Abstract
Aims:
We examined the longitudinal association between change in body composition directly measured by computed tomography (CT) and future insulin sensitivity.
Methods:
This was a prospective study with 10 years of follow-up with 297 Japanese-American without diabetes. Intra-abdominal fat area (IAFA) and abdominal subcutaneous fat area (SCFA), and thigh SCFA were measured by CT. Insulin sensitivity was calculated by HOMA-IR and the Matsuda index.
Results:
Baseline and change in IAFA were significantly and independently associated with change in HOMA-IR and Matsuda index during follow-up. In multivariate analysis, IAFA and 10-year change in IAFA (Δ IAFA) was significantly and positively associated with 10-year HOMA-IR (p <0.001) and significantly and negatively associated with 10-year Matsuda index (p <0.001). The association with Matsuda index though was non-linear and best modeled as a quadratic function (Δ IAFA + Δ IAFA2). No significant associations in multivariate analyses were seen between thigh SCFA and insulin sensitivity or abdominal SCFA and HOMA-IR but an increase in abdominal SCFA was associated with diminished insulin sensitivity measured by the Matsuda index.
Conclusions:
An increase in visceral adiposity predicts diminished insulin sensitivity over 10 years of follow-up independent of the size of this adipose depot at baseline.
Keywords: Visceral fat, Insulin sensitivity, HOMA-IR, Matsuda Index
Introduction
Insulin resistance plays a key role in the development of multiple conditions associated with higher risk of cardiovascular disease [1]. It is defined as reduced action of insulin on glucose and lipid metabolism in adipose tissue, skeletal muscle and liver [2–4]. Insulin resistance occurs more frequently in the presence of excess adiposity, is closely linked to metabolic syndrome features including dyslipidemia, hypertension, and type 2 diabetes, and is associated with multiple serious medical conditions including atherosclerosis, cancer, and sleep apnea [4–7].
Although insulin resistance is seen more commonly in obesity, it also occurs in normal weight or overweight, and does not always accompany obesity [8,9]. These inconsistencies may be explained in part by the recognition that body fat distribution is an important determinant of insulin sensitivity, with intra-abdominal fat playing the most important role [10,11]. Ross et al. reported a cross-sectional association between IAF area (cm2) and mass (kg) and reduced insulin sensitivity in obese men and premenopausal women [12,13]. Baneiji et al. reported that greater IAF volume measured by computed tomography (CT) was independently associated with insulin sensitivity measured using euglycemic-hyperinsulinemic clamps in a cross-sectional study [14,15]. We have reported both cross-sectional and longitudinal associations between CT-measured IAF area and insulin sensitivity in Japanese Americans, with greater visceral fat area associated with lower concurrent and future insulin sensitivity [16,17]. We have further shown that accumulation of IAF over 5 years was related to higher risk of the development of type 2 diabetes [18].
It remains less clear in clinical studies whether change in IAF directly results in long-term changes in insulin sensitivity. Goto et al. reported in a lifestyle intervention study that over 1-year, a reduction in visceral fat was associated with an improvement in insulin sensitivity in an obese middle-aged population [19]. Goodpaster et al. reported that the percent change in visceral adiposity related most clearly to improve insulin sensitivity in obese sedentary subjects participating in a caloric-restriction weight loss intervention with measurement of IAF by CT pre- and post-intervention separated by four months [19,20]. However, no epidemiological research exists on the long-term association between change in body composition measured by CT and future insulin sensitivity. We therefore examined this association in a community based longitudinal observational study of Japanese Americans followed over 10 years.
Research design and methods
Study population
The study population consisted of men and women enrolled in the Japanese-American Community Diabetes Study, a cohort of second- (Nisei) and third-generation (Sansei) Japanese Americans of 100% Japanese ancestry. A detailed description of the selection and recruitment of the study subjects has been published previously [22]. In brief, participants in this study were chosen from healthy volunteers through community-wide recruitment and were representative of Japanese-American residents of King County, Washington, USA, in age composition, residential distribution, and parental immigration pattern from 1983 to 1988. Subjects with infectious diseases, autoimmune or malignant disorders were not included in this community-cohort. Among the total of 658 subjects in the original cohort, we excluded subjects who had a fasting plasma glucose ≥ 7.0 mmol/L, 2-h plasma glucose after 75-g OGTT ≥ 11.1 mmol/L, or were taking oral hypoglycemic medications or insulin at baseline, 5 year, or 10 year follow-up. There remained 310 participants after these exclusions, of whom 13 had missing data on key covariates, leaving 297 for this analysis who were evaluated by the study protocol at baseline, 5 year and 10 year follow-up (Figure 1). The study received approval from the University of Washington Human Subjects Division and all subjects provided written informed consent (Institutional Review Board number: 35082).
Figure 1.
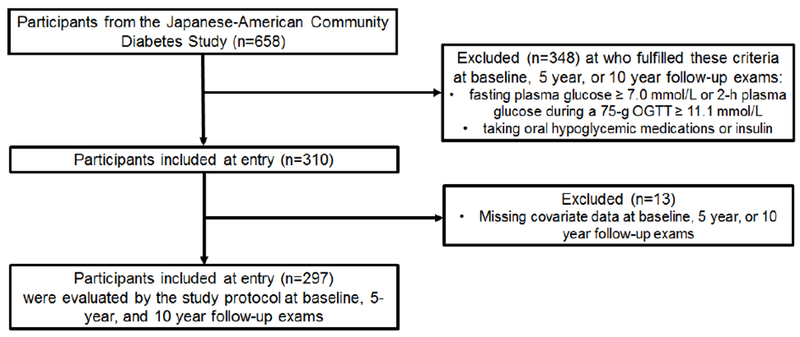
Flow diagram of subjects included and excluded in the analysis.
