Abstract
Introduction
Malnutrition is common in patients with acute kidney injury (AKI), particularly in those requiring renal replacement therapy (RRT). Use of RRT removes metabolic waste products and toxins, but it will inevitably also remove useful molecules such as micronutrients, which might aggravate malnutrition. The RRT modalities vary in mechanism of solute removal; for example, intermittent hemodialysis (IHD) uses diffusion, continuous veno-venous hemofiltration (CVVH) uses convection, and sustained low-efficiency diafiltration (SLEDf) uses a combination of these.
Methods
We assessed micronutrient and amino acid losses in 3 different RRT modalities in patients with AKI (IHD, n = 27; SLEDf, n = 12; CVVH, n = 21) after correction for dialysis dose and plasma concentrations.
Results
Total losses were affected by modality; generally CVVH >> SLEDf > IHD (e.g., amino acid loss was 18.69 ± 3.04, 8.21 ± 4.07, and 5.13 ± 3.1 g, respectively; P < 0.001). Loss of specific trace elements (e.g., copper and zinc) during RRT was marked, with considerable heterogeneity between RRT types (e.g., +849 and +2325 μg/l lost during SLEDf vs. IHD, respectively), whereas effluent losses of copper and zinc decreased during CVVH (effect size relative to IHD, −3167 and −1442 μg/l, respectively). B vitamins were undetectable in effluent, but experimental modeling estimated 40% to 60% loss within the first 15 minutes of RRT.
Conclusion
Micronutrient and amino acid losses are marked during RRT in patients with AKI, with variation between RRT modalities and micronutrients.
Key words: acute kidney injury, amino acids, B vitamins, malnutrition, renal replacement therapy, trace elements
The prevalence of disease-related malnutrition (DRM) in AKI has been estimated at up to 42% and is an independent predictor of mortality.1 Comorbidities, prolonged hospital stay, and RRT may exacerbate malnutrition. RRT may be required in AKI to remove metabolic waste products and toxins, and to regulate fluid and electrolyte balance. Inevitably, mechanisms by which RRT removes unwanted substances will also remove some essential solutes such as micronutrients and amino acids.2, 3
Many RRT modalities are now available to support patients with severe AKI. These vary in duration of treatment (intermittent to continuous) and mechanism of solute clearance (diffusion, convection, or combination). These differences are likely to affect micronutrient losses. Despite high risk of DRM in patients with AKI and the huge financial burden of AKI to health services,4 little research has been published on micronutrient losses in acute RRT. We studied micronutrient losses in 3 RRT modalities routinely used in our renal and intensive care units: (i) IHD, (ii) SLEDf, (iii) CVVH. These treatments vary in duration (intermittent, intermediate, and continuous, respectively) and mechanism of solute removal (diffusion [IHD], convection [CVVH], or combination [SLEDf]). Specific micronutrients and amino acids were selected for investigation based on their physiological and nutritional importance, especially in critical illness, and on whether their physical properties suggested removal primarily by diffusion or convection. Those fulfilling both criteria were of particular interest, such as the amino acids glutamine, arginine, and taurine. Micronutrients of interest included the trace elements zinc, selenium, iron and manganese, and several B vitamins. We also wished to quantify losses of water-soluble B vitamins: thiamine (B1), pyridoxine (B6), folic acid (B9), and cobalamin (B12). All are relatively small water-soluble molecules (B12, 1355 g/mol) so extensive losses during RRT are likely. Patients requiring long-term RRT for end-stage renal disease usually receive supplements containing thiamine and other water-soluble vitamins,5 but the extent of losses between different RRT modalities is not known.
To describe micronutrient and amino acid losses in patients with AKI receiving different types of RRT (IHD, SLEDf, and CVVH), we conducted a prospective, observational study. We hypothesized first that water-soluble, physiologically free molecules in plasma (including trace elements, amino acids, and B vitamins) are lost in clinically significant amounts in acute RRT; and second, that nutrient losses differ qualitatively and quantitatively between RRT modalities because of different mechanisms of solute clearance.
Methods
Expanded descriptions of materials and methods are available in Supplementary Materials and Methods.
Clinical Study and Sampling Protocol
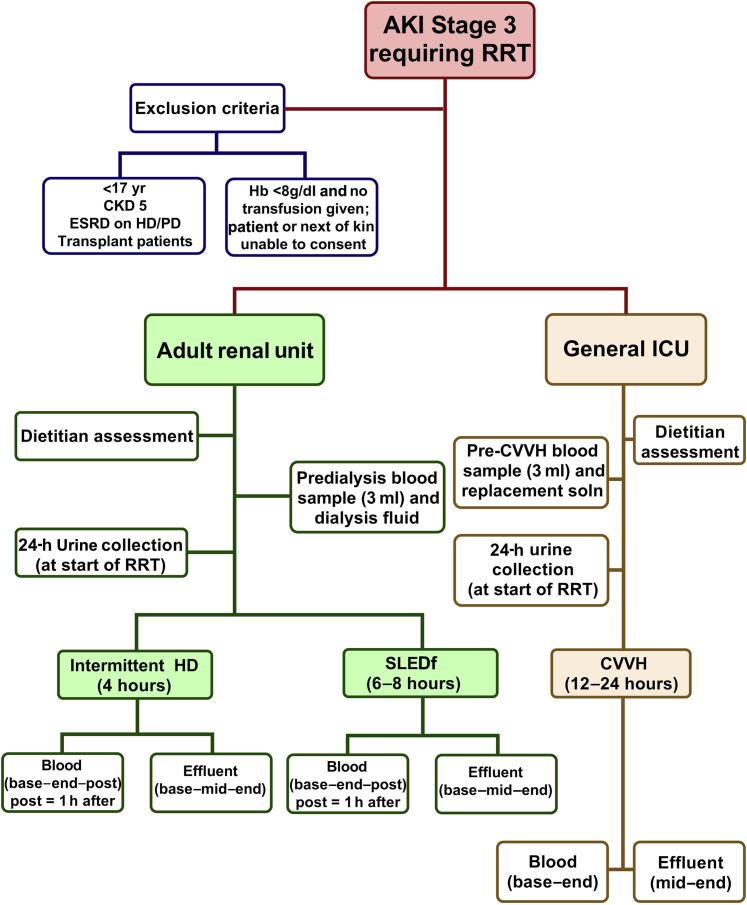
Regional ethics committee approval and sponsorship from Nottingham University Hospitals NHS Trust were obtained. Patients with AKI were eligible for recruitment once a plan for RRT was made. The inclusion and exclusion criteria and general study sampling protocol are illustrated in Figure 1. For blood sampling, a first blood sample was taken before RRT commencement, and plasma was obtained and stored appropriately until analysis (“base”). Further regular sampling occurred at intervals during each RRT session, with the “mid” or “end” sample designated post hoc as those samples taken mid-way or at the end of the session (e.g., at 2 and 4 hours for a 4-hour IHD treatment; at 3 and 6 hours for a 6-hour SLEDf treatment; or at 12 and 24 hours for a 24-hour CVVH treatment). The first 2 RRT sessions were included in the study, but if only a single session was required, no further samples were taken. For effluent sampling, spot-samples were obtained from the effluent port of each RRT machine on a schedule similar to that of blood sampling. “Baseline” effluent was considered as the first sampled dialysate after machine priming had occurred, before patient attachment. For CVVH, a sample of the effluent was taken from each discarded 5-L bag at 12 and 24 hours after commencing treatment.
Figure 1.
Flowchart outlining study design. AKI, acute kidney injury; CVVH, continuous veno-venous hemofiltration; ESRD, end-stage renal disease; HD, hemodialysis; HD/PD, hemodialysis/peritoneal dialysis; ICU, intensive care unit; IHD, intermittent hemodialysis; RRT, renal replacement therapy; SLEDf, sustained low-efficiency diafiltration.
Analysis of Free Amino Acids
Analysis of free amino acids in plasma and effluent (490 μl) used an Amino Acid Analyser (Biochrom 20; Pharmacia LKB, Biochrom Ltd., Cambridge, UK) after deproteinization and derivatization, as described previously.6 Values for 32 α-amino acids were determined as μmoles/l but were mass corrected to μg/l for absolute quantification of loss.
Analysis of Major and Trace Elements
Elemental analysis was conducted on 500 μl of plasma or effluent by inductively coupled plasma−mass spectrometry (iCAP Q, ThermoFisher Scientific Inc., Waltham, MA), as described previously.7, 8 Certified reference materials (CRMs) were included in duplicate for each inductively coupled plasma−mass spectrometry batch run (×60−120 samples) and were SeroNorm L-2 (REF203105, LOT0903107) and SeroNorm L-2 (REF210705, LOT1011645; LGC, Middlesex, UK) for plasma and urine, respectively. Operational parameters were quality controlled within and between different runs. The limit of detection (LOD), limit of quantification (LOQ), and measured values for ultrapure water, tap-water and filtrate (PrismaSol) are presented in Table 1.
