Introduction
Keloids are a recurrent benign neoplastic condition that result from a traumatic injury to the skin in susceptible individuals. Keloid tissue extends beyond the margins of the injured site; this characteristic distinguishes it from hypertrophic scars. Individuals of African, Hispanic, or Asian descent appear to be at increased risk for keloids.1 Keloids tend to be asymptomatic but may cause pain or itching and have functional, aesthetic, or psychosocial impact on patients.2 The extent of scar formation is influenced by the duration and extent of the inflammatory phase of wound healing, which is longer in patients with hidradenitis suppurativa (HS). The prevalence of keloids among African-American and biracial individuals in the US population is higher than that among white patients.3 Some cutaneous areas are more prone to keloid scar development, likely caused by the vulnerability to trauma such as ear lobes. Other types of direct skin causes include body piercings, burns, lacerations, and surgical wounds. Keloids can also result from inflammatory processes including acne and folliculitis.4
HS is a chronic inflammatory skin disease characterized by persistent or recurrent flares of inflamed painful nodules, sinus tracts, and scars in the intertriginous areas.5 The prevalence of HS is estimated at 1% worldwide with a female/male ratio of 3:1.6 The pathogenesis of HS is not completely understood. Follicular occlusion is believed to initiate the process, trapping commensal bacteria within the follicle. The rupture of the pilosebaceous unit and activation of the innate immune system leads to a chronic tissue inflammation that is difficult to extinguish.7
Characteristic HS lesions commonly heal with different types of scars. Atrophic scars are shallow and, in some cases, cribriform, whereas hypertrophic scars can present as firm plaques or rope-like scars. In individuals prone to keloid, chronic inflammatory HS lesions may lead to keloid formation that may contain both mixed of inflammatory lesions and scars.
Two reported cases exist in the literature of keloids in HS and successful treatment with adalimumab.8, 9 We present a case series of 10 more patients with keloid formation in HS wounds and discuss the clinical presentation and therapeutic options.
Case series
We identified 47 patients with keloids from September 2018 to February 2019. Only 10 patients had keloid formation at the location of HS lesions (Table I). Patients with keloids at distant sites from HS lesions or appearing after intralesional steroid injections were excluded.
Table I.
Characteristics of HS patients with keloids
| Case | Age | Sex | Ethnicity | Keloids location | Hurley stage | Keloids treatment |
|---|---|---|---|---|---|---|
| 1 | 50 | Female | Asian | Chest | II | Intralesional triamcinolone |
| 2 | 36 | Male | African | Chest, axillae, neck | III | Intralesional triamcinolone |
| 3 | 30 | Female | Middle Eastern | Back | I | None |
| 4 | 25 | Female | Caucasian | Back | III | Intralesional triamcinolone |
| 5 | 45 | Female | Asian | Chest | I | Intralesional triamcinolone |
| 6 | 63 | Male | African | Chest, axillae, inguinal | III | Intralesional triamcinolone |
| 7 | 26 | Female | Middle Eastern | Shoulder | I | Intralesional triamcinolone |
| 8 | 32 | Female | Caucasian | Axillae | II | None |
| 9 | 42 | Female | African | Chest | I | None |
| 10 | 22 | Female | Caucasian | Axillae, thighs and back | II | Intralesional triamcinolone |
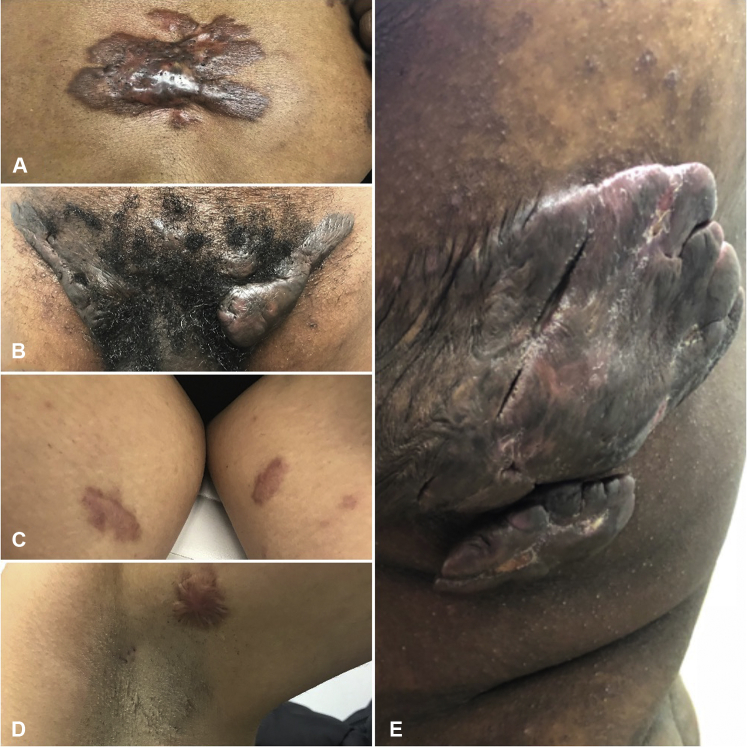
We identified 8 women and 2 men, ages 25 to 63 years. Patient ethnicities were African,3 Asian,2 Middle Eastern,2 and white.2 The most frequent site affected was the chest (60% of cases) followed by the axillae. Some patients had extensive keloids in multiple sites such as one patient (case 6) in the mid chest, bilateral axilla, and inguinal (Fig 1, A and B); one patient (case 10) on the bilateral axilla and medial thighs (Fig 1, C and D); and one patient (case 2) on the back and trunk (Fig 1, E). All patients had a history of HS in the area before keloid formation. The severity of HS using Hurley staging methods included mild-to-severe disease.10 Four patients had stage I, 3 had stage II, and 3 had stage III disease. Patients were treated with intralesional injection of triamcinolone or had received no specific treatment for keloids. Three of 10 patients received adalimumab for treatment of HS with subsequent reduction in both keloidal size and pruritus.