Clinical and laboratory examination
All evaluations were performed at the General Clinical Research Center, University of Washington Medical Center. At baseline, a complete physical examination was performed, and personal medical history obtained. Family history of diabetes was considered positive if any first-degree relative had diabetes. Biochemical measurements were performed as reported previously [23]. All blood samples were obtained following an overnight fast of at least 10 hours. Plasma glucose was measured by the hexokinase method using an autoanalyzer (University of Washington, Department of Laboratory Medicine, Seattle, WA). Plasma insulin was measured by radioimmunoassay (Immunoassay Core, Diabetes Research Center, University of Washington, Seattle, WA). BMI was computed as weight in kilograms divided by height in meters squared (kg/m2). HOMA-IR and Matsuda index were used as approximations of insulin sensitivity at baseline, 5 years, and 10 years in the study. HOMA-IR was calculated from fasting plasma glucose and fasting plasma insulin [24], and Matsuda index was calculated from the OGTT [25]. HOMA-IR was calculated as [fasting glucose (mmol/l) × fasting insulin (μU/ml)] / 22.5 [24]. The Matsuda index was calculated as {10,000/square root of [fasting glucose (mg/dl) × fasting insulin (μU/ml) × [((fasting glucose × 15 + glucose 30 minute×30 + glucose at 60 minnute×45 + glucose at 120 minute×30))/120 × ((fasting insulin× 15 + insulin at 30 minute×30 + insulin at 60 minute×45 + insulin at 120 minute×30))/120 during OGTT]} [25]. A modified Matsuda index was calculated based on the 0, 30, 60, and 120 min values [26]. Single 10-mm slice CT scans were performed at the level of the umbilicus to measure the abdominal subcutaneous fat area (SCFA) and intraabdominal fat area (IAFA) and at the midway between the greater trochanter and superior margin of the patella to measure thigh SCFA. CT scans were analyzed using density contour software (Standard GE 8800 computer software). Attenuation range for identification of fat was −250 to −50 Hounsfield Units. The following cross-sectional areas (cm2) were measured at baseline and 10-year follow-up: visceral fat area (within the confines of the transversalis fascia), left total thigh area, and left thigh subcutaneous fat area. Changes in IAFA, abdominal SCFA and thigh SCFA were calculated by subtracting baseline fat area from fat area at the 10-year follow-up. All CT area measurements were performed by one investigator. The intra-observer variability for multiple measurements by a single observer of a single CT scan ranged from 0.2 to 1.4%.
Statistical Analysis.
Continuous variables are expressed as means ± standard deviation (SD), and categorical variables as numbers and percentages. We used univariate linear regression analysis to estimate coefficients between HOMA-IR or Matsuda index and participant characteristics at baseline as well as change in body composition during follow-up. Multivariable linear regression analysis was used to adjust for covariates. All multivariable models included both the baseline measures of insulin sensitivity (either HOMA-IR or Matsuda index) and the baseline measure of the fat depot of interest. Analysis of residuals was performed to examine model fit and adherence to regression assumption. The dependent insulin sensitivity variables were logarithmically transformed due to non-normality and the robust variance estimator was used to obtain unbiased standard errors for statistical testing in the presence of heteroskedasticity. To assess nonlinearity, quadratic transformations of change in fat depot were inserted into models predicting insulin sensitivity. Assessment for multicollinearity using the variance inflation factor (VIF) was performed with VIFs exceeding 10 considered evidence for presence of multicollinearity [27]. Interaction between sex and IAFA in relation to change in insulin sensitivity was examined using interaction analysis. Analyses were performed using Stata/MP, version 15.1 (Stata Corp., College Station, Texas, USA). Two-sided P -values of ≤0.05 were considered statistically significant.
Results
Baseline characteristics of the study subjects
A total of 297 participants met the inclusion/exclusion criteria and were included in this analysis. Baseline characteristics and fat depot changes of study subjects are shown in Table 1. There was a slight male predominance (52.2%) with participants on average 50 years of age and on average neither overweight nor obese (mean BMI 23.8 kg/m2). Mean HOMA-IR and Matsuda index were 2.85 and 3.9, respectively. Baseline mean IAFA, abdominal SCFA, and thigh SCFA were 72.2 cm2, 153.2 cm2, and 65.2 cm2, respectively. Over 10-years of follow-up, IAFA and abdominal SCFA increased on average, whereas 10-year thigh SCFA did not significantly change.
Table 1.