Table 1.
Limits of detection, quantification and baseline reference samples for inductively coupled plasma−mass spectrometry
| Major element (ppm, mg L-1) | CRM Seronorm L2 Plasma (% recovery) | CRM Seronorm L2 Urine (% recovery) |
LOD (ppb) | LOQ (ppb) | Ultrapure water (18 MΩ cm) | Tap water | Filtration fluid (PrismaSol) |
|---|---|---|---|---|---|---|---|
| Sulphur | 1335 (105) | 671 (101) | 8.5 | 21.3 | 13.41 ± 0.93 | 37.4 ± 2.14 | <LOQ |
| Calcium | 119 (102) | 119 (94) | 0.08 | 0.20 | <LOD | 52.0 ± 1.54 | 48.5 ± 4.4 |
| Phosphorus | 110 (113) | 807 (102) | 0.08 | 0.22 | <LOD | 0.95 ± 0.11 | <LOQ |
| Sodium | 3531 (102) | 2815 (89) | 0.08 | 0.21 | 0.88 ± 0.45 | 35.8 ± 1.90 | 2804 ± 165 |
| Magnesium | 33.9 (96) | 78 (89) | 0.02 | 0.05 | 0.04 ± 0.00 | 10.4 ± 0.54 | 13.8 ± 1.3 |
| Potassium | 221 (104) | 1921 (92) | 0.09 | 0.22 | 0.48 ± 0.07 | 6.76 ± 0.34 | 127 ± 13 |
| Trace element (ppb, μg/l) | |||||||
| Zinc | 1532 (115) | 1281 (104) | 11.0 | 27.5 | 1.9 ± 0.19 | 489 ± 273 | 94.8 ± 27.8 |
| Iron | 2150 (95) | 13.9 (147) | 0.70 | 1.76 | 9.83 ± 2.7 | 17.7 ± 2.5 | 15.0 ± 5.5 |
| Copper | 1925 (91) | 56.3 (108) | 0.12 | 0.29 | 1.53 ± 0.22 | 100 ± 32 | 4.5 ± 2.5 |
| Manganese | 14.5 (106) | 9.3 (109) | 0.09 | 0.23 | 2.12 ± 0.79 | 1.12 ± 0.15 | <LOD |
| Strontium | 110 (107) | 120 (97) | 0.09 | 0.22 | 0.45 ± 0.11 | 247 ± 2 | 22.4 ± 2.0 |
| Selenium | 136 (106) | 71.7 (117) | 0.34 | 0.86 | 0.79 ± 0.41 | 10.0 ± 3.4 | 2.0 ± 0.5 |
| Chromium | 5.7 (101) | 30.1 (95) | 0.15 | 0.39 | <LOD | <LOD | <LOD |
| Vanadium | 1.1 (149) | 26 (99) | 0.05 | 0.13 | <LOD | <LOD | 0.68 ± 0.06 |
| Molybdenum | 1.21 (149) | 48 (92) | 0.21 | 0.52 | <LOD | <LOD | <LOQ |
| Rubidium | 8.7 (109) | 1150 (113) | 0.10 | 0.25 | 0.20 ± 0.02 | 4.95 ± 0.65 | <LOQ |
| Lithium | 9689 (116) | 100 (98) | 0.05 | 0.13 | <LOD | 7.27 ± 0.58 | <LOQ |
| Cesium | 0.02 (233) | 6.6 (110) | 0.02 | 0.05 | <LOD | 0.15 ± 0.00 | <LOD |
Certified reference materials (CRM) were obtained from LGC Standards (Bury, UK). Limit of detection (LOD) and limit of quantification (LOQ) were calculated from calculating SD of 10 operational blank samples as LOD = 3.25 (Student t test, df = 9, 99% confidence interval) × SD and LOQ = 2.5 × LOD. Elemental composition of ultrapure water (n = 6), tap water (n = 6), or PrismaSol filtration fluid (n = 18) was determined by inductively coupled plasma−mass spectrometry. Percentage recovery is mean recovery from 12 independent runs. Aluminium, arsenic, beryllium, cadmium, cobalt, lead, nickel, silver, titanium, thallium, and uranium were measurable above LOD in plasma but not in effluent. As these elements were not a focus of our hypotheses, values are not reported. Barium and boron were quantifiable but were not included in the National Institute of Standards and Technology (NIST) CRM and are therefore also not reported.
Analysis of B Vitamins
Effluent samples were analyzed by liquid chromatography−mass spectrometry (LC-MS). Chromatography was run on an Agilent 1100 fitted with Phenomenex Luna 5U C18. Mass spectrometry used a Micromass Ultima (Manchester, UK). Vitamin B1 (thiamine hydrochloride), B2 (riboflavin), B3 (nicotinic acid), B6 (pyridoxal hydrochloride), B9 (folic acid), and B12 (cyanocobalamin) were analyzed, interpolated from standard curves (0.25−10 ppm [0.25−10 mg/l]). All B vitamins were purchased from Sigma-Aldrich (Cheshire, UK). Further information on MS parameters and conditions for chromatography are given in Supplementary Table S2.
Statistical Analysis
Continuous data were analyzed by analysis of variance (ANOVA) or Kruskall−Wallis test for skewed data, and are presented as mean and 1 SD or 1 SEM, or estimated standard error of the differences between means (SED) where appropriate. The 95% confidence intervals (CIs) may be approximated as mean ± 2 SED. Comparison of categorical or binomial data was performed by logistic regression and is presented as mean proportions (number [percentage of total]). Comparison of treatment groups in which the data naturally followed an ordinal scale (e.g., measures of nutritional status; mild−moderate−severe) was performed by ordinal logistic regression. For repeated measurements, each individual was included in the model as a nested random effect. All data are presented after correction for covariates (e.g., dialysis dose; urea reduction ratio or solute removal index) and baseline plasma concentration. Skewed data were log10 transformed to normalize residual plots. Time-series data with missing measurements were analyzed by restricted maximum likelihood. More stringent P values were assumed where multiple, potentially nonindependent data were analyzed, as indicated in table footnotes and figure legends. All data were analyzed using Genstat v17 (VSNi, Rothampsted, UK).
Results
Baseline Characteristics of Study Population and RRT Sessions
We achieved a 98% consent rate. The study population tended to be elderly, male, with multiple co-morbidities (Table 2). Baseline patient characteristics between RRT groups were relevant to the determination of covariates for statistical models quantifying the loss of micronutrients. Overall demographics were similar for the 3 groups (Table 2). Patients receiving RRT (CVVH) in the intensive care unit had significantly higher C-reactive peptide, consistent with the higher proportion of sepsis-associated AKI in this group (Table 2). Patients prescribed SLEDf had lower blood pressure and plasma bicarbonate than the other groups (Table 2). Most patients were malnourished (Table 3); for example, 82% were determined to be at high risk by using Malnutrition Universal Screening Tool (MUST 2, a 5-step screening tool to identify adults who are malnourished, at risk for malnutrition, or obese); 71% needed nutritional support according to Nutritional Risk Screening (NRS ≥3; NRS contains the nutritional components of MUST as well as a disease severity grading); and 77% were at least mild-to-moderately malnourished according to Subjective Global Assessment (SGA B or C; SGA assesses 10 factors in history and examination, with classification into 3 categories of nutritional status: A, well nourished; B, moderately well nourished or at risk for malnutrition; C, severely malnourished) (Table 3). Details of RRT sessions are given in Table 4. Effluent volume generated per session was significantly different (IHD, 54 ± 20; SLEDf, 98 ± 20; CVVH, 64 ± 21 L; P < 0.001). Variation in dialysis dose (quantified by urea reduction ratio [URR] and solute removal index [SRI]) between RRT types justified inclusion of either as a covariate (blood and effluent outcomes, respectively) when analyzing nutrient losses.
Table 2.
