Summary
Conversion of sunlight to chemical energy based on photoelectrochemical (PEC) processes has been considered as a promising strategy for solar energy harvesting. Here, we propose a novel platform that converts solar energy into sodium (Na) as a solid-state solar fuel via the PEC oxidation of natural seawater, for which a Na ion-selective ceramic membrane is employed together with photoelectrode (PE)-photovoltaic (PV) tandem cell. Using an elaborately modified bismuth vanadate-based PE in tandem with crystalline silicon PV, we demonstrate unassisted solar-to-Na conversion (equivalent to solar charge of seawater battery) with an unprecedentedly high efficiency of 8% (expected operating point under 1 sun) and measured operation efficiency of 5.7% (0.2 sun) and long-term stability, suggesting a new benchmark for low-cost, efficient, and scalable solid solar fuel production. The sodium turns easily into electricity on demand making the device a nature-friendly, monolithic solar rechargeable seawater battery.
Subject Areas: Electrochemical Energy Conversion, Energy Storage, Materials Characterization
Graphical Abstract

Highlights
-
•
Solar rechargeable battery using natural seawater as a medium was realized
-
•
Solar charge was achieved by metal oxide-based photoelectrodes, especially BiVO4
-
•
BiVO4-c-Si tandem cell achieved unbiased charge with a high efficiency up to 8%
Electrochemical Energy Conversion; Energy Storage; Materials Characterization
Introduction
Utilization of abundant (23,000 TW year−1) solar energy in an efficient, cost-effective manner is essential to leapfrog fossil fuel technologies and secure sustainable, climate-friendly future of our society (Cook et al., 2010, Kim et al., 2019, Lewis, 2016, Roger et al., 2017, Seitz et al., 2014). Yet its intermittent feature poses a huge challenge toward practical, large-scale dissemination (Kurtz et al., 2017, Pellow et al., 2015, Sivula and van de Krol, 2016, Yang et al., 2011). In this regard, converting solar energy into easily storable chemical energy is the most effective strategy as adeptly exemplified by the natural photosynthesis that captures sunlight and stores it in chemical bonds. Similarly, artificial photosynthetic systems include production of H2 by photoelectrochemical (PEC) water splitting and of carbon-based chemicals by CO2 reduction (Blankenship et al., 2011, Montoya et al., 2016, Nocera, 2017, Sivula and van de Krol, 2016). Such solar conversion to gaseous or liquid fuels production at a grid scale, however, still requires further advancements in solar-to-chemical conversion efficiencies (ηSTC), device stability, and cost (Montoya et al., 2016, Nocera, 2017, Pellow et al., 2015, Seitz et al., 2014). In addition, the effective storage of gaseous fuels like H2 is rather difficult.
Alternatively, solar-driven electric energy can be stored as chemical energy in electrochemical energy storage (EES) systems, such as rechargeable batteries or supercapacitors, by connecting with photovoltaic (PV) devices (Um et al., 2017, Xu et al., 2015a, Xu et al., 2015b). This simple method, however, has critical drawbacks, such as the high cost stemming from two separate modules and the unavoidable loss of overall energy conversion efficiency (Roger et al., 2017, Xu et al., 2015a, Yang et al., 2011). Recently, solar rechargeable cells (SRCs), which integrate a PEC cell and an EES cell into a single device, have been revived as a promising means for sunlight utilization (Cheng et al., 2017, Li et al., 2016a, Liao et al., 2016, Liu et al., 2016, Nikiforidis et al., 2016). The photo-generated electricity from a PEC component is stored in situ in chemical bonds within an EES component (photo-charging), and the chemical energy can be utilized to generate electric power on demand (discharging). Most of the reported SRCs have employed dissolved redox-active species, such as iodine, bromine, vanadium, or sulfur as a redox mediator due to their fast redox reaction kinetics (Li et al., 2017, Yu et al., 2016), with the photoelectrodes (PEs) immersed in electrolytes. More recently, quinone-based redox flow batteries (RFBs) charged by dual PEs of p/n-Si (Li et al., 2016a, Liao et al., 2016) or Ta3N5 combined with GaN/p-Si (Cheng et al., 2017) have demonstrated highly efficient solar-to-chemical energy conversion. Notwithstanding, the use of highly corrosive acidic electrolytes and expensive membranes or the environmental impact (toxicity) of redox couples themselves (e.g., bromine) still remain to be resolved for large-scale deployment. In addition, such SRCs including RFBs typically have a low discharge voltage less than 1.5 V, because of small difference in the formal potentials between redox-active anolyte and catholyte (Azevedo et al., 2016, Cheng et al., 2017, Li et al., 2016a, Liao et al., 2016).
Here, we report a novel approach to solar fuel production, where earth-abundant seawater is utilized as the infinite medium to store the sunlight energy in chemical bonds of highly electropositive sodium (Na) metal (−2.71 V versus standard hydrogen electrode), which is reduced from Na+ ions present in seawater (∼0.5 M), as a result of solar-driven PEC seawater splitting. To this end, we design a monolithically integrated system, named solar rechargeable seawater battery that employs a Na superionic conducting ceramic membrane (NASICON, Na3Zr2Si2PO12) to separate a charge storage electrode (Na metal anode) in an organic electrolyte from a PE and a cathode immersed in seawater while allowing only Na-ion transport between the two compartments, as illustrated in Figure 1A.
Figure 1.
Cell Configuration and Photo-charging Process of the Solar Seawater Battery
(A) The cell structure of a solar rechargeable seawater battery, which employs a NASICON ceramic membrane to separate a charge storage electrode (Na metal anode) from a photoelectrode (PE) and a cathode immersed in seawater.
(B) Energy diagram of the photo-charging process, where OEC-loaded BiVO4 PE is employed for solar seawater oxidation at the cathode compartment. The energy level is expressed with two different scales relative to reversible hydrogen electrode (RHE) of seawater (pH∼8) and the redox potential of Na/Na+. The PEC seawater splitting on the PE significantly reduces the potential required for battery charging (path 1 versus path 2).
