Abstract
Introduction
Prior studies found renal disease was common among HIV-infected outpatients. We updated incident renal disease estimates in this population, comparing those with and without tenofovir exposure.
Methods
We conducted a retrospective analysis of the DC Cohort, a longitudinal study of HIV patients in Washington, DC, from 2011 to 2015. We included adults prescribed antiretroviral therapy (ART) with baseline glomerular filtration rate (GFR) ≥15 ml/min per 1.73 m2. We defined renal disease as 50% decrease in GFR or doubled serum creatinine (Cr) within 3 months. We defined cumulative viral load as area under the curve (AUC) of log10 transformed longitudinal HIV RNA viral load (VL). Correlates of time to incident renal disease were identified using Cox proportional hazard regression models, adjusted for demographics and known risk factors for kidney disease.
Results
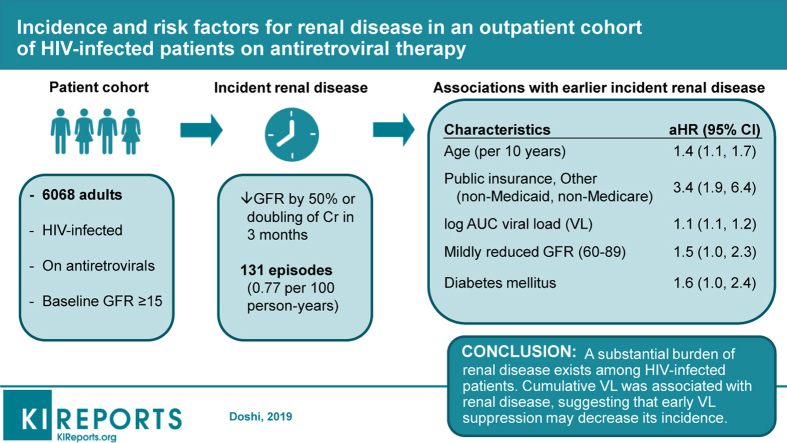
Among 6068 adults, 77% were Black and median age was 48 years. Incident renal disease rate was 0.77 per 100 person-years (95% confidence interval [CI]: 0.65–0.9). Factors associated with renal disease were age (adjusted hazard ratio [aHR]: 1.4; CI 1.1–1.7 per 10 years), public non-Medicaid, non-Medicare insurance (aHR: 3.4; CI: 1.9–6.4), AUC VL (aHR: 1.1; CI: 1.1–1.2), diabetes mellitus (aHR: 1.6; CI: 1.0–2.4), and mildly reduced GFR (60–89 ml/min per 1.73 m2) (aHR: 1.5; CI: 1.0–2.3); recent tenofovir exposure was not associated with renal disease (aHR: 0.7; CI: 0.5–1.1).
Conclusion
Our study revealed a substantial burden of renal disease among HIV patients. Cumulative VL was associated with renal disease, suggesting that early VL suppression may decrease its incidence.
Keywords: cumulative viral load, HIV, hypertension, renal disease
Graphical abstract
Kidney disease has been commonly reported among patients infected with HIV. Among HIV-infected persons cared for at an outpatient infectious diseases clinic in the US from 2000 to 2002, the estimated incidence of renal failure was 5.9 cases per 100 person-years.1 Another study in a London clinic from 1998 to 2005 estimated the incidence of acute kidney injury among HIV-infected outpatients to be 2.7 per 100 person-years. Black race has been associated with kidney injury among both HIV-infected2 and HIV-uninfected3 persons, with HIV-associated nephropathy occurring almost exclusively among persons of African descent. In the United States, HIV disproportionately affects the Black population; in 2012, 41% of Americans living with HIV identified as Black.4 Although the incidence of HIV-associated nephropathy has decreased in the era of ART,5 the number of HIV-infected persons with comorbidities associated with kidney disease, such as hypertension, has increased due, in part, to longer life expectancies.6, 7 Between 22% and 73% of patients in the prior studies of renal disease incidence were prescribed ART. As a greater proportion of patients are prescribed ART, updated estimates of renal disease among HIV-infected patients are needed.
Current US guidelines recommend that ART be given to all HIV-infected patients.8 Randomized controlled trials demonstrated that immediate initiation of ART improves morbidity and mortality even in persons with CD4+ cell counts >500.9, 10 Early ART may decrease immune activation, leading to persistent benefits after viral suppression is achieved. HIV actively replicates in kidney cells, and even low levels of viremia have been associated with declines in renal function.11 Cumulative plasma HIV burden may be a better measure of immune system activation12 that can have deleterious effects on the kidney compared with a single baseline VL measurement. Decreasing this immune activation with early and sustained HIV VL suppression may decrease the risk of renal disease, outweighing the risks posed by ART.
The antiretroviral drug tenofovir disoproxil fumarate (TDF) is nephrotoxic; results from a meta-analysis found that ART regimens containing TDF were associated with a mildly increased risk of acute renal failure compared with those without TDF (risk difference: 0.7%; 95% CI: 0.2–1.2).13 Tenofovir alafenamide has similar efficacy to TDF despite lower plasma levels of tenofovir, resulting in improved reported renal safety profiles.14, 15 Since approval of tenofovir alafenamide by the Food and Drug Administration in 2015,16 the Department of Health and Human Services HIV guidelines recommended 5 initial ART regimens for treatment-naïve patients that did not include TDF.8 Prior versions of the guidelines included TDF in all recommended initial regimens; providers now have the option of starting patients at risk of renal disease on a non–TDF-containing regimen.
In addition to TDF, hypertension has been shown to be an independent risk factor for renal disease among HIV-infected persons.2 HIV-infected adults are 32% more likely to have hypertension than HIV-uninfected adults, and this risk is increased even after controlling for factors such as race.17 Despite these findings, no studies have examined the effects of specific antihypertensive medications on the renal function of HIV-infected persons. According to the Eighth Joint National Committee guidelines for hypertension management, thiazide-type diuretics are recommended as first-line treatment, regardless of race, and HIV status does not affect the selection of therapy.18 The exact mechanism by which thiazide diuretics lower blood pressure is unknown, but an initial reduction in extracellular fluid and plasma volume can lead to volume contraction.19 Prerenal etiologies account for an estimated 38% of renal failure in HIV-infected patients.1 It is unknown whether the mild prerenal state induced by thiazide therapy predisposes patients to tenofovir-induced nephrotoxicity.