Patient characteristics at admission stratified by type of renal replacement therapy
| Characteristic | All (n = 72) | IHD (n = 33) | SLEDf (n = 15) | CVVH (n = 24) | P valuea |
|---|---|---|---|---|---|
| Age, yr | 65 ± 13 | 66 ± 14 | 70 ± 11 | 62 ± 12 | 0.17 |
| Weight, kg | 84.9 ± 21.8 | 91.4 ± 24.2 | 77.4 ± 20.2 | 80.6 ± 16.9 | 0.057 |
| BMI | 26.8 ± 6.1 | 27.6 ± 6.3 | 25.7 ± 7.6 | 26.4 ± 4.8 | 0.47 |
| Male | 54 (75) | 25 (75) | 10 (66) | 19 (79) | 0.43 |
| Systolic blood pressure, mm Hg | 128 ± 27 | 140 ± 25 | 99 ± 20 | 121 ± 23 | <0.001 |
| Diastolic blood pressure, mm Hg | 68 ± 12 | 75 ± 11 | 59 ± 9 | 62 ± 9 | <0.001 |
| Ethnicity (% Caucasian) | 71 (99) | 33 (100) | 14 (93) | 24 (100) | – |
| Inotropic support (% inotropes) | 24 (33) | 0 | 0 | 24 (100) | – |
| Comorbidities | |||||
| Septic AKI | 38 (52) | 10 (30) | 8 (53) | 20 (83) | <0.001 |
| Multiple AKI causes | 25 (35) | 8 (24) | 6 (40) | 11 (45) | 0.21 |
| CKD | 31 (43) | 16 (48) | 9 (60) | 6 (25) | 0.06 |
| Diabetes | 35 (43) | 18 (54) | 8 (53) | 9 (37) | 0.40 |
| Hypertension | 41 (56) | 22 (66) | 8 (53) | 11 (45) | 0.27 |
| Serum parameters | |||||
| Creatinine, μmol/l | 575 ± 313 | 692 ± 307 | 592 ± 388 | 404 ± 175 | <0.001 |
| Urea, mmol/l | 34.3 ± 15.5 | 36.7 ± 9.6 | 30.5 ± 12.5 | 29.8 ± 15.2 | 0.08 |
| Sodium, mmol/l | 133 ± 7 | 132 ± 7 | 133 ± 7 | 135 ± 7 | 0.28 |
| Potassium, mmol/l | 5.24 ± 1.00 | 5.15 ± 1.0 | 5.57 ± 0.95 | 5.15 ± 1.03 | 0.25 |
| Bicarbonate, mmol/l | 17.8 ± 5.9 | 17.7 ± 5.5 | 14.8 ± 5.7 | 19.9 ± 5.9 | 0.019 |
| Phosphate, mmol/l | 2.37 ± 1.05 | 2.56 ± 1.02 | 2.59 ± 1.19 | 1.99 ± 0.96 | 0.08 |
| Albumin, g/l | 22.5 ± 7.7 | 23.8 ± 6.9 | 22.8 ± 8.9 | 20.4 ± 7.8 | 0.21 |
| C-reactive peptide, mg/l | 140 ± 118 | 114 ± 112 | 112 ± 99 | 196 ± 122 | 0.01 |
AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; SLED, sustained low-efficiency diafiltration.
Data are mean ± 1 SD for continuous variables and number of patients (percentage of group total) positive for each category.
Statistical differences between groups of patients on admission were assessed by Kruskall−Wallis One-way analysis of variance for continuous variables and χ2 test for categorical data. All data analyses were conducted using Genstat v17 (VSNi, Rothampsted, UK). Statistical significance was accepted at P < 0.05.
Table 3.
Nutritional status of study participants stratified by type of renal replacement therapy
| Characteristic | All (n = 73) | IHD (n = 33) | SLEDf (n = 15) | CVVH (n = 24) | P valuea |
|---|---|---|---|---|---|
| Dietetic assessment | |||||
| MUST 0 | 11 (15) | 7 (21) | 2 (13) | 2 (8) | 0.06 |
| 1 | 2 (3) | 2 (6) | 0 (0) | 0 (0) | — |
| 2 | 60 (82) | 25 (73) | 13 (87) | 22 (92) | 0.13 |
| NRS ≤2 | 21 (29) | 16 (47) | 2 (13) | 3 (12) | <0.001 |
| NRS ≥3 | 52 (71) | 18 (53) | 13 (87) | 21 (88) | 0.55 |
| SGA A | 17 (23) | 10 (29) | 3 (20) | 4 (17) | 0.09 |
| B | 36 (49) | 20 (59) | 7 (47) | 9 (38) | 0.03 |
| C | 20 (28) | 4 (12) | 5 (33) | 11 (46) | 0.21 |
| Serum parameters | |||||
| Plasma glucose, mmol/l | 6.97 ± 3.58 | 7.13 ± 3.87 | 7.54 ± 4.02 | 6.55 ± 3.06 | 0.78 |
CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; MUST, Malnutrition Universal Screening Tool; NRS, Nutritional Risk Screening; SLED, sustained low-efficiency diafiltration.
Values are mean ± 1 SD for continuous data and number (% of group total) for categorical data.
Statistical differences between groups of patients on admission were assessed by Kruskall−Wallis 1-way analysis of variance for continuous data and χ2 test for categories of nutritional assessment tools. All data analyses were conducted using Genstat v17 (VSNi, UK). Statistical significance was accepted at P < 0.05. Subjective Global Assessment (SGA) tool assessed using a 7-point scale: A (6−7), well nourished; SGA B (3−5), mild-to-moderately malnourished; SGA C (1−2), severely malnourished. Malnutrition Universal Screening Tool (MUST) on a 3-point scale: MUST 0 = low risk, MUST 1 = medium risk, MUST 2 = high risk of malnutrition; Nutritional Risk Screening (NRS) was assessed using a 6-point scale with ≤2 points denoting nutritional support not indicated and NRS ≥3 denoting nutritional support indicated.
Table 4.
Renal replacement therapy characteristics of study participants
| Characteristic | IHD (n = 33) | SLEDf (n = 15) | CVVH (n = 24) | P valuea |
|---|---|---|---|---|
| RRT (First session, T1) | ||||
| Prescribed RRT time, min | 120 ± 5 | 344 ± 42 | 1440 ± 0 | — |
| Actual RRT time, min | 122 ± 22 | 282 ± 105 | 1225 ± 341 | — |
| Blood flow rate, ml/min | 207 ± 17 | 214 ± 29 | 234 ± 25 | <0.001 |
| Plasma flow rate, ml/min | 145 ± 13 | 148 ± 21 | 169 ± 25 | <0.001 |
| Effluent flow rate, ml/min | 462 ± 121 | 209 ± 26 | 35 ± 0b | — |
| Serum urea pre-RRT, mmol/l | 36.7 ± 9.6 | 35.9 ± 24.1 | 29.8 ± 15.2 | 0.03 |
| Serum urea post-RRT, mmol/l | 28.5 ± 8.4 | 22.7 ± 24.3 | 18.7 ± 9.0 | <0.001 |
| Urea reduction ratio (URR) | 0.31 ± 0.14 | 0.54 ± 0.18 | 0.38 ± 0.16 | <0.001 |
| Kt/v | 0.41 ± 0.21 | 0.94 ± 0.42 | 0.85 ± 0.41 | <0.001 |
| Solute removal index (SRI) | 0.28 ± 0.14 | 0.45 ± 0.51 | 0.51 ± 0.21 | <0.001 |
| RRT (second session, T2) | (n = 11) | (n = 4) | (n = 15) | |
| Prescribed RRT time, min | 180 ± 26 | 360 ± 0 | 1440 ± 0 | — |
| Actual RRT time, min | 185 ± 30 | 323 ± 56 | 1244 ± 319 | — |
| Blood flow rate, ml/min | 225 ± 25 | 203 ± 5 | 234 ± 28 | — |
| Plasma flow rate, ml/min | 159 ± 24 | 140 ± 7 | 140 ± 58 | — |
| Effluent flow rate, ml/min | 516 ± 65 | 200 ± 0 | 35 ± 0b | — |
| Serum urea pre-RRT, mmol/l | 25.8 ± 11.6 | 25.8 ± 19.5 | 13.6 ± 5.1 | — |
| Serum urea post-RRT, mmol/l | 15.7 ± 8.0 | 10.0 ± 6.4 | 10.1 ± 4.1 | — |
| Urea reduction ratio | 0.17 ± 0.09 | 0.35 ± 0.17 | 0.38 ± 0.16 | — |
| Kt/v | 0.21 ± 0.11 | 0.51 ± 0.25 | 0.83 ± 0.34 | — |
| Solute removal index | 0.40 ± 0.11 | 0.26 ± 0.01 | 1.40 ± 0.93 | — |
CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; RRT, renal replacement therapy; SLEDf, sustained low-efficiency diafiltration. SRI, solute removal index; URR, urea reduction ratio; solute removal index.
