Abstract
Introduction
Novel anticancer therapies include anti–programmed cell death protein-1 (PD-1) and anti–programmed death ligand-1 (PD-L1) drugs. These novel medications have side effects in different organs, including the kidney. The most common adverse effect in the kidney is acute interstitial nephritis (AIN). No diagnostic criteria are available to distinguish AIN associated with anti–PD-1 therapy from other AINs.
Methods
Kidney biopsy specimens from patients on anti–PD-1 therapy were stained with antibodies to PD-1 and PD-L1. Herein we report morphologic and immunohistochemical findings in 15 patients who received anti–PD-1 therapy and developed acute kidney injury requiring a kidney biopsy.
Results
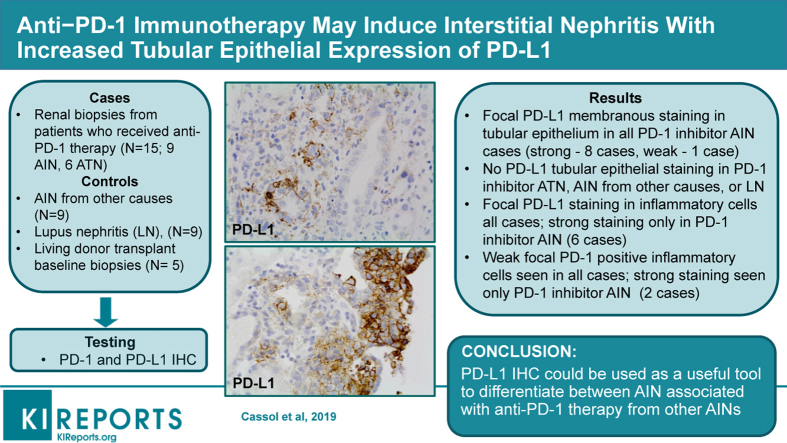
Among these patients, 9 had AIN and 6 had no AIN but showed acute tubular necrosis (ATN). Immunohistochemistry with antibodies to PD-1 and PD-L1 was performed on all of these biopsy specimens and on 9 randomly selected biopsy specimens with AIN from patients who did not receive anti–PD-1 medications, as well as 9 patients with lupus nephritis and active-appearing interstitial inflammation. There was weak staining for PD-1 in T cells in all patients with AIN and lupus; however, tubular epithelial cell membrane staining for PD-L1 was seen only in patients with anti–PD-1 therapy−associated AIN, and not in patients with anti–PD-1 therapy−associated ATN, and not in those with AIN secondary to other medications, or patients with lupus nephritis.
Conclusion
We propose that immunohistochemistry with PD-L1 could be a useful tool to differentiate AIN associated with anti–PD-1 therapy from other AINs.
Keywords: immunohistochemistry, interstitial nephritis, PD-1 antagonists, renal pathology
Graphical abstract
Novel advantages in cancer therapy have brought new classes of drugs such as immune checkpoint inhibitors, including PD-1 antagonists. PD-1 is a transmembrane cell receptor that is expressed on effector T cells, B cells, monocytes, natural killer T cells, and other immune system cell types.1 The PD-1 receptor binds to PD-L1 or PD-L2. These ligands are expressed on cancer cells, and engagement of PD-L1 with its receptor PD-1 on T cells inhibits T-cell proliferation. Another receptor that is expressed on T cells is cytotoxic T-lymphocyte-associated protein 4 (CTLA4). Cytotoxic T-lymphocyte-associated protein 4 acts as an “off” switch when bound to other receptors (such as CD80 or CD86) on the surface of antigen-presenting cells; it is homologous to the T-cell costimulatory protein CD28, and it competes with CD28 for its ligand, B7, and inhibits T-cell stimulation. Novel immune checkpoint inhibitors enhance tumor-directed immune responses by inhibiting CTLA-4 and PD-1/PD-L1. These drugs include CTLA-4 antagonist (ipilimumab) and PD-1 antagonists (nivolumab and pembrolizumab), and they show good efficacy in the treatment of various malignancies, including melanoma, non–small cell lung carcinoma, renal cell carcinoma, Hodgkin lymphoma, and other malignancies.
Unfortunately, these novel anti–PD-1 medications show adverse side effects in different organs, and the incidence rate of such adverse effects is quite high, reaching up to 30%.2, 3 Although renal adverse effects of anti–PD-1 therapy are uncommon, several cases of renal complications in patients who received anti–PD-1 treatment have been reported.4, 5 Among these renal complications, interstitial nephritis (with and without granulomas) is the most common one, but other complications, such as acute tubular necrosis, thrombotic microangiopathy, and minimal change disease, have been reported.1, 6, 7, 8, 9 Recognition and differential diagnosis of anti–PD-1 therapy−associated renal disease in a kidney biopsy specimen could be a challenge for a renal pathologist.
We hypothesized that expression of PD-1 and/or PD-L1 may be different in patients who developed renal complications while receiving an anti–PD-1 therapy. PD-L1 is expressed on antigen-presenting cells such as tumor cells, but also by vascular endothelial cells, astrocytes, and pancreatic islet cells.1, 4 PD-L1 increased expression was reported in the renal tubules in patients with systemic lupus erythematous, but only in occasional tubules in the normal kidney.10, 11 No PD-1 expression was detected by immunohistochemistry in normal kidney.10
Herein we report our renal pathology laboratory experience with morphologic and PD-1/PD-L1 immunohistochemical findings in kidney biopsy specimens from patients on anti–PD-1 therapy.