The primary goal of this analysis was to describe the incidence of renal disease in treatment-experienced HIV-infected patients in an outpatient setting, and to examine if renal disease was associated with a recent TDF prescription. The secondary goal was to identify risk factors for renal disease among HIV patients on ART, most of whom were virally suppressed. Updating estimates and identifying correlates of disease may help guide clinical and programmatic efforts to prevent and treat kidney disease in high-risk populations.
Methods
Setting and Subjects
This analysis used data from the DC Cohort, a longitudinal, observational cohort study that collects clinical data on HIV-infected patients receiving care in 14 clinics in Washington, DC. Participants’ data were abstracted from medical records and entered into a web-based data entry system called Discovere (Cerner Corporation, Kansas City, MO). The study protocol, consent forms, and research instruments were approved by the George Washington University Institutional Review Board, the DC Department of Health Institutional Review Board, and the Institutional Review Boards of the individual study sites. Details of the DC Cohort study design have been described previously.20
Eligibility Criteria
We included HIV-infected adults (≥18 years old) who consented into the DC Cohort from January 1, 2011, through March 31, 2015, and were prescribed ART for ≥14 days. We excluded patients with <2 serum Cr laboratory values during the study period, a baseline GFR <15 ml/min per 1.73 m2, or chronic kidney disease (CKD) stage V or end-stage renal disease (as defined using International Classification of Diseases, Ninth Revision codes 585.5 or 585.6) at study entry. We also excluded any patient whose ART ended before consent because information before enrollment was scarce, limiting our ability to determine previous episodes of renal disease. The index date (start of analytic period) was 14 days after the start of ART. If patients were on ART for ≥14 days at the time of study consent, then the index date was the date of consent. Data were censored at the earliest of any of the following: onset of renal disease, 28 days after ART discontinuation, loss to follow-up, death, or June 15, 2015.
Outcomes
GFR was calculated using the Modification of Diet in Renal Disease equation.21 There is considerable variability in how prior studies defined renal disease. We modified the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria for the outpatient setting in order to differentiate more acute changes from worsening CKD. The RIFLE classification defines acute kidney injury as an increase in serum Cr by 2 times the baseline, or a decline in GFR by >50% within 7 days, or an abrupt decrease in urine output.22 Because patients in the outpatient clinic setting rarely have blood tests repeated within a week or urine output measured, we defined renal disease as a decrease in GFR by >50%, or a doubling of serum Cr within 3 months at any time during the analytic period. Similar to prior studies, we did not require the laboratory value to be repeated given that such a dramatic change in renal function would not be expected to be spurious.23, 24 We chose 3 months as a cutoff because the Kidney Disease Outcomes Quality Initiative defines CKD as lasting for ≥3 months.25
Exposure Ascertainment and Covariates
For the purposes of descriptive statistics (Table 1), we compared 2 groups: patients prescribed TDF versus those who had never been prescribed TDF at any point during the study period. For multivariate analysis, exposure to TDF was treated as time-varying. Incident renal disease was considered associated with TDF only if it occurred within a time range that the patient was prescribed the drug: from 14 days after TDF initiation to 28 days after TDF was stopped. Cumulative VL burden was measured as viremia copy-years. AUC VL was measured as time-varying during the study period. Cumulative VL was calculated as the AUC of longitudinal log10 transformed VL measurements taken from the start of observation to either time of incident renal disease or end of observation period. We normalized patient-level AUC VL by each patient’s respective length of follow-up time.
Table 1.
Demographics and clinical features of HIV-infected patients on ART ≥ 14 days enrolled in the DC Cohort, 2011–2015
| Sociodemographic and clinical characteristics | All Patients | Ever TDF | Never TDF | Pa | |||
|---|---|---|---|---|---|---|---|
| Total patients included in study, n | 6068 | 5039 | 1029 | ||||