(C) Redox potential (Eredox)-voltage saved (Vsaved) plot of various types of solar rechargeable batteries using redox mediators from literatures. The Vsaved was calculated by subtracting the conduction band edge (ECB/e) of photoelectrodes from the Eredox of redox mediators.
We have developed a cost-effective and ecofriendly seawater battery in the last few years (Abirami et al., 2016, Kim et al., 2014, Kim et al., 2016c). Like the discharge process of a typical secondary battery, the chemical energy stored in Na(s) turns to electricity.
Charge:
Discharge:
Overall reaction for charge and discharge will be expressed as:
Specifically, the Na metal anode is oxidized to Na+ ions, which are then transported into seawater through the NASICON membrane, whereas the reduction reaction of dissolved oxygen occurs on the cathode in seawater, powering an external load. On the other hand, the cell can be charged at a significantly reduced voltage by applying a suitable PE that enables the PEC seawater oxidation, when compared with typical electric charging based on seawater electrolysis. The energy diagram of the photo-charging process of the cell is depicted in Figure 1B, where an oxygen-evolving catalyst (OEC)-loaded BiVO4 is exemplified as a PE for our scheme. We have picked BiVO4 to be the major model PE for this work owing to its superior performance among metal oxide-based photoanodes (Kim and Lee, 2019). Upon illumination, photons (λ < 516 nm) captured by BiVO4 generate electron-hole pairs. The photo-generated holes in the valence band (VB) are transferred to the PE-seawater junction, oxidizing seawater to O2. Meanwhile, the electrons excited to the conduction band (CB) flow toward the anode through the external circuit, which decreases the potential required for Na+ reduction following Na ion-transport from the seawater into the anode, as much as the energy difference between the Fermi level of the PE (∼0.05 VRHE) and the redox potential of O2/H2O () in seawater (1.23 VRHE).
Thus the photo-driven process at the PE-seawater interface enables the cell to be charged at ∼2.25 V versus Na/Na+, resulting in a theoretical potential gain of ∼1.23 V (∼35%). Furthermore, we demonstrate spontaneous, unbiased photo-charging of the device with BiVO4-based PE-c-Si PV tandem cell, achieving a record-high ηSTC of up to 8% under 1 sun illumination, which set a new benchmark for solar-to-chemical (Na) energy conversion. To set the scope of this work, previously reported SRCs and their performance in expected voltage saving (Vsaved) versus redox potential (Eredox) are compared with ours in Figure 1C and Table S1.
Results
Selection and Analysis of Photoanodes for PEC Seawater Splitting
We first tested the PEC seawater splitting activity of cheap and environmentally benign metal oxide semiconductor films, such as TiO2, WO3, Fe2O3, and BiVO4, as PE candidates for efficient photolysis of seawater as described in Supplemental Information, Figures S1–S5 and 2. These materials have been extensively studied for PEC splitting of water, but rarely of seawater.
Figure 2.
Performance of Semiconductor Oxide Photoelectrodes (TiO2, WO3, Fe2O3, BiVO4) for Seawater Battery
(A–C) (A) J-V curves of TiO2, WO3, Fe2O3, and H, 1% Mo:BiVO4 photoelectrode in three electrode configurations for Pt metal rod (left side) and Na coin cell as counterelectrode (two-electrode system, right side); (B) galvanostatic photo-charging at 0.01 and 0.1 mA for the photoelectrodes and Pt rod; (C) J-t curves for photo-charge at 0.1 mA (per electrode) with and without light illumination (denoted as on and off). The geometric area of photoelectrodes was 0.20 cm2, and the Pt rod was 3 cm in length.
The J-V curves of the five PEs (together with the Pt rod electrode) relative to the Pt counterelectrode (left) and the Na counterelectrode (seawater cell; right) are shown in Figure 2A. The current density at 3.48 V (versus Na/Na+) was 3.65 mA/cm2 for NiFeOx/H, 1% Mo:BiVO4, 0.78 mA/cm2 for H, 1% Mo:BiVO4, 0.55 mA/cm2 for TiO2, 0.46 mA/cm2 for WO3, and 0.11 mA/cm2 for Fe2O3. We tested galvanostatic photo-charging of the battery with the PEs at currents of 0.01 mA and 0.1 mA upon 1 sun illumination (Figure 2B). The actual charge voltages of these PEs are measured to be 3.32 V for Fe2O3, 3.05 V for WO3, 2.70 V for TiO2 and H, 1% Mo:BiVO4, and 2.62 V for NiFeOx/H, 1% Mo:BiVO4 at a low current density of 0.05 mA/cm2; the theoretical potential requirement is considered as at least 3.68 V (a minimum overpotential of 0.2 V assumed) to achieve photo-charge, which gives a potential saving of 0.36–1.06 V by using a light absorber. The photo-charge at a higher current density of 0.5 mA/cm2 (0.1 mA per 0.20 cm2PE) requires a higher potential, although the value is still mostly lower than 3.68–3.8 V for Fe2O3, 3.35 V for WO3, 3.11 V for TiO2, 3.11 V for H, 1% Mo:BiVO4, and 2.91 V for NiFeOx/H, 1% Mo:BiVO4. The on-off tests clearly showed that the potential saving was achieved by a light-induced process on the PEs (Figure 2C).
Compared with previous reports on the photo-charge of redox couple-based SRCs (only 0.001–0.01 mA/cm2) (Li et al., 2016b, Li et al., 2017, Yu et al., 2014), our system with the NiFeOx/H, 1% Mo:BiVO4 PE showed significantly higher photocurrent densities (∼3 mA/cm2). In addition, the redox mediator (O2/H2O) of our system (pH-neutral natural seawater) (Li et al., 2017) is nature friendly compared with existing SRCs, such as I−/I3− (light absorber—N179/TiO2, Yu et al., 2014; TiO2, Li et al., 2016b; Fe2O3, Nikiforidis et al., 2016) and VO2+/VO2+ (light absorber—CdS/CdSe, Azevedo et al., 2016). Previous demonstrations of O2/H2O redox-based SRCs achieved modest potential savings, but TiO2 (3.2–3.0 eV, Jcharge ∼0.01 mA/cm2) (Kim et al., 2016a) and C3N4 (2.7 eV, Jcharge ∼ 0.05 mA/cm2) (Liu et al., 2016) cannot achieve such a high current density as we did with BiVO4 (2.4 eV, Jcharge >1.0–3.0 mA/cm2). Another important aspect of our BiVO4 PE is transparency that other previous studies could not have (Li et al., 2017, Liu et al., 2016). Above its practical optical band gap (indirect, 2.4 eV, 516 nm), it has near 75% transmittance (Figure S1). Such merit benefits construction of a PE-PV tandem device for unbiased photo-charge of the system, which is demonstrated in a later section. Quinone redox batteries photo-charged by Si (Li et al., 2016a) and Ta3N5 PEs (Cheng et al., 2017) with current densities of ∼10 mA/cm2 only showed superior performance to our system (∼3 mA/cm2), but their systems require high alkalinity of the electrolyte.