Characteristics of study subjects
| Baseline Characteristics | Mean or Percentage (Standard Deviation) |
|---|---|
| N | 297 |
| Age (years) | 49.9 (±11.6) |
| Female sex (%) | 47.8% |
| Family history | 30.4% |
| BMI (kg/m2) | 23.9 (±3.2) |
| Fasting Plasma Glucose (mmol/L) | 5.0 (±0.5) |
| Post-Load 120 min Glucose (mmol/L) | 6.9 (±1.6) |
| Fasting Insulin (pmol/L) | 88.8 (±44.5) |
| Post-Load 120 min Insulin (pmol/L) | 557.0 (±548.6) |
| Total Cholesterol (mmol/L) | 5.8 (±1.0) |
| Triglyceride (mmol/L) | 1.5 (±1.2) |
| HDL Cholesterol (mmol/L) | 1.5 (±0.4) |
| LDL Cholesterol (mmol/L) | 3.6 (±0.9) |
| Baseline HOMA-IR | 2.9 (±1.6) |
| Baseline Matsuda Index | 3.5 (±1.8) |
| Baseline intra-abdominal fat area (IAFA) (cm2) | 73.1 (±44.8) |
| 10-year IAFA change (cm2) | 16.6 (±34.5) |
| Baseline abdominal subcutaneous fat area (SCFA) (cm2) | 156.2 (±80.4) |
| 10-year abdominal SCFA change (cm2) | 27.9 (±47.3) |
| Baseline thigh SCFA (cm2) | 66.1 (±32.3) |
| 10-year thigh SCFA change (cm2) | −0.95 (±22.3) |
| Follow-up Characteristics | Mean or Percentage (Standard Deviation) |
| Fasting Plasma Glucose (mmol/L) | 5.3 (±0.5) |
| Post-Load 120 min Glucose (mmol/L) | 7.7 (±1.6) |
| Fasting Insulin (pmol/L) | 93.1 (±44.3) |
| Post-Load 120 min Insulin (pmol/L) | 507.4 (315.7) |
| Total Cholesterol (mmol/L) | 5.8 (±1.0) |
| Triglyceride (mmol/L) | 1.6 (±1.0) |
| HDL Cholesterol (mmol/L) | 1.5 (±0.4) |
| LDL Cholesterol (mmol/L) | 3.6 (±0.9) |
| 10-year HOMA-IR | 3.4 (±1.7) |
| 10-year Matsuda Index | 3.2 (±1.6) |
IAFA, intra-abdominal fat area, SCFA, subcutaneous fat area; HOMA-IR, HOMA for insulin resistance;
Correlation between baseline adiposity or body fat distribution or their changes over 10 years and insulin sensitivity (HOMA-IR, Matsuda Index) at 10 years
The univariate analysis of baseline measurements revealed that BMI, abdominal circumference, HOMA-IR, Matsuda index, IAFA, and abdominal SCFA were significantly associated with 10-year HOMA-IR and Matsuda index in univariate models (Table 2). We found that baseline IAFA was significantly and positively associated with HOMA-IR and negatively associated with Matsuda index at 10 years. Abdominal SCFA also showed a significant positive association with HOMA-IR and a negative association with Matsuda index at 10 years. On the other hand, there were no significant associations between HOMA-IR or Matsuda index at 10 years and baseline age or thigh SCFA. Next, we examined relationships between changes from baseline to 10 years in IAFA, abdominal SCFA, and thigh SCFA and 10year HOMA-IR or Matsuda index by univariate regression analysis (Table 2). Changes from baseline to 10 years in IAFA, abdominal SCFA, and thigh SCFA were not significantly associated with 10-year HOMA-IR, but 10-yr change in IAFA was significantly and negatively associated with 10-year Matsuda index. No significant associations were seen between 10-yr Matsuda index and either 10-yr change in abdominal SCFA or thigh SCFA.
Table 2.
Univariate linear regression of continuous predictors of 10-year HOMA-IR and Matsuda Index showing regression coefficients
| 10-year HOMA-IR | 10-year Matsuda Index | |||
|---|---|---|---|---|
| Coeff. | p value | Coeff. | p value | |
| Age, baseline | 0.0244 | 0.060 | −0.0184 | 0.006 |
| BMI, baseline | 0.3697 | <0.001 | −0.1851 | <0.001 |
| Abdominal circumference, baseline | 0.1526 | <0.001 | −0.0790 | <0.001 |
| HOMA-IR, baseline | 0.3722 | <0.001 | −0.3373 | <0.001 |
| Matsuda Index, baseline | −0.8413 | <0.001 | 0.5447 | <0.001 |
| IAFA, baseline | 0.0268 | <0.001 | −0.0149 | <0.001 |
| 10-yr change in IAFA | 0.0056 | 0.193 | −0.0067 | 0.004 |
| Abdominal SCFA, baseline | 0.0108 | <0.001 | −0.0065 | <0.001 |
| 10-yr change in abdominal SCFA | −0.0016 | 0.630 | −0.0021 | 0.209 |
| Thigh SCFA, baseline | −0.0049 | 0.322 | 0.0015 | 0.554 |
| 10-yr change in thigh SCFA | 0.0055 | 0.455 | −0.0035 | 0.340 |
Coeff., coefficient; IAFA, intra-abdominal fat area, SCFA, subcutaneous fat area; HOMA-IR, HOMA for insulin resistance;
Correlation coefficients between HOMA-IR or Matsuda index at 10–11 years follow-up and measures of body fat or metabolic characteristics at baseline are shown in Table 3. All measures of regional adiposity at baseline were significantly correlated with HOMA-IR and Matsuda index except thigh fat at 10-year follow-up in the expected directions with higher fat areas positively associated with HOMA-IR and negatively with Matsuda index.
Table 3.