| Years of observation, median (IQR) | 3.2 (2.0, 4.6) | 3.3 (2.1, 4.6) | 3.1 (1.7, 4.6) | <0.01 | |||
| Sex, n (%) | 0.02 | ||||||
| Male, M | 4329 | 71 | 3620 | 72 | 709 | 69 | |
| Female, F | 1628 | 27 | 1319 | 26 | 309 | 30 | |
| Transgender: M to F | 105 | 2 | 94 | 2 | 11 | 1 | |
| Transgender: F to M | 6 | <1 | 6 | <1 | 0 | <1 | |
| Race/ethnicity, n (%) | 0.24 | ||||||
| Non-Hispanic Black | 4675 | 77 | 3866 | 77 | 809 | 79 | |
| Non-Hispanic White | 835 | 14 | 696 | 14 | 139 | 14 | |
| Hispanic | 311 | 5 | 271 | 5 | 40 | 4 | |
| Other/Unknownb | 247 | 4 | 206 | 4 | 41 | 4 | |
| Age, yr, median (IQR) | 47.9 (38.1, 55.1) | 46.7 (37.0, 54.0) | 52.4 (44.5, 59.1) | <0.01 | |||
| HIV transmission, n (%) | <0.01 | ||||||
| MSM | 2326 | 38 | 2027 | 40 | 299 | 29 | |
| High-risk heterosexual | 2015 | 33 | 1669 | 33 | 346 | 34 | |
| IDU | 484 | 8 | 370 | 7 | 114 | 11 | |
| Other/Unknownc | 1243 | 20 | 973 | 19 | 270 | 26 | |
| Primary insurance, n (%) | <0.01 | ||||||
| Medicare | 799 | 13 | 571 | 11 | 228 | 22 | |
| Medicaid | 2075 | 34 | 1740 | 35 | 335 | 33 | |
| Private | 1555 | 26 | 1315 | 26 | 240 | 23 | |
| Public, Other (non-Medicaid, non-Medicare) | 776 | 13 | 659 | 13 | 117 | 11 | |
| Other | 705 | 12 | 613 | 12 | 92 | 9 | |
| Unknown | 158 | 3 | 141 | 3 | 17 | 2 | |
| CD4 count,d cells/mm3, n (%) | 0.15 | ||||||
| < 200 | 629 | 10 | 541 | 11 | 88 | 9 | |
| 200–349 | 910 | 15 | 743 | 15 | 167 | 16 | |
| ≥ 350 | 4419 | 73 | 3663 | 73 | 756 | 73 | |
| Unknown | 110 | 2 | 92 | 2 | 18 | 2 | |
| Viral load,d copies/ml, n (%) | 0.06 | ||||||
| 0–399 | 4964 | 82 | 4089 | 81 | 875 | 85 | |
| 400–999 | 166 | 3 | 146 | 3 | 20 | 2 | |
| 1000–9999 | 286 | 5 | 246 | 5 | 40 | 4 | |
| 10,000–99,999 | 358 | 6 | 301 | 6 | 57 | 6 | |
| ≥100,000 | 179 | 3 | 157 | 3 | 22 | 2 | |
| Unknown | 115 | 2 | 100 | 2 | 15 | 1 | |
| Log10 AUC VL, median (IQR)e | 7.5 (6.8, 12.2) | 7.5 (6.9, 12.5) | 7.4 (6.7, 11.4) | <0.01 | |||
| GFR, ml/min per 1.73 m2, n (%) | <0.01 | ||||||
| Normal, ≥ 90 | 3146 | 52 | 2801 | 56 | 345 | 34 | |
| Mildly reduced, 60–89 | 2479 | 41 | 2036 | 40 | 443 | 43 | |
| Moderately reduced, 45–59 | 319 | 5 | 172 | 3 | 147 | 14 | |
| Moderate-severely reduced, 30–44 | 90 | 1 | 24 | 0 | 66 | 6 | |
| Severely reduced, 15–29 | 34 | 1 | 6 | 0 | 28 | 3 | |
| Median (IQR) | 91.2 (76.4, 106.8) | 93.0 (79.3, 108.2) | 78.0 (61.6, 97.3) | <0.01 | |||
| HBV coinfection at index date, n (%) | 230 | 4 | 209 | 4 | 21 | 2 | <0.01 |
| HCV coinfection at index date, n (%) | 838 | 14 | 637 | 13 | 201 | 20 | <0.01 |
| Diabetes at index date, n (%) | 688 | 11 | 515 | 10 | 173 | 17 | <0.01 |
| Hypertension at index date, n (%) | 1874 | 31 | 1419 | 28 | 455 | 44 | <0.01 |
| Use of NSAIDS at index date, n (%) | 488 | 8 | 398 | 8 | 90 | 9 | 0.36 |
| Use of diuretics at index date, n (%) | 296 | 5 | 222 | 4 | 74 | 7 | <0.01 |
| Use of aminoglycosides at index date, n (%) | 3 | <1 | 2 | <1 | 1 | <1 | 0.43 |
| Incident renal disease during follow-up, n (%) | 131 | 2 | 93 | 2 | 38 | 4 | <0.01 |
GFR, glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; IDU, intravenous drug use; IQR, interquartile range; MSM, men who have sex with men; NSAIDs, nonsteroidal anti-inflammatory drugs; TDF, tenofovir disoproxil fumarate.
P values are derived from χ2 or Fisher exact tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Other/Unknown race/ethnicity includes Asian, American Indian/Alaskan Native, Hawaiian/Pacific Islanders, and those of mixed race.
Other/Unknown HIV transmission risk group includes participants who were perinatally infected and those with blood/coagulation disorders.
Measurements are the closest value to the index date from values documented 6 months before to 12 months post-index date.
Corresponds to the log of the area under the curve of longitudinal viral load measurements taken from the start of observation to the end of observation.
Statistical Analysis
Demographic and clinical characteristics were compared by TDF status using Wilcoxon rank sum test for continuous variables and Pearson χ2 test or Fisher exact test for categorical variables. To identify factors associated with renal disease, univariate and multivariable Cox proportional hazard regression models were used adjusting known risk factors for renal disease1, 2, 24, 26, 27, 28 and sociodemographic characteristics: age, sex, Black race, mode of HIV transmission, insurance payer, CD4 <200 cells/mm3 at enrollment, AUC VL, baseline GFR, coinfection with hepatitis B, coinfection with hepatitis C (HCV), diabetes mellitus, hypertension, and use of nonsteroidal anti-inflammatory drugs.