Among the PEs tested, elaborately modified BiVO4 exhibited the best performance in J-V curves of PEC seawater splitting and seawater battery charge (Figure 2A) and galvanostatic photo-charging (Figures 2B and 2C), and thus naturally became our choice for the PE. For its fabrication, a modified metal-organic decomposition method was employed to obtain 1 atom % Mo-doped BiVO4 film on F-doped SnO2 (FTO) glass, which was further treated with H2 generated by NaBH4 decomposition to improve the bulk charge transfer characteristics. The extensive characterization data of the H, 1% Mo:BiVO4 PE including ultraviolet-visible and X-ray diffraction are available in our recent works (Kim et al., 2016b, Pan et al., 2018). Furthermore, we loaded NiFeOx OEC on the BiVO4 PE by photo-assisted electrodeposition. Electron microscopic images revealed that NiFeOx-loaded, hydrogen-treated, Mo-doped BiVO4 (denoted as NiFeOx/BiVO4 or NiFeOx/H, 1% Mo:BiVO4) PE featured a porous morphology consisting of connected particles (100–200 nm), on which ∼5-nm-thick NiFeOx layer was formed (Figures S5 and S6).
The basic driving force of the solar charging is PEC oxidation of seawater by a photoanode,
| 2H2O + 4hvb+ → O2 + 4H+ |
where hvb+ is the hole generated in the VB of the semiconductor photoanode upon light absorption. The performance of the NiFeOx/BiVO4 PE for PEC seawater splitting under simulated AM 1.5G sunlight (100 mWcm−1) is shown in Figure 3. The NiFeOx/BiVO4 PE showed a reduced onset potential by 0.35 VRHE compared with the bare BiVO4 PE in seawater. Although the NiFeOx/BiVO4 PE exhibited a slightly lower PEC performance in seawater (4.8 mA cm−2 at 1.23 VRHE) than in 0.1 M potassium phosphate (KPi), a typical electrolyte for water splitting (5.0 mA cm−2) (Figures 3A–3C), these values of current density are still comparable to those of reported benchmark metal oxide light absorbers (Kim et al., 2015, Lee and Choi, 2017, Sivula and van de Krol, 2016). It is noteworthy that the current densities recorded by our NiFeOx/BiVO4 are actually the best ever reported for seawater splitting, surpassing the previous record of 2.16 mA cm−2 by RhOx/3% Mo:BiVO4 at 1.0 VRHE (Luo et al., 2011). The NiFeOx/BiVO4 PE exhibited a stable performance for 24 h producing O2 and H2 gases with a molar ratio of 1:2 (Figures 3D and 3E), which is comparable to previous best BiVO4 PEs operated in KPi buffer for 30–50 h (Kim and Choi, 2014, Kim et al., 2015). No notable change in the morphology was found after PEC operation for 24 h (Figures 3F, 3G, S2, and S3). The energy-dispersive X-ray and X-ray photoelectron spectroscopic data indicated that the chemical state of NiFeOx is in an oxyhydroxide form, i.e., NiFeOxHy, and oxidation states of all elements in NiFeOx/BiVO4 PE remain unaltered throughout the PEC reactions (Figures S4–S6, Table S2).
Figure 3.
PEC Performance of NiFeOx/BiVO4 PE under Simulated Sunlight for Seawater Splitting
(A) J-V curves of BiVO4 PE with and without the NiFeOx co-catalyst in KPi buffer (pH 7.0) and natural seawater (pH 8.0).
(B and C) Corresponding (B) surface charge separation efficiencies (ηsurf) and (C) IPCE values.
(D) Photocurrent generation at a constant potential of 1.03 VRHE. The inset shows the J-V curves before and after the stability test for 24 h.
(E) Gas evolution in natural seawater of a PEC cell composed of the BiVO4 PE and Pt rod counterelectrode at an applied bias of 0.9 VRHE (geometric area of the PE = 0.25 cm2).
(F and G) (F) A scanning electron micrograph and (G) a transmission electron microscopic image after the stability test.
We performed controlled experiments to study the effect of electrolyte components on the PEC performance of the NiFeOx/BiVO4 PE by varying the concentration of NaCl or Na2SO4, both of which are known to be rather less effective in PEC water oxidation (Figures S7 and S8) (Shinagawa et al., 2017). To understand better this effect of electrolyte and the role of NiFeOx OEC overlayer, photoelectrochemical impedance spectroscopy (PEIS) was performed in Figures S9–S11. Loading OEC can provide a higher quasi-Fermi level (|Fermi level – quasi-Fermi level| = photovoltage) or a higher photovoltage by passivating defective surface states and providing alternative pathway for hole transfer through OEC instead of surface of the semiconductor, which tends to become recombination centers for holes. Maximum capacitance for surface states (Css) and minimum resistance at semiconductor||electrolyte interface (RCT) determined by PEIS can be indicators that show how hole transfer dynamics changes with OEC loading (Wang et al., 2016). With OEC applied, surface state shifts closer to the Fermi level of BiVO4 (Figure S10) by the “passivation effect” and the potential difference between Fermi level and quasi-Fermi level gets wider (Ma et al., 2014, Ma et al., 2016). Also, RCT values throughout the anodic potential region markedly decrease owing to reduced recombination and facile hole transfer to electrolyte (Figures S10A versus S10C). The maximal Css increases because the NiFeOx could function as a hole storage layer as well as provide alternative active sites for water oxidation, therefore reducing the overpotential compared with the bare BiVO4 photoanode. In seawater, potentials for maximum Css and lowest RCT are all anodically shifted by ∼0.2 V (Figures S10B versus S10D), which is equivalent to the extent that J-V curve shifts relative to those in KPi. However, there is a noticeable difference in terms of the position of maximum Css and minimum RCT for KPi (potentials of maximum Css: < 0.3 VRHE, minimum RCT: 0.45 VRHE) and seawater (potentials of max. Css: 0.45 VRHE, min. RCT: 0.66 VRHE), which indicates that hole transfer from NiFeOx requires a higher potential (by 0.2 V) and is more sluggish in seawater. Interestingly, above 0.6–0.7 VRHE, CSS and RCT values are almost the same for NiFeOx/BiVO4 both in KPi and seawater, which indicates that enough potential is applied for NiFeOx/BiVO4 to drive water oxidation.