Methods
The study has been approved by the Institutional Review Board at the Ohio State University. The Renal Pathology Laboratory biopsy database at the Ohio State Wexner Medical Center (OSUWMC) was searched for biopsy specimens from patients who received an anti–PD-1 therapy between 1 January 2016 and 31 July 31 2018. Kidney biopsy morphologic findings, demographic data, and clinical history were reviewed. Immunoperoxidase staining with antibodies to PD-1 (Clone NAT105, Cell Marque, Rocklin, CA) and PD-L1 (Clone 22C3, Dako, Troy, MI) was performed on sections of paraffin-embedded tissue using the standard stainers (Leica Bond III, Melbourne, Australia) for PD-1 and Dako Link 48 (Santa Clara, CA; for PD-L1). The stainings were performed according to the manufacturer protocols. For PD-1, antigen retrieval was performed by using the ER2 (ethylenediamine tetraacetic acid ) retrieval for 20 minutes; primary antibody was diluted at 1:25 and incubated with primary antibody for 30 minutes; and detection was done using the Leica Bond Polymer Refine kit (catalog no. DS9800). For PD-L1, antigen retrieval was performed by using the PT Link Low pH retrieval for 20 minutes; the primary antibody was used directly from the kit and incubated for 30 minutes; and detection was done using the Dako Flex HRP detection kit (catalog no. SK006). Staining with the antibody to CD3 (Dako, Troy, MI; catalog no. A0452) was performed on sections that were prestained by immunoperoxidase with either anti–PD-1 or anti–PD-L1 (as described above). Primary antibodies (1:100 dilution) were incubated for 1 hour at room temperature; slides were washed and incubated with secondary antibodies (Dylight 594 conjugated with Affinipure Donkey anti-rabbit IgG (H+L) (Jackson Labs, West Grove, PA, catalog no. 711-515-152) at 1:50 dilution for 30 minutes.
For comparison studies, kidney biopsy specimens with morphologic features of AIN from patients who did not receive anti–PD-1 therapy and from patients with lupus nephritis and active-appearing interstitial inflammation were used. Normal baseline biopsies from living renal allograft donors were used as negative control.
For statistical analysis, a χ2 test was used to determine whether there is a significant difference in the observed frequencies between the study groups.
Results
We identified 15 kidney biopsy specimens from patients on PD-1 inhibitor therapy in our database. Demographic data on these patients are shown in Table 1. There were 10 male and 5 female patients, all Caucasian, with a mean age of 60.8 ± 10.5 years (range, 43−77 years).
Table 1.
Demographic data and PD-1 treatment in patients who underwent kidney biopsy for impaired kidney function
| Case | Age (yr) | Sex | Race | Primary malignancy | PD-1 inhibitor | Dose | Duration prior to kidney biopsy (mo) | |
|---|---|---|---|---|---|---|---|---|
| Interstitial nephritis | 1 | 68 | F | a | Lung Ca | pembrolizumab | 200 mg q 20 days | 4 |
| 2 | 63 | M | C | Bladder Ca | nivolumab | 3 mg/kg q 15 days | 0.5 | |
| 3 | 43 | M | C | Renal cell Ca | nivolumab | 3 mg/kg q 15 days | 22 | |
| 4 | 68 | M | C | Melanoma | pembrolizumab/ipilimumab | 200 mg q 20 days 3 mg/kg q 15 days |
9 | |
| 5 | 67 | F | C | Lung Ca | pembrolizumab | 200 mg q 20 days | 16 | |
| 6 | 77 | F | C | Renal cell Ca | nivolumab | 3 mg/kg q 15 days | 4 | |
| 7 | 49 | F | C | Lung Ca | pembrolizumab | 200 mg q 20 days | 10 | |
| 8 | 41 | M | C | Melanoma | nivolumab/ipilimumab | 3 mg/kg q 15 days | 15 | |
| 9 | 62 | F | C | Lung Ca | pembrolizumab | 200 mg q 20 days | 11 | |
| No interstitial nephritis | 10 | 59 | M | C | Melanoma | pembrolizumab | 200 mg q 20 days | 1 |
| 11 | 59 | M | C | Renal cell Ca | nivolumab | 3 mg/kg q 15 days | 2 | |
| 12 | 56 | M | C | Melanoma | nivolumab/ipilimumab | 3 mg/kg q 15 days | 0.5 | |
| 13 | 62 | M | a | Melanoma | nivolumab/ipilimumab | 3 mg/kg q 15 days | 2 | |
| 14 | 63 | M | C | Melanoma | nivolumab | 3 mg/kg q 15 days | 17 | |
| 15 | 75 | M | C | Melanoma | nivolumab | 3 mg/kg q 15 days | 5 | |
C, Caucasian; Ca, cancer; F, female; M, male; PD-1, programmed cell death protein-1.
Data unavailable.
