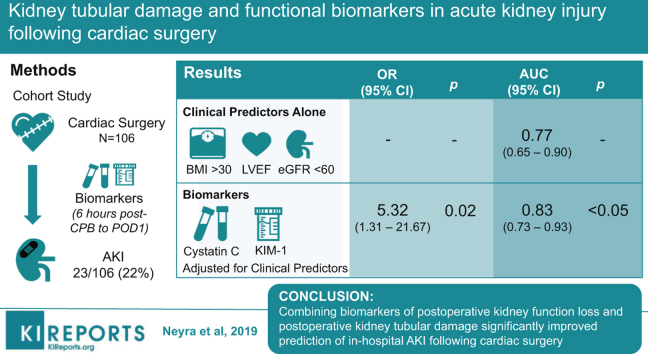
Abstract
Background
Cardiac surgery–associated acute kidney injury (AKI) is associated with increased morbidity and mortality. We examined the utility of combining biomarkers of kidney function loss (serum cystatin C) and kidney tubular damage (urine neutrophil gelatinase-associated lipocalin [NGAL] and Kidney Injury Molecule-1 [KIM-1]) for the prediction of post–cardiac surgery AKI.
Methods
Single-center prospective cohort study of 106 adults undergoing coronary artery bypass grafting and/or valve surgery with cardiopulmonary bypass (CPB). Primary outcome was postoperative in-hospital AKI defined by serum creatinine (SCr)–Kidney Disease: Improving Global Outcomes criteria. Biomarkers were measured preoperatively, 6 hours after CPB and on postoperative days (PODs) 1 to 4.
Results
A total of 23 subjects (21.7%) developed AKI. After adjusting for preoperative left ventricular ejection fraction, body mass index >30 kg/m2, and estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2, the combination of peak serum cystatin C and peak urine KIM-1/creatinine (Cr) (6 hours post-CPB to POD 1) above optimal cutoff significantly associated with postoperative AKI (odds ratio [OR]: 5.32; 95% confidence interval [CI]: 1.31–21.67; P = 0.020). This biomarker combination significantly improved the performance of the clinical model for the prediction of postoperative AKI (area under the curve [AUC]: 0.77, 95% CI: 0.65–0.90 for the clinical model alone versus 0.83, 95% CI: 0.73–0.93 for the clinical model with the addition of biomarker data, P = 0.049).
Conclusions
Combining biomarkers of postoperative kidney function loss and postoperative kidney tubular damage significantly improved prediction of in-hospital AKI following cardiac surgery. Future large, multicenter studies are warranted to assess whether panels of biomarkers reflecting distinct pathobiology can be used to guide interventions and improve short- and long-term outcomes in patients undergoing cardiac surgery.
Keywords: acute kidney injury, biomarkers, critical care, major adverse kidney events, prediction, thoracic surgery
Graphical abstract
AKI after cardiac surgery is associated with increased morbidity,1, 2, 3, 4, 5 mortality,6, 7, 8, 9 and health care costs.10, 11 It occurs in approximately 20% of cardiac surgery patients and is the second leading cause of in-hospital AKI.12, 13 Even small increases (<50% from baseline) in SCr after cardiac surgery have been associated with higher postoperative mortality.14 Diagnosis of cardiac surgery–associated AKI using SCr may occur 2 to 3 days after AKI onset, as glomerular filtration rate must decline significantly before SCr rises,15 postoperative hemodilution can mask detection of SCr elevation,16 and monitoring of urine output as a marker of AKI is insensitive due to diuresis from medication and physiologic effects post-CPB.
Ability to detect AKI early after cardiac surgery could lead to interventions that mitigate kidney damage and preserve kidney function.17 Although individual AKI biomarkers have been studied for early detection of post–cardiac surgery AKI,18, 19 few studies have assessed the utility of combining biomarkers of kidney function loss and kidney tubular damage.20 In addition, little is known about the utility of non-SCr AKI biomarkers for predicting post-AKI outcomes.
This study measured serum cystatin C,21, 22 urine NGAL,23 and urine KIM-124 before and after cardiac surgery. The primary hypothesis was that combining biomarkers of kidney function loss and tubular damage measured in the early postoperative period will improve prediction of in-hospital post–cardiac surgery AKI. An exploratory secondary hypothesis of this study was that these biomarker combinations will predict death, renal replacement therapy (RRT), or having a ≥25% reduction in postoperative eGFR in reference to preoperative eGFR at approximately 30 days after cardiac surgery.
Methods
Study Population
A total of 116 patients scheduled for coronary artery bypass graft and/or valve surgery on CPB at the University of Texas Southwestern Medical Center in Dallas, TX, underwent prospective enrollment into this observational cohort study (clinicaltrials.gov NCT01258231). Exclusion criteria for enrollment included inability to give informed consent, age <20 years, preoperative hematocrit <25%, or known infection with HIV or hepatitis C virus. All surgeries (n = 116) were performed at Clements University Hospital from May 5, 2015, through March 10, 2017. Ten enrolled subjects were excluded from study analyses because they required preoperative RRT (n = 2), they withdrew from the study before postoperative day 4 (n = 3), or they did not have blood and urine samples for analysis (n = 5). The study was approved by the University of Texas Southwestern Institutional Review Board, and all subjects provided written informed consent.
Clinical Data
Patient characteristics, medications, comorbidities, surgical characteristics, and postoperative events during primary surgical hospitalization were collected using a standardized case report form. Post–hospital discharge SCr values (value closest to 30 days postsurgery from routine clinical care medical records) were obtained retrospectively by medical record review.
Study Definitions
Peak biomarker measurements were defined for 2 different ranges of postoperative time points: (i) peak of postoperative hour 6 after CPB and the morning of POD 1; and (ii) peak of postoperative hour 6 after CPB and measurements on the mornings of PODs 1 to 4.
Diabetes was defined as requiring preoperative insulin or oral hypoglycemic medication. Preoperative anemia was defined by the World Health Organization cutoffs of <12 g/dl in women and <13 g/dl in men.25 Preoperative beta-blocker use was defined as administration within 24 hours preoperatively. Preoperative history of thyroid disease was defined as history of hypothyroidism or hyperthyroidism and/or taking thyroid hormone replacement therapy at the time of surgery. Other preoperative medications were analyzed if found on the patient’s home medication list on the same day of surgery or on the patient’s hospital medication list if the patient was hospitalized before surgery.
Serum and Urine Biomarker Data
Serum and urine samples were obtained preoperatively, hour 6 after CPB, and daily on PODs 1 to 4. Serum samples were centrifuged at 1227g for 15 minutes at 4 °C. Urine samples were centrifuged at 626g for 30 minutes at 4 °C. Serum and urine aliquots were stored at −80 °C until thawed for batch analysis. Urine creatinine concentrations were measured by capillary electrophoresis. Enzyme-linked immunosorbent assays were used to measure serum cystatin C (R&D Systems, Minneapolis, MN), urine KIM-1 (R&D Systems), and urine NGAL (BioPorto Diagnostics, Hellerup, Denmark). The lower limit of detection for urine KIM-1 was 0.156 ng/ml. Of the total of 598 urine samples, only 6 values were below this limit, and these values were analyzed as being 0.156 ng/ml. Measurements of cystatin C and NGAL were not below the lower limits of detection for their respective assays. Urine NGAL and KIM-1 values were normalized by urine Cr level.