This rather unexpected behavior should be explained with another observation—transient photocurrent (TPC) appears in the low-bias region for NiFeOx/BiVO4. The “spike” appears when hole resides in excess on the surface of semiconductor, indicating a sluggish hole transfer from semiconductor to electrolyte and a large amount of holes residing at this interface (Liu et al., 2014). In Figures S8A and S8B, TPC appearing at 0.4 VRHE is minute and almost the same for BiVO4 in KPi and seawater, but a very large TPC appears for NiFeOx/BiVO4 only in seawater. This suggests that seawater affects the efficacy of NiFeOx on BiVO4 and the effect is much more dominant at low biases (0.3–0.6 VRHE) than at high biases (above 0.8 VRHE), which is consistent with the onset potential of bare BiVO4 (∼0.8 VRHE). Thus, for NiFeOx, hole transfer at low biases is more sluggish in seawater than KPi. From PEIS analysis, the hole storage capability and the surface impedance when hole transfer is initiated are almost the same, thus the characteristic of NiFeOx itself is unchanged in different electrolytes. However, hole transfer at NiFeOx||electrolyte depends on the electrolyte, i.e., KPi is better than seawater in water oxidation kinetics and has a dominant effect in the low-bias region.
The above considerations have been presented schematically in Figure S11. For bare BiVO4, electrolytes of KPi and seawater bring almost no difference in J-V curves, capacitance, or impedance. However, for NiFeOx/BiVO4, a large difference in the efficacy of the co-catalyst is in the low-bias region. Thus for the case of KPi, hole transfer from NiFeOx to electrolyte is facile throughout the bias region (Figure S11C). In seawater, BiVO4 itself cannot participate in direct hole transfer from BiVO4 to the electrolyte, but NiFeOx instead takes holes and transfers them to electrolyte, although it is more sluggish than in KPi. However, when a high bias is applied, direct hole transfer from BiVO4 to electrolyte is also possible (Figure S11D).
Photoelectrode-Driven Solar Rechargeable Seawater Battery
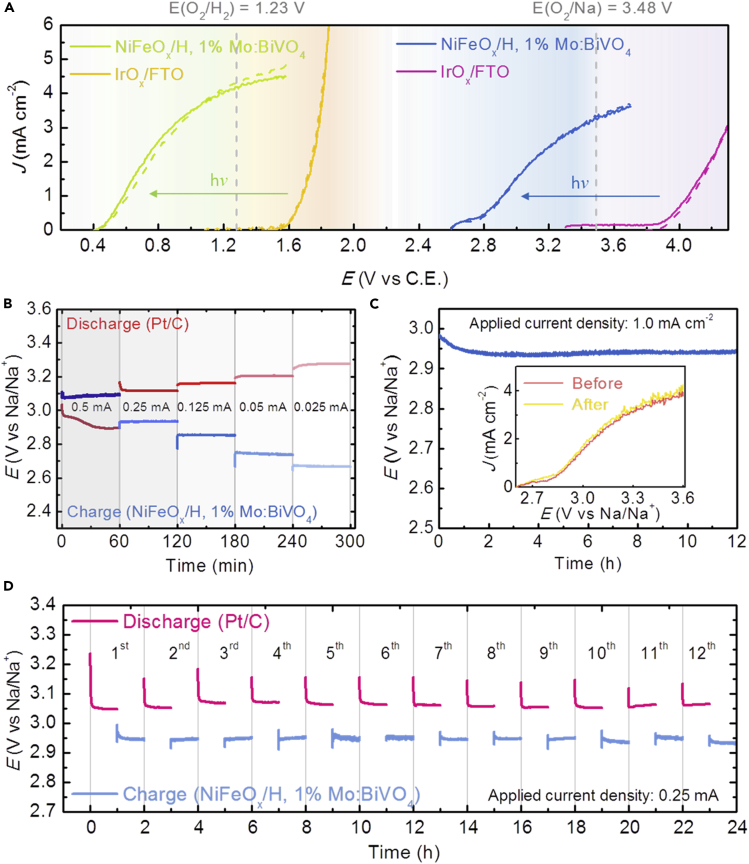
The developed PE is now applied to solar charging of the seawater battery. In Figure 4A, we examined photo-charge of the solar seawater battery (picture images in Figure S12) by employing the optimized NiFeOx/BiVO4 PE in comparison with a well-known OEC, IrOx/FTO without light absorber (Figure S8). The IrOx/FTO showed an onset potential of 1.55 VRHE for the electrochemical seawater splitting and of 3.87 V versus Na/Na+ for Na+ reduction in the seawater cell. On the other hand, the photo-driven oxygen evolution reaction on the optimized NiFeOx/BiVO4 PE in seawater started at 0.35 VRHE for water splitting and at 2.55 V versus Na/Na+ for Na reduction. Although the onset potential (2.55 V) for charging the solar-seawater cell was a little larger than the value expected from the CB potential of BiVO4 (∼2.25 V), the BiVO4 PE clearly reduced the potential required for charging, resulting in almost ∼1.3 V of potential saving compared with IrOx/FTO, which was comparable to the theoretical potential difference (∼1.28 V) between the flat band potential (EFB) of the PE and the in seawater. It also represents the largest value among the cheap and stable metal oxide-based light absorbers reported in the literature, and is comparable to those of expensive and unstable CdTe or GaAs (Mayer, 2017).