Nine patients had AIN as the main pathologic diagnosis on the kidney biopsy specimen (group 1), and 6 patients had different morphologic findings, including acute tubular necrosis (ATN, 5 cases) and advanced diabetic nephropathy (1 case) (group 2). All patients received PD-1 inhibitors, including nivolumab, and pembrolizumab, either alone or in combination with the CTLA4 inhibitor ipilimumab (Table 1). Underlying malignancies included metastatic malignant melanoma (7 cases), lung carcinoma (4 cases), renal cell carcinoma (3 cases), and bladder carcinoma (1 case). The immunotherapy treatment and its duration are shown in Table 1. Other immune-related adverse effects were observed in 4 of the 9 patients with AIN (1 patient developed autoimmune diabetes, 2 patients developed hypothyroidism, and 1 patient had colitis and hepatitis). Of the 6 patients on PD-1 inhibitor without AIN, 1 patient had diffuse skin rash that was severe enough to warrant temporary discontinuation of immunotherapy, and another patient had myositis. The duration of the treatment prior to the kidney biopsy varied between 10 days and 22 months (7.3 ± 7.0 months). In patients who developed AIN while on PD-1 treatment (Table 1, cases 1−9), the duration of the treatment had a tendency to be longer than in those who did not have interstitial nephritis (Table 1, cases 9−15) (10.2 ± 6.8 months and 4.6 ± 6.3 months, respectively, P = 0.1325).
Treatment with medications that potentially can induce interstitial nephritis (such as proton pump inhibitors (PPIs) and nonsteroid antiinflammatory drugs (NSAIDs)12 is shown in Table 2. There was no significant difference between the patients who had AIN in the kidney biopsy specimen and those who did not for either PPI (χ2 = 0.110, P = 0.7402) or NSAID use (χ2=1.091, P = 0.2963). Interestingly, patients who developed ATN without AIN had more commonly a history of antibiotics use than those who had AIN on a kidney biopsy specimen (χ2 = 4.000, P = 0.0455). None of the patients in group 1 had imaging studies with contrast at least 1 month prior to the kidney biopsy, whereas in group 2, two patients had recent exposure to the contrast media, which could have contributed to the ATN (χ2 = 2.400, P = 0.1213).
Table 2.
Risk factors for developing acute interstitial nephritis or acute tubular necrosis
| Case | PPIs | NSAIDs | Antibiotics | Chemotherapy (conventional) | Contrast imaging studies | Hypotension | Sepsis | |
|---|---|---|---|---|---|---|---|---|
| Interstitial nephritis | 1 | a | a | a | a | a | None | None |
| 2 | None | None | None | None | None | None | None | |
| 3 | None | None | None | None | 2 mo prior | None | None | |
| 4 | None | None | None | None | 2 wk prior | None | None | |
| 5 | Omeprazole | None | None | Carboplatin | 2 mo prior | None | None | |
| 6 | Omeprazole | None | None | None | 1 mo prior | None | None | |
| 7 | a | a | a | Carboplatin 6 mo prior |
a | None | None | |
| 8 | Omeprazole | None | None | None | 1 mo prior | None | None | |
| 9 | a | a | a | Carboplatin 9 mo prior |
a | None | None | |
| No interstitial nephritis | 10 | None | None | Amoxicillin 10 days prior | None | 1 mo prior | None | None |
| 11 | Pantoprazole | None | Cefazolin 2 mo prior |
None | None | None | None | |
| 12 | a | Naproxen | None | None | Recent | None | None | |
| 13 | Pantoprazole | None | Fluconazole | None | 1 mo prior | None | None | |
| 14 | None | None | None | None | 3 wk prior | None | None | |
| 15 | Omeprazole | None | None | None | None | None | None | |
NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors.
Data unavailable.
Laboratory data are provided in Table 3. All patients experienced acute kidney injury that was the indication for the kidney biopsy. Baseline serum creatinine (1.13 ± 0.5 mg/dl) increased to 3.2 ± 1.4 mg/dl (P = 0.0001) at presentation. Patients had proteinuria (2.6 ± 4.6 g/g) and microscopic hematuria (large [3+] in 2 of 12 patients, but either small [1+] or negative hematuria in 10 other patients) (Table 3). Leucocyte esterase and an increased number of white blood cells in the urine were noted in 2 of 6 patients (data not available for all patients) in group 1 and in 2 of 6 patients in group 2 (χ2 = 0.000, P = 1.000).
Table 3.
Renal function and urinalysis data in patients with anti–PD-1 treatment
| Case | Serum creatinine, mg/dl |
Urine protein/creatinine ratio, g/g | Urinalysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | At presentation | Hematuria | Leukocyte esterase | WBCs | Tubular casts | Tubular epithelial cells | |||
| Interstitial nephritis | 1 | 0.8 | 4.3 | 1.9 | a | a | a | a | a |
| 2 | 1.5 | 4.1 | 3.8 | Large | Moderate | >20 | None | None | |
| 3 | 1.03 | 2.16 | 0.1 | Small | Negative | 0−5 | None | None | |
| 4 | 0.9 | 2.9 | a | a | Negative | 0−5 | Hyaline | None | |
| 5 | 0.7 | 3.8 | 1.5 | Small | Small | 6−9 | None | Trace | |
| 6 | 1.0 | 2.3 | 0.4 | Negative | Negative | 0-5 | None | 1+ | |
| 7 | 1.17 | 3.0 | 0.26 | a | a | a | a | a | |
| 8 | 0.9 | 1.4 | 0.3 | Negative | Negative | 0−5 | None | 1+ | |
| 9 | 0.7 | 2.3 | Mild | Small | a | a | a | a | |
| No interstitial nephritis | 10 | 0.71 | 0.97 | 0.4 | Negative | Negative | 0−5 | Hyaline | None |
| 11 | 2.4 | 3.76 | 2.4 | Large | Trace | 10−19 | None | 1+ | |
| 12 | 1.1 | 5.0 | 1.0 | Small | Negative | 6−10 | Granular | None | |
| 13 | 1.9 | 6.5 | 1.5 | Negative | Trace | 0−5 | None | None | |
| 14 | 1.3 | 1.98 | 13.1 | Small | Negative | 0−5 | None | None | |
| 15 | 0.9 | 1.5 | 0.6 | Small | Negative | 0−5 | None | None | |
PD-1, programmed cell death protein-1; WBC, white blood cells.