Correlation coefficients between insulin resistance measures at the 10- to 11-year follow-up and measures of body fat or metabolic characteristics at baseline
| 10-year HOMA-IR |
10-year Matsuda Index |
|||
|---|---|---|---|---|
| Coeff. | p value | Coeff. | p value | |
| Age | −0.0229 | 0.690 | −0.1081 | 0.060 |
| Metabolic variables at baseline | ||||
| Fasting plasma glucose | 0.3018 | <0.001 | −0.3374 | <0.001 |
| Fasting plasma insulin | 0.5232 | <0.001 | −0.4193 | <0.001 |
| HOMA-IR | 0.5761 | <0.001 | −0.4621 | <0.001 |
| Matsuda Index | −0.4303 | <0.001 | 0.5413 | <0.001 |
| 2-h glucose | 0.0178 | 0.757 | −0.0834 | 0.147 |
| Adipose tissue variables at baseline | ||||
| Intra-Abdominal Fat Area | 0.3543 | <0.001 | −0.4086 | <0.001 |
| Abdomen Subcutaneous Fat Area | 0.3237 | <0.001 | −0.2913 | <0.001 |
| Thigh fat | 0.0546 | 0.344 | −0.0009 | 0.987 |
| Waist circumference | 0.3971 | <0.001 | −0.3727 | <0.001 |
| BMI | 0.4130 | <0.001 | −0.3212 | <0.001 |
Coeff., coefficient; IAFA, intra-abdominal fat area, SCFA, subcutaneous fat area; HOMA-IR, HOMA for insulin resistance;
Association of fat areas and their changes over 10 years with insulin sensitivity (HOMA-IR, Matsuda Index) at 10 years, adjusting for covariates
We conducted multivariable analyses to assess the associations between 10-year change in IAFA, abdominal SCFA, and thigh SCFA and 10-year insulin sensitivity while adjusting for covariates (Table 4). Age did not show any significant correlations with HOMA-IR or Matsuda Index in all models. In Model 1, baseline IAFA, 10-year change in IAFA and each baseline insulin sensitivity index were significantly associated with each 10-year insulin sensitivity index adjusted for age, gender, family history of diabetes, and BMI (Table 4). This relationship remained significant when further adjusted for baseline and 10-year change in abdominal SCFA, and baseline and 10-year change in thigh SCFA (Table 4, Models 2 and 3). In Model 2 (Table 4), 10-year change in abdominal SCFA was also significantly and negatively associated with 10-year Matsuda index.
Table 4.
Multiple linear regression analysis of the associations between change in fat depots and HOMA-IR and Matsuda Index at 10- to 11-year follow-up, adjusted for covariates including BMI
| Independent variables from baseline in the model | Dependent Variable | |||
|---|---|---|---|---|
| 10-year HOMA-IR | 10-year Matsuda Index | |||
| β | p-value | β | p-value | |
| Model 1 | ||||
| Age | −0.002667 | 0.247 | 0.000808 | 0.684 |
| Female | 0.017029 | 0.762 | 0.002308 | 0.962 |
| Positive family history of diabetes | −0.018039 | 0.704 | −0.011035 | 0.803 |
| IAFA, baseline | 0.003423 | <0.001 | −0.006612 | <0.001 |
| 10-year Change in IAFA | 0.003175 | <0.001 | −0.003534 | <0.001 |
| BMI, baseline | 0.013890 | 0.183 | −0.007395 | 0.399 |
| HOMA-IR, baseline | 0.118381 | <0.001 | ||
| Matsuda Index, baseline | 0.115637 | <0.001 | ||
| IAFA2 | 0.000018 | 0.007 | ||
| R-squared | 0.375900 | 0.433600 | ||
| Model 2 | ||||
| Age | −0.001997 | 0.409 | −0.000109 | 0.956 |
| Female | 0.026140 | 0.723 | 0.014707 | 0.821 |
| Positive family history of diabetes | −0.008498 | 0.857 | −0.027883 | 0.515 |
| IAFA, baseline | 0.003574 | <0.001 | −0.008288 | <0.001 |
| 10-year Change in IAFA | 0.002648 | 0.002 | −0.002272 | 0.003 |
| Abdominal SCFA, baseline | −0.000261 | 0.647 | 0.000145 | 0.766 |
| 10-year Change in Abdominal SCFA | 0.000848 | 0.180 | −0.001962 | 0.001 |
| BMI, baseline | 0.016761 | 0.233 | −0.006690 | 0.587 |
| HOMA-IR, baseline | 0.122345 | <0.001 | ||
| Matsuda Index, baseline | 0.120003 | <0.001 | ||
| IAFA2 | 0.000025 | 0.001 | ||
| R-squared | 0.381000 | 0.458600 | ||
| Model 3 | ||||
| Age | −0.003093 | 0.202 | 0.000411 | 0.396 |
| Female | 0.095811 | 0.342 | −0.088020 | 0.141 |
| Positive family history of diabetes | −0.013076 | 0.786 | −0.017572 | 0.816 |
| IAFA, baseline | 0.003296 | <0.001 | −0.002844 | <0.001 |
| 10-year Change in IAFA | 0.003160 | <0.001 | −0.003531 | <0.001 |
| Thigh SCFA, baseline | −0.001468 | 0.287 | 0.001949 | 0.072 |
| 10-year Change in thigh SCFA | −0.000047 | 0.970 | −0.000423 | 0.534 |
| BMI, baseline | 0.023770 | 0.073 | −0.020959 | 0.055 |
| HOMA-IR, baseline | 0.116287 | <0.001 | ||
| Matsuda Index, baseline | 0.115350 | <0.001 | ||
| IAFA2 | 0.000021 | 0.003 | ||
| R-squared | 0.37740 | 0.44410 | ||
IAFA, intra-abdominal fat area, SCFA, subcutaneous fat area; HOMA-IR, HOMA for insulin resistance;
Models 1-3 were repeated with abdominal circumference used instead of BMI as a covariate (Table 5). Similar statistically significant associations between baseline and change in fat depots and 10-year insulin sensitivity indexes were seen as in Table 4, as well as significant associations between the baseline and 10-year insulin sensitivity measurement. Baseline IAFA was significantly associated with an increased 10-year HOMA-IR and a decreased 10-year Matsuda index. No significant association was seen between baseline abdominal SCFA or baseline thigh SCFA and 10-year HOMA-IR or Matsuda index. Change in IAFA over 10 years was significantly associated with an increased 10-year HOMA-IR and a decreased 10-year Matsuda index. However, no significant association was seen between 10-year change in abdominal SCFA or thigh SCFA and 10-year HOMA-IR. There was a significant negative association between 10-year change in abdominal SCFA and 10-year Matsuda index, but no association between 10-year thigh SCFA and 10-year HOMA-IR (Table 5, Model 5). The quadratic transformation of Δ IAFA (Δ IAFA2) when inserted into Models 1-6 was not significantly associated with 10-year HOMA-IR, but it was significantly and positively associated with Matsuda index. No evidence of multicollinearity was seen in any multivariable model (Tables 4 and 5) as all variance inflation factors were < 4.