Study Outcomes
The primary study outcome was in-hospital postoperative AKI defined by the SCr–Kidney Disease: Improving Global Outcomes criteria comparing postoperative SCr values with preoperative SCr measured closest to the time of surgery. These criteria were an increase in postoperative SCr of ≥0.3 mg/dl within 48 hours, or >1.5-fold increase in SCr during the 7 days following surgery or during primary surgical hospitalization if hospital stay was less than 7 days.26 A secondary study outcome was major adverse kidney events (MAKEs), defined as postoperative death, or the need for RRT during the 30 days following surgery, or having ≥25% reduction in postoperative eGFR in reference to preoperative eGFR (determined by the post–hospital discharge routine clinical care SCr value available closest to 30 days after surgery). If no postdischarge SCr value was available, the last SCr measured during primary surgical hospitalization was used. Preoperative baseline eGFR was determined by the Chronic Kidney Disease–Epidemiology Collaboration equation.27
Statistical Analysis
Statistical analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC). P values were 2-tailed for all analyses. Data for preoperative and peak postoperative serum cystatin C, urine KIM-1, and urine NGAL were right-skewed in distribution. Continuous biomarker data were therefore log10 transformed to normalize distributions before additional analyses. Clinical variables were selected a priori as potential predictors of postoperative AKI and MAKE. Chi-square, t-tests, Fisher’s exact, and nonparametric Wilcoxon tests were used to assess univariate difference in clinical and biomarker variables between patients who did and did not develop in-hospital postoperative AKI.
Logistic regression was used to assess univariate associations of AKI biomarkers with primary and secondary outcomes. Two different clinical models for predicting postoperative AKI were selected a priori and assessed for benefit of adding information to the study’s AKI biomarker data. Model 1 included preoperative eGFR <60 ml/min per 1.73 m2, preoperative left ventricular ejection fraction, and obesity (body mass index >30 kg/m2). These variables also have been reported in prior studies as risk factors for AKI after cardiac surgery.28, 29 An alternative model 1 was additionally assessed in which preoperative eGFR and body mass index were included in the model as continuous variables. Model 2 consisted of the Cleveland Clinic score, a preoperative risk prediction score for predicting AKI-RRT following cardiac surgery.30 Peak biomarker levels were assessed alone for association with postoperative AKI and were then added separately and then together (cystatin C plus either NGAL or KIM-1) to model 1 and model 2. Receiver operating characteristics analysis was used to determine optimal cutoffs of peak biomarker levels before considering duplets of biomarkers. The point on the receiver operating characteristic curve that was closest to the left-upper corner of unit square was selected as the optimal cutoff value for the respective biomarker. The 2 better performing biomarkers were then combined as follows: (i) at least 1 biomarker was above the receiver operating characteristic cutoff value (combination 1), or (ii) both biomarkers were above the receiver operating characteristic cutoff value (combination 2). These combinations of biomarkers were assessed as predictors of postoperative AKI. Performance of the AKI biomarkers and their combinations in predicting the AKI outcome were assessed using change in AUC, integrated discrimination improvement, and net reclassification improvement. For predicting the study’s MAKE outcome, biomarker data were assessed using logistic regression with adjustment for the occurrence of postoperative in-hospital AKI (SCr–Kidney Disease: Improving Global Outcomes criteria).
Results
A total of 106 patients were included in the study’s analyses. The incidence of postoperative AKI was 21.7% (n = 23). Stage 1 AKI occurred in 16 (74%), stage 2 AKI occurred in 5 (22%), and stage 3 AKI in 1 patient (4%). Table 1 compares characteristics between patients who did and did not develop postoperative in-hospital AKI. Patients who suffered from AKI were more frequently obese, had lower preoperative left ventricular ejection fraction, and had lower urine output during the first 24 hours after surgery when compared with those without AKI. In addition, patients with AKI stayed longer in the hospital than patients without AKI: median (interquartile range) 9 (7, 13) versus 6 (5, 8) days, P < 0.001.
Table 1.
Patient characteristics of 106 subjects who underwent cardiac surgery with CPB stratified by the occurrence of postoperative in-hospital AKI
| All n = 106 | AKI n = 23 | No-AKI n = 83 | P | |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Age, yr, mean ± SD | 61 ± 12 | 62 ± 12 | 61 ± 12 | 0.83 |
| Female, % | 29 (27.4) | 6 (26.1) | 23 (27.7) | 0.88 |
| Race, % | 0.70 | |||
| White | 68 (64.1) | 13 (56.5) | 55 (66.3) | |
| Black | 14 (13.2) | 4 (17.4) | 12 (14.5) | |
| Other | 24 (22.6) | 6 (26.1) | 16 (19.3) | |
| Obesity, BMI >30 kg/m2, % | 50 (47.2) | 18 (78.3) | 32 (38.6) | <0.001 |
| Diabetes mellitus, % | 35 (33.0) | 7 (30.4) | 28 (33.7) | 0.77 |