Data unavailable.
Morphologic findings in kidney biopsy samples are summarized in Table 4. All cases with AIN had diffuse active interstitial inflammatory cell infiltrates associated with interstitial edema. There were no glomerular proliferative lesions in any of the biopsy specimens. Immunofluorescence showed mild segmental staining for either IgG or IgA in the mesangium (in 2 of 9 patients with interstitial nephritis, and in 4 of 6 patients with no interstitial nephritis), but electron microscopy did not show electron-dense immune-type deposits in those biopsy specimens. The main morphologic finding in 6 patients without interstitial nephritis was ATN. Two of these 6 biopsy specimens showed mild and focal interstitial inflammation that was disproportionately mild relative to the tubular epithelial cell injury and did not fulfill criteria to diagnose AIN. The underlying chronic kidney injury was mild to moderate, with interstitial fibrosis and tubular atrophy not exceeding 50% in any of these biopsy specimens.
Table 4.
Kidney biopsy findings in patients with PD-1 treatment
| Case | Number of glomeruli |
Immunofluorescence | IFTA | Interstitial inflammation | ||
|---|---|---|---|---|---|---|
| Total | Globally sclerosed/obsolescent, number (%) | |||||
| Interstitial nephritis | 1 | 10 | 1 (10) | Negative | 1+ | Diffuse, active |
| 2 | 7 | 2 (29) | Negative | 2+ | Diffuse, active | |
| 3 | 15 | 0 | Negative | 1+ | Diffuse, active | |
| 4 | 8 | 0 | Focal mesangial IgG | 0 | Diffuse, active | |
| 5 | 20 | 2 (10) | Focal mesangial IgG | 1+ | Diffuse, active | |
| 6 | 22 | 2 (9) | Negative | 1+ | Diffuse, active | |
| 7 | 19 | 0 | Negative | 2+ | Diffuse, active | |
| 8 | 13 | 2 (15) | Negative | 1+ | Diffuse, active | |
| 9 | 68 | 2 (3) | Negative | 2+ | Diffuse, active | |
| No interstitial nephritis | 10 | 23 | 6 (26) | Focal mesangial IgA | 1+ | Mild, focal |
| 11 | 6 | 1 (17) | Focal mesangial IgA | 1+ | Not significant | |
| 12 | 9 | 0 | Focal mesangial IgG | 1+ | Not significant | |
| 13 | 49 | 22 (45) | Focal mesangial IgA | 2+ | Not significant | |
| 14 | 19 | 10 (53) | Negative | 3+ | Mild, focal | |
| 15 | 7 | 1 (14) | Negative | 2+ | Mild, focal | |
IFTA, interstitial fibrosis and tubular atrophy; PD-1, programmed cell death protein-1.
Grading for ITFA is performed using a semiquantitative scale of 0 to 3+. Score 0 was designated for IFTA < 10% of the renal cortex, score 1+ for IFTA between 10% and 25%, score 2+ for IFTA between 25% and 50%, and 3+ for IFTA > 50%.
To identify possible pathogenic mechanisms of the PD-1-inhibitor−associated AIN, we stained all kidney biopsy specimens from patients on anti–PD-1 therapy with antibodies to PD-1 and PD-L1 by immunohistochemistry. In addition, we stained 9 randomly selected kidney biopsy specimens with a diagnosis of AIN from patients who were not treated with a PD-1 inhibitor. In light of a previous reports showing PD-L1 tubular overexpression in systemic lupus erythematous patients,10, 11 we also stained 9 biopsy samples of patients with lupus nephritis and active-appearing interstitial inflammation. Etiologies of AIN in the control group included antibiotics in 3 cases, proton pump inhibitors in 3 cases, NSAIDs in 2 cases, and antipsychotics in 1 case. Interstitial inflammatory cell infiltrates in these biopsy specimens contained increased eosinophils, which, in combination with the clinical history, suggested that these were cases of allergic interstitial nephritis
Five pre-perfusion baseline biopsy specimens from living donors were used as negative controls.