Table 5.
Multiple linear regression analysis of the associations between change in fat depots and HOMA-IR and Matsuda Index at 10- to 11-year follow-up, adjusted for covariates including abdominal circumference
| Independent variables from baseline in the model | Dependent Variable | |||
|---|---|---|---|---|
| 10-year HOMA-IR | 10-year Matsuda Index | |||
| β | p-value | β | p-value | |
| Model 4 | ||||
| Age | −0.00332 | 0.145 | 0.00133 | 0.500 |
| Female | 0.00100 | 0.986 | 0.00895 | 0.854 |
| Positive family history of diabetes | −0.01636 | 0.734 | −0.01320 | 0.767 |
| IAFA, baseline | 0.00373 | <0.001 | −0.00704 | <0.001 |
| 10-year Change in IAFA | 0.00319 | <0.001 | −0.00356 | <0.001 |
| Abdominal circumference, baseline | 0.00183 | 0.661 | −0.00039 | 0.915 |
| HOMA-IR, baseline | 0.01958 | <0.001 | ||
| Matsuda Index, baseline | 0.11670 | <0.001 | ||
| IAFA2 | 0.00002 | 0.006 | ||
| R-squared | 0.374 | 0.43300 | ||
| Model 5 | ||||
| Age | −0.00260 | 0.275 | 0.00015 | 0.940 |
| Female | −0.01172 | 0.874 | 0.04605 | 0.472 |
| Positive family history of diabetes | −0.00811 | 0.866 | −0.02972 | 0.491 |
| IAFA, baseline | 0.00380 | <0.001 | −0.00850 | <0.001 |
| 10-year Change in IAFA | 0.00259 | 0.002 | −0.00229 | 0.003 |
| Abdominal SCFA, baseline | 0.00001 | 0.987 | −0.00018 | 0.763 |
| 10-year Change in Abdominal SCFA | 0.00095 | 0.131 | −0.00202 | 0.001 |
| Abdominal circumference, baseline | 0.00162 | 0.800 | 0.00187 | 0.745 |
| HOMA-IR, baseline | 0.12616 | <0.001 | ||
| Matsuda Index, baseline | 0.12018 | <0.001 | ||
| IAFA2 | 0.00003 | 0.001 | ||
| R-squared | 0.379 | 0.45880 | ||
| Model 6 | ||||
| Age | −0.00382 | 0.122 | 0.00246 | 0.222 |
| Female | 0.03680 | 0.686 | −0.07154 | 0.322 |
| Positive family history of diabetes | −0.01459 | 0.766 | −0.00916 | 0.838 |
| IAFA, baseline | 0.00362 | <0.001 | −0.00712 | <0.001 |
| 10-year Change in IAFA | 0.00308 | <0.001 | −0.00338 | <0.001 |
| Thigh SCFA, baseline | −0.00074 | 0.559 | 0.00164 | 0.163 |
| 10-year Change in thigh SCFA | 0.00023 | 0.855 | −0.00094 | 0.446 |
| Abdominal circumference, baseline | 0.00399 | 0.418 | −0.00412 | 0.326 |
| HOMA-IR, baseline | 0.12348 | <0.001 | ||
| Matsuda Index, baseline | 0.11640 | <0.001 | ||
| IAFA2 | 0.00002 | 0.004 | ||
| R-squared | 0.373 | 0.424 | ||
IAFA, intra-abdominal fat area, SCFA, subcutaneous fat area; HOMA-IR, HOMA for insulin resistance;
Additional models assessed presence of interaction. Since IAF is known to differ by sex, we tested whether the association between IAF differed by sex through insertion of an IAF*sex interaction term into Models 1 to 6. No significant interaction was seen between IAF and sex when this term was inserted into these regression models. Also, there were no significant interactions between 10-year change in IAF and sex in all models (data not shown).
Discussion
These prospective data demonstrated that both 10-year increase in IAFA and baseline IAFA were significantly and independently associated with a decreased insulin sensitivity at 10 years as measured by either HOMA-IR or Matsuda Index. A negative association was seen between 10-year change in abdominal SCFA and Matsuda Index only, but no association was found between baseline abdominal SCFA and HOMA-IR or Matsuda Index. These results do not support an association between thigh SCFA or thigh SCFA change and future change in insulin sensitivity. These results suggest that both reducing IAFA or limiting IAFA gain over time may result in greater insulin sensitivity than otherwise would have occurred with unchecked IAFA gain.
Our previous studies have shown that greater visceral adiposity at baseline was associated with future increased insulin resistance at 10 years [17], and this research further demonstrates an independent role as well for visceral fat accumulation in the development of worsening insulin sensitivity.