| Hypertension, % | 21 (19.8) | 21 (91.3) | 64 (77.1) | 0.15 |
| Hypercholesterolemia, % (n = 105) | 72 (68.6) | 18 (78.3) | 54 (65.9) | 0.26 |
| Tobacco smoking, % | 0.73 | |||
| Current smoker | 9 (8.5) | 2 ( 8.7) | 7 ( 8.4) | |
| Past smoker | 54 (50.9) | 10 (43.5) | 44 (53.0) | |
| Never smoker | 43 (40.6) | 11 (47.8) | 32 (38.6) | |
| Baseline eGFR, ml/min per 1.73 m2, mean ± SD | 71.0 ± 23.3 | 71 ± 24 | 79 ± 23 | 0.18 |
| Baseline eGFR < 60 ml/min per 1.73 m2 | 23 (21.7) | 6 (26.1) | 17 (20.5) | 0.58 |
| Left ventricular ejection fraction %, mean ± SD | 57 ± 9.9 | 50 ± 12 | 59 ± 8 | <0.001 |
| History of preoperative arrhythmia, % | 16 (15.1) | 5 (21.7) | 11 (13.3) | 0.33 |
| History of heart failure, % | 32 (30.2) | 9 (39.1) | 23 (27.7) | 0.29 |
| History of thyroid disease, % | 12 (11.3) | 1 (4.3) | 11 (13.3) | 0.46 |
| Anemia, % | 35 (33.0) | 7 (30.4) | 28 (33.7) | 0.77 |
| Cleveland Clinic preoperative score,30 median (IQR) | 2 (1, 4) | 3 (1, 5) | 2 (1, 4) | 0.40 |
| Preoperative medications | ||||
| ACEI or ARB, % | 65 (61.3) | 18 (78.3) | 47 (56.6) | 0.09 |
| Loop diuretic, % | 36 (34.0) | 11 (47.8) | 25 (30.1) | 0.11 |
| Statin, % | 76 (71.7) | 17 (73.9) | 59 (71.1) | 0.79 |
| Beta blocker (within 24 h of surgery), % | 58 (54.7) | 15 (65.2) | 43 (51.8) | 0.25 |
| Calcium channel blocker, % | 24 (22.6) | 4 (17.4) | 20 (24.1) | 0.58 |
| Aspirin, % | 71 (67.0) | 16 (69.6) | 55 (66.3) | 0.77 |
| Nonaspirin platelet inhibitor, % | 10 (9.4) | 2 (8.7) | 8 (9.6) | 1.00 |
| Surgical, intraoperative, and postoperative characteristics | ||||
| CPB time, minutes, median (IQR) | 121 (104, 161) | 125 (105, 180) | 121 (103, 161) | 0.48 |
| Aortic cross-clamp time, minutes, median (IQR) | 85 (70, 113) | 87 (72, 114) | 85 (68, 112) | 0.49 |
| Type of cardiac surgery, % | 0.36 | |||
| CABG only | 43 (40.6) | 10 (43.5) | 33 (39.8) | |
| CABG plus valve | 11 (10.4) | 4 (17.4) | 7 (8.4) | |
| Valve only | 52 (49.1) | 9 (39.1) | 43 (51.8) | |
| Reoperative cardiac surgery, % | 12 (11.3) | 4 (17.4) | 8 (9.6) | 0.29 |
| Nadir intraoperative hemoglobin, g/dl, mean ± SD | 7.8 ± 1.5 | 7.4 ± 1.5 | 7.9 ± 1.5 | 0.16 |
| Intraoperative PRBC transfusion, % | 33 (31.1) | 7 (30.4) | 26 (31.3) | 0.94 |
| PRBC transfused intraoperative + 24 h after surgery, % | 44 (41.5) | 12 (52.2) | 32 (38.6) | 0.24 |
| ICU length of stay, d, median (IQR) | 2 (1, 4) | 4 (2, 6) | 2 (1, 3) | <0.001 |
| Hospital length of stay, d, median (IQR) | 7 (6, 9) | 9 (7, 13) | 6 (5, 8) | <0.001 |
| Urine output first 24 h after surgery, liters, median (IQR) | 1.72 (1.29, 2.10) | 1.29 (1.10, 1.72) | 1.86 (1.51, 2.17) | 0.004 |
ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin-II receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; PRBC, packed red blood cell.
Data are shown as n (%) for categorical variables and mean ± SD or median (IQR = 25th and 75th percentile) for continuous variables.
The study’s postoperative MAKE outcome occurred in 15.1% (n = 16) of patients. Of these 16 patients, 2 patients died within 30 days after surgery, with 1 of these requiring RRT before death. One patient required RRT within 30 days after surgery and survived. The other 13 subjects with MAKE did not require RRT but had postoperative eGFR that did not return to within 25% of preoperative value. The median (interquartile range) days to available postoperative SCr (closest to 30 days after surgery) was 35 (14, 80) days. The MAKE outcome was more frequently encountered in patients who suffered from postoperative in-hospital AKI (30.4% of patients with AKI patients vs. 10.8% of patients without AKI; P = 0.02).
Perioperative Serum and Urine Biomarker Levels in Patients With and Without Postoperative AKI
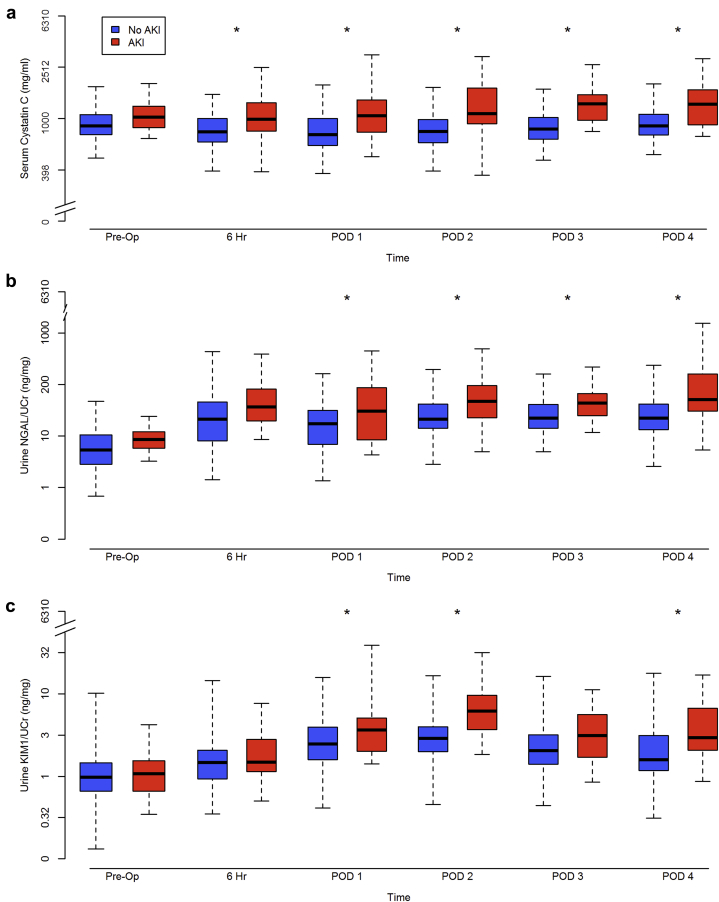
Box plots of log10 biomarker measurements across the perioperative time points stratified by the occurrence of AKI versus no-AKI are represented in Figure 1. Biomarker levels were differentially and significantly elevated at 6 hours post-CPB (serum cystatin C) or POD 1 (urine NGAL/Cr and KIM-1/Cr) in patients with versus without AKI (Figure 1).
Figure 1.
Perioperative time-varying biomarker levels stratified by the occurrence of postoperative in-hospital acute kidney injury (AKI). (a) Serum cystatin C; (b) urine neutrophil gelatinase-associated lipocalin (NGAL)/creatinine (Cr); and (c) urine kidney injury molecule 1 (KIM-1)/Cr. *P < 0.05 comparison between patients with AKI (red) and without AKI (blue). The y-axis denotes biomarker measurements and is log-scaled; the x-axis denotes study perioperative time points. Edges of each box plot represent the lower and upper limits of the interquartile range and lines across the middle of the box plots represent median values. For the 23 patients with AKI and the 83 patients without AKI, serum cystatin C was measured preoperatively (n = 23/n = 83), hour 6 post–cardiopulmonary bypass (CPB) (n = 21/n = 79), postoperative day (POD) 1 (n = 23/n = 83), POD 2 (n = 22/n = 81), POD 3 (n = 21/n = 82), and POD 4 (n = 22/n = 78); urine biomarkers were measured preoperatively (n = 23/n = 80), hour 6 post-CPB (n = 20/n = 80), POD 1 (n = 23/n = 83), POD 2 (n = 23/n = 78), POD 3 (n = 19/n = 78), and POD 4 (n = 22/n = 69).
