications for skeletal muscle blood flow control and the effects of ageing. J Physiol 2012;590:6277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwamoto A, Kajikawa M, Maruhashi T, Iwamoto Y, Oda N, Kishimoto S, Matsui S, Kihara Y, Chayama K, Goto C, Noma K, Aibara Y, Nakashima A and Higashi Y. Vascular Function and Intima-media Thickness of a Leg Artery in Peripheral Artery Disease: A Comparison of Buerger Disease and Atherosclerotic Peripheral Artery Disease. J Atheroscler Thromb 2016;23:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Liu K, Spring B, Tian L, Domanchuk K, Kibbe M, Zhao L, Lloyd Jones D, Liao Y, Gao Y and Rejeski WJ. Unsupervised exercise and mobility loss in peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brendle DC, Joseph LJO, Corretti MC, Gardner AW and Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. American Journal of Cardiology 2001;87:324–329. [DOI] [PubMed] [Google Scholar]
- 25.Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P and Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2008;294:R975–82. [DOI] [PubMed] [Google Scholar]
- 26.Hoier B, Rufener N, Bojsen-Moller J, Bangsbo J and Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol 2010;588:3833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera S, Mody SH, Woodman RC and Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 28.Jaff MR, Dale RA, Creager MA, Lipicky RJ, Constant J, Campbell LA and Hiatt WR. Anti-chlamydial antibiotic therapy for symptom improvement in peripheral artery disease: prospective evaluation of rifalazil effect on vascular symptoms of intermittent claudication and other endpoints in Chlamydia pneumoniae seropositive patients (PROVIDENCE-1). Circulation 2009;119:452–8. [DOI] [PubMed] [Google Scholar]
- 29.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT and Investigators CS. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott MM, Ades PA, Dyer A, Guralnik JM, Kibbe M and Criqui MH. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 1237 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery PS and Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11. [DOI] [PubMed] [Google Scholar]
- 32.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y and Criqui MH. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol 2011;57:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR and Ferrucci L. Six-Minute Walk Is a Better Outcome Measure Than Treadmill Walking Tests in Therapeutic Trials of Patients With Peripheral Artery Disease. Circulation 2014;130:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solway S, Brooks D, Lacasse Y and Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest 2001;119:256–70. [DOI] [PubMed] [Google Scholar]
- 35.Luck JC, Miller AJ, Aziz F, Radtka JF 3rd, Proctor DN, Leuenberger UA, Sinoway LI and Muller MD. Blood pressure and calf muscle oxygen extraction during plantar flexion exercise in peripheral artery disease. J Appl Physiol (1985) 2017;123:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]