Figure 4.
Photo-charge Performance of the Solar Seawater Battery with the NiFeOx/BiVO4 Photoelectrode
(A) J-V curves of three-electrode configuration with a Pt rod counterelectrode and two-electrode configuration (seawater cell) with a Na counterelectrode. Dotted curves are for backward bias scanning, and the vertical lines indicate the theoretical potential of water oxidation versus reversible hydrogen electrode and E(Na/Na+).
(B) Rate capability for photo-charging and discharging (using a Pt/C-coated cathode of 1.0 cm2) at different currents of 0.025–0.5 mA.
(C) Long-term stability of the solar seawater battery during photo-charging at a current density of 1.0 mA cm−2; the inset shows J-V curves before and after the test.
(D) Cycling performance at a current of 0.25 mA; a photo-charge current (+0.25 mA per 0.25 cm2) and a discharge current (−0.25 mA per 1.0 cm2).
The galvanostatic photo-charge and discharge were tested at different currents for 1 h each in Figure 4B, where the discharge process was performed using a 20 wt. % Pt/C-coated carbon electrode (see Methods). Discharge voltages of 3.27–2.89 V were measured for 0.025–0.5 mA of currents, and photo-charge voltages of 2.67–3.09 V at 0.025–0.5 mA of electric charge currents. It should be noted that the charge voltage for our solar rechargeable seawater battery was lower than that of the discharge voltage at current conditions below 0.5 mA, owing to the solar-driven seawater oxidation process by the PE, achieving apparent voltage efficiencies (Vdischarge/Vcharge) of 106%–122% unlike usual batteries, for which Vdischarge is always smaller than Vcharge. The photo-charge uses solar energy to earn such a voltage increase to the extent of “potential saving” as described already.
The long-term photo-charging stability of the solar seawater cell with the BiVO4 PE was evaluated at 1.0 mA cm−2. As shown in Figure 4C, following an initial small decrease of the photo-charge voltage, it remained at ∼2.95 V over 12 h without any appreciable decay. The J-V curves of the NiFeOx/BiVO4 PE also remained unchanged before and after the stability test (the inset). We further examined photo-charge/discharge cycling performance of the cell at a current of 0.25 mA for 1 h each (Figure 4D). The cell cycled in a stable manner with a photo-charge voltage of ∼2.95 V and a discharge voltage of ∼3.12 V for 12 cycles (total 24 h), showing an average voltage efficiency of 106%. This indicates that our NiFeOx/BiVO4 PE could achieve stable solar charge of the seawater battery with immensely increased voltage efficiency relative to usual electric charge, demonstrating an efficient solar-to-Na conversion.
The sunlight intensity is a critical parameter that affects the performance of PE, and thus, we investigated the effect of light intensity by varying it from 0.1 to 2.0 sun (Figure S13). For practical application of solar-seawater battery charge, there are many places or times of the day where solar energy intensity is lower than the standard condition of 1 sun (100 mW cm−2). Although the photocurrent density generated in photo-charging was naturally reduced with decreasing light intensity, the solar energy conversion efficiency, i.e., photocurrents normalized by the incident light intensity, was considerably higher at a lower light intensity (Figure S13). For example, surface charge separation efficiency (ηsurf) significantly increases at attenuated light intensity (Figure S14), indicating that the hole recombination rate at semiconductor||electrolyte interface is greatly reduced, especially at the low-bias region. Thus, at 0.5 VRHE, the 0.1 sun condition showed nearly three times less recombination than under 2.0 sun. Chopping illumination showed less transient current (evolution of spike due to the recombination of surface accumulated charges, Figure S15) at low biases under attenuated light intensity, indicating that less amounts of holes accumulated on the surface of the NiFeOx/BiVO4 PE. As low amounts of incident photons generate less amounts of holes at the surface, they give rise to a low kinetic barrier for hole transfer to the electrolyte. Such characteristics pose a significant merit of the present system in practical operation under circumstances wherein solar energy intensity is low.
The actual overload for solar rechargeable seawater battery can be also reduced, because the overall current density is reduced by attenuated light intensity, which leads to an improved applied bias photon-to-current efficiency calculated by dividing the photocurrent with the incident light intensity (Figure S13). In the present device configuration, a photocurrent above 3 mA seems to induce inefficient photo-charging due to the overpotentials arising from the anode compartment, such as the resistance of NASICON membrane and the overpotential of Na reduction reaction. Of course, the problem could be easily resolved by increasing the capacity of the anode part.
Photoelectrode-PV Tandem Cell for Unbiased Solar-Powered Seawater Battery
Finally, we realized solar-energy-only-driven charging of the seawater battery by using a PE-PV tandem cell as depicted in Figures 5A and S16. To the best of our knowledge, this is the first dual light absorber, 4 photons (D4) scheme applied for aquatic Na-O2 cell, which produces Na as a solid solar fuel (electricity equivalent) instead of common gas fuels like H2. For PVs, side-by-side series-connected 7 pieces of crystalline silicon solar cell (7p c-Si) or 3 pieces lead halide (MAPbI3) perovskite solar cell (PSC) were used (details in Figures S17–S19), which showed open-circuit voltages (VOC) of ∼3.65 and ∼3.25 V under BiVO4 PE, respectively. Operating points (Jop) and solar-to-chemical conversion efficiency (ηSTC) of these BiVO4 PE-PVs under 1 sun condition were 2.29 mAcm−2 and 8.0% for 7p c-Si, whereas they were Jop = 1.64 mA cm−2 and ηSTC = 5.7% for 3p PSC (Figure 5B). Despite the relatively lower estimated ηSTC and insufficient stability of PSC, its low cost makes it a promising option for practical systems (Kim et al., 2019). In any case, these are unprecedentedly high efficiencies for SRCs outperforming recently reported devices of the integrated RFBs with p/n Si photoanode and photocathode (5.44–5.9%) (Li et al., 2016b, Liao et al., 2016), or Ta3N5 photoanode-GaN/Si photocathode (3.0%) (Cheng et al., 2017). The ηSTC is also higher than solar-to-hydrogen conversion efficiency (ηSTH) with the same PE-PV tandem assemblies; ηSTH of 5.5% and 3.05% for the PE-1p PSC and the PE-1p c-Si, respectively, in 0.1 M KPi (Figure S20), and even higher than the reported most efficient metal oxide-based PE-PV tandem cell for water splitting (BiVO4-Fe2O3-c-Si, ηSTH of 7.7%) (Kim et al., 2016b). More recently, systems of higher efficiency have been demonstrated: triple junction III/V solar cell jointed with RFB with ηSTC of 14.1% (Li et al., 2018) and PSC solar cell with DC-DC converter—Li ion battery of 9.8% (Ashim et al., 2017). However, our seawater battery system still has practical advantages of an environmentally benign electrolyte, a stable metal oxide light absorber, and a simple PV module.