Values are mean ± 1 SD for continuous data, and number (percentage of of group total) for categorical data. Only a proportion of study participants received a second RRT session (IHD, n = 11, SLED-F, n = 4, CVVH, n = 15) in this cohort. Hence information is included for comparison (to first session, T1) but was not formally compared.
Statistical differences for T1 were assessed by Kruskall−Wallis 1-way analysis of variance for continuous data. All data analyses were conducted using Genstat v17 (VSNi, UK).
Fixed filtration fluid rate (ml/kg per minute).
Amino Acid Losses
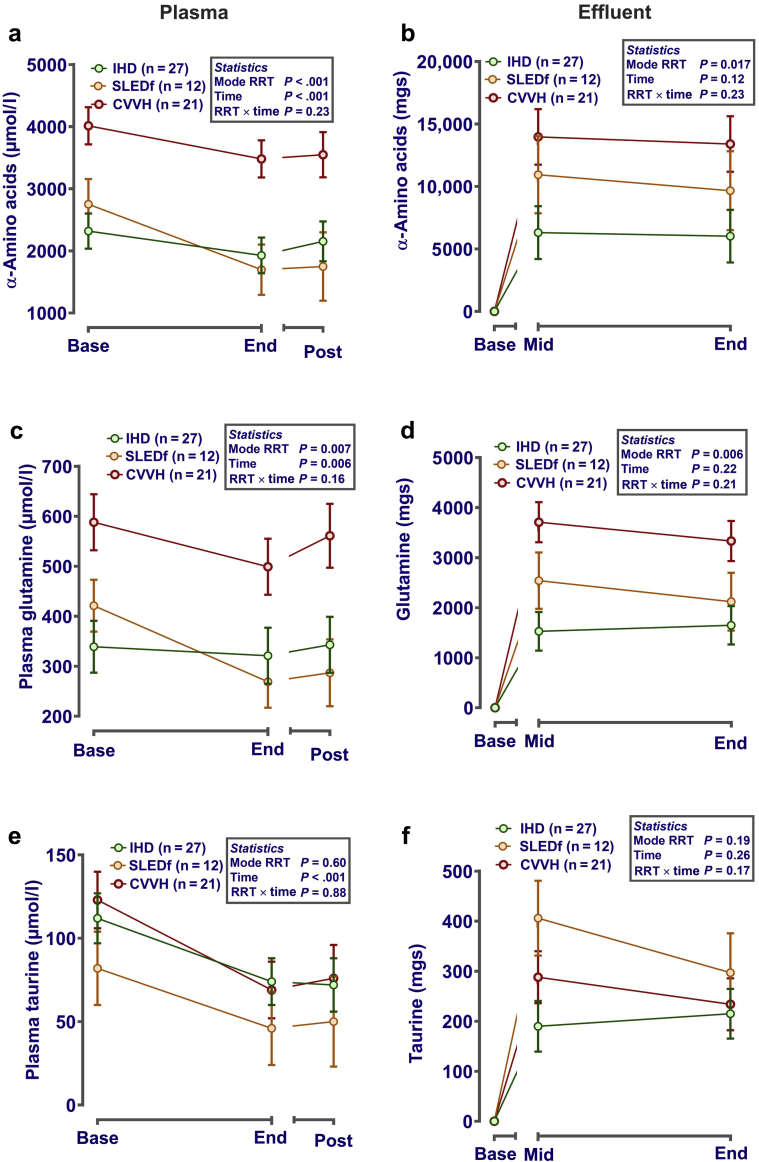
Plasma concentrations of all α-amino acids at baseline were similar for IHD and SLEDf but considerably higher for CVVH (Figure 2a). By the end of each RRT session, plasma concentration of amino acids had reduced, but tended to rebound by 1 to 2 hours after RRT (for IHD and SLED-F, CVVH for reference only; Figure 2a). This pattern was similar for all measured amino acids when considered separately, but was most obvious in those present at highest concentrations in plasma (e.g., glycine and glutamine) (Figure 2c and Supplementary Table S1). Minor differences in this pattern were noted for less prevalent plasma amino acids such as taurine (Figure 2e). No significant differences were noted between subgroups of amino acids (e.g., branched chain, essential, or acidic groups). Corrected for plasma concentration and dose of dialysis, loss of amino acids was significantly influenced by modality (CVVH >>> SLED-F >> IHD) (Figure 2b), with estimated loss being similar whether sampling was performed midway or at the end of each session. Again, the pattern of overall loss was largely driven by amino acids at highest concentration in plasma, such as glutamine (Figure 2d). Nevertheless, some variation in the pattern of loss between RRT types was again noted for less abundant amino acids, such as taurine (Figure 2f and Supplementary Table S1).
Figure 2.
Plasma amino acids and effluent loss during different modes of renal replacement therapy (RRT). Plasma (a,c,e) and effluent (b,d,f) were sampled before, at the end, and 1 to 2 hours after the end of each RRT session. α-Amino acids (×20 standard proteogenic plus a further 18 amino acids) were measured after deproteinization and derivatization using a Biochrom 20 (Biochrom, Cambridge, UK). Data (mean ± SE) are presented corrected (i.e., included as covariates in the statistical model) for dose-of-dialysis (urea reduction ratio for plasma levels; solute removal index for effluent losses) and plasma concentration (for calculation of effluent losses only). If necessary, to normalize residual error before statistical analysis, data were log10 transformed. Graphs were generated in GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA). Analysis was by repeated-measures analysis of variance or mixed-effect models, as appropriate, with RRT mode and time as fixed effects and patient ID as a nested random effect, using Genstat v18 (VSNi, Rothampsted, UK). Statistical significance was accepted at P < 0.05. CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; SLEDf, sustained low-efficiency diafiltration.
Trace Element Losses
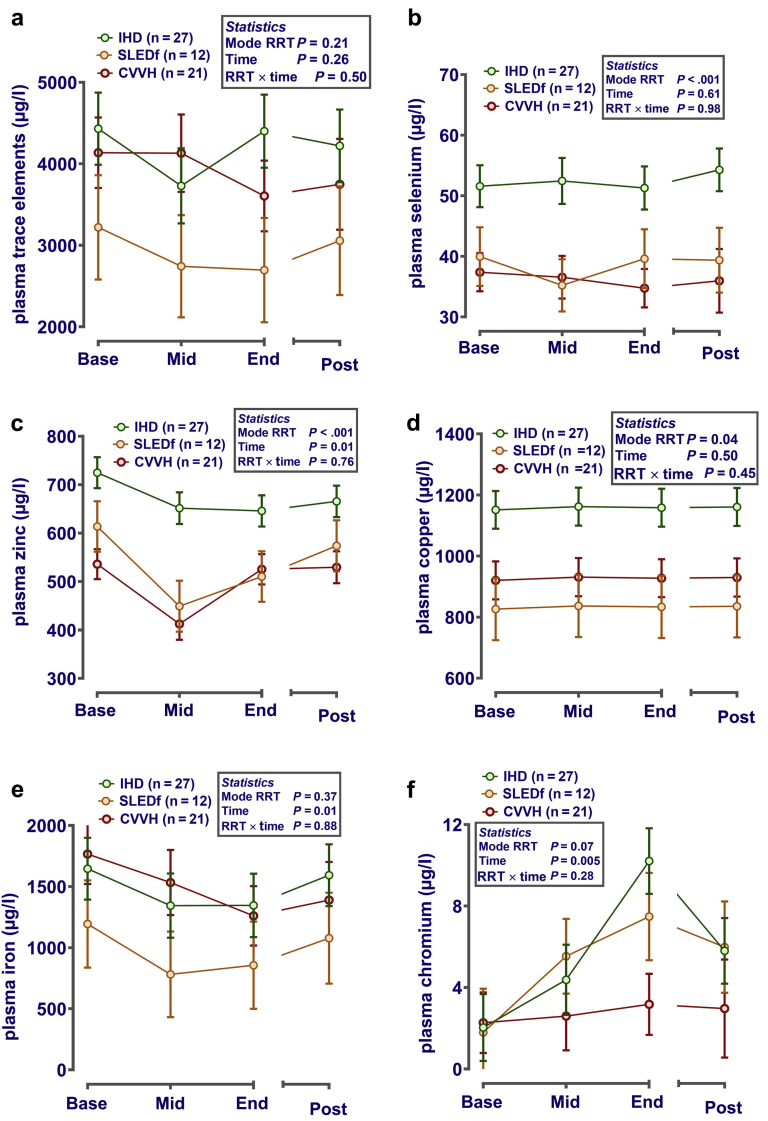
Total measured plasma trace element composition was similar between RRT groups at baseline and did not change appreciably during or after the first RRT session (Figure 3a). This is likely attributable to patient heterogeneity for overall plasma trace element profile. Analysis of individual trace elements revealed some clear effects. Plasma selenium (Figure 3b), zinc (Figure 3c), and copper (Figure 3d) were markedly higher in patients receiving IHD compared with other modalities. Duration of RRT, regardless of modality, reduced plasma cesium (pooled means; from 1.11 to 0.70 ± 0.30 μg/l; Ptime < 0.001), iron (Figure 3e), molybdenum (pooled means; from 3.79 to 2.74 ± 0.41 μg/l; Ptime= 0.01), and rubidium (pooled means; from 242 to 158 ± 8.7 μg/l; Ptime < .001) but increased plasma chromium (Figure 3f). Significant reduction in plasma concentration of trace elements by the end of an RRT session, relative to the patient’s baseline values, suggests net loss from the plasma pool. Trace elements in plasma that did not vary with RRT modality or time were (all μg/l, pooled median [IQR]): Mn (1.14 [0.62–1.68]), Sr (32.8 [27.0−37.9]), and Va (1.66 [0.69−2.24]).
