Introduction
Genetic kidney diseases are rare situations resulting from mutations in genes expressed in major structures of the kidney, including podocytes, tubular cells, and basement membrane.1 More recently, a new field of monogenic inflammatory diseases has been identified2; these immune-mediated diseases can affect all organs, including the kidney. Here we report on a young girl diagnosed with lupus nephritis and carrying a mutation in the COPA gene encoding for coatomer protein complex subunit alpha.
Case Presentation
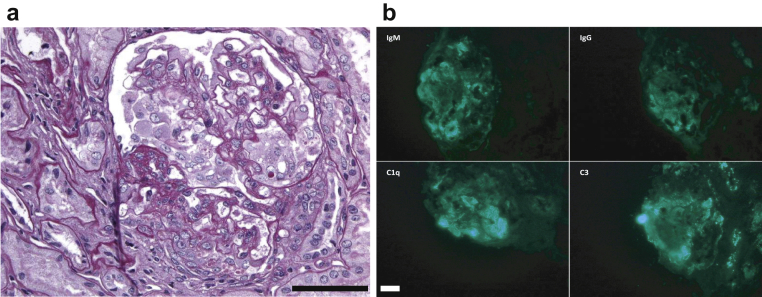
A 10-year-old girl was referred because of fever, facial edema, and fatigue. Upon admission, her blood pressure was 117/70 mm Hg and her temperature was 39 °C. Clinical examination revealed facial edema. Her medical history was unremarkable. Two other second-degree relatives were suspected of having systemic vasculitis because of lung hemorrhage and antineutrophil cytoplasmic antibody positivity. Initial laboratory testing performed at the emergency department revealed acute renal failure with increased serum creatinine at 106 μmol/L, a urinary protein-to-creatinine ratio of 1.76 g/g, and microscopic hematuria with >1×106 red blood cells/ml. C-reactive protein was 3 mg/l, and the erythrocyte sedimentation rate was 52 mm. The hemoglobin level was 9 g/dl. Tests for antinuclear antibodies, antineutrophil cytoplasmic antibody, and antiglomerular basement membrane were negative, and C3 and C4 complement levels were 1.23 g/l and 0.39 g/l, respectively. Infectious screening was normal, and a kidney biopsy was performed. Light microscopy and immunofluorescence studies were consistent with lupus nephritis (Figure 1). Treatment of putative lupus nephritis was started with a steroid pulse, i.v. cyclophosphamide, and hydroxychloroquine. Proteinuria persisted in the following months and angiotensin-converting enzyme inhibitors were introduced, but the disease was progressive.
Figure 1.
Kidney biopsy specimen of patient 1 (case index). (a) Proliferative lesions associated with focal and segmental glomerulosclerosis. (b) Immunofluorescence study revealed endomembranous deposits of C1q, C3, IgM, and IgG (bar = 50 µm).
Transient arthralgia of the knee was later reported without joint lesions or synovitis. Two additional biopsies were performed and showed the same immunopathologic pattern with progression of chronic lesions. Mycophenolate mofetil was introduced but without significant impact on the chronic course of the disease and she developed end-stage renal failure. While living donor transplantation was being considered, exploration in the father, age 50 years, revealed chronic proteinuria. A kidney biopsy revealed membranous glomerulonephritis. Meanwhile, the 2 second-degree relatives were diagnosed with COPA syndrome, a monogenic disease presenting as antineutrophil cytoplasmic antibody–associated vasculitis. The patient and her father then underwent screening for COPA mutation, and they both carried the familial mutation. Diagnosis of COPA syndrome was made.