Association of Postoperative Peak Biomarker Levels With Postoperative In-hospital AKI
Table 2 shows the association of individual peak biomarker levels and combinations of biomarkers with postoperative in-hospital AKI.
Table 2.
Logistic regression assessment of clinical parameters and peak postoperative biomarker levels (independent variables) with the development of postoperative in-hospital AKI (dependent variable)
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
|---|---|---|---|---|
| Clinical model 1 | ||||
| Preoperative eGFR < 60 ml/min per 1.73 m2 | 1.27 (0.35–4.58) | 0.71 | ||
| Preoperative LVEF % | 0.92 (0.87–0.97) | 0.003 | ||
| BMI > 30 kg/m2 | 5.72 (1.77–18.52) | 0.004 | ||
| Clinical model 2 | ||||
| Cleveland Clinic score | 1.14 (0.91–1.42) | 0.27 | ||
| Biomarkers | Postoperative 6 h–POD 1 | Postoperative 6 h–POD 4 | ||
| Serum cystatin C | 3.81 (1.53–9.48) | 0.004 | 6.93 (2.59–18.59) | <0.001 |
| Urine NGAL/Cr | 1.17 (0.97–1.45) | 0.10 | 1.33 (1.05–1.67) | 0.016 |
| Urine KIM-1/Cr | 1.60 (1.06–2.42) | 0.026 | 2.23 (1.41–3.54) | <0.001 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 6.20 (1.71–22.48) | 0.006 | 7.98 (1.98–25.97) | 0.003 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 4.14 (1.50–11.43) | 0.006 | 7.14 (2.54–20.11) | <0.001 |
| Biomarkers adjusted by clinical model 1 | ||||
| Serum cystatin C | 4.09 (1.11–15.09) | 0.035 | 8.87 (2.34–33.58) | 0.001 |
| Urine NGAL/Cr | 1.22 (0.92–1.61) | 0.17 | 1.41 (1.03–1.94) | 0.030 |
| Urine KIM-1/Cr | 1.43 (0.87–2.36) | 0.16 | 2.07 (1.21–3.57) | 0.008 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 5.32 (1.31–21.67) | 0.020 | 6.16 (1.51–25.05) | 0.011 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 3.64 (1.01–13.15) | 0.049 | 4.97 (1.45–17.05) | 0.011 |
| Biomarkers adjusted by clinical model 2 | ||||
| Serum cystatin C | 4.78 (1.56–14.61) | 0.006 | 9.99 (3.12–32.06) | <0.001 |
| Urine NGAL/Cr | 1.16 (0.93–1.45) | 0.19 | 1.32 (1.02–1.69) | 0.033 |
| Urine KIM-1/Cr | 1.56 (1.02–2.38) | 0.041 | 2.22 (1.38–3.57) | 0.001 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 6.02 (1.61–22.55) | 0.008 | 7.15 (1.89–27.00) | 0.004 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 3.94 (1.35–11.44) | 0.012 | 7.03 (2.39–20.70) | <0.001 |
AKI, acute kidney injury; BMI, body mass index (kg/m2); CI, confidence interval; Cr, creatinine; eGFR, estimated glomerular filtration rate (ml/min per 1.73 m2); KIM-1, kidney injury molecule 1; LVEF, left ventricular ejection fraction (%); NGAL, neutrophil gelatinase-associated lipocalin; POD, postoperative day.
Clinical model 1 consisted of 3 preoperative clinical parameters that either were significantly associated with AKI in univariate analysis (preoperative LVEF% and BMI >30 kg/m2) or were clinically relevant for the model (preoperative eGFR <60 ml/min per 1.73 m2). Clinical model 2 was the Cleveland Clinic score validated for predicting AKI–renal replacement therapy following cardiac surgery.30 Duplets of biomarkers were combined as follows: (i) at least 1 biomarker above the cutoff value (combination 1), or (ii) both biomarkers above the cutoff value (combination 2). Biomarker data were log10 transformed and the odds ratios reported correspond to 2-fold higher levels in patients with versus without postoperative AKI.
Serum Cystatin C
Patients with a 2-fold higher peak serum cystatin C (from 6 hours post-CPB to POD 1) were 3.8 times more likely to develop postoperative AKI (OR 3.81; 95% CI: 1.53 – 9.48; P = 0.004). The association remained statistically significant after adjusting for model 1 (adjusted OR: 4.09; 95% CI: 1.11–15.09; P = 0.035) and model 2 (adjusted OR: 4.78; 95% CI: 1.56–14.61; P = 0.006). Two-fold higher peak serum cystatin C (from 6 hours post-CPB to POD 4) was also significantly associated with increased likelihood of postoperative AKI by itself and after adjustment by clinical models 1 and 2 (Table 2).
Urine NGAL/Cr
Two-fold higher peak urine NGAL/Cr (from 6 hours post-CPB to POD 1) was not significantly associated with an increased likelihood of postoperative AKI (OR: 1.17; 95% CI: 0.97–1.45; P = 0.10). The association remained nonsignificant after adjusting for models 1 and 2. A 2-fold higher peak urine NGAL/Cr (from 6 hours post-CPB to POD 4) was, however, significantly associated with an increased likelihood of postoperative AKI in unadjusted and adjusted models (Table 2).
Urine KIM-1/Cr
A 2-fold higher peak urine KIM-1/Cr (from 6 hours post-CPB to POD 1) was significantly associated with an increased likelihood of postoperative AKI by itself (OR: 1.60; 95% CI: 1.06–2.42; P = 0.026) and after adjustment for model 2 (adjusted OR: 1.56; 95% CI: 1.02–2.38; P = 0.041) but not model 1. Similarly, 2-fold higher peak urine KIM-1/Cr (from 6 hours post-CPB to POD 4) was also significantly associated with the occurrence of postoperative AKI (OR: 2.23; 95% CI: 1.41–3.54; P < 0.001). The association remained significant after adjusting for model 1 (adjusted OR: 2.07; 95% CI: 1.21–3.57; P = 0.008) and model 2 (adjusted OR: 2.22; 95% CI: 1.38–3.57; P = 0.001) (Table 2).
Development of Biomarker Cutoffs for Postoperative In-hospital AKI
Cutoffs of peak serum cystatin C and peak urine KIM-1/Cr levels from 6 hours after separating from CPB to POD 1 or POD 4 for the prediction of postoperative in-hospital AKI were identified. The cutoffs exhibited good sensitivity (range: 0.65–0.78) and specificity (range: 0.65–0.70). Duplets of the biomarkers were then combined as follows: (i) at least 1 biomarker above the cutoff value (combination 1), or (ii) both biomarkers above the cutoff value (combination 2).