Figure 3.
Plasma trace elements of renal replacement therapy (RRT). Plasma was sampled before (base), during (mid), at the end (end) and 1 to 2 hours after (post) each RRT session for measurement of trace elements by inductively coupled plasma−mass spectrometry. Data (mean ± SE) for each spot-sampled trace element in plasma are presented after correction (i.e., included as a covariate) for dose-of-dialysis (plasma urea reduction ratio). If necessary, to normalize residual error before statistical analysis, data were log10 transformed. Statistical analysis was by repeated-measures analysis of variance or mixed-effect models, as appropriate, with RRT mode and time as fixed effects and patient ID as a nested random effect, using Genstat v18 (VSNi, Rothampsted, UK). Statistical significance was accepted at P ≤ 0.002 (adjusted for the number of comparisons). (a) The sum of all measurable trace elements greater than the limit of quantification (Cs, Cr, Cu, Fe, Li, Mn, Mo, Rb, Se, Sr, V, Zn). (b–f) Individual elements of a priori interest that exhibit differences between modalities (b–d, Se, Zn, and Cu), or an effect of time (i.e., [e] decreases Fe and [f] increases Cr). CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; SLEDf, sustained low-efficiency diafiltration.
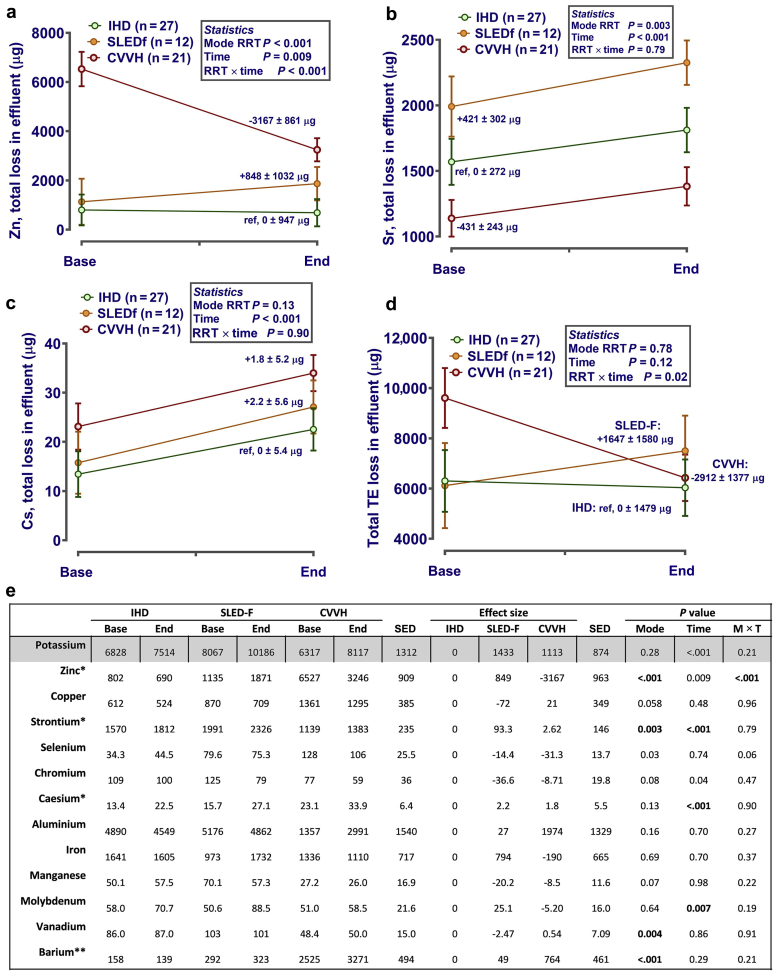
For calculation of net effluent trace element loss attributable to RRT, the mass of each element present in effluent above limits of quantification multiplied by effluent volume was considered, corrected for plasma concentration and dose of dialysis (solute removal index). In addition, the quantity of trace element present in effluent at baseline, that is when sampled after filtration but before patient attachment, multiplied by effluent volume, was subtracted from all values. Loss of major elements (S, Ca, P, Na, Mg, and K) was calculated, but data are not shown because the study hypotheses did not consider these elements. In total, net losses of 13 of 32 trace elements between modalities of RRT were assessed, with significance levels adjusted accordingly. For effluent zinc, no significant net loss above baseline occurred in IHD (Figure 4a), but removal as a result of SLEDf was noted (average increase of 848 ± 1032 μg in effluent concentration from base to end) (Figure 4a). Apparent gain of zinc (i.e., gain to patient) resulted during CVVH (i.e., net decrease in effluent Zn from base to end) (Figure 4a). A statistically significant loss of strontium occurred with all modalities of RRT (Figure 4b), with effluent levels different between modalities at baseline (P for effect of RRT mode = 0.003). The latter effect was also observed for vanadium, which was lower in effluent of CVVH patients (Figure 4e). For cesium (Figure 4c) and molybdenum (Figure 4e), an effect of time only was noted. Trace elements in effluent above LOQ, but not significantly influenced by modality or duration of RRT, were Cr, Cu, Fe, Mn, and Se (Figure 4e). In summary, net loss of trace elements to effluent as a consequence of RRT was negligible for IHD (net loss −369 ± 1094 μg), statistically significantly higher for SLEDf (+1787 ± 1603 μg), but negligible to reversed for CVVH (net loss −1099 ± 1012 μg) (Figure 4d).
Figure 4.
Effluent trace elements during different modes of renal replacement therapy (RRT). (a−d) Concentrations of trace elements were measured in spot samples of postfilter baseline effluent after “priming” of each RRT machine before the patient was connected (“BASE”) or at the end of each session (“END”). Total losses were estimated by multiplying measured concentrations in BASE or END sample (μg/l) by the total volume in liters of effluent produced for each patient for that session. Data are estimated mean after correction (i.e., included as covariates in the statistical model) for dose-of-dialysis (solute removal index) and plasma concentration. If necessary, to normalize residual error before analysis, data were log10 transformed. Analysis was by repeated-measures analysis of variance or mixed-effect models, as appropriate, with RRT mode and time as fixed effects and patient ID as a nested random effect, using Genstat v18 (VSNi, Rothampsted, UK). Estimated effect size was calculated by the statistical package with IHD as referent category for the effects of mode and time (as indicated on each graph). Statistical significance was accepted at P ≤ 0.003 (adjusted for the number of comparisons). (e) Table: potassium included for orientation of RRT effects, as it is known that sustained low-efficiency diafiltration (SLED-F) removes more K+ than intermittent hemodialysis (IHD). *Data for graphs also are included in the table for comparison to other trace elements. **Barium levels were quantifiable, but since values are not included in the certified reference materials (CRM) (Seronorm L2 Urine), data should be interpreted with caution. CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; SED, standard error of the differences between means.