For the multivariable models, we used the serum Cr test date when renal disease was first noted as the right censor time point. To assess the adequacy of this approach, we performed a sensitivity analysis using a middle point approach, in which the midpoint between the serum Cr test date when renal disease was first noted and the immediate prior serum Cr test date was used as the imputed outcome time. For all statistical tests, a 2-tailed P value <0.05 was considered statistically significant. Analyses were performed using SAS v9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics
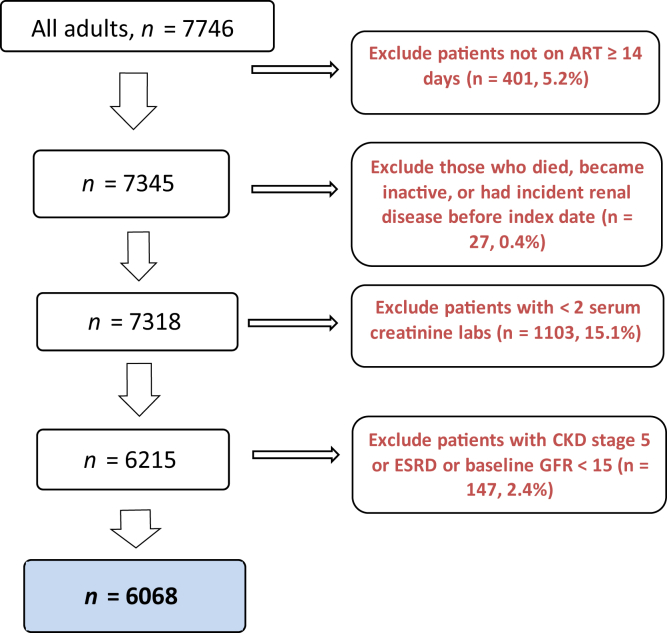
Among 7746 patients, 6068 (78%) were included in this analysis (Figure 1) for a total of 12,202 person-years of follow-up. Most patients were male (71%), identified as non-Hispanic Black (77%), and had a median age of 48 years (interquartile range 38–55) (Table 1). The most common risk factor for HIV transmission was men having sex with men (38%) followed by high-risk heterosexual behavior (33%) and injection drug use (IDU) (8%). Most patients had well-controlled HIV infection: 82% had VL <400 copies/ml at study entry, whereas only 3% had VL >100,000 copies/ml; similarly, 88% had a CD4 count ≥200 cells/mm3. A normal baseline GFR (≥90 ml/min per 1.73 m2) was observed in 52% of patients and 93% had a baseline GFR ≥60. The median baseline GFR was 91 ml/min per 1.73 m2. Few patients were prescribed nephrotoxic medications during the study, such as nonsteroidal anti-inflammatory drugs (8%), diuretics (5%), and aminoglycosides (<1%). The median number of serum Cr laboratory values resulted per patient in the cohort was 8; the mean number of serum Cr laboratory values was 5.4 per patient-year (data not shown).
Figure 1.
Patients included in the analysis of incident renal disease among HIV-infected patients in the DC Cohort, 2011–2015.
Most patients (83%) had been prescribed TDF at some point during the study period. Table 1 compares patients who had ever been prescribed TDF with those never prescribed TDF. Patients prescribed TDF were younger (median age 47 vs. 52 years, P < 0.01) and had higher baseline GFR (median 93.0 vs. 78.0, P < 0.01). Viral suppression was similar between the 2 groups (81% vs. 85%, P = 0.06). Compared to those without TDF exposure, a smaller proportion of patients prescribed TDF had diabetes mellitus (10% vs. 17%, P < 0.01), hypertension (28% vs. 44%, P < 0.01), HCV infection (13% vs. 20%, P < 0.01), and were prescribed a diuretic (4% vs. 7%, P < 0.01). Only 50% of patients with hypertension were prescribed antihypertensive medications (data not shown). Among patients on antiretroviral and antihypertensive medications, 77.5% were prescribed diuretics, including 61.9% on thiazide diuretics. A greater proportion of patients on a TDF regimen had hepatitis B infection (4.1% vs. 2.1%, P < 0.01).
Incidence of Renal Disease
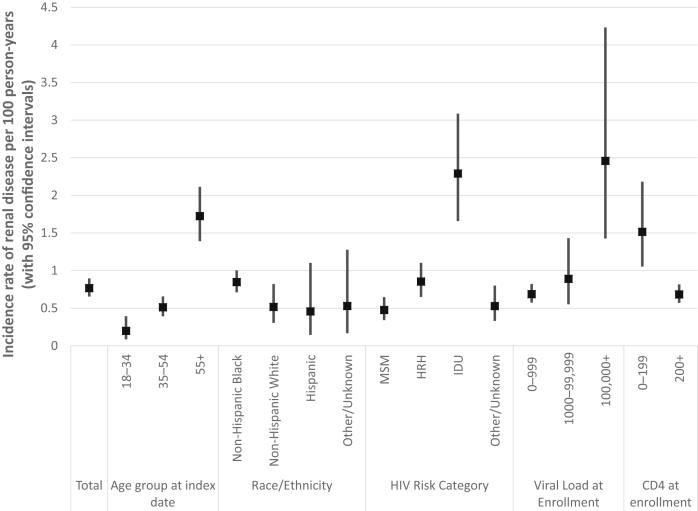
We observed 131 episodes of new-onset renal disease during our study period. The overall incidence rate (IR) of renal disease was 0.77 cases per 100 person-years (CI: 0.65–0.90) (Figure 2). Patients aged 55 and older (IR: 1.72; CI: 1.39–2.11) and those with IDU as an HIV risk category (IR: 2.29; CI: 1.66–3.09) had higher renal disease incidence rates. Patients with poorly controlled or advanced HIV disease, as evidenced by baseline VL >100,000 copies/mL (IR: 2.46; CI: 1.43–4.23) and CD4 count <200 cells/mm3 (IR: 1.52; CI: 1.05–2.18) also had higher incidence rates of renal disease.
Figure 2.
Incidence rate of renal disease among adults receiving antiretroviral therapy, the DC Cohort, 2011–2015. HRH, high-risk heterosexual (contact); IDU, injection drug use; MSM, men who have sex with men.
Renal Disease Risk Factors
For the multivariable Cox proportional hazard model, the assumption in proportional hazards over time for patients ever on TDF versus those never on TDF was first graphically examined with Kaplan-Meier curve and then statistically tested using χ2 in the Cox regression model. No visual, nor strong statistical, evidence of disproportionality in hazard was found between the 2 patient groups.
After controlling for potential confounders, older patients (aHR per 10 years: 1.4; CI: 1.1–1.7) and diabetic individuals (aHR: 1.6; CI: 1.0–2.4) were more likely to experience an episode of renal disease (Table 2). Among insurance categories, Public, Other (non-Medicaid, non-Medicare) insurance was associated with renal disease (aHR: 3.4; CI: 1.9–6.4) compared with private insurance. Patients with higher AUC VL were more likely to experience renal disease (aHR:1.1; CI: 1.1–1.2). When compared with baseline GFR >90 mL/min per 1.73 m2, mildly reduced renal function (GFR 60–89 mL/min per 1.73 m2) was associated with renal disease (aHR: 1.5; CI: 1.0–2.3). Further reductions in baseline GFR also were associated with a shorter time to renal disease, although these were not statistically significant likely because the comparison groups had fewer patients. IDU (aHR: 1.9; CI: 1.0–3.5) and HCV infection (aHR: 1.5; CI: 1.0–2.4) were marginally associated with renal disease. To account for possible collinearity between HCV and IDU, we performed the McNemar χ2 test for independence and found them to be associated with each other (P < 0.01). Similarly, CD4 count <200 cells/mm3 at study entry (compared with ≥200) (aHR: 1.5; CI: 0.9–2.4), hypertension (aHR: 1.4; CI: 1.0–2.1), and recent TDF exposure (aHR: 0.7; CI: 0.5–1.1) did not reach statistical significance for association with renal disease.