Association of Combinations of Peak Biomarker Levels With Postoperative In-hospital AKI
Combinations of peak serum cystatin C and peak urine KIM-1/Cr from 6 hours post-CPB to POD 1 were significantly associated with the likelihood of developing postoperative AKI (OR: 6.20; 95% CI: 1.71–22.48; P = 0.006 for combination 1 and OR: 4.14; 95% CI: 1.50–11.43; P = 0.006 for combination 2). Similarly, combinations of peak serum cystatin C and peak urine KIM-1/Cr from 6 hours post-CPB to POD 4 were also significantly associated with the development of postoperative AKI. These associations remained significant after adjustment by models 1 and 2 (Table 2). Further, the observed associations were concordant in direction and magnitude when eGFR and body mass index were included in model 1 as continuous instead of dichotomous variables.
Utility of Combining Kidney Function and Kidney Tubular Injury Biomarkers for Assessment of Postoperative In-hospital AKI
Metrics of performance for the prediction of postoperative AKI when postoperative peak biomarker levels (alone or in combination) were added to the clinical models are represented in Tables 3 and 4. Among the 2 clinical models, model 1 exhibited the best performance. The addition of peak serum cystatin C and peak urine KIM-1/Cr (combination 1) from 6 hours post-CPB to POD 1 significantly improved the performance of model 1 for the prediction of postoperative in-hospital AKI (AUC: 0.77; 95% CI: 0.65–0.90 for clinical model 1 alone vs. 0.83, 95% CI: 0.73–0.93 for model 1 with the addition of biomarker data; P = 0.049, Table 4). Importantly, this combined model using clinical and biomarker data significantly improved the risk reclassification of postoperative AKI over clinical model 1 alone, as evident by net reclassification improvement and integrated discrimination improvement metrics (Table 4).
Table 3.
Utility of peak postoperative biomarker levels expressed as area under the receiver operating characteristics curve for the prediction of postoperative in-hospital AKI
| Postoperative 6 h–POD 1 |
Postoperative 6 h–POD 4 |
|||
|---|---|---|---|---|
| AUC (95% CI) | Pa | AUC (95% CI) | Pa | |
| Clinical model 1 | 0.77 (0.65–0.90) | ref | 0.77 (0.65–0.90) | ref |
| + Serum cystatin C | 0.82 (0.70–0.93) | 0.088 | 0.85 (0.74–0.95) | 0.057 |
| + Urine NGAL/Cr | 0.80 (0.68–0.92) | 0.15 | 0.81 (0.69–0.93) | 0.16 |
| + Urine KIM-1/Cr | 0.80 (0.67–0.92) | 0.19 | 0.83 (0.72–0.94) | 0.064 |
| + Combination 1 Serum cystatin C + urine KIM-1/Cr |
0.83 (0.73–0.93) | 0.049 | 0.83 (0.73–0.94) | 0.036 |
| + Combination 2 Serum cystatin C + urine KIM-1/Cr |
0.80 (0.68–0.91) | 0.32 | 0.81 (0.68–0.93) | 0.24 |
| Clinical model 2 | 0.56 (0.42–0.70) | ref | 0.56 (0.42–0.70) | ref |
| + Serum cystatin C | 0.69 (0.56–0.82) | 0.14 | 0.78 (0.66–0.90) | 0.019 |
| + Urine NGAL/Cr | 0.62 (0.48–0.76) | 0.21 | 0.68 (0.55–0.81) | 0.075 |
| + Urine KIM-1/Cr | 0.63 (0.50–0.76) | 0.29 | 0.74 (0.63–0.85) | 0.023 |
| + Combination 1 Serum cystatin C + urine KIM-1/Cr |
0.68 (0.56–0.80) | 0.006 | 0.68 (0.56–0.80) | 0.007 |
| + Combination 2 Serum cystatin C + urine KIM-1/Cr |
0.64 (0.49–0.78) | 0.10 | 0.71 (0.57–0.84) | 0.010 |
AKI, acute kidney injury; AUC, area under the receiving operating characteristic curve; CI, confidence interval; Cr, creatinine; KIM-1, kidney injury molecule 1; NGAL, neutrophil gelatinase-associated lipocalin; POD, postoperative day.
Clinical model 1 consisted of 3 preoperative clinical parameters that either were significantly associated with AKI in univariate analysis (preoperative left ventricular ejection fraction percent and body mass index >30 kg/m2) or were clinically relevant for the model (preoperative estimated glomerular filtration rate <60 ml/min per 1.73 m2). Clinical model 2 was the Cleveland Clinic score validated for predicting AKI–renal replacement therapy following cardiac surgery.30 Duplets of biomarkers were combined as follows: (i) at least 1 biomarker above the cutoff value (combination 1), or (ii) both biomarkers above the cutoff value (combination 2). Biomarker data were log10 transformed.
P value denotes comparison between clinical model + biomarker(s) vs clinical model alone.
Table 4.