B Vitamin Losses
Methods for simultaneous measurement of the B-vitamin series (1, 2, 3, 6, 9, and 12) were optimized to quantify losses at <10 ppb (i.e., <10 μg/l), but no measurable values were detected in effluent for any modality at any time point (50 samples). Reasons could include the following: (i) dilution of signal (i.e., by 50−100 L of effluent); (ii) conversion to alternative metabolites not discriminated on targeted mass spectrometry (e.g., thiamine pyrophosphate is the main form of Vit B1 [Mwt; 425.31 g/mol], but thiamine mono/triphosphate and adenosine thiamine diphosphate are other active forms [Mwt 345.33 and 674.5, respectively]); or (iii) B vitamins are not removed to effluent by RRT. The last is unlikely, as the filtration coefficient for micromolecules ≤1000 Kd is ∼1.0 and Vit B12, the largest B vitamin, with Mwt 1355 g/mol. Adsorption to the RRT circuit and dialyzer/filter is a possible explanation. This would represent net loss to the patient but would not appear in the effluent. We therefore generated preliminary data using an in vitro model of RRT (using an HDF440 filter for CVVH) with supraphysiological levels of B vitamins added to replacement solution (Prismasol for CVVH [Baxter, Thetford, UK]) to demonstrate net filtration and adsorption (Supplementary Figure S1A and B). After only 5 minutes of pseudo-filtration, there was a marked difference in B vitamin concentration of replacement solution and effluent, accountable only by adsorption to the circuit and/or filter (Supplementary Figure S1A and B). Similar results were obtained for trace elements (Supplementary Figure S1C) and amino acids (Supplementary Figure S1D). However, elution of the used dialyzers and filters with an acidified ethanol solution (after rinsing and clearing of blood) (Supplementary Figure S1E) recovered only minimal (<1 g for all modes of RRT) quantities of amino acids (Supplementary Figure S1F).
First Versus Second Session of RRT
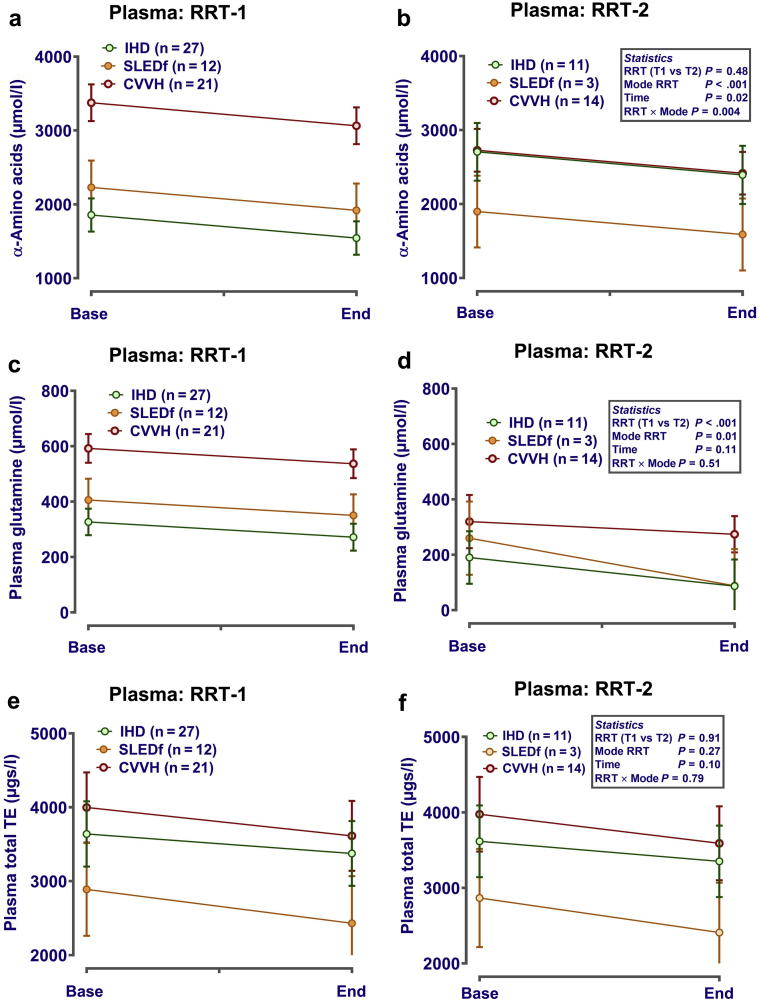
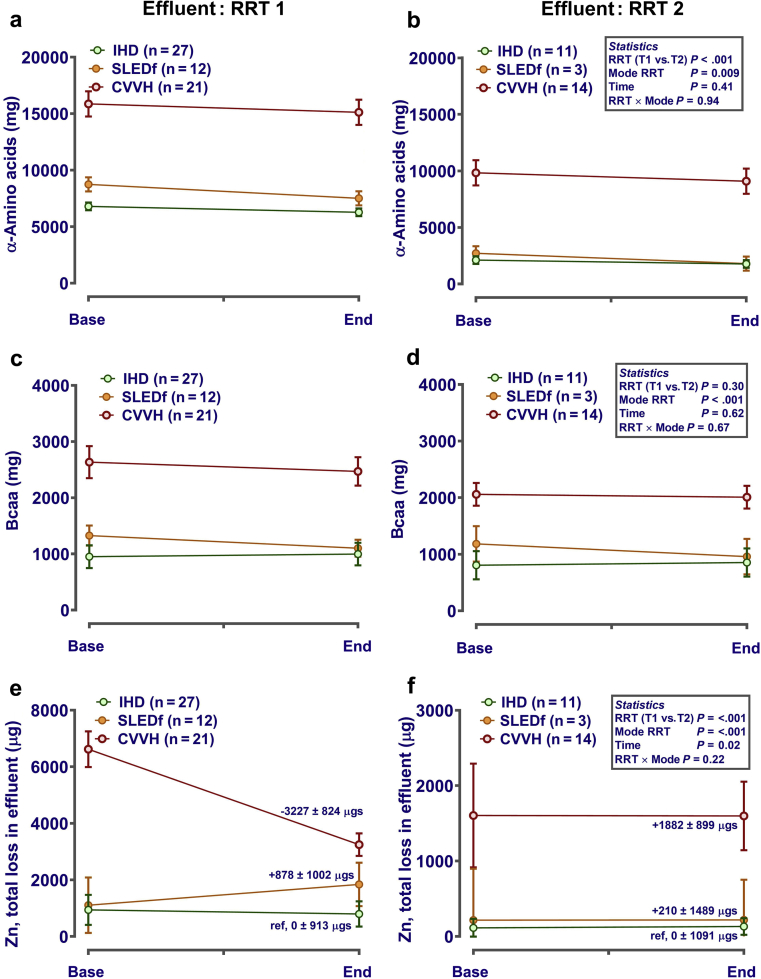
Only 28 patients (IHD, n = 11; SLED-F, n = 3; CVVH, n = 14) received a second RRT session. For those patients, variability of end-points was generally increased, but pattern of micronutrient change during RRT was similar (Figures 5 and 6). For example in plasma, amino acid concentrations tended to decline during each session (Figure 5a and b), with some declining further between the end of the first and the start of the second RRT session (e.g., plasma glutamine) (Figure 5c and d). Concentration of trace elements in plasma again was largely unaffected by RRT (Figure 5e and f). Effluent losses of micronutrients generally followed the pattern observed during the first session, but with lower total losses (e.g., amino acids) (Figure 6a and b). This was not the case for all amino acid groups, with little apparent effect on branched-chain amino acids (isoleucine, leucine, and valine) (Figure 6c and d). Similarly, comparison of effluent trace element loss during RRT2 between modalities followed a pattern similar to that of RRT1, with slightly lower losses (e.g., for zinc) (Figure 6e and f).
Figure 5.
Plasma amino acids and trace elements during the first renal replacement therapy (RRT) session (RRT1) and second RRT session (RRT2). Plasma was sampled before (base) and at the end (end) of each RRT session. Micronutrients and amino acids were measured in spot samples of plasma as described in the Methods. Data (mean ± SE) are presented corrected (i.e., included as covariates in the statistical model) for dose-of-dialysis (plasma urea reduction ratio) with or without log10 transformation if necessary. Analysis was by repeated-measures analysis of variance or mixed-effect models (Genstat v18; VSNi, Rothampsted, UK), as appropriate, with RRT mode, time, and session (first or second) as fixed effects. Patient ID was included as a nested random effect. Statistical significance was accepted at P ≤ 0.002 (adjusted for the number of comparisons). The data emphasize that our broad assumptions on the change in plasma micronutrients during RRT1 are largely applicable during RRT2, despite moderate depletion of micronutrient status. (a,b) Change in total plasma amino acid concentration for RRT-1 and RRT-2, respectively; (c,d) data for plasma glutamine; (e,f) data for plasma total trace element concentration. CVVH, continuous veno-venous hemofiltration; IHD, intermittent hemodialysis; SLEDf, sustained low-efficiency diafiltration.
Figure 6.