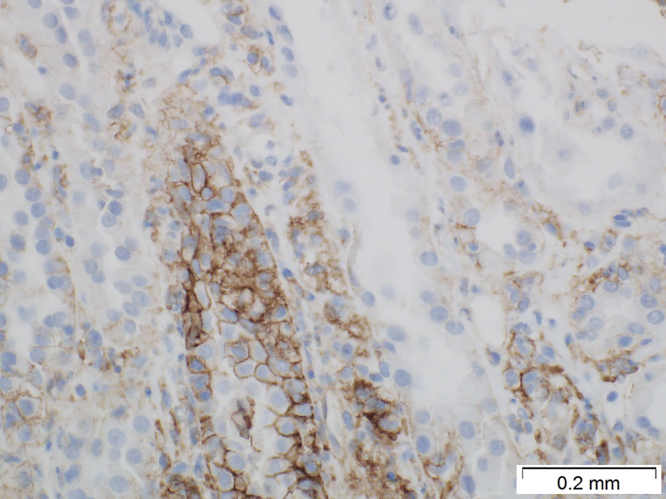
The immunohistochemistry findings are summarized in Table 5, and representative images are shown in Figure 1 and Figure 2. All biopsy specimens from patients who were treated with a PD-1 inhibitor and who had AIN showed focal cell membrane staining for both PD-1 and PD-L1. The PD-1 staining was mostly weak and focal (1%−2% of the total number of inflammatory cells) and restricted to inflammatory cells (double staining with CD3 and PD-1 showed that the majority of inflammatory cells with positive staining were CD3-positive T cells; Figure 3). There was no significant difference in the PD-1 pattern of staining among the 4 groups (both groups of patients with anti–PD-1 therapy, control AIN, and lupus nephritis) (χ2 = 5.08, P = 0.166). In contrast, PD-L1 staining was mostly strong and was seen not only in inflammatory cells (CD3-positive and PD-L1−positive T cells) (Figure 3), but also focally along the cell membranes of tubular epithelial cells within the areas of interstitial inflammation (Figures 1 and 3). The tubules that showed positive staining did not appear to be atrophic, as they did not show thickened tubular basement membrane. There was significant difference in the pattern of PD-L1 staining between patients on anti–PD-1 therapy and AIN that was not associated with PD-1, especially with regard to tubular epithelial cell membrane staining, which was present only in patients on anti–PD-1 therapy and AIN (χ2 = 23.00, P < 0.001) (Figure 2). In addition, strong PD-L1 staining in inflammatory cells was present only in patients on anti–PD-L1 therapy with AIN but not in other patients (χ2 = 19.6, P < 0.001) (weak focal (<2%) PD-L1 staining in inflammatory cells was seen in 2 patients in groups 2 and 3 each and in 1 patient in group 4). Most of the biopsy specimens contained renal cortex only, so the pattern of staining in the renal medulla could not be assessed.
Table 5.
Immunohistochemistry findings in kidney biopsy specimens
| Cases | PD-1 |
PDL-1 |
||||||
|---|---|---|---|---|---|---|---|---|
| Inflammatory cells |
Inflammatory cells |
Tubular epithelial cells |
||||||
| Intensity/% | Pattern | Intensity/% | Pattern | Intensity/% | Pattern | |||
| PD-1 inhibitor therapy | Interstitial nephritis (group 1) | 1 | Strong/20 | Diffuse | Strong/30 | Diffuse | Strong/50 | Focal |
| 2 | Weak/5 | Focal | Strong/5 | Focal | Strong/15 | Focal | ||
| 3 | Weak/15 | Diffuse | Weak/10 | Focal | Weak/10 | Focal | ||
| 4 | Weak/10 | Focal | Strong/10 | Focal | Strong/15 | Focal | ||
| 5 | Weak /10 | Diffuse | Weak/5 | Focal | Strong/5 | Focal | ||
| 6 | Weak/5 | Focal | Strong/5 | Focal | Strong/5 | Focal | ||
| 7 | Weak/1 | Focal | Weak/1 | Focal | Weak/3 | Focal | ||
| 8 | Weak/10 | Diffuse | Strong/20 | Focal | Strong/40 | Focal | ||
| 9 | Strong/10 | Diffuse | Strong/5 | Focal | Strong/5 | Focal | ||
| No interstitial nephritis (group 2) | 10 | Weak/2 | Focal | Negative | Negative | Negative | Negative | |
| 11 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 12 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 13 | Weak/2 | Focal | Weak/2 | Focal | Negative | Negative | ||
| 14 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 15 | Weak/5 | Focal | Weak/1 | Focal | Negative | Negative | ||
| No history of PD-1 inhibitor therapy | Interstitial nephritis | 16 | Weak/10 | Focal | Negative | Negative | Negative | Negative |
| 17 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 18 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 19 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 20 | Strong/20 | Focal | Negative | Negative | Negative | Negative | ||
| 21 | Weak/5 | Focal | Weak/1 | Focal | Negative | Negative | ||
| 22 | Weak/10 | Diffuse | Negative | Negative | Negative | Negative | ||
| 23 | Weak/5 | Focal | Weak/1 | Focal | Negative | Negative | ||
| 24 | Weak/5 | Focal | Negative | Negative | Negative | Negative | ||
| Lupus nephritis with active interstitial inflammation | 25 | Moderate/10 | Diffuse | Negative | Negative | Negative | Negative | |
| 26 | Weak/1 | Focal | Negative | Negative | Negative | Negative | ||
| 27 | Weak/1 | Focal | Negative | Negative | Negative | Negative | ||
| 28 | Strong/5 | Focal | Negative | Negative | Negative | Negative | ||
| 29 | Weak/<1 | Focal | Weak/1 | Focal | Negative | Negative | ||
| 30 | Moderate/2 | Focal | Negative | Negative | Negative | Negative | ||
| 31 | Weak/2 | Focal | Negative | Negative | Negative | Negative | ||
| 32 | Weak/5 | Focal | Negative | Negative | Negative | Negative | ||
| 33 | Negative | Negative | Negative | Negative | Negative | Negative | ||
| Baseline | 34 | Negative | Negative | Negative | Negative | Negative | Negative | |
| 35 | Weak/<1 in PTC | Very focal | Negative | Negative | Negative | Negative | ||
| 36 | Negative | Negative | Negative | Negative | Negative | Negative | ||
| 37 | Negative | Negative | Negative | Negative | Negative | Negative | ||
| 38 | Negative | Negative | Negative | Negative | Weak/<1 | Very focal | ||
PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; PTC, peritubular capillaries.