Table 2.
Likelihood of earlier time to incident renal disease among patients prescribed ART, the DC Cohort, Washington, DC, 2011–2015 (N = 6068; 131 experienced incident renal disease)
| Sociodemographic and clinical characteristicsa | Univariate |
Multivariableb |
||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| Age (per 10 yr) | 1.9 (1.7–2.3) | <0.01 | 1.4 (1.1–1.7) | <0.01 |
| Female sex at birth | 0.6 (0.4–1.0) | 0.06 | 0.9 (0.5–1.5) | 0.65 |
| Non-Hispanic black race/ethnicity | 1.8 (1.1–2.8) | 0.02 | 0.9 (0.5–1.5) | 0.61 |
| HIV transmission risk | ||||
| MSM | Referent | Referent | ||
| High-risk heterosexual | 2.0 (1.3–3.1) | <0.01 | 1.5 (0.9–2.6) | 0.13 |
| IDU | 5.3 (3.3–8.6) | <0.01 | 1.9 (1.0–3.5) | 0.05 |
| Other/Unknown | 1.1 (0.6–2.0) | 0.85 | 1.0 (0.5–2.0) | 0.93 |
| Insurance | ||||
| Medicare | 2.2 (1.1–4.4) | 0.02 | 1.1 (0.5–2.2) | 0.81 |
| Medicaid | 1.5 (0.8–2.8) | 0.20 | 1.0 (0.5–2.0) | 0.91 |
| Private | Referent | Referent | ||
| Public, Other (non-Medicaid, non-Medicare) | 7.4 (4.3,12.8) | <0.01 | 3.4 (1.9–6.4) | <0.01 |
| Other | 0.2 (0.0–1.5) | 0.11 | 0.2 (0.0–1.2) | 0.07 |
| Unknown | 0.7 (0.1–5.5) | 0.76 | 0.8 (0.1–5.9) | 0.81 |
| CD4 < 200 cells/mm3 (vs. ≥200) | 2.1 (1.3–3.2) | <0.01 | 1.5 (0.9–2.4) | 0.14 |
| log AUC VLc | 1.1 (1.1–1.2) | <0.01 | 1.1 (1.1–1.2) | <0.01 |
| GFR (ml/min per 1.73 m2) | ||||
| Normal (≥ 90) | Referent | Referent | ||
| Mildly reduced (60–89) | 1.7 (1.2–2.5) | 0.01 | 1.5 (1.0–2.3) | 0.04 |
| Moderately reduced (45–59) | 3.4 (1.9–6.1) | <0.01 | 1.9 (1.0–3.5) | 0.05 |
| Moderate-severely reduced (30–44) | 5.1 (2.2,12.0) | <0.01 | 1.8 (0.7–5.0) | 0.23 |
| Severely reduced (15–29) | 8.6 (2.7,27.6) | <0.01 | 3.0 (0.9,10.0) | 0.08 |
| Hepatitis B coinfection | 1.4 (0.6–3.1) | 0.47 | 1.6 (0.7–3.6) | 0.29 |
| Hepatitis C coinfection | 3.4 (2.3–4.8) | <0.01 | 1.5 (1.0–2.4) | 0.08 |
| Diabetes mellitus | 2.8 (1.9–4.2) | <0.01 | 1.6 (1.0–2.4) | 0.04 |
| Hypertension | 2.3 (1.7–3.3) | <0.01 | 1.4 (1.0–2.1) | 0.08 |
| NSAID use | 1.4 (0.8–2.6) | 0.23 | 1.1 (0.6–2.1) | 0.67 |
| Recent exposure to TDFd | 0.5 (0.4–0.8) | <0.01 | 0.7 (0.5–1.1) | 0.10 |
aHR, adjusted hazard ratio; ART, antiretroviral therapy; AUC VL, area under the curve of HIV viral load; CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; IDU, injection drug use; MSM, men who have sex with men; NSAIDs, nonsteroidal anti- inflammatory drugs; TDF, tenofovir disoproxil fumarate.
All variables measured at index date except for TDF exposure and AUC of VL, which were time-varying covariates during the study period.
All variables shown in this table were included in the multivariate model.
The log AUC VL was calculated as the log of the cumulative area under the curve of longitudinal HIV viral load measurements taken from the start of observation to either time of incident renal disease or end of observation period.
Incident renal disease was considered associated with TDF exposure only if it occurred within a time range that the patient was prescribed the drug: from 14 days after TDF was initiated to 28 days after TDF was stopped.
Sensitivity analyses between the right and middle point censoring approaches with the multivariable Cox regression models showed no significant differences in parameter estimates and thus led to the same conclusions on covariate effects as stated previously (data not shown).
Discussion
We provide estimates of renal disease in a large, urban, multicenter cohort of HIV-infected patients. These estimates were derived during an era when, compared with prior studies, early initiation of safer, more-effective ART was recommended. Using a strict definition adapted from the RIFLE classification, we found a substantial burden of renal disease among patients prescribed ART. Higher cumulative VL was associated with renal disease; however, in a population in which most patients were virally suppressed, traditional risk factors, such as advanced age and lower baseline renal function, were also associated with renal disease.