Figure 5.
Unassisted Photo-charging of Solar Seawater Battery by a PE-PV Tandem Cell
(A) Scheme of solar rechargeable seawater battery with NiFeOx/BiVO4 PE in tandem with PSC or c-Si PVs for unassisted solar charging.
(B) Energy diagram of BiVO4 PE and 7p c-Si or 3p PSC PV for unassisted solar charging. VPV indicates photovoltage of individual solar cell and VPV module for whole module's photovoltage.
(C) Overlap of the J-V curves of the solar seawater cell and the PVs placed behind the PE under simulated 1 sun, showing the operating points (the PE active area: 0.25 cm2; the PV active area: 2.27 cm2).
(D and E) (D) Estimated solar-to-chemical conversion efficiency (ηSTC) at the estimated operating points and actual operation of the tandem devices under various light intensities when compared with the solar-to-hydrogen conversion efficiency (ηSTH) achieved by similar light absorbers under 1.0 sun condition (Figure S20). (E) Unassisted photo-charging of the solar-seawater tandem device with the 7p Si for 8 h under 0.2 sun. The inset shows the J-V curves before and after employing the tandem cell with c-Si PV (illuminated area: 2.30 cm2).
(F) BiVO4 PE-c-Si PV tandem assembly under natural sun. Conditions: solar intensity (85–45 mW/cm2), seawater (Ilsan beach, Ulsan, Republic of Korea [GPS 35.497005, 129.430996, pH∼8.0], active area: 2.7 cm2 for the PE, charge time: nearly 4 h). Charge storage electrode: desodiated hard carbon anode; see Transparent Methods and Figure S23. The discharge test, where a Pt/C-loaded carbon electrode was used as cathode, was conducted by powering a red light-emitting diode bulb (see Video S1).
In actual unassisted solar charging operation, we found that the photocurrent generated under 1 sun with the PE-PV tandem cell (∼5 mA) overloaded the Na storage (anode) compartment. Thus, we conducted unbiased charging under attenuated light intensities (0.1–0.3 sun) (Figures 5C, S25, and S21). The c-Si tandem system enabled spontaneous seawater battery charge without any external bias under the attenuated light, showing good reproducibility close to the expected operating points with the maximum unbiased photocurrent of 0.7 mA at 0.2 sun, achieving an actual ηSTC of ∼5.7%. Owing to low capacity of Na coin cell, ηSTC of PE-PV using PSC (Figure S22) was lower than expectation (Figure S20). Increasing overall size and reducing resistance of Na coin cell will greatly metigate such loss. The device operated in a stable manner for 8 h without notable degradation of the Jph (Figure 5E and the inset), suggesting a unique and promising way for low-cost, efficient, and scalable solar energy deployment. We also tested a stand-alone mode of the solar-seawater tandem device as a solar seawater battery under real sunlight, demonstrating the spontaneous, unbiased photo-charging and successful powering of a red light-emitting diode bulb (Figure 5F and Video S1).
Discussion
Our solar seawater tandem device is distinct from existing solar fuel production systems as much as it performs energy conversion from sunlight to Na in the form of a dense solid equivalent to electricity, instead of the usual gas or liquid fuels by harnessing the most earth-abundant natural resource, seawater, which plays roles in providing the Na+ ion source as well as mediating seawater battery charging via seawater splitting. The monolithically combined system is more efficient relative to the simple connection of PV to the battery. As demonstrated in Figure S24, the simple connection of 9p c-Si as PV device (OEC-PV) with an IrO2 electrode under attenuated 0.1 sun showed ηSTC of 1.18%, whereas our PE-PV device showed nearly three times higher efficiency (ηSTC = 3.92%). As PE has a larger band gap and a high photovoltage by itself (1.3 V), it is energetically more favorable to combine dual light absorbers (PV + PE) instead of PV only for maximizing solar energy harvesting. This approach to solar-to-Na (or electricity) production is considered intrinsically more efficient than solar-to-hydrogen production owing to better match of current density and potential (Figure S24). Thus, in solar hydrogen production by a PE-PV tandem cell, low photocurrents of the front photoanode limit the efficiency (Kim et al., 2016b). However, this seawater battery charge needs high voltage, whereas the current density is a low priority, and photoanodes provide higher photovoltage than most known PV devices. We were able to operate completely bias-free, spontaneous solar charge of seawater battery for 8 h. However, for practical applications, it should be much longer than 10 years (Rongé et al., 2015). Previous studies on the stability of PEs for water oxidation demonstrated stability for less than 2,000 h (Sun et al., 2015, Zhou et al., 2016), and thus the stability issue should be addressed in further developments of PEC devices in addition to efficiency.
Finally, solar-to-energy conversion efficiencies achieved by various light absorbers and battery systems are compared in Figure S25. It demonstrates that our novel solar rechargeable seawater battery shows a top efficiency, although it is made of earth-abundant, cheap, and nature-friendly materials and thus holds a high prospective for practical applications.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by the Basic Science Grant (NRF-2018R1A2A1A05077909), Climate Change Response project (2015M1A2A2074663, NRF-2017M1A2A2087630), Korea Center for Artificial Photosynthesis (KCAP, No. 2009-0093880), Korea-China Key Joint Research Program (2017K2A9A2A11070341) funded by MSIT, and Project No. 10050509 and KIAT N0001754 funded by MOTIE of Republic of Korea.