Figure 1.
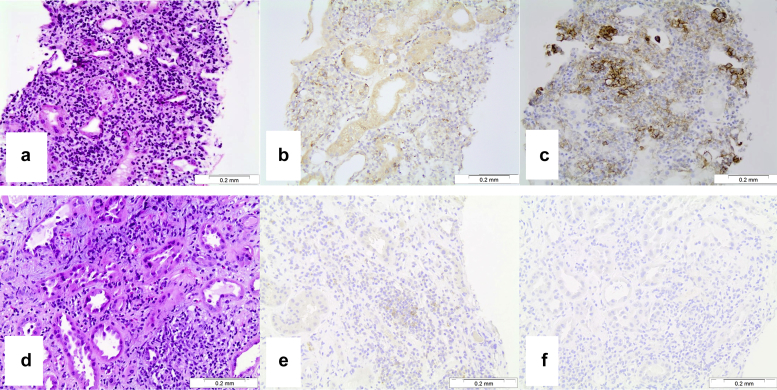
Histologic findings in the kidneys in patients with interstitial nephritis. (a) Active interstitial inflammatory cell infiltrates in a patient on anti–programmed cell death protein-1 (PD-1) therapy; hematoxylin and eosin, original magnification ×200. (b) Immunohistochemistry with an antibody to PD-1 shows moderate diffuse staining in inflammatory cells in a patient on anti–PD-1 therapy; original magnification ×200. (c) Immunohistochemistry with an antibody to programmed death ligand-1 (PD-L1) shows focal strong membrane staining in inflammatory cells and tubular epithelial cells in a patient on anti–PD-1 therapy; original magnification ×200. (d) Active interstitial inflammatory cell infiltrates in a patient who did not receive anti–PD-1 therapy; hematoxylin and eosin, original magnification ×200. (e) Immunohistochemistry with the antibody to PD-1 shows weak focal staining in inflammatory cells in a patient who did not receive anti–PD-1 therapy; original magnification ×200. (f) Immunohistochemistry with the antibody to PD-L1 did not show staining in a patient who did not receive anti–PD-1 therapy; original magnification ×200.
Figure 2.
Patients with acute interstitial nephritis (AIN) associated with anti–programmed cell death protein-1 (PD-1) therapy show positive membrane staining in tubular epithelial cells for programmed death ligand-1 (PD-L1). Representative photograph of immunohistochemistry staining with an antibody to PD-L1 in a patient on anti–PD-1 therapy and with morphologic findings of AIN in a kidney biopsy specimen. Original magnification ×400.
Figure 3.
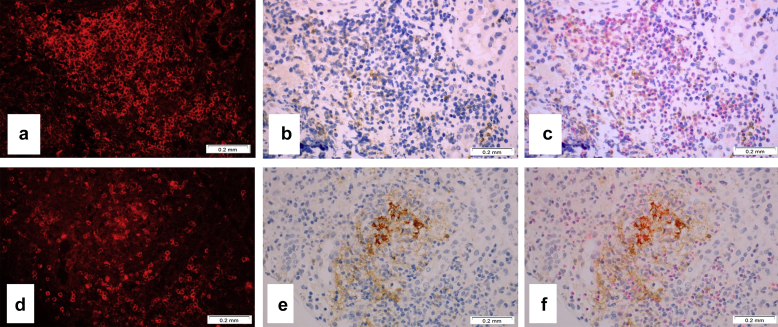
Characterization of programmed cell death protein-1 (PD-1)− and program death ligand-1 (PDL-1)−positive cells. (a) Immunofluorescence staining with the antibody to CD3 shows numerous T cells; original magnification ×400. (b) Same area as in panel (a) shows PD-1−positive cells; immunoperoxidase, original magnification ×400. (c) Combined image from panels (a) and (b) shows that PD-1−positive cells are CD3-positive T lymphocytes; original magnification ×400. (d) Immunofluorescence staining with the antibody to CD3 shows numerous T cells; original magnification ×400. (e) Same area as in panel (d) shows programmed death ligand-1 (PD-L1)−positive cells; immunoperoxidase, original magnification ×400. (f) Combined image from panels (a) and (b) shows that some PD-L1−positive cells are CD3-positive T lymphocytes, whereas other PD-L1−positive cells appear to be tubular epithelial cells; original magnification ×400.
A single case (case 7) showed only weak focal staining for both markers, but the amount of active interstitial inflammation in that biopsy specimen was smaller than in other cases (the patient was treated with steroids prior to the kidney biopsy, which could have reduced the inflammation in the kidney; all other patients did not have records of steroid treatment prior to the kidney biopsy). Interestingly, patients who were on a PD-1 inhibitor therapy and did not have interstitial nephritis; they did not have relevant staining for PD-L1 in the kidney biopsy specimens. Thus, PD-L1 was negative in tubular epithelial cells in all kidney biopsy specimens from those patients, and it was weak and focal (less than 2%) in 2 of 6 biopsy specimens in the inflammatory cells (Table 5, cases 16−24). There was negative or weak and focal staining (in 1 case) for PD-1 in a few inflammatory cells in areas of fibrosis.