Our study updates the clinical findings on renal disease in the outpatient HIV setting. We found renal disease incidence to be 0.77 episodes per 100 person-years. Our estimate differs substantially from prior studies, but direct comparisons are difficult due to the lack of a standard definition. An analysis of the HIV Outpatient Study data from 2001 to 2005 among patients on ART found the incidence rate of renal disease (defined as a doubling of serum Cr within 1 year) was 0.71 and 0.36 per 100 person-years among TDF-exposed and TDF-unexposed patients.23 However, because this latter study excluded patients with baseline kidney disease (Cr clearance <50 ml/min), it likely underestimated incident renal disease in the general HIV cohort. A study of patients attending a single North Carolina HIV clinic from 1998 to 2005 defined acute renal failure as an increase in serum Cr by >1.36 mg/dl or >50% above baseline or a reduction in GFR by >40% within 3 months; the reported incidence was 2.7 per 100 person-years.24 In another study of HIV-infected patients in a single outpatient setting from 2000 to 2002 by Franceschini et al.,1 acute renal failure was defined as a 50% decrease in GFR or an increase in Cr by 0.5 mg/dl if the baseline was ≤1.9; an increase in Cr by 1.0 if the baseline was 2.0 to 4.9, or an increase in Cr by 1.5 if the baseline was ≥5.0; they reported an incidence of 5.9 per 100 person-years.1 When applying the latter, broader definition to our data, our renal disease incidence was lower than prior estimates (1.76 episodes per 100 person-years [CI 1.59–1.96]).
In the study by Franceschini et al.,1 the high acute renal failure incidence may be because only 68% ever received ART and 38% had VL >10,000 copies/ml. In our study, although patients with higher cumulative VL had shorter time to incident renal disease, most patients had achieved virologic suppression at baseline. Similarly, few patients in our study had immunologic failure: 10% had CD4 counts <200 at enrollment.
Despite better control of HIV, our estimates still exceeded the upper estimate of community-based incidence of acute renal failure found in a study of non–HIV-infected Kaiser Permanente patients from 1996 to 2003 (0.55 per 100 person-years).29 The Kaiser cohort was also not directly comparable because all patients had private insurance, the study included acute renal failure during hospitalizations, and used the broader acute renal failure definition described previously. However, their denominator included outpatients and provides the best available estimate of renal disease incidence rate in the general community. Multiple factors may have contributed to the high burden of renal disease in our cohort despite mostly successful ART. Our study population was composed of mostly Black patients, who are at higher risk of kidney disease.30, 31, 32 Sepsis-associated kidney injury and nephrotoxicity of HIV itself may contribute to the higher incidence of renal failure, but the risk for both are lowered by viral suppression.7, 24
Viral replication has been implicated in kidney function decline, independent of CD4 count.11, 33 Prior studies have linked higher VLs to renal disease, although this finding was based on a single VL measurement.1, 2 We found that every log increase in cumulative VL was associated with a 10% increased risk of renal disease. This suggests that earlier time to viral suppression could lead to lower renal disease incidence. Based on recent trials, guidelines now recommend starting ART on all HIV-infected patients, regardless of baseline CD4 count or viral load. Most notably, the START study was a multinational randomized control trial of ART-naïve adults that showed a reduction in serious events and death in early initiation of ART compared with deferred initiation.10 The primary composite outcome in the START trial included end-stage renal disease and death from renal disease, but excluded less severe kidney disease. Future studies should explore the impact of earlier ART initiation on renal function.
The presence of CKD was a predictor of incident renal disease in our cohort, with a trend toward higher risk as baseline GFR declined. Lower GFR has been correlated with acute renal failure in patients with HIV in studies conducted during pre- and early ART eras.2, 27 This relationship between CKD and acute renal failure has been long recognized in non-HIV populations.34 As patients with HIV live longer,6 they will be increasingly faced with comorbidities such as diabetes mellitus and hypertension. These known risk factors will likely increase the prevalence of CKD in this population. Process measures such as measurement of proteinuria and early nephrology referral have been shown to improve outcomes in patients with CKD.35 Biomarkers may aid in early detection and halt progression of CKD.36 A focus on process measures and biomarkers for CKD may improve outcomes in HIV-infected patients, even in those with suppressed VL.
Moreover, HCV coinfection and IDU had a trend toward association with renal disease. HCV coinfection has been previously associated with acute renal failure in patients with HIV.1 An estimated 16% of patients with HIV in the United States are coinfected with HCV; among IDUs with HIV, most are coinfected with HCV.37 In our analysis, collinearity of HCV and IDU may make it difficult to tease out their independent effects on renal disease. HCV coinfection may contribute to renal disease in multiple ways: liver disease predisposes to infection, diuretic therapy for ascites can reduce renal perfusion, and cirrhosis can result in hepatorenal syndrome.1 Active HCV infection itself is associated with higher rates of nephritis, nephrotic syndrome, and nephrosis.38 Curative treatment for HCV has been shown to be associated with resolution of cryoglobulinemia complications, including glomerulonephritis.39 Antiviral treatment of HCV is associated with improved renal outcomes overall.40 Given that patients coinfected with HIV/HCV have more rapid progression of liver fibrosis than their HCV mono-infected counterparts,41, 42 HCV treatment with coinfection is paramount.
Unsurprisingly, most (83%) patients in our cohort had been prescribed TDF at some point during the study period. After adjustment for other risk factors, we found no association between recent TDF exposure and renal disease. This is likely because clinicians followed guidelines and avoided TDF in patients with a GFR <60 or who were at risk for renal disease; only 3% of cohort patients ever prescribed TDF had a baseline GFR <60 ml/min per 1.73 m2. TDF has been associated with some loss of kidney function in a large proportion (63%) of patients,43 but the estimated reduction in Cr clearance is mild (mean 3.92 ml/min).13 Overt renal failure due to TDF is rare; one study reported 0.3% of patients developed renal failure.44 When TDF-associated kidney injury occurs, it has usually been reported with concomitant medications that either increase TDF levels (such as protease inhibitors) or are also nephrotoxic.13, 45 In our cohort, few patients were on nonsteroidal anti-inflammatory drugs (6%), or aminoglycosides (<1%), but 41% were on TDF and a protease inhibitor.