Improvement in the discrimination of the clinical model for the prediction of postoperative in-hospital AKI by combining the 2 clinical models used for the study with peak postoperative biomarker levels following cardiac surgery (peak levels from 6 hours post–cardiopulmonary bypass to postoperative day 1): the IDI and NRI are reported
| IDI | IDI events (95% CI) | IDI non-events (95% CI) | Absolute IDI (95% CI) | P |
|---|---|---|---|---|
| Clinical model 1 + peak of postoperative 6 h–POD 1 biomarkers | ||||
| Serum cystatin C | 0.038 (−0.005 to 0.081) | 0.011 (−0.008 to 0.029) | 0.048 (0.002–0.095) | 0.042 |
| Urine NGAL/Cr | 0.016 (−0.010 to 0.042) | 0.004 (−0.006 to 0.015) | 0.021 (−0.008 to 0.049) | 0.15 |
| Urine KIM-1/Cr | 0.018 (−0.010 to 0.046) | 0.005 (−0.007 to 0.017) | 0.023 (−0.007 to 0.053) | 0.14 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 0.040 (−0.004 to 0.085) | 0.011 (−0.010 to 0.032) | 0.051 (0.002–0.101) | 0.041 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 0.030 (−0.010 to 0.069) | 0.008 (−0.008 to 0.024) | 0.038 (−0.005 to 0.081) | 0.081 |
| Clinical model 2 + peak of postoperative 6 h–POD 1 biomarkers | ||||
| Serum cystatin C | 0.070 (0.005–0.135) | 0.019 (−0.003 to 0.042) | 0.089 (0.021–0.158) | 0.011 |
| Urine NGAL/Cr | 0.017 (−0.007 to 0.040) | 0.013 (−0.007 to 0.033) | 0.004 (−0.008 to 0.016) | 0.16 |
| Urine KIM-1/Cr | 0.043 (−0.003 to 0.089) | 0.034 (−0.009 to 0.076) | 0.009 (−0.008 to 0.027) | 0.066 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 0.059 (0.025–0.093) | 0.016 (−0.009 to 0.041) | 0.075 (0.033–0.117) | 0.001 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 0.052 (−0.000 to 0.104) | 0.014 (−0.001 to 0.035) | 0.066 (0.010–0.122) | 0.021 |
| NRI | NRI (95% CI) | % of events correctly reclassified | % of no-events correctly reclassified | P |
| Clinical model 1 + peak of postoperative 6 h–POD 1 biomarkers | ||||
| Serum cystatin C | 0.56 (0.12–1.00) | 30.44 (−10.43 to 71.30) | 25.30 (3.79–46.82) | 0.018 |
| Urine NGAL/Cr | 0.41 (−0.05 to 0.86) | 13.04 (−27,83 to 53.91) | 27.71 (6.20–49.23) | 0.084 |
| Urine KIM-1/Cr | 0.41 (−0.05 to 0.86) | 13.04 (−27.83 to 53.91) | 27.71 (6.20–49.23) | 0.084 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 0.73 (0.38–1.08) | 73.91 (33.04–1.15) | −1.20 (−22.72 to 20.31) | 0.002 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 0.56 (0.12–0.99) | −13.04 (−53.91 to 27.83) | 68.68 (47.16–90.19) | 0.018 |
| Clinical model 2 + peak of postoperative 6 h–POD 1 biomarkers | ||||
| Serum cystatin C | 0.67 (0.24–1.10) | 39.13 (−1.74 to 80.00) | 27.71 (6.20–49.23) | 0.005 |
| Urine NGAL/Cr | 0.20 (−0.26 to 0.66) | 4.35 (−36.52 to 45.22) | 15.66 (−5.85 to 37.18) | 0.40 |
| Urine KIM-1/Cr | 0.27 (−0.19 to 0.73) | 4.35 (−36.52 to 45.22) | 22.89 (1.38–44.41) | 0.25 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 0.66 (0.29–1.04) | 65.22 (24.35–1.06) | 1.20 (−2.03 to 22.72) | 0.005 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 0.56 (0.12–0.99) | −13.04 (−53.91 to 68.68) | 68.68 (47.16–90.19) | 0.018 |
AKI, acute kidney injury; CI, confidence interval; Cr, creatinine; IDI, integrated discrimination improvement; KIM-1, kidney injury molecule 1; NGAL, neutrophil gelatinase-associated lipocalin; NRI, net reclassification improvement; POD, postoperative day.
Clinical model 1 consisted of 3 preoperative clinical parameters that either were significantly associated with AKI in univariate analysis (preoperative left ventricular ejection fraction percent and body mass index >30 kg/m2) or were clinically relevant for the model (preoperative estimated glomerular filtration rate <60 ml/min per 1.73 m2). Clinical model 2 was the Cleveland Clinic score validated for predicting AKI–renal replacement therapy following cardiac surgery.30 Duplets of biomarkers were combined as follows: (i) at least 1 biomarker above the cutoff value (combination 1), or (ii) both biomarkers above the cutoff value (combination 2). Biomarker data were log10 transformed.
Association of Postoperative Peak Biomarker Levels With Postoperative MAKE
Table 5 shows associations of peak biomarker levels and their combinations with the likelihood of developing postoperative MAKE. Cutoffs of biomarker levels from 6 hours after separating from CPB to POD 1 or POD 4 for the prediction of postoperative MAKE were identified. The combination of peak serum cystatin C and peak urine NGAL/Cr from 6 hours post-CPB to POD 4 (OR: 3.96; 95% CI: 1.06–14.86; P = 0.041 for combination 1 and OR: 10.29; 95% CI: 3.14–33.69; P < 0.001 for combination 2), and the combination of peak serum cystatin C and peak urine KIM-1/Cr from 6 hours post-CPB to POD 4 (OR 4.30; 95% CI: 1.29–14.36; P = 0.018 for combination 1 and OR 5.40; 95% CI: 1.59–18.37; P = 0.007 for combination 2) both significantly associated with the likelihood of developing postoperative MAKE (Table 5). When these associations with MAKE were additionally adjusted for occurrence of in-hospital postoperative AKI, the combination of both high peak serum cystatin C and high peak urine NGAL/Cr (combination 2; OR: 8.65; 95% CI: 2.34–32.05; P = 0.001) remained significantly associated with MAKE (Table 5).
Table 5.
Logistic regression assessment of postoperative in-hospital AKI and peak postoperative biomarker levels (independent variables) with the development of postoperative MAKE (dependent variable)
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
|---|---|---|---|---|
| Clinical model | ||||
| Postoperative AKI | 3.60 (1.17–11.09) | 0.026 | ||
| Biomarkers | Postoperative 6 h–POD 1 | Postoperative 6 h–POD 4 | ||
| Serum cystatin C | 1.47 (0.57–3.76) | 0.42 | 2.31 (0.95–5.60) | 0.065 |
| Urine NGAL/Cr | 1.14 (0.91–1.44) | 0.26 | 1.28 (0.99–1.65) | 0.060 |
| Urine KIM-1/Cr | 1.76 (1.10–2.83) | 0.019 | 1.72 (1.09–2.72) | 0.020 |
| Combination 1 Serum cystatin C + urine NGAL/Cr | 1.08 (0.36–3.24) | 0.89 | 3.96 (1.06–14.86) | 0.041 |
| Combination 2 Serum cystatin C + urine NGAL/Cr | 2.40 (0.77–7.48) | 0.13 | 10.29 (3.14–33.69) | <0.001 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 1.91 (0.57–6.39) | 0.29 | 4.30 (1.29–14.36) | 0.018 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 2.10 (0.68–6.49) | 0.20 | 5.40 (1.59–18.37) | 0.007 |
| Biomarkers adjusted by postoperative AKI | ||||
| Serum cystatin C | 1.06 (0.38–2.92) | 0.92 | 1.63 (0.60–4.42) | 0.33 |
| Urine NGAL/Cr | 1.10 (0.87–1.41) | 0.43 | 1.22 (0.93–1.58) | 0.15 |
| Urine KIM-1/Cr | 1.63 (1.00–2.68) | 0.052 | 1.53 (0.94–2.49) | 0.090 |
| Combination 1 Serum cystatin C + urine NGAL/Cr | 0.77 (0.24–2.51) | 0.66 | 2.91 (0.71–11.86) | 0.14 |
| Combination 2 Serum cystatin C + urine NGAL/Cr | 1.90 (0.58–6.24) | 0.29 | 8.65 (2.34–32.05) | 0.001 |
| Combination 1 Serum cystatin C + urine KIM-1/Cr | 1.40 (0.39–5.00) | 0.61 | 3.32 (0.93–11.83) | 0.064 |
| Combination 2 Serum cystatin C + urine KIM-1/Cr | 1.50 (0.45–5.04) | 0.51 | 3.63 (0.84–15.65) | 0.084 |
AKI, acute kidney injury; CI, confidence interval; Cr, creatinine; KIM-1, kidney injury molecule 1; MAKE, major adverse kidney events as defined in the Methods section; NGAL, neutrophil gelatinase-associated lipocalin; POD, postoperative day.