Author Contributions
J.H.K. conceived the idea, performed synthesis and conducted electrochemical analysis for photoelectrodes and solar battery system, and characterized the materials. S.M.H and J.H. prepared Na coin cell and seawater battery testing instrument; I.H. prepared and characterized silicon solar cell and its module under supervision of K.S.; J.H.K. synthesized Fe2O3 and TiO2; Y.H.J. prepared and characterized perovskite solar cell; Y.K. and J.S.L. supervised the project. J.H.K., S.M.H., Y.K., and J.S.L co-wrote the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.024.
Contributor Information
Jin Hyun Kim, Email: kihi6783@unist.ac.kr.
Youngsik Kim, Email: ykim@unist.ac.kr.
Jae Sung Lee, Email: jlee1234@unist.ac.kr.
Supplemental Information
References
- Abirami M., Hwang S.M., Yang J., Senthilkumar S.T., Kim J., Go W.-S., Senthilkumar B., Song H.-K., Kim Y. A metal–organic framework derived porous cobalt manganese oxide bifunctional electrocatalyst for hybrid Na–Air/Seawater batteries. ACS Appl. Mater. Interfaces. 2016;8:32778–32787. doi: 10.1021/acsami.6b10082. [DOI] [PubMed] [Google Scholar]
- Ashim G., Ke C., Reza K., Saad A.S., Geetha V., Rajesh P., Roya N., Qiquan Q. Highly efficient perovskite solar cell photocharging of lithium ion battery using DC–DC booster. Adv. Energy Mater. 2017;7:1602105. [Google Scholar]
- Azevedo J., Seipp T., Burfeind J., Sousa C., Bentien A., Araújo J.P., Mendes A. Unbiased solar energy storage: photoelectrochemical redox flow battery. Nano Energy. 2016;22:396–405. [Google Scholar]
- Blankenship R.E., Tiede D.M., Barber J., Brudvig G.W., Fleming G., Ghirardi M., Gunner M.R., Junge W., Kramer D.M., Melis A. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science. 2011;332:805–809. doi: 10.1126/science.1200165. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Fan W., He Y., Ma P., Vanka S., Fan S., Mi Z., Wang D. Photorechargeable high voltage redox battery enabled by Ta3N5 and GaN/Si dual-photoelectrode. Adv. Mater. 2017;29:1700312–1700319. doi: 10.1002/adma.201700312. [DOI] [PubMed] [Google Scholar]
- Cook T.R., Dogutan D.K., Reece S.Y., Surendranath Y., Teets T.S., Nocera D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010;110:6474–6502. doi: 10.1021/cr100246c. [DOI] [PubMed] [Google Scholar]
- Kim G., Oh M., Park Y. Solar-rechargeable battery based on photoelectrochemical water oxidation: solar water battery. Sci. Rep. 2016;6:33400. doi: 10.1038/srep33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-K., Mueller F., Kim H., Bresser D., Park J.-S., Lim D.-H., Kim G.-T., Passerini S., Kim Y. Rechargeable-hybrid-seawater fuel cell. NPG Asia Mater. 2014;6:e144. [Google Scholar]
- Kim J.H., Hansora D., Sharma P., Jang J.-W., Lee J.S. Toward practical solar hydrogen production – an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019;48:1908–1971. doi: 10.1039/c8cs00699g. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Jang J.-W., Jo Y.H., Abdi F.F., Lee Y.H., van de Krol R., Lee J.S. Hetero-type dual photoanodes for unbiased solar water splitting with extended light harvesting. Nat. Commun. 2016;7:13380. doi: 10.1038/ncomms13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Lee J.S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 2019;31:e1806938. doi: 10.1002/adma.201806938. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Choi K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science. 2014;343:990–994. doi: 10.1126/science.1246913. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Ping Y., Galli G.A., Choi K.-S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015;6:8769. doi: 10.1038/ncomms9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kim H., Park S., Seo I., Kim Y. Na ion- conducting ceramic as solid electrolyte for rechargeable seawater batteries. Electrochim Acta. 2016;191:1–7. [Google Scholar]
- Kurtz S., Haegel N., Sinton R., Margolis R. A new era for solar. Nat. Photon. 2017;11:3–5. [Google Scholar]
- Lee D.K., Choi K.-S. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy. 2017:53–60. [Google Scholar]
- Lewis N.S. Research opportunities to advance solar energy utilization. Science. 2016;351:aad1920. doi: 10.1126/science.aad1920. [DOI] [PubMed] [Google Scholar]
- Li Q., Li N., Liu Y., Wang Y., Zhou H. High-safety and low-cost photoassisted chargeable aqueous sodium-ion batteries with 90% input electric energy savings. Adv. Energy Mater. 2016;6:1600632–1600637. [Google Scholar]
- Li Q., Liu Y., Guo S., Zhou H. Solar energy storage in the rechargeable batteries. Nano Today. 2017;16:46–60. [Google Scholar]
- Li W., Fu H.-C., Li L., Cabán-Acevedo M., He J.-H., Jin S. Integrated photoelectrochemical solar energy conversion and organic redox flow battery devices. Angew. Chem. Int. Ed. 2016;55:13104–13108. doi: 10.1002/anie.201606986. [DOI] [PubMed] [Google Scholar]
- Li W., Fu H.-C., Zhao Y., He J.-H., Jin S. 14.1% efficient monolithically integrated solar flow battery. Chem. 2018;4:2644–2657. [Google Scholar]