Patients who were not treated with a PD-1 inhibitor but who had AIN showed a different degree of PD-1 staining, which was mostly weak and focal in inflammatory cells, with only 1 case showing prominent inflammatory cell staining. There was no relevant staining for PD-L1 (only 2 cases showed weak staining in 1% of inflammatory cells; no kidney biopsy had tubular epithelial cell–membrane staining). Cases of lupus nephritis showed similar findings, with mostly weak and focal staining for PD-1 in inflammatory cells. Only 2 cases showed moderate PD-1 staining in 10% of inflammatory cells, and weak focal staining was observed in some patients for PD-L1 in inflammatory cells (Table 5, cases 25−33).
In the majority of baseline renal allograft biopsy specimens from living donors, both PD-1 and PD-L1 were negative. In 1 case, PD-1 staining was seen in a few inflammatory cells in the peritubular capillaries, and in another case focal mild PD-L1 staining in occasional tubular epithelial cells was observed (Table 5, cases 34−38).
Treatment for AIN included steroids with taper in 6 of the 9 patients (for whom information was available). Two patients were maintained on immunotherapy and 4 patients discontinued. No patients were rechallenged. Patients with ATN were managed symptomatically (2 with diuretics and 1 with temporary dialysis) and a short course of steroids (3 patients) (not all follow-up medical records are available). Two patients continued immunotherapy, and 2 discontinued and were not rechallenged because of stable disease. Kidney function was restored in 4 of 6 patients (with follow-up medical records available) with AIN and in 3 of 5 patients with ATN. Unfortunately, 5 patients from the study groups (3 patients in group 1 and 2 patients in group 2) did not survive the cancer.
Discussion
To the best of our knowledge, this is the first case series reporting PD-1/PD-L1 immunohistochemistry staining patterns in kidney biopsy specimens from patients on PD-1 inhibitor immunotherapy. Previous reports have described renal complications of PD-1 inhibitors that included interstitial nephritis and ATN.7, 13, 14, 15 In this study, we describe morphologic features of AIN in 9 patients and ATN in 6 patients on anti–PD-1 immunotherapy. These 2 groups were distinct by clinical presentation and kidney biopsy findings. Most significantly, tubular epithelial cell membrane staining for PD-L1 was seen only in patients treated with PD-1 inhibitors who had AIN, but not in those who had ATN, nor in patients with AIN associated with other medications, nor in patients with lupus nephritis.
Clinically, although AIN developed usually after several months on anti–PD-1 therapy, ATN tended to present at much shorter intervals after therapy initiation. Indeed, of 9 patients with AIN, there was only 1 case (Table 1, case 2) in which the patient presented with acute kidney injury shortly after the beginning of anti–PD-1 therapy; in the other 8 cases, acute kidney injury developed 4 months or later while the patient was on anti–PD-1 therapy. This time frame is consistent with other series reported, which also describe a delay between PD-1 inhibition and development of AIN.12
On the other hand, of 6 patients without AIN, only 1 patient (Table 1, case 14) presented at late stages after the anti–PD-1 therapy began. All other 5 patients developed acute kidney injury within 2 months of anti–PD-1 therapy. Arguably, the early case of AIN could represent a form of allergic interstitial nephritis in response to the PD-1 inhibitor or other medications, whereas the late case of ATN could represent an acute kidney injury secondary to other causes not associated with the PD-1 inhibitor. The pathologic basis for such difference could be that it requires some time for anti–PD-1 inhibitors to alter the immune system and to modify PD-1−expressing T cells. Modified T cells may attack different organs, including the kidney. The study by Hoffman et al.2 described side effects of anti–PD-1 medications in different organs based on an analysis of 496 patients treated for melanoma. The rate of such complications was as high as 28% (138 patients). These immune-related adverse effects of anti–PD-1 medications appear at different times after the beginning of the PD-1 therapy. Early adverse effects are usually seen in endocrine system (0.2−2 months after the beginning of therapy), whereas late adverse effects may occur 6 to 10 months after and often involve the skin, gastrointestinal tract, or kidneys.2 Renal complications are usually rare; in the study by Hoffman et al., they were noted in 2 of 138 patients (1.4%) who developed adverse effects while being on anti–PD-1 inhibitors.2 Other reports demonstrate similar rates of kidney side effects in patients on anti–PD-1 therapy, which are close to 1%.16, 17 We did not find significant differences between patients who did and did not develop interstitial nephritis with the use of other medications, such as PPIs and NSADs, that potentially could contribute to acute kidney injury. Therefore, the interstitial nephritis that we observed in the current series is independent of other risk medications and is related to anti–PD-1 medications. Among the patients who developed ATN but not interstitial nephritis, there was 1 patient among the 6 patients who was taking NSAIDs prior to the biopsy, as compared to none of the 6 patients (data are not available for some patients) who developed AIN. Interestingly, antibiotics were more commonly used in patients who developed ATN without interstitial nephritis than in those who had interstitial nephritis on kidney biopsy (Table 2). None of the patients in both study groups had a history of hypotension or sepsis that could explain the ATN prior to the kidney biopsy.