Limited research has been published that reports on longitudinal associations between visceral fat accumulation and outcomes related to change in insulin sensitivity. A study of a multiethnic sample of obese adults showed increased abdominal visceral adiposity, but not abdominal subcutaneous or general adiposity, was associated with the development of pre-diabetes or diabetes over a 7-year follow-up period [28]. A possible explanation for this finding is that insulin resistance change played a role in the development of pre-diabetes and diabetes in this cohort. We previously have similarly demonstrated that accumulation of visceral fat over 5 years was found to also be an independent predictor of the future development of type 2 diabetes in Japanese Americans, independent of baseline adiposity levels [18]. A study in 30 Canadian women without diabetes showed that changes in abdominal visceral adiposity over a 7-year follow-up period were directly associated with changes in fasting plasma insulin and glucose and insulin areas under the curve during OGTT after control for total body fat mass [29]. No adjustment though was performed for baseline visceral fat area or for other fat depot areas. A longitudinal 3-year study of Japanese men also found that changes in abdominal visceral adiposity were directly associated with metabolic risk factor measurements (fasting plasma glucose, triglycerides, HDL cholesterol, systolic and diastolic blood pressure) [30].
Since the advent of CT with excellent reproducibility of subcutaneous and visceral adipose tissue area measurements [31], evidence has accumulated to support an association between lower insulin sensitivity and greater IAFA. Several studies have examined the relationship between insulin sensitivity and various adipose tissue regions in both human and animal models [28,32–36]. We have demonstrated that greater visceral adiposity predicts lower insulin sensitivity [17]. Research on a rat animal model has demonstrated that the development of insulin resistance can be significantly reduced in aging rats by preventing the age-dependent accumulation of IAFA [21] through surgical removal of visceral fat which also has been shown to reverse hepatic insulin resistance [37]. Research has demonstrated that visceral adipose depots can be reduced in humans, and that changes in these depots are associated with change in insulin resistance [21,38,39]. Furthermore, a recent study by Goto et al. of obese Japanese subjects showed that a 1-year decrease in BMI and visceral fat area through a lifestyle intervention program was associated with improvement in insulin sensitivity as measured by HOMA-IR and Matsuda Index [19]. Such research argues in favor of a causal association between visceral adiposity and insulin lower insulin sensitivity. Goto et al. found that a decrease in the subcutaneous fat area was significantly associated with an increase in Matsuda Index, but not significantly associated with change in HOMA-IR, in accordance with our findings. These results may reflect the differences in the physiologic relevance of these surrogate markers of insulin resistance. HOMA-IR may be more reflective of hepatic insulin resistance, while Matsuda Index is more indicative of whole-body insulin sensitivity [25].
Another possible mechanism by which visceral adiposity loss improves insulin sensitivity and prevents diabetes development may be by favorably altering adipokine and inflammatory profiles. Specifically, visceral fat levels are inversely correlated with high molecular weight (HMW) adiponectin, which is the biologically active form of adiponectin [40]. Low adiponectin levels are correlated with inflammation and insulin resistance [41,42]. Decreased visceral adiposity may, therefore, increase HMW adiponectin levels and result in improved insulin sensitivity. Also, excessive free fatty acids from increased adipose tissue could contribute to insulin resistance; abdominal visceral adipose depots could contribute more to this effect due to their proximity to the hepatic portal system [43]. Further studies could help elucidate the mechanism by which observed changes in visceral adiposity levels result in changes in insulin sensitivity.
The association of IAFA with insulin resistance is well known, but associations between SCFA and insulin sensitivity are less frequently reported and inconsistent. Rie et al. showed that IAFA and abdominal SCFA had strong associations with insulin resistance as measured using HOMA-IR and Matsuda index in middle-aged Japanese in a cross-sectional study [44] while IAFA but not subcutaneous abdominal fat was associated with insulin resistance as measured using Matsuda index in Asian Indians without diabetes also examined cross-sectionally [45]. Greater IAFA was more strongly correlated with lower insulin sensitivity measured by HOMA-IR than SCFA in Chinese subjects with pre-diabetes [33]. This inconsistent association between insulin sensitivity and IAFA and SCFA may reflect differences in adipocyte biology between these two fat depots, differences in study design, or other factors. IAFA may contribute to insulin resistance through both adipocyte hypertrophy and macrophage- or B-cell mediated inflammation, while SCFA exhibits adipocyte hypertrophy, but no changes in cell-mediated immunity [46]. Moreover, the Matsuda index may have greater ability to detect lower insulin sensitivity than HOMA-IR, which may also explain the discordance in findings between Matsuda index and HOMA-IR at 10 years regarding associations between SCFA and change in insulin sensitivity. One report of a positive association between the ratio of abdominal SCFA to IAFA and Matsuda index is similar to our results [47]. Also, Matsuda index has been shown to be a more sensitive index for the detection of lower insulin sensitivity than HOMA-IR in Finnish offspring without diabetes of parents with type 2 diabetes. Matsuda index showed differences in insulin sensitivities between subjects with normal compared to impaired fasting glucose while HOMA-IR did not [48]. Furthermore, these investigators also demonstrated a higher correlation between Matsuda index and insulin sensitivity measured by IVGTT than HOMA-IR [48].
This study has several limitations. Surrogate measurements estimated insulin sensitivity at baseline and 10 years in the study; direct measures would be more accurate. However, these indices have previously been shown to have good correlation with a gold standard marker of insulin sensitivity, the euglycemic insulin clamp [24,25]. Due to the reduced time points available for the calculation of the Matsuda Index (specifically, the lack of the 90-minute OGTT time point), a modified Matsuda Index was calculated; however, using fewer OGTT time points still provides a valid estimate of whole-body insulin sensitivity that agrees well with the composite index calculated with more time points [26]. Another potential limitation of this study includes small sample size and its use of a Japanese American cohort, possibly limiting its generalizability to other ethnic/racial backgrounds. Testing in other populations would ensure generalizability of these findings. Additionally, as with any observational study, the potential exists for confounding by unmeasured variables.