- Liao S., Zong X., Seger B., Pedersen T., Yao T., Ding C., Shi J., Chen J., Li C. Integrating a dual-silicon photoelectrochemical cell into a redox flow battery for unassisted photocharging. Nat. Commun. 2016;7:11474. doi: 10.1038/ncomms11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Shi J., Zhang F., Chen Z., Han J., Ding C., Chen S., Wang Z., Han H., Li C. A tantalum nitride photoanode modified with a hole-storage layer for highly stable solar water splitting. Angew. Chem. Int. Ed. 2014;53:7295–7299. doi: 10.1002/anie.201404697. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li N., Liao K., Li Q., Ishida M., Zhou H. Lowering the charge voltage of Li-O2 batteries via an unmediated photoelectrochemical oxidation approach. J. Mater. Chem. A. 2016;4:12411–12415. [Google Scholar]
- Luo W., Yang Z., Li Z., Zhang J., Liu J., Zhao Z., Wang Z., Yan S., Yu T., Zou Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Envrion. Sci. 2011;4:4046–4051. [Google Scholar]
- Ma Y., Kafizas A., Pendlebury S.R., Le Formal F., Durrant J.R. Photoinduced absorption spectroscopy of CoPi on BiVO4: the function of CoPi during water oxidation. Adv. Funct. Mater. 2016;26:4951–4960. [Google Scholar]
- Ma Y., Pendlebury S.R., Reynal A., Le Formal F., Durrant J.R. Dynamics of photogenerated holes in undoped BiVO4 photoanodes for solar water oxidation. Chem. Sci. 2014;5:2964–2973. [Google Scholar]
- Mayer M.T. Photovoltage at semiconductor–electrolyte junctions. Curr. Opin. Electrochem. 2017;2:104–110. [Google Scholar]
- Montoya J.H., Seitz L.C., Chakthranont P., Vojvodic A., Jaramillo T.F., Nørskov J.K. Materials for solar fuels and chemicals. Nat. Mater. 2016;16:70–81. doi: 10.1038/nmat4778. [DOI] [PubMed] [Google Scholar]
- Nikiforidis G., Tajima K., Byon H.R. High energy efficiency and stability for photoassisted aqueous lithium–iodine redox batteries. ACS Energy Lett. 2016;1:806–813. [Google Scholar]
- Nocera D.G. Solar fuels and solar chemicals industry. Acc. Chem. Res. 2017;50:616–619. doi: 10.1021/acs.accounts.6b00615. [DOI] [PubMed] [Google Scholar]
- Pan L., Kim J.H., Mayer M.T., Son M.-K., Ummadisingu A., Lee J.S., Hagfeldt A., Luo J., Grätzel M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018:412–420. [Google Scholar]
- Pellow M.A., Emmott C.J.M., Barnhart C.J., Benson S.M. Hydrogen or batteries for grid storage? A net energy analysis. Energy Envrion. Sci. 2015;8:1938–1952. [Google Scholar]
- Roger I., Shipman M.A., Symes M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017;1:0003. [Google Scholar]
- Rongé J., Bosserez T., Huguenin L., Dumortier M., Haussener S., Martens J.A. Solar hydrogen reaching maturity. Oil Gas Sci. Technol. 2015;70:863–876. [Google Scholar]
- Seitz L.C., Chen Z., Forman A.J., Pinaud B.A., Benck J.D., Jaramillo T.F. Modeling practical performance limits of photoelectrochemical water splitting based on the current state of materials research. ChemSusChem. 2014;7:1372–1385. doi: 10.1002/cssc.201301030. [DOI] [PubMed] [Google Scholar]
- Shinagawa T., Ng M.T.-K., Takanabe K. Electrolyte engineering towards efficient water splitting at mild pH. ChemSusChem. 2017;10:4155–4162. doi: 10.1002/cssc.201701266. [DOI] [PubMed] [Google Scholar]
- Sivula K., van de Krol R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016;1:15010. [Google Scholar]
- Sun K., Saadi F.H., Lichterman M.F., Hale W.G., Wang H.-P., Zhou X., Plymale N.T., Omelchenko S.T., He J.-H., Papadantonakis K.M. Stable solar-driven oxidation of water by semiconducting photoanodes protected by transparent catalytic nickel oxide films. Proc. Natl. Acad. Sci. U S A. 2015;112:3612. doi: 10.1073/pnas.1423034112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um H.-D., Choi K.-H., Hwang I., Kim S.-H., Seo K., Lee S.-Y. Monolithically integrated, photo-rechargeable portable power sources based on miniaturized Si solar cells and printed solid-state lithium-ion batteries. Energy Envrion. Sci. 2017;10:931–940. [Google Scholar]
- Wang Z., Fan F., Wang S., Ding C., Zhao Y., Li C. Bridging surface states and current-potential response over hematite-based photoelectrochemical water oxidation. RSC Adv. 2016;6:85582–85586. [Google Scholar]
- Xu J., Chen Y., Dai L. Efficiently photo-charging lithium-ion battery by perovskite solar cell. Nat. Commun. 2015;6:8103. doi: 10.1038/ncomms9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Li S., Zhang H., Shen Y., Zakeeruddin S.M., Graetzel M., Cheng Y.-B., Wang M. A power pack based on organometallic perovskite solar cell and supercapacitor. ACS Nano. 2015;9:1782–1787. doi: 10.1021/nn506651m. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang J., Kintner-Meyer M.C.W., Lu X., Choi D., Lemmon J.P., Liu J. Electrochemical energy storage for green grid. Chem. Rev. 2011;111:3577–3613. doi: 10.1021/cr100290v. [DOI] [PubMed] [Google Scholar]
- Yu M., McCulloch W.D., Huang Z., Trang B.B., Lu J., Amine K., Wu Y. Solar-powered electrochemical energy storage: an alternative to solar fuels. J. Mater. Chem. A. 2016;4:2766–2782. [Google Scholar]
- Yu M., Ren X., Ma L., Wu Y. Integrating a redox-coupled dye-sensitized photoelectrode into a lithium–oxygen battery for photoassisted charging. Nat. Commun. 2014;5:5111. doi: 10.1038/ncomms6111. [DOI] [PubMed] [Google Scholar]
- Zhou X., Liu R., Sun K., Papadantonakis K.M., Brunschwig B.S., Lewis N.S. 570 mV photovoltage, stabilized n-Si/CoOx heterojunction photoanodes fabricated using atomic layer deposition. Energy Envrion. Sci. 2016;9:892–897. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.