Comparison of effluent amino acid and trace element loss during the first renal replacement therapy (RRT) session (RRT1) and the second renal replacement session (RRT2). Effluent was sampled before, mid-way through, and at the end of each RRT session. Concentrations of micronutrients were measured in spot samples of baseline effluent (i.e., after “priming” each dialyser; intermittent hemodialysis [IHD], sustained low-efficiency diafiltration [SLED-f]) or in replacement solution (e.g., PrismaSol for continuous veno-venous hemofiltration [CVVH]). Total losses were estimated by multiplying all measured concentrations (μg/l) by the total volume of effluent produced for each patient. Data (mean ± SE) are presented corrected (i.e., included as covariates in the statistical model) for dose-of-dialysis (urea reduction ratio for plasma levels; solute removal index for effluent losses) and plasma concentration (for calculation of effluent losses only). If necessary, data were log10 transformed before statistical analysis to normalize residual error. Graphs were generated in GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA). Analysis was by repeated-measures analysis of variance or mixed-effect models (Genstat v18; VSNi, Rothampsted, UK), as appropriate, with RRT mode, time, and session as fixed effects. Patient ID was included as a nested random effect. Statistical significance was accepted at P ≤ 0.002 (adjusted for the number of comparisons). bcaa, branched chain amino acids.
Discussion
We have confirmed a high prevalence of disease-related malnutrition (DRM) in patients with AKI requiring RRT. We demonstrated that acute RRT results in significant losses of amino acids and micronutrients. Such losses may contribute to DRM. We show for the first time that these losses vary both qualitatively and quantitatively with the type of RRT. These data raise the possibility that tailoring of nutritional supplements to the mode of RRT might be indicated in the future, if further evidence is acquired.
Loss of Amino Acids
Three different methods of nutritional assessment were used before RRT.9, 10 The proportion of patients at risk for malnutrition was very high, 82% in this study using MUST. Contributing factors to pre-RRT DRM in AKI patients are likely to include (i) reduced food intake, because of anorexia or malabsorption; (ii) increased catabolism associated with acute illness and sepsis; and (iii) increased gastrointestinal losses. Micronutrient loss from RRT has previously been considered; however, before the present study, no study had compared 3 different modalities. We demonstrate marked amino acid loss for all 3 modalities, with the greatest losses for CVVH (∼14−22 g per session), followed by SLEDf (∼7−10 g) and then IHD (∼3−6 g). This difference between modalities remained, albeit attenuated, after adjustment for the delivered dose of RRT and baseline plasma amino acid concentration (both differed considerably between RRT groups). It is likely that plasma amino acid levels were greatest in the CVVH group due to enteral or parenteral supplements.11 These data suggest that amino acids are lost more from convection-based RRT than from diffusion-based treatment. Modes of RRT using a combination of both mechanisms were predictably intermediate for amino acid loss. Previous studies have demonstrated significant amino acid loss during CVVH12, 13 and also during hemodialysis,14 but the latter studied patients with end-stage renal disease, which has important pathophysiological differences from AKI and should not be compared directly. We suggest that nutritional assessment of patients receiving RRT might need to take into account the mechanism of solute removal. Specifically, convection-based RRT, which is increasingly being used in renal units, may have a greater effect on metabolism and nutritional homeostasis than diffusion-based RRT.
Reductions in total serum/plasma amino acids during RRT, as observed in this study (with the exception of IHD) are common, but some ambiguity exists.14, 15 Such variability may relate to differences in the study cohorts in terms of their nutritional or metabolic state. Sampling of the plasma amino acid pool is a snapshot of overall protein turnover (composite of protein assimilation/supplementation vs. degradation). For individual amino acids, those at relatively high concentration in plasma (e.g., glutamine, glycine) tended to dominate the overall pattern of loss between modes of RRT. For some such as taurine, however, important differences were noted, such as more being lost in SLEDf than in CVVH (a decrease of ∼30−50 μmol/l from a baseline of 62 ± 45 μmol/l). Acute or chronic taurine deficiency has been associated with heart failure.16 We were particularly interested in assessing losses of essential and conditionally essential amino acids because we assumed that their deficiency might be more likely to result in adverse clinical consequences. Losses varied with mode of RRT; however, in SLEDf, for example, plasma concentration decreased from 821 to 545 ± 114 μmol/l, illustrating the effect that RRT can have on important micronutrients that can be restored only through diet.
Loss of Trace Elements
Loss of trace elements was variable between elements and between modes of RRT for the same element. Differing behaviors of individual elements are not surprising, as they vary in size, charge, protein binding, and movement between extra- and intracellular compartments. We were particularly interested in selenium, copper, zinc, and iron because of their roles in immune response to critical illness and in oxidative stress,17 which is a feature of AKI.18 Small clinical and in vitro studies have demonstrated loss of trace elements in hemofiltrate.19, 20, 21 Tonelli et al. suggested that marginal Se status is strongly associated with risk of hospitalization and death, although this was a study of incident hemodialysis patients with end-stage renal disease rather than AKI.22 Compared with published reference ranges for healthy adults, baseline plasma Se, Cu, and Zn levels were low in our study, again consistent with DRM in AKI and critical illness.23, 24 Zn effluent losses varied markedly between RRT modalities, with adjusted loss greatest for CVVH. Rate of loss appeared to increase with duration in SLEDf but remain unchanged for IHD. A possible explanation is that Zn is removed more by convection because this mechanism can remove larger, protein-bound molecules to some extent. Furthermore, our in vitro data suggest that Zn adsorption could be considerable (Supplementary Figure S1C), and this would remain unaccounted for in any analyses.
Loss of B Vitamins
We optimized methods to detect B vitamins in the parts per billion (ppb) range but were unable to detect any above (>) the limit of detection (LOD) in effluent. We attribute this to the dilution effect of large effluent volumes. B-vitamin deficiency can occur in patients with end-stage renal disease who are receiving regular hemodialysis. Standard practice is to prescribe supplements of water-soluble vitamins. It is not known whether clinically significant losses of water-soluble vitamins occur in RRT for AKI, for which patients usually undergo only a limited number of RRT sessions and are likely to have different pretreatment pathophysiological status. We assume that significant vitamin loss does occur based on molecular size, charge, and lack of protein binding, but we have been unable to quantify it. Our in vitro study, using supraphysiological but easily measurable concentrations of B vitamins added to filtrate, suggested that significant adsorption to the hemofilter occurs. Such an effect would partly explain the undetectable levels in RRT effluent.
Limitations of the Study
This study has several limitations. First, it is an observational study in which prescription and details of each RRT were determined by clinicians independent of the study. RRT modality, duration, pump speeds, and delivered dose therefore varied. We could not randomize patients to RRT groups, and we recruited the fewest patients to the SLEDf group. It was not surprising that the CVVH group, recruited from intensive care units, had the worst baseline clinical nutritional status. Second, we measured only plasma levels of nutrients, which may not reflect total body status of a nutrient. The volume of distribution, degree of protein binding, and kinetics of transfer between fluid compartments is likely to vary between the nutrients that we studied. We have adjusted our results for plasma concentrations, but we have not attempted to incorporate 2-compartment kinetic modeling into our calculations. It might be that 2-compartment modeling would be appropriate for some individual nutrients, including those for which we noted a degree of post-RRT rebound in plasma concentration (such as glutamine). We acknowledge that comparison between RRT modalities is particularly complex for such solutes. Nevertheless, we believe that we have adopted a pragmatic approach to the calculation of total nutrient losses and comparison of these losses between RRT modalities.
Another limitation was the inability to quantify enteral and parenteral input of individual nutrients, although all patients in the CVVH group received some supplementation. We provided indirect evidence for adsorption of some micronutrients from an in vitro experiment, but we were unable to quantify the extent of adsorption of amino acids, trace elements, or B vitamins in the clinical study. This will be an important challenge in future studies. It will also be of interest to study differences in adsorption of micronutrients to different RRT membrane types, for example, polyacrylonitrile (a component of the AN69 hemofilter) and polysulphone (a component of the FX60 dialyzer). Markedly different degrees of adsorption of specific micronutrients between membrane types might confound interpretation of the corresponding effluent concentrations.
Our data derived mainly from patients receiving their first and only session of RRT. Only 28 of 72 patients in our cohort required a second RRT session, so our data may reflect qualitative and quantitative differences at this time, rather than being representative of a prolonged schedule of RRT. However, our analysis of patients receiving a second RRT session broadly corresponded to their first, with evidence for gradual nutritional depletion. The observation that less than 40% of patients required a second RRT session was perhaps surprising, but it reflected other observational data collected from our unit in a larger patient cohort. One possible explanation would be a low threshold for initiation of RRT, which was a clinical decision independent of the study. Such an approach might increase the reported renal recovery rate. It was not surprising that the highest rate of second RRT was in the CVVH group (15 of 24 patients), as these were the most unwell patients.