Although hypertension has been previously identified as a risk factor for kidney disease in patients with HIV,2 we did not find a strong association between hypertension and renal disease. We did not take into account control of blood pressure, which would likely affect this association. In a separate subanalysis, we examined the effect of diuretics on renal disease, positing that the diuretic-induced prerenal state may make patients more susceptible to the nephrotoxic effects of TDF. There was no increase in renal disease among patients prescribed diuretics, even when given concomitantly with TDF. This may be partly explained by the fact that plasma volumes return to normal 4 to 6 weeks after initiation of thiazide diuretics; their antihypertensive effects are maintained via vasodilatory pathways.19 We did not always have the date that medications were started, so we are unable to comment on the risk of renal disease in the few weeks after diuretic initiation when plasma volumes are reduced.
Similarly, we did not find a statistically significant association between Black race and renal disease. HIV-associated nephropathy almost exclusively occurs in Black patients with low CD4 counts and uncontrolled HIV infection.46 Although our study population was mostly Black, the high rates of viral suppression and low proportion of patients with CD4 count <200 likely explains part of the decreased racial disparity in incident renal disease.
The association that we found between Public, Other insurance and renal disease may have multiple explanations. Those with Public, Other insurance had a higher frequency of laboratory monitoring, improving our ability to detect a rise in Cr within 3 months (and thus meet our outcome definition). There may be other confounding variables that we were unable to adjust for, such as severity of comorbidities and hospital admissions, that were associated with insurance provider. For example, if patients with poorly controlled diabetes were more likely to have Public, Other insurance than those with well-controlled diabetes, this would likely result in higher rates of renal disease, explaining the apparent association between insurance status and renal disease.
Our study has other limitations that are inherent to analysis of cohort data. We did not have data before consent into the cohort; we are thus unable to factor in variables such as nadir CD4+ cell count or baseline VL before ART initiation. Patients hospitalized with community-onset renal disease would not have been captured in our analysis. Medication data were extracted from electronic health records; we did not confirm adherence via drug-level measurement or pharmacy data. Over-the-counter medications, such as nonsteroidal anti-inflammatory drugs, may not have been captured. Similarly, we did not have data on duration of TDF exposure. However, most cases of TDF-associated tubular dysfunction have been reported within the first year45; prolonging exposure to TDF did not increase the risk of incident renal disease substantially in prior studies. Also, as mentioned previously, future analyses of risk factors for renal disease in patients with HIV should account for control of hypertension.
Despite these limitations, our study provides updated estimates on incident renal disease in a large HIV cohort that is representative of the urban population in Washington, DC. In a population with mostly successful viral suppression, we found a substantial burden of renal disease among patients with HIV, underscoring the need for continued vigilance in monitoring kidney function and addressing modifiable risk factors.
Disclosure
All the authors declared no competing interests.
Acknowledgements
Supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (UO1 AI69503-03S2). Data in this manuscript were collected by the DC Cohort Study Group with investigators and research staff located at or by the following: the Cerner Corporation (Thilakavathy Subramanian, Jeffery Binkley, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, and Jeff Naughton); the Children’s National Medical Center Adolescent (Lawrence D'Angelo) and Pediatric (Natella Rahkmanina) clinics; the Senior Deputy Director of the DC Department of Health HAHSTA (Michael Kharfen); the Family and Medical Counseling Service (Angela Wood and Michael Serlin); Georgetown University (Princy Kumar); George Washington University Medical Faculty Associates (David Parenti); George Washington University Department of Epidemiology and Biostatistics (Amanda Castel, Alan Greenberg, Anne Monroe, Lindsey Powers Happ, Maria Jaurretche, Brittany Lewis, James Peterson, and Naji Younes); the Howard University Adult Infectious Disease Clinic (Ronald Wilcox), and Pediatric Clinic (Sohail Rana); Kaiser Permanente Mid-Atlantic (Michael Horberg); La Clinica Del Pueblo (Ricardo Fernandez); MetroHealth (Annick Hebou); the National Institutes of Health (Carl Dieffenbach and Henry Masur); Providence Hospital (Jose Bordon); Unity Health Care (Gebeyehu Teferi); Veterans Affairs Medical Center (Debra Benator); Washington Hospital Center (Maria Elena Ruiz); and Whitman-Walker Health (Deborah Goldstein, David Hardy). We would like to thank Dr. Scott Cohen (Division of Kidney Diseases and Hypertension, George Washington University) for his valuable input. We would also like to acknowledge the research assistants at all the participating sites, and the DC Cohort Community Advisory Board. Preliminary results of this study were initially presented as a poster at IDWeek 2015, October 7–11, 2015, San Diego, CA. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Contributor Information
Saumil Doshi, Email: saumil.doshi@medstar.net.
DC Cohort Executive Committee:
Thilakavathy Subramanian, Jeffery Binkley, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, Jeff Naughton, Lawrence D'Angelo, Michael Kharfen, Angela Wood, Michael Serlin, Princy Kumar, David Parenti, Amanda Castel, Alan Greenberg, Anne Monroe, Lindsey Powers Happ, Maria Jaurretche, Brittany Lewis, James Peterson, Naji Younes, Ronald Wilcox, Sohail Rana, Michael Horberg, Ricardo Fernandez, Annick Hebou, Carl Dieffenbach, Henry Masur, Jose Bordon, Gebeyehu Teferi, Debra Benator, Maria Elena Ruiz, Deborah Goldstein, and David Hardy
Appendix
The members of the DC Cohort Executive Committee are as follows:
Thilakavathy Subramanian, Jeffery Binkley, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, Jeff Naughton, Lawrence D'Angelo, Michael Kharfen, Angela Wood, Michael Serlin, Princy Kumar, David Parenti, Amanda Castel, Alan Greenberg, Anne Monroe, Lindsey Powers Happ, Maria Jaurretche, Brittany Lewis, James Peterson, Naji Younes, Ronald Wilcox, Sohail Rana, Michael Horberg, Ricardo Fernandez, Annick Hebou, Carl Dieffenbach, Henry Masur, Jose Bordon, Gebeyehu Teferi, Debra Benator, Maria Elena Ruiz, Deborah Goldstein, and David Hardy.