In conclusion, this study provides evidence that, independent of baseline body composition, 10-year change in visceral adiposity is significantly associated with insulin sensitivity, with greater accumulation related to greater resistance. Given that lifestyle interventions have been shown to reduce the size of the visceral fat depot, our study suggests that such interventions may be important in limiting the worsening of insulin resistance, and thereby preventing conditions associated with it, such as features of the metabolic syndrome and type 2 diabetes.
Acknowledgments
We are grateful to the King County Japanese-American community for support and cooperation.
Funding
National Institutes of Health grants DK-31170 and HL-49293. This work was supported by facilities and services provided by the Diabetes Research Center (DK-017047), Clinical Nutrition Research Unit (DK-035816), and the General Clinical Research Center (RR-000037) at the University of Washington. The funding entities had no role in the conduct of this study or interpretation of its results. VA Puget Sound provided support for the participation of E.J.B. and S.E.K. in this research.
Abbreviations list
- Coeff.
coefficient
- IAFA
intra-abdominal fat area
- SCFA
subcutaneous fat area
- HOMA-IR
HOMA for insulin resistance
- OGTT
oral glucose tolerance test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
References
- [1].McFarlane SI, Banerji M, Sowers JR. Insulin Resistance and Cardiovascular Disease 1. J Clin Endocrinol Metab 2001;86:713–8. doi: 10.1210/jcem.86.2.7202. [DOI] [PubMed] [Google Scholar]
- [2].Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996;334:374–81. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- [3].Sowers JR. Effects of Insulin and IGF-I on Vascular Smooth Muscle Glucose and Cation Metabolism. Diabetes 1996;45:S47–51. doi: 10.2337/diab.45.3.S47. [DOI] [PubMed] [Google Scholar]
- [4].Reaven GM. Role of Insulin Resistance in Human Disease. Diabetes 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- [5].Von Eyben F, Mouritsen E, Holm J, Montvilas P, Dimcevski G, Suciu G, et al. Intra-abdominal obesity and metabolic risk factors: a study of young adults. Int J Obes Relat Metab Disord 2003;27:941–9. doi: 10.1038/sj.ijo.0802309. [DOI] [PubMed] [Google Scholar]
- [6].Bell DSH. Heart Failure: The frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003;26:2433–41. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- [7].Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- [8].Banerji MA, Lebovitz HE. Insulin-Sensitive and Insulin-Resistant Variants in NIDDM. Diabetes 1989;38:784–92. doi: 10.2337/diab.38.6.784. [DOI] [PubMed] [Google Scholar]
- [9].Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 1997;100:1166–73. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pouliot M-C, Despres J-P, Nadeau A, Moorjani S, Prud’Homme D, Lupien PJ, et al. Visceral Obesity in Men: Associations With Glucose Tolerance, Plasma Insulin, and Lipoprotein Levels. Diabetes 1992;41:826–34. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- [11].Tchemof A, Lamarche B, Prud’homme D, Nadeau A, Mooijani S, Labrie F, et al. The Dense LDL Phenotype: Association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care 1996;19:629–37. doi: 10.2337/diacare.19.6.629. [DOI] [PubMed] [Google Scholar]
- [12].Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab 2002;87:5044–51. doi: 10.1210/jc.2002-020570. [DOI] [PubMed] [Google Scholar]
- [13].Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Metab 2002;282:E657–63. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- [14].Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body Composition, Visceral Fat, Leptin, and Insulin Resistance in Asian Indian Men. J Clin Endocrinol Metab 1999;84:137–44. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- [15].Banerji MA, Lebowitz J, Chaiken RL, Gordon D, Krai JG, Lebovitz HE. Relationship of visceral adipose tissue and glucose disposal is independent of sex in black NIDDM subjects. Am J Physiol 1997;273:E425–32. doi: 10.1152/ajpendo.1997.273.2.E425. [DOI] [PubMed] [Google Scholar]
- [16].Boyko EJ, Leonetti DL, Bergstrom RW, Newell-Morris L, Fujimoto WY. Visceral adiposity, fasting plasma insulin, and lipid and lipoprotein levels in Japanese Americans. Int J Obes Relat Metab Disord 1996;20:801–8. [PubMed] [Google Scholar]
- [17].Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 2008;57:1269–75. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- [18].Wander PL, Boyko EJ, Leonetti DL, McNeely MJ, Kahn SE, Fujimoto WY. Change in Visceral Adiposity Independently Predicts a Greater Risk of Developing Type 2 Diabetes Over 10 Years in Japanese Americans. Diabetes Care 2013;36:289–93. doi: 10.2337/dcl2-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goto M, Morita A, Goto A, Deura K, Sasaki S, Aiba N, et al. Reduction in Adiposity, β-Cell Function, Insulin Sensitivity, and Cardiovascular Risk Factors: A Prospective Study among Japanese with Obesity. PLoS One 2013;8:e57964. doi: 10.1371/joumal.pone.0057964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 1999;48:839–47. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- [21].Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 2002;51:2951–8. [DOI] [PubMed] [Google Scholar]
- [22].Fujimoto WY, Leonetti DL, Kinyoun JL, Shuman WP, Stolov WC, Wahl PW. Prevalence of Complications Among Second-Generation Japanese-American Men With Diabetes, Impaired Glucose Tolerance, or Normal Glucose Tolerance. Diabetes 1987;36:730–9. doi: 10.2337/diab.36.6.730. [DOI] [PubMed] [Google Scholar]