Discussion
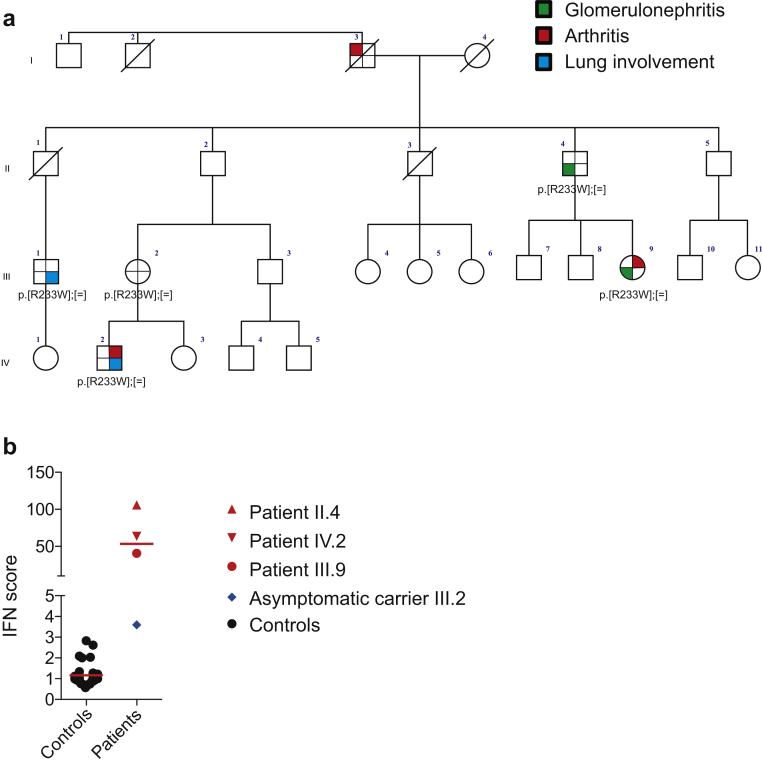
COPA syndrome represents a newly recognized autosomal-dominant cause of autoinflammatory/autoimmune disease characterized by variable expression and a high frequency of clinical nonpenetrance. Arthritis, interstitial lung disease, and glomerulonephritis represent the main characteristic features.3 The COPA syndrome occurs as a result of a heterozygous mutation in the COPA gene encoding for the alpha subunit of the COPI coatomer protein complex. The COPI protein complex is involved in the trafficking of membranes containing proteins and lipids from the Golgi to the endoplasmic reticulum.4 To date, the link between intracellular trafficking and immune dysregulation has not been characterized, but endoplasmic reticulum stress and the activation of the unfolded protein response have been evocated.4 Here, the family history was informative (Figure 2a). Despite the absence of symptoms in the siblings of the father, a phenotypic link between the cousins, the father, and index case was suspected, and genetic screening for the known familial COPA mutation confirmed that both the father and the index case were heterozygous for the same amino acid substitution (p.Arg233His), a mutation already described as causal in 2 families.3 The family tree in this pedigree illustrates the variable expression and incomplete penetrance of COPA syndrome, suggesting that additional environmental or genetic modifiers might influence the phenotype. To date, 31 cases of COPA syndrome have been reported, 14 of which presented with renal lesions; the main histologic features are crescentic glomerulonephritis and focal mesangial hypercellularity with immune complex deposits, ranging from isolated IgA deposits to “full-house” immunofluorescence (IgM, IgG, and C1q deposits).
Figure 2.
Genetic and immunologic description. (a) Family tree of the index case (III.9) with mutation segregation and clinical features. (b) Type I interferon (IFN) assessment in whole blood of members of the family. The IFN signature was increased in all symptomatic patients carrying the familial mutation.
The patient benefited from a live-donor transplantation from her mother, and renal function was satisfactory 3 months after transplantation. Remarkably, patient IV.2 displayed a positive response to rituximab, suggesting a possible contribution of B cells to some aspect of the disease.
Underlying pathogenesis of COPA syndrome is still unknown, but a positive type I interferon signature has been identified in some patients with COPA syndrome.5 We also explored type I interferon signaling in the peripheral blood of different members of the family and identified a positive transcriptomic signature in all symptomatic individuals tested (Figure 2b). Of note, type I interferon is a key cytokine in systemic lupus erythematosus. Taken together, a history of arthritis, interstitial lung disease, and immune-mediated glomerulonephritis with dominant inheritance is evocative of COPA syndrome (Table 1). Type I interferon assessment might represent an additional biologic marker of this syndrome and an explanatory factor for the overlapping features of COPA syndrome with both lupus renal immunopathology and the phenotype associated with gain-of-function of the gene-encoding Stimulator of Interferon Genes (STING).6 Thus Janus kinase inhibitors or type I interferon inhibition may represent promising therapies in this context.
Table 1.
Teaching points
| COPA syndrome is an inherited disease presenting with systemic autoimmunity from antineutrophil cytoplasmic antibody vasculitis to lupus phenotype |
| Expressivity and penetrance are variable in a single family |
| Type I interferon is increased in symptomatic patients and may play a role in the pathogenesis of the disease |
COPA, coatomer protein.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was funded by the French Society for Rheumatology (SFR) and a grant from the Agence Nationale de la Recherche (ANR14-CE14-0026).
References
- 1.Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–1295. doi: 10.1016/S0140-6736(10)60236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belot A. Are all pediatric-onset inflammatory diseases genetically driven? [article in French] Arch Pediatr. 2015;22:1103–1106. doi: 10.1016/j.arcped.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Watkin L.B., Jessen B., Wiszniewski W. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet. 2015;47:654–660. doi: 10.1038/ng.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vece T.J., Watkin L.B., Nicholas S. Copa syndrome: a novel autosomal dominant immune dysregulatory disease. J Clin Immunol. 2016;36:377–387. doi: 10.1007/s10875-016-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpi S., Tsui J., Mariani M. Type I interferon pathway activation in COPA syndrome. Clin Immunol. 2018;187:33–36. doi: 10.1016/j.clim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Jeremiah N., Neven B., Gentili M. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124:5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]