Immunohistochemistry showed that the staining patterns in biopsy samples with AIN from patients who were on anti–PD-1 therapy and those who were not, were different. Thus, although PD-1−positive inflammatory cells were seen in both groups, strong staining for PD-L1 was seen only in biopsy specimens from patients with AIN on anti–PD-1 therapy and not in patients with AIN and no history of anti–PD-1 therapy, nor in lupus nephritis patients (Table 5). Moreover, the PD-L1 staining pattern was distinct in patients who developed AIN while on anti–PD-1 therapy, as it was positive not only in inflammatory cells but also in tubular epithelial cells (Figures 1 and 2). This may be an important contributing factor to the pathogenesis of AIN, suggesting that PD-L1−positive tubular epithelial cells may mobilize modified PD-1−positive inflammatory cells.11 Interestingly, despite previous reports of positive PD-L1 staining in the tubular epithelium of patients with lupus nephritis,10, 11 we were unable to replicate those findings. One possible explanation is that the antibodies used in those studies were different from the one that we used. Specifically, 1 of the studies used a noncommercial antibody,11 which was also noted to stain tubular epithelial cells of healthy controls, and the other study used an anti–PD-L1 antibody that had been optimized for flow cytometry, only with a longer incubation time (16 hours).10
It is theoretically possible that the PD-1 inhibitors could reduce tolerance to other drugs known to cause AIN, thereby allowing AIN to develop. Our data did not show significant associations between PPIs, NSAIDs, and AIN in patients on anti–PD-1 therapy. In fact, it appears that antibiotic use was more frequent in patients who developed ATN but not interstitial nephritis (Table 2). However, we do not know whether anti–PD-1 therapy could reduce tolerance to other drugs or to other substances that are not usually recorded (such as over-the-counter medications or herbal medications).
We herein propose that immunohistochemistry with PD-L1 antibody could be a novel marker to differentiate between cases of AIN associated with PD-1 antibodies and AIN of other etiologies. In patients who received anti–PD-1 therapy and showed AIN on a kidney biopsy specimen, if PD-L1 staining is positive not only in inflammatory cells but also in the tubular epithelial cells, then AIN is probably secondary to the PD-1 antagonist. If PD-L1 staining is negative or is seen only in inflammatory cells within areas of fibrosis, then it is probably an AIN associated with other etiologies than anti–PD-1 therapy. Staining for PD-1 appears to be nonspecific, as it was seen in inflammatory cells irrespective of the AIN etiology (Table 5). The time frame between the beginning of anti–PD-1 therapy and kidney function deterioration is another hint that could help in the differential diagnosis. Based on our experience, cases of anti–PD-1 therapy−associated AIN usually occur after 4 months of the anti–PD-1 therapy, which is supported by other observations.2 Of note, 1 of 9 patients had AIN shortly after initiation of anti–PD-1 therapy; however, this may reflect a different pathogenesis of AIN, such as one related to drug nephrotoxicity rather than to modifications in the host immune system.
In conclusion, anti–PD-1 therapy−associated adverse effects in the kidneys are rare, but serious complications of novel anticancer therapy. Such complications should be recognized by renal pathologists and detailed clinical history and immunohistochemistry with anti–PD-L1 antibodies can help in their differential diagnosis.
Disclosure
All the authors declared no competing interests.
References
- 1.Sury K., Perazella M.A., Shirali A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol. 2018;14:571–588. doi: 10.1038/s41581-018-0035-1. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann L., Forschner A., Loquai C. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti–PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Sosa A., Lopez Cadena E., Simon Olive C. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918764628. 1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perazella M.A., Shirali A.C. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018;29:2039–2052. doi: 10.1681/ASN.2018050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami N., Motwani S., Riella L.V. Renal complications of immune checkpoint blockade. Curr Probl Cancer. 2017;41:100–110. doi: 10.1016/j.currproblcancer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao B.X., Lin N.J., Wang S.X. Minimal change disease associated with anti–PD1 immunotherapy: a case report. BMC Nephrology. 2018;19:156. doi: 10.1186/s12882-018-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzedine H., Mateus C., Boutros C. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2017;32:936–942. doi: 10.1093/ndt/gfw382. [DOI] [PubMed] [Google Scholar]
- 8.Kitchlu A., Fingrut W., Avila-Casado C. Nephrotic syndrome with cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70:581–585. doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Cortazar F.B., Marrone K.A., Troxell M.L. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding H.L., Wu X.F., Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115:184–191. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Bertsias G.K., Nakou M., Choulaki C. Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum. 2009;60:207–218. doi: 10.1002/art.24227. [DOI] [PubMed] [Google Scholar]
- 12.Shirali A.C., Perazella M.A., Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 13.Tabei A., Watanabe M., Ikeuchi H. The analysis of renal infiltrating cells in acute tubulointerstitial nephritis induced by anti-PD-1 antibodies: a case report and review of the literature. Intern Med. 2018;57:3135–3139. doi: 10.2169/internalmedicine.0444-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manohar S., Kompotiatis P., Thongprayoon C. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34:108–117. doi: 10.1093/ndt/gfy105. [DOI] [PubMed] [Google Scholar]
- 15.Wanchoo R., Karam S., Uppal N.N. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45:160–169. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 16.Robert C., Schachter J., Long G.V. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 17.Topalian S.L., Sznol M., McDermott D.F. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]