Fig 1.
Keloid spectrums in HS. Case 6 shows keloids in the mid chest (A) and bilateral inguinal regions (B). Case 10 shows keloidal plaques in bilateral medial thighs (C) and left axilla (D). Note the double-ended comedones. Case 2 has large extensive keloids on the left side of the chest (E).
Discussion
We present the largest series of keloids developing in the setting of HS. HS is a chronic inflammatory skin disease characterized by inflamed painful nodules, tracts, and scars. Medical treatment of HS consists of antimicrobial and biologic medications, but none are curative; consensus guidelines have recently been generated. It is thought that medical therapy combined with surgical therapy will lead to the most effective outcomes. Patients often do not respond consistently to a single treatment modality; the presence of keloids in the setting of HS lesions presents a greater therapeutic dilemma.
The etiology of keloids is far better understood than HS. There are several theories linking keloid formation to fibroblast dysfunction with an overproduction of type-I procollagen and higher levels of growth factors including vascular endothelial growth factor, transforming growth factor β1 and β2, and platelet-derived growth factor.10 Keloid fibroblasts have lower rates of apoptosis and show a downregulation of apoptosis-related genes, including p53.11 Neutrophils and macrophages release proinflammatory cytokines such as interleukin (IL)-1 and IL-6 and tumor necrosis factor (TNF)-α, which increase the level of reactive oxygen species and lead to extracellular matrix and cell damage. This process of keloid formation occurs at the site of tissue injury as an exaggerated response to scar formation.
The relationship between HS and keloid formation is unknown. HS, however, is associated with extensive scar formation and sinus tracts; thus, we hypothesize that the pathway underlying tissue destruction and scar formation in HS triggers keloid formation. HS has been associated with upregulation of proinflammatory cytokines including IL-1, similar to keloids, which may also play a role in scar formation. It is uncertain whether the scarring pathways overlap or represent 2 distinct pathways with the HS scarring cascade triggering keloid formation.
Treating a patient with both HS and keloids within the same area is complex. In the setting of no residual HS inflammation, but only HS scar with keloid formation, it is likely that these lesions may respond to traditional keloid therapeutic modalities such as intralesional corticosteroid injection.12 However, keloids that occur in the chronically inflamed HS lesions require a different approach that targets not only keloid reduction and prevention but also addresses the underlying HS process leading to scar formation. In patients with active HS, intralesional steroids are commonly used in the management of localized flares.13 However, the impact of intralesional steroids in the setting of combined keloids and HS has yet to be studied.
Biologic therapy may also play a role in the management of keloid in the setting of HS. TNF-α induces nuclear factor κB, which is known to play a role in the pathogenesis of keloids by stimulating fibroblast proliferation.14 Intralesional etanercept (anti–TNF-α) has been shown to reduce pruritus and size of keloids.15 However, the role of biologics in reduction of keloid size could be linked to early control of inflammation, which will decrease the risk of keloid formation and also help decrease the bulk of keloid mass containing inflammatory lesions.
While this article is, to our knowledge, the largest study described in the literature, our ability to make concrete treatment recommendations for combined HS and keloid lesions is difficult. We recommend a trial of biologic therapy and/or intralesional corticosteroid injection especially in the setting of ongoing inflammation. We hypothesize that the HS-triggered acute inflammation, which leads to scar formation and subsequent keloid, may resolve.