- [23].Bergstrom RW, Leonetti DL, Newell-Morris LL, Shuman WP, Wahl PW, Fujimoto WY. Association of plasma triglyceride and C-peptide with coronary heart disease in Japanese-American men with a high prevalence of glucose intolerance. Diabetologia 1990;33:489–96. [DOI] [PubMed] [Google Scholar]
- [24].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [25].Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- [26].DeFronzo RA, Matsuda M. Reduced Time Points to Calculate the Composite Index. Diabetes Care 2010;33:e93–e93. doi: 10.2337/dcl0-0646. [DOI] [PubMed] [Google Scholar]
- [27].Belsley DA, Kuh E, Welsch RE. Regression Diagnostics. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 1980. doi: 10.1002/0471725153. [DOI] [Google Scholar]
- [28].Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional Adiposity and the Risk of Prediabetes and Type 2 Diabetes in Obese Adults. JAMA 2012;308:1150. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lemieux S, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP. Seven-Year Changes in Body Fat and Visceral Adipose Tissue in Women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care 1996;19:983–91. doi: 10.2337/diacare.19.9.983. [DOI] [PubMed] [Google Scholar]
- [30].Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Yokoyama T, Mizoue T, et al. Effect of longitudinal changes in visceral fat area and other anthropometric indices to the changes in metabolic risk factors in Japanese men: the Hitachi Health Study. Diabetes Care 2012;35:1139–43. doi: 10.2337/dc11-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thaete FL, Colberg SR, Burke T, Kelley DE. Reproducibility of computed tomography measurement of visceral adipose tissue area. Int J Obes Relat Metab Disord J Int Assoc Study Obes 1995;19:464–7. [PubMed] [Google Scholar]
- [32].Miyazaki Y, DeFronzo RA. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc Diabetol 2009;8:44. doi: 10.1186/1475-2840-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu L, Feng J, Zhang G, Yuan X, Li F, Yang T, et al. Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in Chinese subjects with pre-diabetes. Curr Med Res Opin 2018;34:123–9. doi: 10.1080/03007995.2017.1364226. [DOI] [PubMed] [Google Scholar]
- [34].Hirose H, Takayama M, Iwao Y, Kawabe H. Effects of Aging on Visceral and Subcutaneous Fat Areas and on Homeostasis Model Assessment of Insulin Resistance and Insulin Secretion Capacity in a Comprehensive Health Checkup. J Atheroscler Thromb 2016;23:207–15. doi: 10.555l/jat.30700. [DOI] [PubMed] [Google Scholar]
- [35].de Mutsert R, Gast K, Widya R, de Koning E, Jazet I, Lamb H, et al. Associations of Abdominal Subcutaneous and Visceral Fat with Insulin Resistance and Secretion Differ Between Men and Women: The Netherlands Epidemiology of Obesity Study. Metab Syndr Relat Disord 2018;16:54–63. doi: 10.1089/met.2017.0128. [DOI] [PubMed] [Google Scholar]
- [36].Schousboe JT, Langsetmo L, Schwartz AV, Taylor BC, Vo TN, Kats AM, et al. Comparison of Associations of DXA and CT Visceral Adipose Tissue Measures With Insulin Resistance, Lipid Levels, and Inflammatory Markers. J Clin Densitom 2017;20:256–64. doi: 10.1016/j.jocd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 1999;48:94–8. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- [38].Group DPP (DPP) R. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, et al. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes 2007;56:1680–5. doi: 10.2337/db07-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].KELLY KR, NAVANEETHAN SD, SOLOMON TPJ, HAUS JM, COOK M, BARKOUKIS H, et al. Lifestyle-Induced Decrease in Fat Mass Improves Adiponectin Secretion in Obese Adults. Med Sci Sport Exerc 2014;46:920–6. doi: 10.1249/MSS.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kershaw EE, Flier JS. Adipose Tissue as an Endocrine Organ. J Clin Endocrinol Metab 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- [42].Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: More Than Just Another Fat Cell Hormone? Diabetes Care 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- [43].Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care 2007; 10:142–8. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- [44].Oka R, Yagi K, Sakurai M, Nakamura K, Nagasawa S, Miyamoto S, et al. Impact of Visceral Adipose Tissue and Subcutaneous Adipose Tissue on Insulin Resistance in Middle-Aged Japanese. J Atheroscler Thromb 2012;19:814–22. doi: 10.5551/jat.12294. [DOI] [PubMed] [Google Scholar]
- [45].Sandeep S, Gokulakrishnan K, Velmurugan K, Deepa M, Mohan V. Visceral & subcutaneous abdominal fat in relation to insulin resistance & metabolic syndrome in non-diabetic south Indians. Indian J Med Res 2010; 131:629–35. [PubMed] [Google Scholar]
- [46].Verboven K, Wouters K, Gaens K, Hansen D, Bijnen M, Wetzels S, et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep 2018;8:4677. doi: 10.1038/s41598-018-22962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu J, Liu J, Li H, Liu L, Zheng J, Huang Z, et al. Higher Ratio of Abdominal Subcutaneous to Visceral Adipose Tissue Related with Preservation of Islet β -Cell Function in Healthy Individuals. Int J Endocrinol 2017;2017:1–10. doi: 10.1155/2017/6180904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maki KC, McKenney JM, Farmer MV, Reeves MS, Dicklin MR. Indices of insulin sensitivity and secretion from a standard liquid meal test in subjects with type 2 diabetes, impaired or normal fasting glucose. Nutr J 2009;8:22. doi: 10.1186/1475-2891-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