Clinical model consisted of postoperative in-hospital AKI. Duplets of biomarkers were combined as follows: (i) at least 1 biomarker above the cutoff value (combination 1), or (ii) both biomarkers above the cutoff value (combination 2). MAKE was defined as postoperative death (n = 2, 1 required renal replacement therapy (RRT) before death and 1 met estimated glomerular filtration rate (eGFR) criterion before death), alive with need for RRT during the 30 days following surgery (n = 1), or alive but having ≥25% reduction in postoperative eGFR in reference to preoperative eGFR (n = 13, determined by the post–hospital discharge routine clinical care serum Cr (SCr) value available closest to 30 days after surgery). If no postdischarge SCr value was available, the last SCr measured during primary surgical hospitalization was used.
Discussion
The main finding of our study was that the combination of postoperative biomarkers of kidney function loss and kidney tubular damage measured from 6 hours after separating from CPB to POD 1 significantly added information to preoperative clinical variables for predicting in-hospital AKI after cardiac surgery. This study also found that the combination of kidney function loss and kidney tubular damage biomarkers significantly associated with post–cardiac surgery MAKE, and the combination of increased serum cystatin C and urine NGAL/Cr remained significantly associated with MAKE after adjusting for the occurrence of postoperative in-hospital AKI. Identification of cardiac surgery patients at high risk of AKI or MAKE may help the development and implementation of interventions26 to prevent and mitigate postoperative AKI and promote kidney recovery. Interventions focusing on the optimization of hemodynamics, fluid balance, and the avoidance of nephrotoxic agents or hyperglycemia were shown to be notably effective in reducing the frequency and severity of AKI after cardiac surgery in the PrevAKI trial.31 Our study exhibits an approach to clinical and biomarker data utilization for the identification of patients at high risk of postoperative AKI or MAKE.
The combination of biomarkers of kidney dysfunction (functional loss) and kidney tubular injury (structural damage) constitutes an insufficiently tested and underused approach in research and clinical practice. There is a theoretical benefit of combining novel AKI biomarkers of distinct pathobiological background to outline a more precise phenotype pattern of kidney injury and therefore provide higher performance for AKI risk-stratification, early detection, and AKI course prognostication.16, 32, 33 Several studies have assessed combined biomarker panels for the prediction of AKI in adult34, 35, 36, 37 and pediatric cardiac surgery patients.20 Katagiri and colleagues34 conducted a cohort study of 77 cardiac surgery adult patients to examine the value of combining measurements of urine L-type fatty acid-binding protein and N-acetyl-beta-D-glucosaminidase in the early postoperative period (within the first 12 hours) for the prediction of AKI. They found that this combination of urine biomarkers significantly improved the perioperative clinical model: AUC 0.79 (95% CI: 0.66–0.88) for the clinical model alone and 0.86 (95% CI: 0.74–0.93) for the clinical model considered together with the biomarker panel. Similarly, Prowle and colleagues36 measured (before and within the first 24 hours postoperatively) urine alpha and pi glutathione S-transferases, urine L-type fatty acid-binding protein, urine NGAL, urine hepcidin, and serum cystatin C in a cohort of 93 high-risk adults undergoing CPB to assess their individual or combined predictive value for postoperative AKI and found urine NGAL/Cr (AUC: 0.73; 95% CI: 0.60–0.86) and serum cystatin C (AUC: 0.72; 95% CI: 0.59–0.85) were among the top performers for predicting postoperative AKI. Importantly, in our study we combined biomarkers based on consideration of their distinct pathobiological phenotypes (kidney function and kidney tubular damage). A prior cohort study of 345 pediatric cardiac surgical patients also reported the value of combining plasma cystatin C and urine NGAL/Cr for the prediction of postoperative AKI.20 However, pediatric cardiac surgical patients present for surgery with unique cardiac pathophysiology and with different comorbidities than adult cardiac surgical patients. We therefore examined biomarker combinations of kidney function and kidney tubular damage in adult cardiac surgical patients at 2 postoperative time windows: (i) 6 hours post-CPB through POD 1, and (ii) 6 hours post-CPB through POD 4. The former time window allowed assessment of prediction of postoperative AKI events, whereas the latter allowed the examination of associations between biomarker combinations and AKI events and also MAKE.
Unique from prior studies, we also tested the performance of biomarker combinations for predicting MAKE, which is a composite outcome that has been linked to the development of CKD after AKI and accounts for competing risk of death.38 An alternative approach was used by Arthur and colleagues35 with the goal of examining the progression of AKI after cardiac surgery. Their study included 95 adults with postoperative AKI stage 1 in whom a panel of 32 heterogeneous candidate urinary biomarkers was examined. The primary outcome was worsening AKI (progression to a higher stage of AKI up to 10 days postoperatively) or 30-day death. Two of the top 4 performers were urine NGAL/Cr (AUC: 0.72; 95% CI: 0.59–0.82) and urine KIM-1/Cr (AUC: 0.73; 95% CI: 0.60–0.83). Interestingly, the combination of urine NGAL/Cr and the percentage of change in SCr exhibited great performance for the primary outcome (AUC: 0.82; 95% CI: 0.70–0.90). Similar to our study, the Cleveland Clinic score exhibited only modest predictive performance.
Our study has several distinct strengths. First, we examined the combination of urine biomarkers of kidney tubular damage with serum cystatin C, a biomarker of kidney function loss. Second, we used a pragmatic approach for timing the first postoperative biomarker measurement (6 hours post-CPB), which equates to several hours after intensive care unit admission for most of the patients. This is a very feasible collection time for clinical practice. Third, we assessed the utility of our biomarker data in reference to an established preoperative clinical score30 and to relevant preoperative clinical variables from our own cohort that have been also identified as risk factors for postoperative AKI in prior studies.28, 29, 39 Fourth and finally, we performed an exploratory examination of a composite outcome of postoperative MAKE that has been postulated as a more precise way to assess kidney outcomes in AKI research.38
Our study also has limitations. First, our conclusions are limited by the sample size, the number of overall AKI and MAKE events, and the single-center design. Therefore, future larger studies are warranted to further validate our findings. Second, most AKI in the study was stage 1. Although Stage 1 AKI may represent pre–renal azotemia in some cases, Stage 1 AKI has been associated with adverse long-term outcomes after cardiac surgery.40, 41, 42 Furthermore, the use of single SCr-criterion to evaluate postoperative AKI may have enhanced the performance of cystatin C, as both SCr and cystatin C are mostly functional filtration markers of kidney health. Third, although 87% of AKI events occurred by POD 3, we followed patients for development of AKI through POD 7. Because there are patients who were discharged from the hospital before POD 7, there is a possibility that a few AKI events that occurred after hospital discharge could have been missed. Fourth, our findings might not generalize to susceptible hospitalized patient groups, such as those undergoing major noncardiac surgeries or those who are critically ill nonsurgical patients. Fifth and finally, there other biomarker groups, such as the tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7, that we did not assess in this study.43 However, our study did measure a panel of 3 biomarkers that are widely represented in the cardiac surgical literature, that represent different aspects of AKI pathobiology, and that have readily available commercial enzyme-linked immunosorbent assay kits. Our study findings suggest that further studies of other combinations of biomarkers that represent different aspects of AKI biology appear warranted.