The fact that most RRT sessions in the study represented a first treatment of patients with AKI, together with the observational design with no control over RRT prescription, explains the low blood pump speed for IHD. Indeed it is similar to those for SLEDf and CVVH, although dialysate flow rates are markedly different. It would be of interest to acquire data from several consecutive RRT treatments, likely with increasing blood pump speeds, but this was not feasible within the design of this study.
To our knowledge, this is the first study to compare loss of amino acids and micronutrients in IHD, SLEDf, and CVVH. These 3 RRT modalities are used commonly to manage AKI and were chosen to allow comparison of mechanisms of solute clearance: diffusion, combined diffusion plus convection, and convection. Even though patient numbers were small, this is one of the largest studies investigating loss of micronutrients in acute RRT, a potentially important research area so far largely neglected. The study was not designed to investigate clinical consequences of micronutrient loss, but it has provided data that will facilitate design of such studies. Further work is required to investigate the details of individual micronutrient clearance with different RRT modalities. Thereafter, if larger studies suggest evidence of clinically significant losses and adverse clinical outcomes (or surrogates such as markers of inflammation), a future step might be to design interventional studies with bespoke nutritional supplements.
In conclusion, we have demonstrated significant amino acid and trace element loss in 3 RRT modalities commonly used to manage AKI. The pattern of nutrient losses varies considerably between modalities (convection >> hemodiafiltration > diffusion). The type of RRT used to manage AKI might influence the patients’ risk of malnutrition and their nutritional requirements. Far more research is required in this important area.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This paper presents independent research funded by the NIHR under its Research for Patient Benefit programme, grant reference PB-PG-0613-31042. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Early stages of the work were funded by a pump priming grant from Nottingham University Hospitals Charity. The authors gratefully acknowledge the help and support of Dr. Scott Young for inductively coupled plasma−mass spectrometry analyses, Dr. Liu Miu for B-vitamin analyses, Dr. Dongfang Li for amino acid analyses, and Dr. Jim Craigon for statistical advice on the manuscript (all School of Biosciences, Sutton Bonington Campus, University of Nottingham). We thank nursing staff at NUH Renal and Transplant Unit and Intensive Care Units, Mr. Mike Pikett for technical input with hemodialysis machines, and the patients who kindly consented to participation in the study. We thank the Nottinghamshire Kidney Units Appeal for ongoing support of our research programme.
Author Contributions
MAJD conceived the study; DSG and MAJD designed research; WCO, JCA, DSG, BM, MR, AS, DH, and MAJD conducted the research; DSG, WCO, and MAJD co-wrote the manuscript. All authors critically evaluated the paper. DSG conducted the statistical analyses. DSG and MAJD have primary responsibility for its final content.
Footnotes
Table S1. Change in plasma and effluent amino acids during RRT.
Table S2. LC-MS/MS parameters and limits of detection for B vitamins in effluent.
Figure S1. In vitro model of micronutrient adsorption to RRT circuits. Micronutrient adsorption to RRT circuits: (A) an HDF440 was primed (30 minutes) using standard PrismaSol solution before addition of supraphysiological concentration of B-vitamins (B), trace elements (C), and amino acids (D, all purchased from Sigma-Aldrich). Replacement solution was sampled before (base, –5 minutes) and after (time zero, 0 minutes) the addition of micronutrients and amino acids and subsequently at 5, 30, and 60 minutesof ”pseudoRRT.” Data are mean ± SEM of 3 to 4 independent experiments. (E) In a separate experiment n = 15 spent filters (IHD & SLED-F, Fx60; CVVH, AN69) were rinsed of blood contamination (for 1 to 2 hours with PBS plus 3mM EDTA at 80 mls/minutes) and flushed with an eluting fluid (100 mLs, 50:50 EtOH:water + 0.1M HCl for 15 minutesat 80 mls/minute). The resulting elute (50 to 80 mls) was evaporated to dryness before reconstitution in 1 ml amino acid running buffer for measurement of adsorbed amino acids (F).
Supplementary Methods.
Contributor Information
David S. Gardner, Email: david.gardner@nottingham.ac.uk.
Mark A.J. Devonald, Email: mark.devonald@nuh.nhs.uk.
Supplementary Material
References
- 1.Fiaccadori E., Lombardi M., Leonardi S. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10:581–593. doi: 10.1681/ASN.V103581. [DOI] [PubMed] [Google Scholar]
- 2.Umber A., Wolley M.J., Golper T.A. Amino acid losses during sustained low efficiency dialysis in critically ill patients with acute kidney injury. Clin Nephrol. 2014;81:93–99. doi: 10.5414/CN107982. [DOI] [PubMed] [Google Scholar]
- 3.Btaiche I.F., Mohammad R.A., Alaniz C. Amino acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. 2008;28:600–613. doi: 10.1592/phco.28.5.600. [DOI] [PubMed] [Google Scholar]
- 4.Kerr M., Bedford M., Matthews B. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29:1362–1368. doi: 10.1093/ndt/gfu016. [DOI] [PubMed] [Google Scholar]
- 5.Descombes E., Hanck A.B., Fellay G. Water soluble vitamins in chronic hemodialysis patients and need for supplementation. Kidney Int. 1993;43:1319–1328. doi: 10.1038/ki.1993.185. [DOI] [PubMed] [Google Scholar]
- 6.Dunford L.J., Sinclair K.D., Kwong W.Y. Maternal protein-energy malnutrition during early pregnancy in sheep impacts the fetal ornithine cycle to reduce fetal kidney microvascular development. FASEB J. 2014;28:4880–4892. doi: 10.1096/fj.14-255364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies M., Alborough R., Jones L. Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Sci Rep. 2017;7:17107. doi: 10.1038/s41598-017-17159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray C., Al-Dujaili E.A., Sparrow A.J. Excess maternal salt intake produces sex-specific hypertension in offspring: putative roles for kidney and gastrointestinal sodium handling. PLoS One. 2013;8:e72682. doi: 10.1371/journal.pone.0072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright M., Jones C. Renal Association clinical practice guideline on nutrition in CKD. Nephron Clin Pract. 2011;118(Suppl 1):153–164. doi: 10.1159/000328067. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson M., Capra S., Bauer J. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15:458–464. doi: 10.1016/s0899-9007(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 11.Wolfson M., Jones M.R., Kopple J.D. Amino acid losses during hemodialysis with infusion of amino acids and glucose. Kidney Int. 1982;21:500–506. doi: 10.1038/ki.1982.52. [DOI] [PubMed] [Google Scholar]
- 12.Davenport A., Roberts N.B. Amino acid losses during continuous high-flux hemofiltration in the critically ill patient. Crit Care Med. 1989;17:1010–1014. doi: 10.1097/00003246-198910000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Chua H.R., Baldwin I., Fealy N. Amino acid balance with extended daily diafiltration in acute kidney injury. Blood Purif. 2012;33:292–299. doi: 10.1159/000335607. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt J.J., Hafer C., Spielmann J. Removal characteristics and total dialysate content of glutamine and other amino acids in critically ill patients with acute kidney injury undergoing extended dialysis. Nephron Clin Pract. 2014;126:62–66. doi: 10.1159/000358434. [DOI] [PubMed] [Google Scholar]
- 15.Kihara M., Ikeda Y., Fujita H. Amino acid losses and nitrogen balance during slow diurnal hemodialysis in critically ill patients with renal failure. Intensive Care Med. 1997;23:110–113. doi: 10.1007/s001340050299. [DOI] [PubMed] [Google Scholar]
- 16.Yamori Y., Taguchi T., Hamada A. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valko M., Morris H., Cronin M. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 19.Berger M.M., Shenkin A., Revelly J.-P. Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am J Clin Nutr. 2004;80:410–416. doi: 10.1093/ajcn/80.2.410. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura A.T., Btaiche I.F., Pasko D.A. In vitro clearance of trace elements via continuous renal replacement therapy. J Ren Nutr. 2004;14:214–219. [PubMed] [Google Scholar]
- 21.Tonelli M., Wiebe N., Hemmelgarn B. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. 2009;7:25. doi: 10.1186/1741-7015-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonelli M., Wiebe N., Bello A. Concentrations of trace elements and clinical outcomes in hemodialysis patients: a prospective cohort study. Clin J Am Soc Nephrol. 2018;13:907–915. doi: 10.2215/CJN.11451017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst R., Armah C.N., Dainty J.R. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyland D.K., Dhaliwal R., Suchner U. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.