References
- 1.Franceschini N., Napravnik S., Eron J.J. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005;67:1526–1531. doi: 10.1111/j.1523-1755.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Li Y., Shlipak M.G., Grunfeld C. Incidence and risk factors for acute kidney injury in HIV Infection. Am J Nephrol. 2012;35:327–334. doi: 10.1159/000337151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James M.T., Grams M.E., Woodward M. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66:602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Diagnoses of HIV Infection in the United States and Dependent Areas, 2014. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2014-vol-26.pdf Available at.
- 5.Atta M.G., Lucas G.M., Fine D.M. HIV-associated nephropathy:epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther. 2008;6:365–371. doi: 10.1586/14787210.6.3.365. [DOI] [PubMed] [Google Scholar]
- 6.Samji H., Cescon A., Hogg R.S. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Röling J., Schmid H., Fischereder M. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis. 2006;42:1488–1495. doi: 10.1086/503566. [DOI] [PubMed] [Google Scholar]
- 8.AIDSinfo What’s New in the Guidelines? Adult and Adolescent ARV. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/37/whats-new-in-the-guidelines Available at:
- 9.TEMPRANO ANRS 12136 Study Group. Daniel C., Moh R. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 10.INSIGHT START Study Group. Lundgren J.D., Babiker A.G. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi A.I., Shlipak M.G., Hunt P.W. HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. AIDS. 2009;23:2143–2149. doi: 10.1097/QAD.0b013e3283313c91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugavero M.J., Napravnik S., Cole S.R. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–935. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper R.D., Wiebe N., Smith N. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 14.Sax P.E., Wohl D., Yin M.T. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 15.Gallant J.E., Daar E.S., Raffi F. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3:e158–e165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration FDA approves new treatment for HIV [press release]. Silver Spring, MD. November 5, 2015. https://aidsinfo.nih.gov/news/1617/fda-approves-new-treatment-for-hiv Available at:
- 17.van Zoest R.A., Wit F.W., Kooij K.W. Higher prevalence of hypertension in HIV-1-infected patients on combination antiretroviral therapy is associated with changes in body composition and prior stavudine exposure. Clin Infect Dis. 2016;63:205–213. doi: 10.1093/cid/ciw285. [DOI] [PubMed] [Google Scholar]
- 18.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 19.Shahin M.H., Johnson J.A. Mechanisms and pharmacogenetic signals underlying thiazide diuretics blood pressure response. Curr Opin Pharmacol. 2016;27:31–37. doi: 10.1016/j.coph.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg A.E., Hays H., Castel A.D. Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: technical challenges and innovative solutions. J Am Med Inform Assoc. 2016;23:635–643. doi: 10.1093/jamia/ocv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Diabetes and Digestive and Kidney Diseases Estimating glomerular filtration rate. https://www.niddk.nih.gov/health-information/communication-programs/nkdep/laboratory-evaluation/glomerular-filtration-rate/estimating Available at:
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 23.Young B., Buchacz K., Baker R.K. Renal function in Tenofovir-exposed and Tenofovir-unexposed patients receiving highly active antiretroviral therapy in the HIV Outpatient Study. J Int Assoc Physicians AIDS Care (Chic) 2007;6:178–187. doi: 10.1177/1545109707300676. [DOI] [PubMed] [Google Scholar]
- 24.Roe J., Campbell L.J., Ibrahim F. HIV care and the incidence of acute renal failure. Clin Infect Dis. 2008;47:242–249. doi: 10.1086/589296. [DOI] [PubMed] [Google Scholar]
- 25.Inker L.A., Astor B.C., Fox C.H. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 26.Bpharm S.M., Talbot A., Trottier B. Acute renal failure in four HIV-infected patients: potential association with tenofovir and nonsteroidal anti-inflammatory drugs. Can J Infect Dis Med Microbiol. 2008;19:75–76. doi: 10.1155/2008/370535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim F., Naftalin C., Cheserem E. Immunodeficiency and renal impairment are risk factors for HIV-associated acute renal failure. AIDS. 2010;24:2239–2244. doi: 10.1097/QAD.0b013e32833c85d6. [DOI] [PubMed] [Google Scholar]
- 28.Perazella M.A. Acute renal failure in HIV-infected patients: a brief review of common causes. Am J Med Sci. 2000;319:385–391. doi: 10.1097/00000441-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Hsu C.-Y., McCulloch C.E., Fan D. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi A.I., Rodriguez R.A., Bacchetti P. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol. 2007;18:2968–2974. doi: 10.1681/ASN.2007040402. [DOI] [PubMed] [Google Scholar]
- 31.Jotwani V., Li Y., Grunfeld C. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis. 2012;59:628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas G.M., Lau B., Atta M.G. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalayjian R.C., Franceschini N., Gupta S.K. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008;22:481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu C.Y., Ordoñez J.D., Chertow G.M. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 36.Hayek S.S., Sever S., Ko Y.-A. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman K.E., Rouster S.D., Chung R.T. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 38.Lee M.-H., Yang H.-I., Lu S.-N. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 39.Negro F., Forton D., Craxì A. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Hsu Y.-C., Ho H.J., Huang Y.-T. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 41.Lo Re V., Kallan M.J., Tate J.P. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160:369–379. doi: 10.7326/M13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sulkowski M.S., Mehta S.H., Torbenson M.S. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 43.Laprise C., Baril J.-G., Dufresne S. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56:567–575. doi: 10.1093/cid/cis937. [DOI] [PubMed] [Google Scholar]
- 44.Nelson M.R., Katlama C., Montaner J.S. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–1281. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 45.Izzedine H., Isnard-Bagnis C., Hulot J.S. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS. 2004;18:1074–1076. doi: 10.1097/00002030-200404300-00019. [DOI] [PubMed] [Google Scholar]
- 46.Lucas G.M., Eustace J.A., Sozio S. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]