Conclusions
The combination of postoperative biomarkers of kidney function loss (serum cystatin C) and kidney tubular damage (urine KIM-1/Cr) significantly improved clinical prediction of in-hospital AKI after adult cardiac surgery. Exploratory analyses also suggest that the combination of kidney function loss and kidney tubular damage biomarkers may predict postoperative MAKE. Additional larger studies of cardiac surgical patients are warranted to assess combinations of biomarkers of distinct aspects of AKI pathobiology for the ability to accurately predict early postoperative AKI but also long-term MAKE.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Lauren Wehrmann, BS; Alberto Portillo, MS; and Kenni Landgraff, RN, for their work within the study program. We thank Jia Hwei Ng, MD for preparing the graphical abstract for this manuscript.
This study was supported by the Department of Anesthesiology and Pain Management of UT Southwestern Medical Center (to AAF), NIH NIDDK P30DK079328, UT Southwestern O’Brien Kidney Center (to OWM, and AAF awarded a Pilot and Feasibility Grant from this funding source), and NIDDK R01 DK092461–04S1 (to JAN, M-CH, OWM, and AAF) JAN was supported by the Ben J. Lipps Research Fellowship Program of American Society of Nephrology Foundation for Kidney Research and the UT Southwestern Truelson Fellowship Fund from the Jane Pak Center of Mineral Metabolism and Clinical Research. JAN is currently supported by an Early Career Pilot Grant from the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001998.
Author Contributions
Substantial contribution to conception and design (JAN, M-CH, RDT, MEJ, OWM, AAF), acquisition of data (GEN, SAA), or analysis (AM) and interpretation of data (JAN, AM, RDT, OWM, AAF). Drafting the article (JAN, AAF) or revising it critically for important intellectual content (JAN, M-CH, AM, GEN, SAA, RDT, MEJ, OWM, AAF). Final approval of the version to be published (all authors). Agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (all authors).
References
- 1.Wu V.C., Wu P.C., Wu C.H. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu V.C., Wu C.H., Huang T.M. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla L.S., Amdur R.L., Amodeo S. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakar C.V., Christianson A., Himmelfarb J., Leonard A.C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wald R., Quinn R.R., Adhikari N.K. Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125:585–593. doi: 10.1016/j.amjmed.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Lafrance J.P., Miller D.R. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neyra J.A., Shah S., Mooney R. Contrast-induced acute kidney injury following coronary angiography: a cohort study of hospitalized patients with or without chronic kidney disease. Nephrol Dial Transplant. 2013;28:1463–1471. doi: 10.1093/ndt/gft082. [DOI] [PubMed] [Google Scholar]
- 9.Hobson C., Ozrazgat-Baslanti T., Kuxhausen A. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261:1207–1214. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasta J.F., Kane-Gill S.L., Durtschi A.J. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 11.Brown J.R., Parikh C.R., Ross C.S. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg. 2014;97:111–117. doi: 10.1016/j.athoracsur.2013.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosner M.H., Okusa M.D. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 13.Moore E.M., Simpson J.A., Tobin A., Santamaria J. Preoperative estimated glomerular filtration rate and RIFLE-classified postoperative acute kidney injury predict length of stay post-coronary bypass surgery in an Australian setting. Anaesth Intensive Care. 2010;38:113–121. doi: 10.1177/0310057X1003800119. [DOI] [PubMed] [Google Scholar]
- 14.Kork F., Balzer F., Spies C.D. Minor postoperative increases of creatinine are associated with higher mortality and longer hospital length of stay in surgical patients. Anesthesiology. 2015;123:1301–1311. doi: 10.1097/ALN.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao H., Katz N., Ariyanon W. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3:178–199. doi: 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullough P.A., Bouchard J., Waikar S.S. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: executive summary from the tenth consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:5–12. doi: 10.1159/000349962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alge J.L., Arthur J.M. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–155. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh C.R., Coca S.G., Thiessen-Philbrook H. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meersch M., Schmidt C., Van Aken H. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basu R.K., Wong H.R., Krawczeski C.D. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64:2753–2762. doi: 10.1016/j.jacc.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlipak M.G., Coca S.G., Wang Z. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011;58:366–373. doi: 10.1053/j.ajkd.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yong Z., Pei X., Zhu B. Predictive value of serum cystatin C for acute kidney injury in adults: a meta-analysis of prospective cohort trials. Sci Rep. 2017;7:41012. doi: 10.1038/srep41012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra J., Ma Q., Prada A. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya V.S., Ramirez V., Ichimura T. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 25.Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 27.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan K.E., Byrne J.S., Hudson A. The effect of obesity on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:1622–1628. doi: 10.1016/j.jtcvs.2015.08.082. [DOI] [PubMed] [Google Scholar]
- 29.Mangano C.M., Diamondstone L.S., Ramsay J.G. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Thakar C.V., Arrigain S., Worley S. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 31.Meersch M., Schmidt C., Hoffmeier A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough P.A., Shaw A.D., Haase M. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 33.Endre Z.H., Kellum J.A., Di Somma S. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 34.Katagiri D., Doi K., Honda K. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg. 2012;93:577–583. doi: 10.1016/j.athoracsur.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Arthur J.M., Hill E.G., Alge J.L. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014;85:431–438. doi: 10.1038/ki.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prowle J.R., Calzavacca P., Licari E. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren Fail. 2015;37:408–416. doi: 10.3109/0886022X.2014.1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J.J., Chi N.H., Huang T.M. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit Care. 2018;22:108. doi: 10.1186/s13054-018-2035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellum J.A., Zarbock A., Nadim M.K. What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med. 2017;43:901–903. doi: 10.1007/s00134-017-4732-1. [DOI] [PubMed] [Google Scholar]
- 39.Parikh C.R., Thiessen-Philbrook H. Key concepts and limitations of statistical methods for evaluating biomarkers of kidney disease. J Am Soc Nephrol. 2014;25:1621–1629. doi: 10.1681/ASN.2013121300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu J., Chen R., Liu S. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2016;30:82–89. doi: 10.1053/j.jvca.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Pickering J.W., James M.T., Palmer S.C. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis. 2015;65:283–293. doi: 10.1053/j.ajkd.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Engoren M., Habib R.H., Arslanian-Engoren C. The effect of acute kidney injury and discharge creatinine level on mortality following cardiac surgery*. Crit Care Med. 2014;42:2069–2074. doi: 10.1097/CCM.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 43.Kashani K., Al-Khafaji A., Ardiles T. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]