For patients with isolated keloid scars in the absence of significant inflammation, surgical excision, intralesional injection (corticosteroids, 5-fluorouracil, bleomycin, and interferon), topical therapy (imiquimod, corticosteroids), plus physical modalities (compression, cryotherapy, radiation, silicon sheeting, and laser radiofrequency or light-based therapies) are appropriate.16 Optimal treatment of patients with HS and keloids requires further investigation.
Recurrence rates after keloid excision alone range from 45% to 100%. We believe that the development of keloids in HS patients is caused by underlying tissue inflammation triggered by HS. Ongoing inflammation makes management of keloids in HS patients daunting because scars and keloids continue to remodel during the course of the disease. It is important to counsel the patient on the importance of controlling HS to prevent further scarring and contractures. Longer wound healing and more inflammation tends to result in more pathologic scars and scar formation. In active HS, we recommend a trial of multimodal therapies including intralesional triamcinolone concomitant with systemic antibiotic, biologics, and surgical therapy targeting the microbiome in early diagnosed cases, depending on their disease severity, to shorten the time to healing. Surgery should be used in medically optimized patients, whereas radiation should be reserved for refractory progressive keloids, given the potential for skin cancer development within HS lesions. Scars and contractures from HS have a significant impact on patient quality of life, causing social isolation and embarrassment as well as pain and itching. Clinicians need to be cognizant of scar and keloid formation risk in these patients and provide adequate counseling and support.
Conclusion
Keloid formation in patients with HS likely results from chronic tissue inflammation. Management of keloids in HS patients can be difficult given the chronicity of the skin inflammation and the formation of deep tunnels and tracts. We recommend treatment with intralesional triamcinolone or immunosuppressive therapy including other biologics. Patients require counselling regarding scar formation from HS, particularly in those prone to keloid formation. Further studies are necessary to understand the pathogenesis of keloids in the setting of HS.
Footnotes
Funding sources: None.
Conflicts of interest: Drs Alavi and O'Brien: Abbvie; speakers bureau and members of the National Advisory Committee for HS.
References
- 1.Bayat A., McGrouther D.A., Ferguson M.W. Skin scarring. BMJ. 2003;326(7380):88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock O., Schmid-Ott G., Malewski P., Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433–438. doi: 10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 3.Garg A., Kirby J.S., Lavian J., Lin G., Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crockett D.J. Regional keloid susceptibility. Br J Plast Surg. 1964;17:245–253. doi: 10.1016/s0007-1226(64)80040-0. [DOI] [PubMed] [Google Scholar]
- 5.Jemec G.B. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 6.Revuz J.E., Canoui-Poitrine F., Wolkenstein P. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 7.von Laffert M., Stadie V., Wohlrab J., Marsch W.C. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164(2):367–371. doi: 10.1111/j.1365-2133.2010.10034.x. [DOI] [PubMed] [Google Scholar]
- 8.Singer E. Hidradenitis suppurativa with extensive secondary keloid formation. JAAD. 2017;76(6):AB153. [Google Scholar]
- 9.Scheinfeld N. Treatment of coincident seronegative arthritis and hidradentis supprativa with adalimumab. J Am Acad Dermatol. 2006;55(1):163–164. doi: 10.1016/j.jaad.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Hurley H.J. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk R.K., Roenigk H.H.J., editors. Dermatologic surgery: principles and practice. Marcel Dekker; New York: 1989. pp. 717–743. [Google Scholar]
- 11.Marneros A.G. KTK–cd, pathogenesis, and treatment options. J Dtsch Dermatol Ges. 2004;2(11):905–913. doi: 10.1046/j.1439-0353.2004.04077.x. [DOI] [PubMed] [Google Scholar]
- 12.De Felice B., Ciarmiello L.F., Mondola P. Differential p63 and p53 expression in human keloid fibroblasts and hypertrophic scar fibroblasts. DNA Cell Biol. 2007;26(8):541–547. doi: 10.1089/dna.2007.0591. [DOI] [PubMed] [Google Scholar]
- 13.Riis P.T., Boer J., Prens E.P. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75(6):1151–1155. doi: 10.1016/j.jaad.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Zhu G., Cai J., Zhang J., Zhao Y., Xu B. Abnormal nuclear factor (NF)-kappaB signal pathway and aspirin inhibits tumor necrosis factor alpha-induced NF-kappaB activation in keloid fibroblasts. Dermatol Surg. 2007;33(6):697–708. doi: 10.1111/j.1524-4725.2007.33146.x. [DOI] [PubMed] [Google Scholar]
- 15.Berman B., Patel J.K., Perez O.A. Evaluating the tolerability and efficacy of etanercept compared to triamcinolone acetonide for the intralesional treatment of keloids. J Drugs Dermatol. 2008;7(8):757–761. [PubMed] [Google Scholar]
- 16.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125(2):557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]